- 1Henan Key Laboratory of Child Brain Injury and Henan Pediatric Clinical Research Center, Institute of Neuroscience and Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Center for Brain Repair and Rehabilitation, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 3Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden
Objective: Preterm birth is a leading contributor to childhood morbidity and mortality, and the incidence tends to increase and is higher in developing countries. The aim of this study was to analyze the potential impact of preterm birth in different etiology groups on neonatal complications and outcomes and to gain insight into preventive strategies.
Methods: We performed a retrospective cohort study of preterm infants less than 32 weeks' gestation in the Third Affiliated Hospital of Zhengzhou University from 2014 to 2019. Preterm births were categorized as spontaneous or iatrogenic, and these groups were compared for maternal and neonatal characteristics, neonatal complications, and outcomes. All infants surviving at discharge were followed up at 12 months of corrected age to compare the neurodevelopmental outcomes.
Results: A total of 1,415 mothers and 1,689 neonates were included, and the preterm population consisted of 1,038 spontaneous preterm infants and 651 iatrogenic preterm infants. There was a significant difference in the incidence of small for gestational age between the two groups. Infants born following spontaneous labor presented with a higher risk of intraventricular hemorrhage, whereas iatrogenic preterm birth was associated with higher risk of necrotizing enterocolitis and coagulopathy and higher risk of pathoglycemia. There was no difference in mortality between the two groups. Follow-up data were available for 1,114 infants, and no differences in neurologic outcomes were observed between the two preterm birth subtypes.
Conclusions: Preterm births with different etiologies were associated with some neonatal complications, but not with neurodevelopmental outcomes at 12 months of corrected age.
Introduction
Preterm birth is the leading cause of neonatal mortality and morbidity and is a major risk factor of neurodevelopmental impairment (1, 2). It is estimated that preterm birth occurs in 11% of all worldwide deliveries (3). Even though neonatal intensive care has improved in recent years, there is still a wide range of neurodevelopmental disabilities resulting from very preterm birth (2, 4). Thus, preterm infants and especially very preterm infants remain a challenging problem within the field of perinatology.
Preterm birth can result from many possible etiologies, but the two major clinical etiologies of preterm birth are iatrogenic and spontaneous preterm birth (5). Iatrogenic preterm birth, including labor induction and cesarean delivery without labor, constitutes about 30–40% of all preterm births, and pre-eclampsia/eclampsia and severe intrauterine growth restriction are the common causes (6). Spontaneous preterm birth can result from multiple causes such as infection or inflammation, cervical factors, hemorrhage, decidual senescence, stress, genetics, and sociodemographic factors (7, 8).
Gestational age (GA) at birth is the strongest predictor of neonatal complications and outcomes (9), and all preterm infants presumably experience some risk due to immaturity. In addition, some causes of preterm delivery may be dangerous in themselves beyond the effects related to immaturity. Some studies have investigated the differences between etiologies of preterm birth that might affect neonatal outcomes, for example, iatrogenic preterm birth has been shown to increase neonatal mortality and severe morbidity and to result in worse psychomotor outcomes compared with spontaneous preterm birth (10, 11).
There is a growing awareness of the role of pathologies in preterm mortality and morbidity. Given the heterogeneity within clinical etiologies of preterm birth, it is helpful to examine the etiologies separately. In addition, identifying the specific characteristics of each clinical etiology is essential for developing effective methods for preventing preterm birth (12). There are limited data, however, regarding how the indications for premature delivery affect the infant's outcomes. Thus, the aim of this study was to investigate if there is any link between etiologies of very preterm birth and neurodevelopmental outcomes.
Methods
Study Population
This was a retrospective, hospital-based cohort study carried out at the Third Affiliated Hospital of Zhengzhou University between 1 July 2014 and 30 June 2019. This study included all preterm infants born in this hospital with a GA < 32 weeks. Infants delivered at other hospitals and transferred to the NICU at the Third Affiliated Hospital of Zhengzhou University within 7 days after birth were also included if the detailed pregnancy and delivery records were available. Exclusion criteria included stillbirths, delivery room deaths, malformed fetuses, and infants for whom the pregnancy information was not fully recorded. This study was approved by the ethics committee of Third Affiliated Hospital of Zhengzhou University and was exempt from the requirement for informed consent.
Definition and Data Collection
Newborn infants at GA < 32 weeks were included. The study cohort was classified into spontaneous preterm delivery (due to preterm labor with regular uterine contractions and cervical changes or with preterm rupture of membranes) and iatrogenic preterm delivery (due to any maternal or fetal medical complication necessitating early delivery) (13). Indications for maternal admission and delivery were identified from the hospital admission and delivery records. Clinical information, demographic data, gestation and delivery characteristics, and major neonatal conditions were collected. GA was established based on first-trimester ultrasound estimations in the majority of patients and in a few patients by the last menstrual bleeding before pregnancy. The following parameters were included in the analysis: small for gestational age (SGA, defined as birth weight < 10th percentile for gestational age), asphyxia (14), bronchopulmonary dysplasia (BPD, defined simply by considering the need for oxygen at 28 days after birth) (15), pneumonia, anemia (16), patent ductus arteriosus (17), persistent pulmonary hypertension (18), coagulopathy (19), retinopathy of prematurity (20), necrotizing enterocolitis (NEC, Bell's stage ≥ II) (16), intraventricular hemorrhage (IVH, diagnosed by intracranial ultrasound and graded from 1 to 4 on the Papile scale) (21), periventricular leukomalacia (PVL, diagnosed by intracranial ultrasound or brain magnetic resonance imaging) (22), pathoglycemia (blood sugar < 2.2 mmol/L or > 7 mmol/L), and neonatal death (died before 28 days of life).
Follow-Up
All survivors were assessed at 3, 6, and 12 months of corrected age by trained specialists. Development was evaluated using the Bayley Scales of Infant and Toddler Development-Chinese version, including the psychomotor developmental index and the mental developmental index. Developmental delay was indicated by scores <70 on either scale. The criteria for the diagnosis of cerebral palsy included permanent movement and posture disorders caused by non-progressive disturbances that occurred in the developing fetal or neonatal brain (23). Adverse neurological outcomes included cerebral palsy, blindness (corrected visual acuity <0.05), deafness (hearing loss requiring amplification or worse), and development delay.
Statistical Analysis
Data were analyzed using the SPSS 26.0 software (IBM, Armonk, NY). Descriptive statistics are presented using absolute and relative frequencies for categorical variables, and continuous variables are presented as means ± standard deviations. Categorical data were compared using either Fisher's Exact Test or the chi-square test, and continuous data were analyzed with ANOVA for normally distributed variables and by Kruskal–Wallis tests for non-normally distributed variables. The multivariate analysis was adjusted for GA, birthweight, gender, and delivery model, with the reference group being spontaneous labor onset. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Neonatal Characteristics of Preterm Birth
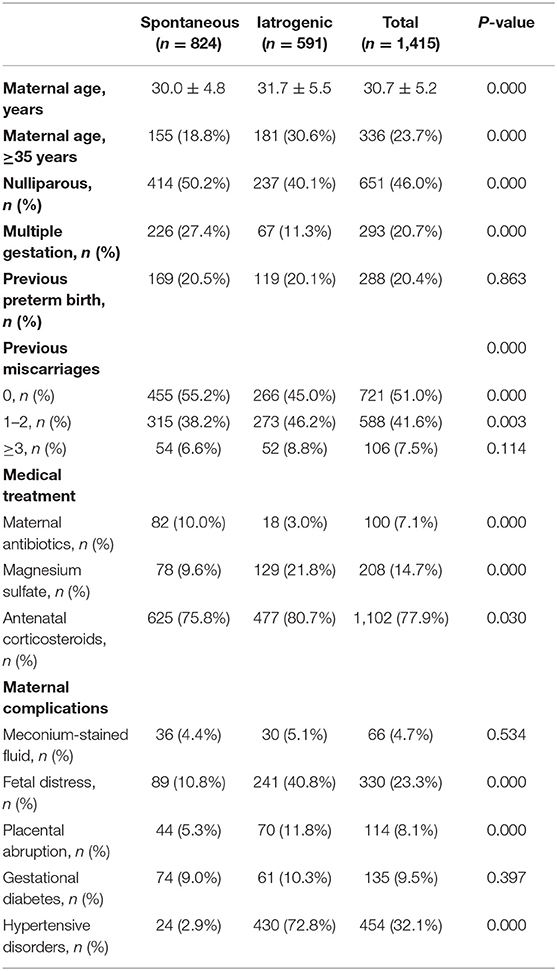
The study population consisted of 1,415 mothers and 1,689 neonates. Among the 1,689 neonates, 1,122 were singletons and 567 were twins or triplets (of which 203 were with assisted reproductive technology). The preterm infants were classified as spontaneous (n = 1,038; 61.5%) or iatrogenic (n = 651; 38.5%). The ratio of gender, incidence of asphyxia, percentage of erythropoietin treatment, and duration of mechanical ventilation showed no differences between the two groups. GA and birth weight were significantly different between the groups, with the iatrogenic group having the higher GA and lower birth weight. The overall prevalence of SGA was 10.0%, which was much higher in the iatrogenic group than that in the spontaneous group (21.7 vs. 2.7%, p < 0.001). In addition, the cesarean section rate in the iatrogenic group was more than twice that in the spontaneous group (94.8 vs. 46.3%, p < 0.001), which indicates that vaginal delivery is not an option for most iatrogenic very preterm infants (Table 1).
Maternal Characteristics of Preterm Birth
Women who underwent an iatrogenic preterm delivery tended to be older than the spontaneous group (p < 0.001). There were also relatively more multiple gestations in the spontaneous group (27.4%) compared to the iatrogenic group (11.3%). As expected, hypertensive disorder was the most common indication for early pregnancy termination in the iatrogenic group (72.8%), followed by fetal distress (40.9%) and placental abruption (11.8%). Meanwhile, women who underwent a spontaneous preterm delivery were more likely to have received antibiotics (10 vs. 3.0%) compared to the iatrogenic group for the treatment of ureaplasma urealyticum infection, chorioamnionitis, and streptococcal infection. The use of magnesium sulfate to prevent eclampsia was higher in the iatrogenic group (21.8%) than in the spontaneous group (9.6%; Table 2).
Neonatal Complications
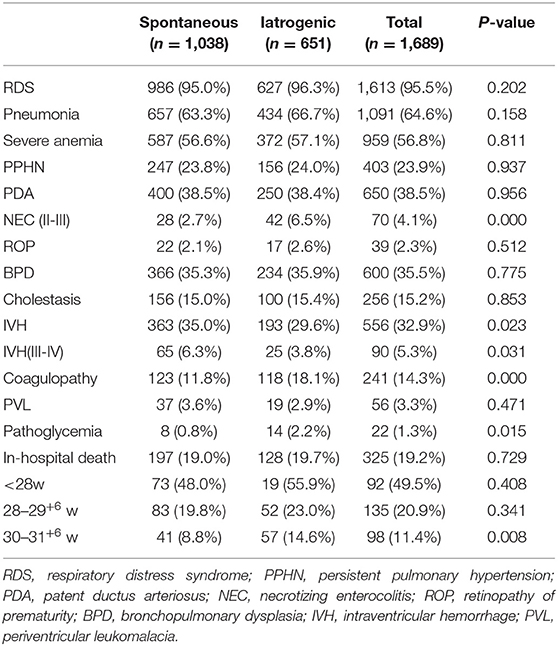
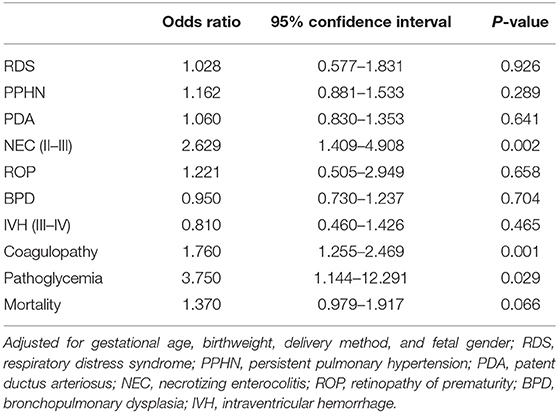
The most common complication in this study cohort was respiratory distress syndrome, but there was no significant difference between the two groups. A greater frequency of NEC (6.5 vs. 2.7%, p < 0.001) and coagulopathy (18.1 vs. 11.8%, p < 0.001) was observed in the iatrogenic group, while the spontaneous group had a greater frequency of IVH (35.0 vs. 29.6%, p = 0.023). In addition, the ratio of pathoglycemia was relatively low, but it occurred more frequently in the iatrogenic group (2.2 vs. 0.8%, p = 0.015) compared to the spontaneous group. There was no difference in neonatal mortality between the two groups for infants < 30 weeks. For infants born at 30–31+6 weeks' gestation, the risk of death was greater in the iatrogenic group than the spontaneous group (14.6 vs. 8.8%, p = 0.008; Table 3). Multivariate logistic regression analysis showed slight changes in neonatal complications (Table 4). Compared to the spontaneous group, the iatrogenic group was associated with higher risk of NEC [OR 2.629, p = 0.002], coagulopathy [OR 1.760, p = 0.001], and pathoglycemia [OR 3.750, p = 0.029].
Adverse Outcomes in Preterm Infants
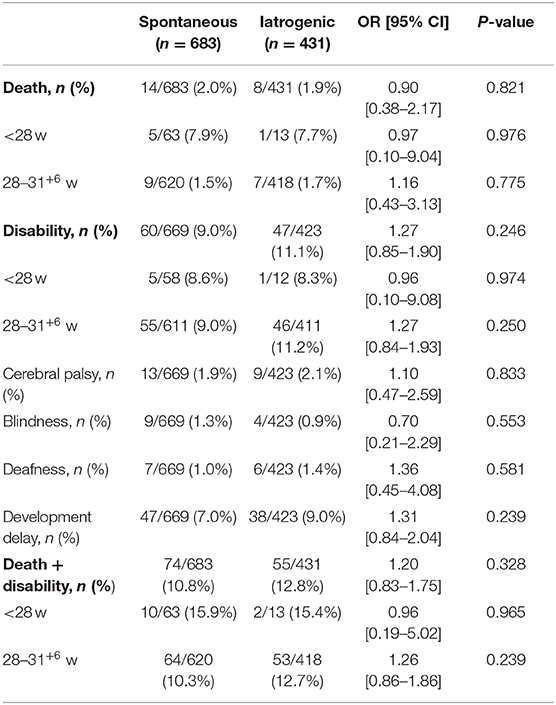
The preterm infants were followed up to 12 months of corrected age. Twenty-two infants died (spontaneous group: 14; iatrogenic group: 8) during the follow-up period, and 250 infants were completely lost to follow-up (spontaneous group: 158; iatrogenic group: 92). Follow-up information was therefore available for 1,114 infants, and no differences in poor outcomes were observed between the two preterm birth subtypes either for GA < 28 weeks or for GA 28–31+6 weeks (Table 5).
Discussion
Preterm delivery is one of the most important causes of perinatal morbidity and mortality. This retrospective study showed that iatrogenic preterm births with GA < 32 weeks were more vulnerable to some complications.
The rate of iatrogenic preterm birth in this study was slightly higher than that described previously (5), which might be due to the fact that preterm births related to medical indications are transferred to tertiary hospitals in China, and this is more common for pregnancy with GA < 32 weeks (24). Neonates in the iatrogenic group were more likely to be low-birth-weight with higher GA because the abnormal intrauterine environment was unfavorable for fetal growth, which is an indication for early pregnancy termination. Women facing increased risk of problems during pregnancy, including hypertension and placental abruption, are more likely to need cesarean section. The prevalence of SGA was higher in the iatrogenic group, which indicated that the fetus was more likely to be affected by pregnancy complications.
When comparing neonatal complications, 13 parameters were selected for descriptive analysis. Although there was no difference between the two groups regarding the incidence of pneumonia, the incidence was over 60% in both groups, which indicated that preterm neonates with GA < 32 weeks were more likely to have had pneumonia diagnosed. Lung inflammation in the neonate may be initiated by events that occur before birth or as a result of respiratory therapies used to treat lung disease. Premature infants are particularly vulnerable because they under-express protective factors like the anti-inflammatory cytokine IL-10 and Clara cell protein (25) and because they have immature immune systems with reduced innate and adaptive immunity, which can greatly affect their ability to fight infection (26). Significant differences were observed between the two groups in terms of the incidence of NEC. Some studies have demonstrated that fetal distress, preeclampsia, birthweight, and cesarean section are associated with NEC, and maternal preeclampsia in particular might be an important risk factor for the development of NEC in premature infants (27). Moreover, our results suggest that infants delivered early on the basis of medical indications might have an increased risk of coagulopathy, and preterm newborns are considered to be at risk of acquired coagulopathy (28). Thus, it appears that maternal complications might have an impact on the development of NEC and coagulopathy. IVH was a common complication among infants in the spontaneous group, which was consistent with prior reports (29, 30). There are several possible explanations for the outcomes of coagulopathy, NEC, and IVH. First, GA plays a vital role, and the GA at birth was slightly higher in the iatrogenic group compared to the spontaneous group. Second, the administration of antenatal corticosteroids (ACS) is the state-of-the-art for reducing adverse neonatal outcomes and can reduce the incidence of both PVL and severe IVH (31). Pregnant women in the iatrogenic group are often admitted to hospital early due to pregnancy symptoms. According to their condition, the doctor will use ACS to promote fetal lung maturation, and this allows them to choose the appropriate timing of pregnancy. In contrast, the spontaneous group has only a short window between admission and birth in which they can be treated with ACS. Third, hypertensive disorders were the major pregnancy complications experienced by the iatrogenic group in the present study, and this was consistent with a previous study (32). Antenatal magnesium sulfate is used in obstetrics as a first line anticonvulsant in the treatment of eclampsia and in preventing the progression of preeclampsia to eclampsia. A high frequency of the use of magnesium sulfate to prevent the occurrence of eclampsia was observed in the iatrogenic group, and some initial studies have suggested that magnesium sulfate may have a protective role against IVH in preterm neonates (33, 34). The finding in this study of an increased risk of pathoglycemia among infants born after iatrogenic preterm delivery is interesting. One study indicated that hypo- and hyperglycemia are common in preterm infants, especially SGA infants and those with low Apgar score (35). Similar to that study, we found that the prevalence of SGA was much higher in the iatrogenic group than in the spontaneous group, and this might explain the difference in the incidence of pathoglycemia we observed between the two groups. However, our study included only the preterm infants with GA < 32 weeks, which is different from other studies. The relevance of this difference appears to be questionable due to the small number of patients from a clinical point of view, and further research is needed.
Numerous studies have evaluated the relationship between preterm birth subtypes and neonatal death risk, but these have reported inconsistent results (29, 30, 36). Similar to prior studies, our study did not detect any significant differences in mortality rates. However, for infants born at 30–31+6 weeks' GA, the risk of death was greater in the iatrogenic group, which indicated that the underlying causes of iatrogenic preterm birth might be diverse at this GA. This reflects the fact that these infants were induced due to severe pregnancy complications, which might have increased their risk of death. For infants with lower GA, there were no differences between the two groups, and it is likely that the shorter GA diminished the significance of the differences in mortality between the groups. We also observed no significant difference in neurological outcome between the spontaneous group and the iatrogenic group. One explanation for this might be that only neonates still alive at follow-up were included in this study, and aggressive medical care was beneficial to the children's outcomes. Whether infants received rehabilitation therapy depended on parental attitudes and socio-economic factors. The small number of children with adverse neurological outcomes in the follow-up cohort, particularly children with cerebral palsy, might also explain this lack of difference between the two groups. In addition, the rate of infants lost to follow-up was not negligible. Therefore, conclusions should be drawn with caution because unknown confounding factors might have accounted for this observation.
Some limitations of this study should be noted. First, our results were restricted to very preterm infants (GA < 32 weeks) in a single maternity hospital, which might limit generalizability. Second, the rate of lost to follow-up was 18%, and it is possible that children with poor outcomes were not followed up, which might have biased the results. Finally, multiple factors are associated with the neurodevelopment outcomes, and not only preterm birth itself and its subtypes, but also medical care and socio-economic factors might affect the outcomes. Despite these limitations, this study has several strengths. This study chose to classify the etiologic types of preterm birth according to the reason for delivery by consulting obstetric records and collecting neonatology records, which ensured the accuracy of the data to the greatest extent possible. The assessment of both neonatal and infancy period outcomes in very preterm infants was another strength. This investigation might provide guidance for future research.
In conclusion, some neonatal complications were associated with etiologic types of very preterm birth. However, we evaluated only the neonatal and infancy period outcomes of the preterm infants in this study. Thus, further research is needed on the long term consequences of preterm birth subtype. Outcomes beyond survival are also important, especially long-term neurological, cognitive, and behavioral consequences. Finally, we strongly support the view that it is the understanding of the association between neonatal complications and preterm birth clinical subtypes that will pave the way to the prevention of adverse outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Third Affiliated Hospital of Zhengzhou University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
CZ designed the study. XC, XZ, WenhL, WendL, YW, and SZ were involved in data collection. XC and XZ analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Funding
This work was supported by National Key Research and Development Program of China (2018YFC1004604), the Department of Science and Technology (171100310200), and Department of Health (SB201901055) of Henan Province, Swedish Research council (2018-02667), ALF (ALFGBG-717791).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Shaw JC, Berry MJ, Dyson RM, Crombie GK, Hirst JJ, Palliser HK. Reduced neurosteroid exposure following preterm birth and its' contribution to neurological impairment: a novel avenue for preventative therapies. Front Physiol. (2019) 10:599. doi: 10.3389/fphys.2019.00599
2. Brumbaugh JE, Hansen NI, Bell EF, Sridhar A, Carlo WA, Hintz SR, et al. Outcomes of extremely preterm infants with birth weight less than 400 g. JAMA Pediatr. (2019) 173:434–45. doi: 10.1001/jamapediatrics.2019.0180
3. Kinney MV, Lawn JE, Howson CP, Belizan J. 15 Million preterm births annually: what has changed this year? Reprod Health. (2012) 9:28. doi: 10.1186/1742-4755-9-28
4. Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. (2018) 60:342–55. doi: 10.1111/dmcn.13675
5. Goldenberg RL, Culhane JF, Iams JD, Romero R. Preterm birth 1 - epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
6. Gyamfi-Bannerman C, Ananth CV. Trends in spontaneous and indicated preterm delivery among singleton gestations in the United States, 2005-2012. Obstet Gynecol. (2014) 124:1069–74. doi: 10.1097/AOG.0000000000000546
7. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. (2014) 345:760–5. doi: 10.1126/science.1251816
8. Cobo T, Kacerovsky M, Jacobsson B. Risk factors for spontaneous preterm delivery. Int J Gynaecol Obstet. (2020) 150:17–23. doi: 10.1002/ijgo.13184
9. Song J, Sun H, Xu F, Kang W, Gao L, Guo J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol. (2016) 80:24–34. doi: 10.1002/ana.24677
10. Richter LL, Ting J, Muraca GM, Synnes A, Lim KI, Lisonkova S. Temporal trends in neonatal mortality and morbidity following spontaneous and clinician-initiated preterm birth in Washington State, USA: a population-based study. BMJ Open. (2019) 9:e023004. doi: 10.1136/bmjopen-2018-023004
11. Nuss EE, Spiegelman J, Turitz AL, Gyamfi-Bannerman C. Childhood neurodevelopment after spontaneous versus indicated preterm birth. Am J Obstet Gynecol MFM. (2020) 2:100082. doi: 10.1016/j.ajogmf.2019.100082
12. Kamath-Rayne BD, DeFranco EA, Chung E, Chen A. Subtypes of preterm birth and the risk of postneonatal death. J Pediatr. (2013) 162:28–34.e2. doi: 10.1016/j.jpeds.2012.06.051
13. Stout MJ, Demaree D, Merfeld E, Tuuli MG, Wambach JA, Cole FS, et al. Neonatal outcomes differ after spontaneous and indicated preterm birth. Am J Perinatol. (2018) 35:494–502. doi: 10.1055/s-0037-1608804
14. Yuan X, Kang W, Song J, Guo J, Guo L, Zhang R, et al. Prognostic value of amplitude-integrated EEG in neonates with high risk of neurological sequelae. Ann Clin Transl Neurol. (2020) 7:210–8. doi: 10.1002/acn3.50989
15. Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. (2018) 16:36. doi: 10.1186/s12967-018-1417-7
16. Wang Y, Song J, Sun H, Xu F, Li K, Nie C, et al. Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial. J Transl Med. (2020) 18:308. doi: 10.1186/s12967-020-02459-w
17. McNamara PJ, Sehgal A. Towards rational management of the patent ductus arteriosus: the need for disease staging. Arch Dis Child Fetal Neonatal Ed. (2007) 92:F424–7. doi: 10.1136/adc.2007.118117
18. Kaestner M, Schranz D, Warnecke G, Apitz C, Hansmann G, Miera O. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT DGPK. Heart. (2016) 102(Suppl 2):ii57–66. doi: 10.1136/heartjnl-2015-307774
19. Saxonhouse MA, Manco-Johnson MJ. The evaluation and management of neonatal coagulation disorders. Semin Perinatol. (2009) 33:52–65. doi: 10.1053/j.semperi.2008.10.007
20. Sun H, Song J, Kang W, Wang Y, Sun X, Zhou C, et al. Effect of early prophylactic low-dose recombinant human erythropoietin on retinopathy of prematurity in very preterm infants. J Transl Med. (2020) 18:397. doi: 10.1186/s12967-020-02562-y
21. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
22. de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. (1992) 49:1–6. doi: 10.1016/S0166-4328(05)80189-5
23. MacLennan AH, Lewis S, Moreno-De-Luca A, Fahey M, Leventer RJ, McIntyre S, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. (2019) 34:472–6. doi: 10.1177/0883073819840449
24. Zou L, Wang X, Ruan Y, Li G, Chen Y, Zhang W. Preterm birth and neonatal mortality in China in 2011. Int J Gynaecol Obstet. (2014) 127:243–7. doi: 10.1016/j.ijgo.2014.06.018
25. Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. (2008) 93:F455–61. doi: 10.1136/adc.2007.121327
26. Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. (2013) 7:79. doi: 10.3389/fnins.2013.00079
27. Ahle M, Drott P, Elfvin A, Andersson RE. Maternal, fetal and perinatal factors associated with necrotizing enterocolitis in Sweden. A national case-control study. PLoS One. (2018) 13:e0194352. doi: 10.1371/journal.pone.0194352
28. Revel-Vilk S. The conundrum of neonatal coagulopathy. Hematol Am Soc Hematol Educ Program. (2012) 2012:450–4. doi: 10.1182/asheducation.V2012.1.450.3798660
29. Dehaene I, Scheire E, Steen J, De Coen K, Decruyenaere J, Smets K, et al. Obstetrical characteristics and neonatal outcome according to aetiology of preterm birth: a cohort study. Arch Gynecol Obstet. (2020) 302:861–71. doi: 10.1007/s00404-020-05673-5
30. Morken NH, Kallen K, Jacobsson B. Outcomes of preterm children according to type of delivery onset: a nationwide population-based study. Paediatr Perinat Epidemiol. (2007) 21:458–64. doi: 10.1111/j.1365-3016.2007.00823.x
31. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2017) 3:CD004454. doi: 10.1002/14651858.CD004454.pub3
32. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. (2005) 365:785–99. doi: 10.1016/S0140-6736(05)71003-5
33. Doyle LW, Crowther CA, Middleton P, Marret S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. (2007) 3:CD004661. doi: 10.1002/14651858.CD004661.pub2
34. Petrova A, Mehta R. Magnesium sulfate tocolysis and intraventricular hemorrhage in very preterm infants. Indian J Pediatr. (2012) 79:43–7. doi: 10.1007/s12098-011-0440-y
35. Yoon JY, Chung HR, Choi CW, Yang SW, Kim BI, Shin CH. Blood glucose levels within 7 days after birth in preterm infants according to gestational age. Ann Pediatr Endocrinol Metab. (2015) 20:213–9. doi: 10.6065/apem.2015.20.4.213
Keywords: preterm birth, spontaneous preterm birth, iatrogenic preterm birth, neonatal outcome, neonatal complication
Citation: Chen X, Zhang X, Li W, Li W, Wang Y, Zhang S and Zhu C (2021) Iatrogenic vs. Spontaneous Preterm Birth: A Retrospective Study of Neonatal Outcome Among Very Preterm Infants. Front. Neurol. 12:649749. doi: 10.3389/fneur.2021.649749
Received: 05 January 2021; Accepted: 01 March 2021;
Published: 23 March 2021.
Edited by:
Raffaele Falsaperla, University Hospital Polyclinic Vittorio Emanuele, ItalyReviewed by:
Thalia Harmony, National Autonomous University of Mexico, MexicoChristian Zammit, University of Malta, Malta
Copyright © 2021 Chen, Zhang, Li, Li, Wang, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changlian Zhu, Y2hhbmdsaWFuLnpodUBuZXVyby5ndS5zZQ==
 Xi Chen
Xi Chen Xiaoli Zhang
Xiaoli Zhang Wenhua Li1
Wenhua Li1 Yong Wang
Yong Wang Shan Zhang
Shan Zhang Changlian Zhu
Changlian Zhu