- 1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, China
Background: The relationship between glycosylated hemoglobin (HbA1c) and prognosis of spontaneous intracerebral hemorrhage (SICH) patients has not been fully elucidated. This study aimed to reveal the relationship between HbA1c levels and short-term mortality after patient admission with SICH.
Methods: It was a large-scale, multicenter, cross-sectional study. From August 1, 2015, to July 31, 2019, a total of 41910 SICH patients were included in the study from the Chinese Stroke Center Alliance (CSCA) program. Finally, we comprehensively analyzed the data from 21,116 patients with SICH. HbA1c was categorized into four groups by quartile. Univariate and multivariate logistic regression analyses were used to assess the association between HbA1c levels and short-term mortality in SICH patients.
Results: The average age of the 21,116 patients was 62.8 ± 13.2 years; 13,052 (61.8%) of them were male, and 507 (2.4%) of them died. Compared to the higher three quartiles of HbA1c, the lowest quartile (≤5.10%) had higher short-term mortality. In subgroup analysis with or without diabetes mellitus (DM) patients, the mortality of the Q3 group at 5.60–6.10% was significantly lower than that of the Q1 group at ≤5.10%. After adjustment for potential influencing factors, the ROC curve of HbA1c can better predict the short-term mortality of patients with SICH (AUC = 0.6286 P < 0.001).
Conclusions: Therefore, we concluded that low or extremely low HbA1c levels (≤5.10%) after stroke were associated with higher short-term mortality in SICH patients, with or without DM.
Introduction
Spontaneous intracerebral hemorrhage (SICH) accounts for 20–30% of all strokes. As a disabling type of stroke with poor prognosis, SICH contributes to an increase in the global burden of the disease (1, 2). The 30-day mortality rate of ICH was 35–52% (3). Half of the deaths occurred in the acute phase, especially in the first 2 days (4, 5).
Data from several studies suggested that hyperglycemia is associated with severe neurological impairment and poor prognosis in SICH patients (6–14). It is recommended that blood glucose levels should be measured and closely monitored to avoid hyperglycemia (15). However, in patients with SICH (16–18), subarachnoid hemorrhage (19), and ischemic stroke (20), early intensive insulin hypoglycemic therapy did not improve functional prognosis. Recent studies demonstrated that hyperglycemia is only the result of severe nervous system damage, which may be caused by a stress response, mainly adrenergic stress, and relative insulin deficiency (21, 22). Thus, it suggests that blood glucose level measured after the onset of SICH is not an ideal prognostic indicator for stroke patients. In contrast, glycosylated hemoglobin (HbA1c) is a measure of average blood glucose across 2–3 months before stroke. HbA1c possesses a high stability than random blood glucose after stroke, without the need to be measured or compared in a specific time (23, 24). Therefore, HbA1c could be considered as a potential biomarker for the prognosis of SICH. Some studies revealed that HbA1c is a better predictor of adverse outcomes in patients with SICH (25–27). However, a study showed that HbA1c is not associated with clinical outcome in patients with SICH (28). The relationship between HbA1c and the prognosis of SICH patients is not yet fully elucidated.
Therefore, our study aimed to investigate the relationship between HbA1c levels and short-term mortality in SICH patients.
Methods
Study Population
From August 1, 2015, to July 31, 2019, a total of 1,006,798 patients diagnosed with cerebral hemorrhage, subarachnoid hemorrhage, acute ischemic stroke, or transient ischemic attack were included in the Chinese Stroke Center Alliance (CSCA) program. The patients were over 18 years old within 7 days of symptom onset. In the CSCA program, there was no follow-up after discharge, and only in-hospital information was recorded. We comprehensively analyzed the data from SICH patients enrolled in the CSCA. We included all the SICH patients. Spontaneous intracerebral hemorrhage refers to intracerebral hemorrhage caused by spontaneous rupture of the cerebral artery, vein, and capillary, excluding traumatic cerebral hemorrhage. We excluded patients with (1) history of previous stroke events; (2) history of abnormal liver function and renal function; (3) bleeding history or tendency; (4) lack data of death; and (5) lack data of HbA1c.
The study was conducted in accordance with guidelines from the Helsinki Declaration. All participating hospitals of CSCA had the right to collect data without informed consent of patients or a waiver of authorization and exemption from their institutional review board.
Clinical Information
During the hospital admission of the selected patients for this study, the following information was collected: demographic information, medical history [atrial fibrillation, coronary heart disease, hypertension, diabetes mellitus, dyslipidemia, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), drinking, smoking, body mass index (BMI), medication history (hypotension, hypoglycemia, anticoagulation, antiplatelet agent), systolic blood pressure (SBP), and diastolic blood pressure (DBP)]. A history of diabetes is defined as a patient who was definitely diagnosed with diabetes before admission.
Baseline Neurological Assessment
The Glasgow Coma Scale (GCS) and National Institutes of Health Stroke Scale (NIHSS) were used to assess neurological deficit at admission. The GCS score was divided into mild coma (13–15) or moderate to severe coma (≤12). The NIHSS score was divided into mild to moderate disability (<16) or severe disability (≥16).
Laboratory Examinations
Venous blood was collected in a vacuum EDTA collection tube, and plasma was separated. HbA1c was assessed within 7 days after admission. Patients with SICH were divided into four groups according to the quartile of HbA1c: Q1, HbA1c ≤5.10%; Q2, HbA1c 5.10–5.60%; Q3, HbA1c 5.60–6.10%; and Q4, HbA1c ≥6.10%. The quartile was based on the data available in the study. There were significant differences in HbA1c levels among different quartile groups. Compared with the previously published data, the quartile level of HbA1c determined in this study is generally low.
Other laboratory tests, including FBG, bun, creatinine, low-density lipoprotein cholesterol (LDL-C), serum homocysteine (HCY), and international normalized ratio (INR) were also collected.
Clinical Outcomes
We analyzed the relationship between HbA1c levels and adverse outcome in patients with SICH. Adverse outcomes were defined as death during hospitalization.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation; categorical variables were presented as count (percentage). The median and quartile range expressed ordinal variables. Group differences were analyzed by the independent sample t-test or the Mann–Whitney U-test for continuous variables and by the chi-squared test for categorical variables. Logistic regression was used to analyze the relationship between different glycosylated hemoglobin levels and short-term mortality. The receiver operating characteristic (ROC) curve was used to evaluate the prognostic value of HbA1c. Sensitivity analysis was used to estimate the effects of potential unmeasured or uncontrolled confounding variables. First, we coded patients who lacked the NIHSS score or GCS score. Then, we analyzed patients with SICH who had NIHSS and GCS scores to determine if the results were similar. Odds ratios (ORs) and 95% confidence interval (95% CI) were expressed for the results and probability values. A two-sided value of P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
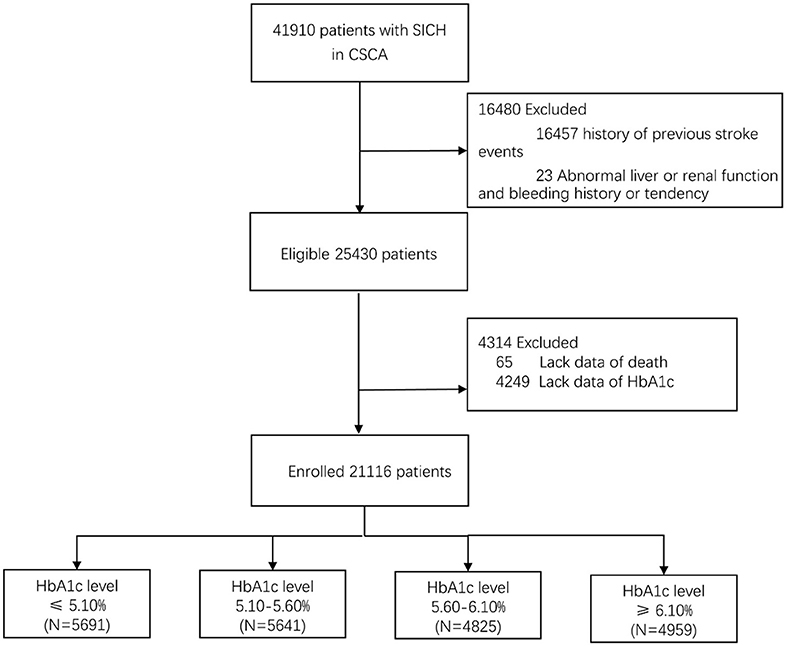
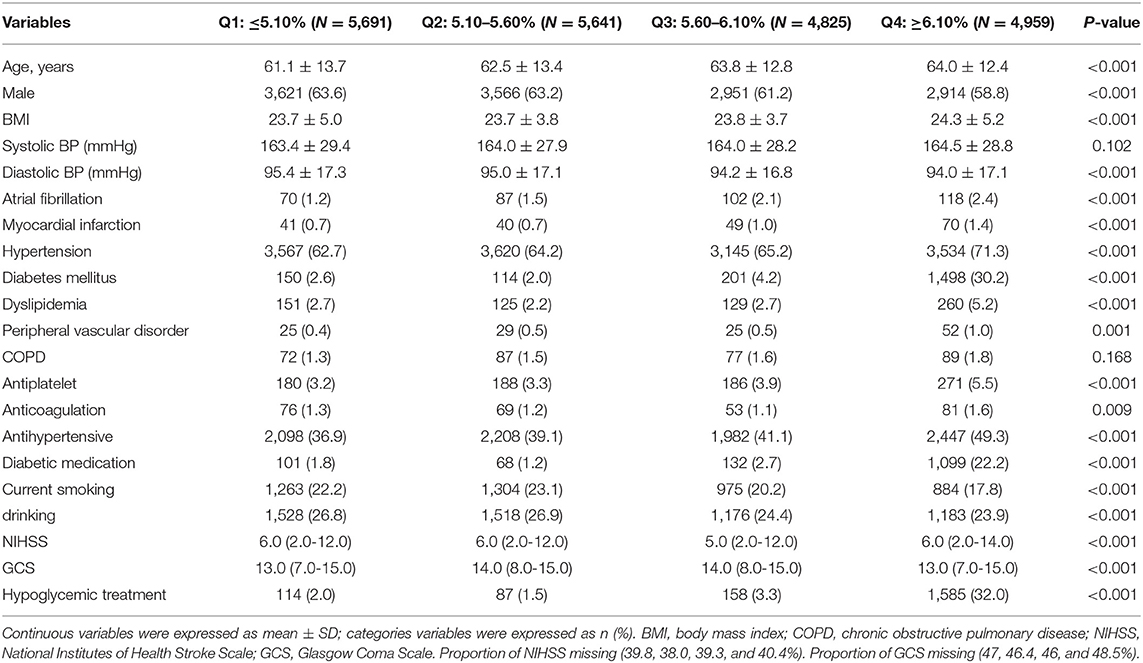
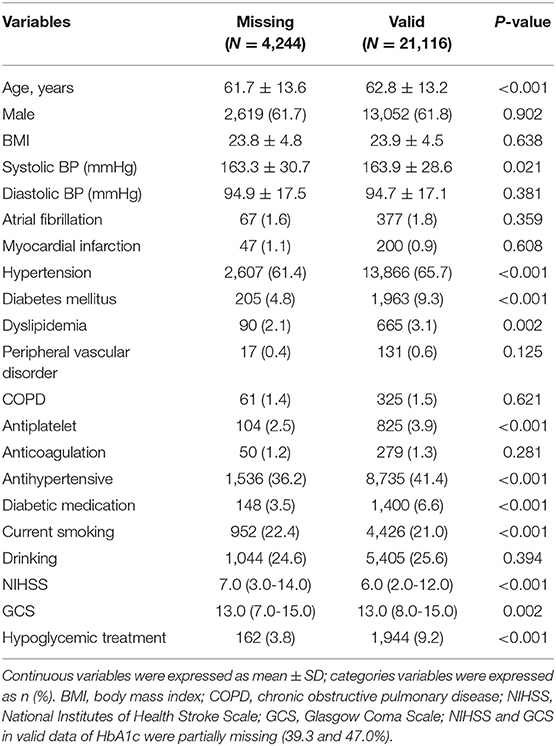
From August 1, 2015, to July 31, 2019, a total of 41,910 SICH patients were included in the study from the CSCA program. We excluded patients with history of previous stroke events, abnormal liver function, renal function, and bleeding history or tendency. A total of 25,430 patients with SICH were enrolled, and 21,116 (83.0%) patients of them had valid data of HbA1c and death while the data for others were missing (Figure 1). The average age of the patients having valid data was 62.8 ± 13.2 years; 13,087 (61.8%) of them were male, and 507 (2.4%) of them died. Age, atrial fibrillation, myocardial infarction, hypertension, diabetes, and hyperlipidemia in patients with HbA1c stratification of ≥6.10% were larger than in patients with HbA1c stratification of ≤5.10%. With regard to sex, the highest quartile group presented relatively few males. Patients with higher HbA1c levels were less likely to drink or smoke and more likely to be obese. Table 1 shows the baseline characteristics of 21,116 patients with different HbA1c levels. Due to the missing HbA1c in more than 15%, a comparison of baseline characteristics between valid and missing HbA1cs was conducted. This comparison revealed significant differences in GCS and NIHSS scores between valid and missing HbA1c. We observed that GCS was relatively low and NIHSS was relatively high in the group lacking HbA1c data (Table 2).
Logistic Regression Analysis
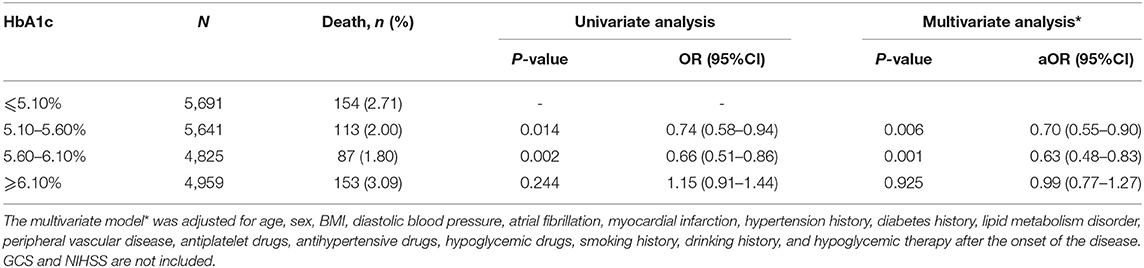
Among the patients with different HbA1c levels, the univariate logistic regression analysis showed that in the HbA1c stratification of 5.10–5.60% and 5.60–6.10% of patients, the short-term mortality rate after admission was drastically lower than that of patients in the ≤5.10% group (OR respectively 0.74 95% CI P = 0.014 and 0.66 95% CI P = 0.002). However, no significant association was found in the ≥6.10% group compared with the ≤5.10% group (OR: 1.15 95% CI, P = 0.244). The results were consistent after the imbalance factors were adjusted (Table 3).
Subgroup Analysis
A stratified analysis of the association between HbA1c levels and short-term mortality after admission in patients of SICH with or without DM was performed. In univariate analysis of the patients with DM, a significant difference of the short-term mortality was found in the 5.60–6.10% group compared with the ≤5.10% group (P > 0.05). However, there was no significant correlation between HbA1c 5.10–5.60% and ≥6.10% groups compared with the ≤5.10% (P > 0.05) group. In the patients without DM, there was a significant correlation between the HbA1c 5.10–5.60% and 5.60–6.10% groups compared with ≤5.10% (P < 0.05). However, compared with the ≤5.10% group, no significant association was found in the ≥6.10% group (P > 0.05). The conclusions were consistent after adjusting the imbalance factors (Table 4).
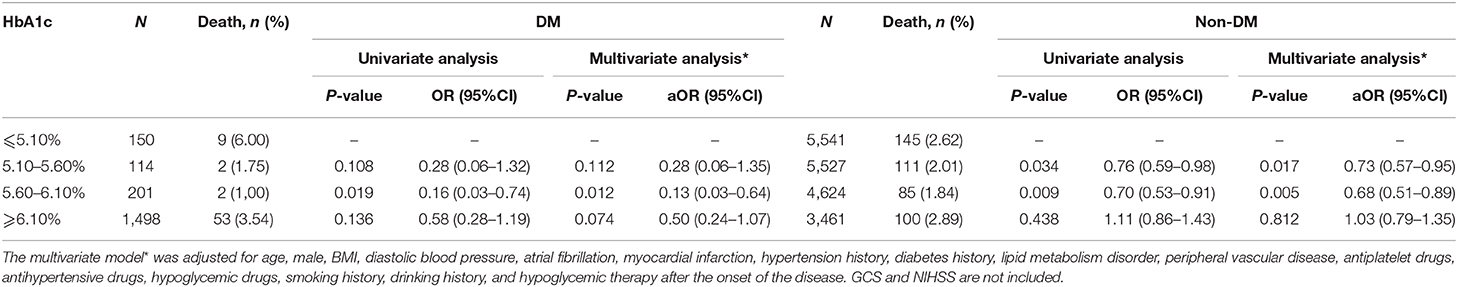
Table 4. Stratified analysis of association between HbA1c levels and short-term mortality after admission in patients of SICH with or without DM.
The ROC Curve
The AUC was 0.5596 (P < 0.001). In addition, the ROC curve of HbA1c combined with age, male, BMI, diastolic blood pressure, atrial fibrillation, myocardial infarction, hypertension history, diabetes history, lipid metabolism disorder, peripheral vascular disease, antiplatelet drugs, antihypertensive drugs, hypoglycemic drugs, smoking history, drinking history, NIHSS score, GCS, and hypoglycemic therapy after the onset of the disease can better predict the short-term mortality of patients with SICH (AUC = 0.6286 P < 0.001).
Discussion
In this large, multicenter, cross-sectional study, we concluded that low or extremely low HbA1c after the stroke is associated with higher short-term mortality after SICH, regardless of whether patients have DM or not.
In accordance with the present results, previous studies have shown that very low HbA1c (<5.0 or <4.0%) is an independent risk factor for all-cause mortality (29, 30). The mechanism between low or very low HbA1c and higher short-term mortality after SICH has not been fully elucidated, which will be speculated as follows. Accounting for 20% of the total body energy consumption, brain energy consumption is mainly through sugar to provide energy. In light of the limited energy storage material, it is easy to be affected by the decrease of substrate supply (31). The increase of blood glucose is regulated by glucagon, adrenaline, and other hormones, of which glucagon and epinephrine mainly act on the liver, promoting the decomposition of liver glycogen into the blood and increasing the gluconeogenesis. Low HbA1c represents that the blood glucose level before stroke is at a low level for a long time, which transfers the blood glucose threshold of adrenaline to a low plasma glucose level, reducing the response level of elevated blood glucose and slowing the response of glucagon (32). It may lead to “brain energy crisis” (18, 33–35) when SICH occurs in this group of people by virtue of the low blood glucose threshold of adrenaline and the slow response of glucagon, hereby resulting in the increase of brain anaerobic metabolism and increasing the short-term mortality. It is found that the metabolic demand is the highest on the third day of intracranial injury (36, 37). If the patient has liver fibrosis or cirrhosis at the same time, the ability of the liver to regulate glucose will be affected. In this case, intrahepatic gluconeogenesis and glycogen decomposition will be further reduced, which will aggravate intracranial glucose deficiency. At the same time, the levels of procoagulant factor and anticoagulant factor were decreased and vitamin K was deficient in patients with abnormal liver function, which may generate larger hematoma volume and higher risk of rebleeding for patients with SICH.
Low or extremely low HbA1c after stroke may be related to the following aspects. To begin with, low or extremely low HbA1c may be associated with potential liver disease (38). Although we excluded patients with a history of liver dysfunction that had been diagnosed before admission, potential liver dysfunction or liver vulnerability may exist in the included population. Additionally, a very low HbA1c level may indicate the long-term weakness and malnutrition before the onset of SICH. Finally, long-term intensive glycemic control may be about low or extremely low HbA1c after SICH. Regular health monitoring should be carried out for people with low or very low HbA1c levels, and the causes of abnormal HbA1c levels should be identified. HbA1c detection at least twice a year is recommended for patients with high risk of ICH, which may benefit them with early intervention. Early intervention refers to the following: (1) It is needed to improve the liver function examination early, being alert for potential liver dysfunction. On the basis of other studies, it has been shown that there is a lag in the increase of liver enzymes after liver function injury. Albumin dysfunction can be adopted as a new potential indicator of liver function injury (39). (2) We should look for malabsorption problems such as eating disorders or celiac disease to improve the nutritional status. (3) Being alert for severe hypoglycemia, we attempt to use appropriate antidiabetic drugs for individualized treatment. Previous randomized controlled trials revealed that intensive glycemic control does not reduce the cardiovascular risk in patients with diabetes (40–42). Meanwhile, strict glycemic control increased the risk of severe hypoglycemia, and the incidence of severe hypoglycemia events related to intensive treatment was two to three times higher than that of the non-intensive treatment group (43–45). From this point of view, long-term blood glucose should not be overcontrolled in diabetic patients with a high risk of SICH (46). Likewise, routine monitoring and routine HbA1c detection at admission are of great significance for patients with SICH to possibly prevent severe damage. At 3 months, the blood glucose threshold of symptoms and neuroendocrine response returned to normal with the recovery of glucagon response (47). For SICH patients with a low HbA1c level, it may be necessary to ensure enough brain energy and actively give non-surgical interventions including hypertonic drugs, antihypertensive drugs, and surgical interventions such as craniotomy or minimally invasive surgery (MIS).
HbA1c is a biomarker used to monitor blood glucose, which can evaluate the average blood glucose status within 2 to 3 months. Compared with other diabetes detection (such as fasting blood glucose or glucose tolerance test), HbA1c measurement does not need fasting, has high stability, is less affected by acute physiological disorders, and is more convenient for clinical application (23, 24). Some studies have shown that HbA1c is related to poor prognosis in patients with SICH (48, 49). These results are consistent with earlier studies indicating that patients with newly diagnosed diabetes based on the HbA1c criteria have a poor long-term prognosis after acute cerebral hemorrhage (50). Small cohort studies suggested that HbA1c is a better predictor of adverse outcomes in patients with SICH (25–27). Besides, HbA1c can better predict the symptomatic hemorrhagic transformation of acute ischemic stroke after thrombolysis (26, 51). These findings support the results of a large cohort study showing a J-type relationship between HbA1c and the risk of SICH. The lowest risk observed in HbA1c was 6.5% (52). Taken together, HbA1c levels might affect the incidence and prognosis of an acute cerebral hemorrhage, which is consistent with our findings. However, our outcome is contrary to a previous study showing that HbA1c was not associated with clinical outcome in patients with SICH (28).
At the same time, our study showed that when HbA1c was 5.60–6.10%, compared with patients without DM, DM patients with reasonable pre-stroke glycemic control had a lower mortality rate after SICH, but there was no statistical difference (1.00 vs. 1.84%, p = 0.543). This positive relationship could be linked to speculations that basement membrane thickening and endothelial cell proliferation caused by long-term diabetes before stroke reduce the risk of rebleeding after cerebral vascular rupture. In diabetic patients, increased levels of coagulation and plasminogen activator inhibitors are associated with the decreased fibrinolytic activity, as well as reduced bleeding volume and risk of rebleeding (53). However, this evidence contradicts previous studies that diabetes is a predictor of poor outcome after SICH (7, 54, 55).
There are several limitations in our current research. First of all, there is no follow-up data in this study, so long-term prognosis data are not available. At the same time, there were no imaging data and blood samples. The volume and location of the hematoma were not recorded. Secondly, in this study, patients without or with HbA1c measurement presented major baseline differences (Table 1). At the same time, the correlation between HbA1c and mortality was not statistically significant in the sensitivity analysis among patients with GCS and NIHSS scores (full details are given in Appendices 1, 2). The reason may be that patients with missing values have more severe neurological deficits and higher mortality, leading to possible selection bias.
Conclusion
In summary, this study mainly discusses the effect of low HbA1c on the prognosis of patients with SICH and expounds the possible mechanism. This study demonstrates that low or extremely low HbA1c is proportionally related to higher short-term mortality after SICH, regardless of whether patients have DM or not. Low or extremely low HbA1c may be associated with liver disease, long-term malnutrition, and excessive glycemic control in diabetes. It is very important for clinical decision-making to find the cause of low or extremely low HbA1c. These findings and their clinical significance need to be evaluated by future randomized clinical trials.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PL and LC: study design, analysis and interpretation, and primary responsibility for writing the manuscript. YW, KK, HG, ZL, LL, YW, and XZ: study design, data interpretation, critical revision of the manuscript for important intellectual content, and supervision of the study. HG: data statistics. All authors: contributed to the article and approved the submitted version.
Funding
This study was supported by the (1) Ministry of Science and Technology of the People's Republic of China (National Key R&D Programme of China, 2017YFC1310901, 2018YFC1312903), (2) Beijing Municipal Science and Technology Commission (D171100003017002, Z181100001818001), and (3) Beijing Municipal Committee of Science and Technology (Z201100005620010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the staff and participants of the CSCA (Chinese Stroke Center Alliance) study for their contribution.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.648907/full#supplementary-material
References
1. Nordic Burden of Disease Collaborators. Life expectancy and disease burden in the Nordic countries: results from the global burden of diseases, injuries, and risk factors study 2017. Lancet Public Health. (2019) 4:e658–9. doi: 10.1016/S2468-2667(19)30224-5
2. Wang YJ, Li ZX, Gu HQ, Zhai Y, Jiang Y, Zhao XQ, et al. China stroke statistics 2019: a report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. (2020) 5:211–39. doi: 10.1136/svn-2020-000457
3. Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. (2009) 40:394–9. doi: 10.1161/STROKEAHA.108.523209
4. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. (2007) 38:2001–23. doi: 10.1161/STROKEAHA.107.183689
5. Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. (2009) 40:3567–73. doi: 10.1161/STROKEAHA.109.556324
6. Zheng J, Yu Z, Ma L, Guo R, Lin S, You C, et al. Association between blood glucose and functional outcome in intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. (2018) 114:e756–65. doi: 10.1016/j.wneu.2018.03.077
7. Saxena A, Anderson CS, Wang X, Sato S, Arima H, Chan E, et al. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage: the INTERACT2 study. Stroke. (2016) 47:682–8. doi: 10.1161/STROKEAHA.115.011627
8. Bejot Y, Aboa-Eboule C, Hervieu M, Jacquin A, Osseby GV, Rouaud O, et al. The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke. (2012) 43:243. doi: 10.1161/STROKEAHA.111.632950
9. Wu TY, Putaala J, Sharma G, Strbian D, Tatlisumak T, Davis SM, et al. Persistent hyperglycemia is associated with increased mortality after intracerebral hemorrhage. J Am Heart Assoc. (2017) 6:e005760. doi: 10.1161/JAHA.117.005760
10. Tapia-Perez JH, Gehring S, Zilke R, Schneider T. Effect of increased glucose levels on short-term outcome in hypertensive spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. (2014) 118:37–43. doi: 10.1016/j.clineuro.2013.12.018
11. Tan X, He J, Li L, Yang G, Liu H, Tang S, et al. Early hyperglycaemia and the early-term death in patients with spontaneous intracerebral haemorrhage: a meta-analysis. Intern Med J. (2014) 44:254–60. doi: 10.1111/imj.12352
12. Appelboom G, Piazza MA, Hwang BY, Carpenter A, Bruce SS, Mayer S, et al. Severity of intraventricular extension correlates with level of admission glucose after intracerebral hemorrhage. Stroke. (2011) 42:1883–8. doi: 10.1161/STROKEAHA.110.608166
13. Stead LG, Jain A, Bellolio MF, Odufuye A, Gilmore RM, Rabinstein A, et al. Emergency department hyperglycemia as a predictor of early mortality and worse functional outcome after intracerebral hemorrhage. Neurocrit Care. (2010) 13:67–74. doi: 10.1007/s12028-010-9355-0
14. Wang G, Zhang J. Hematoma expansion: clinical and molecular Predictors and corresponding pharmacological treatment. Curr Drug Targets. (2017) 18:1367–76.
15. Cao Y, Yu S, Zhang Q, Yu T, Liu Y, Sun Z, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of intracerebral haemorrhage. Stroke Vasc Neurol. (2020) 5:396–402. doi: 10.1136/svn-2020-000433
16. Bellolio MF, Gilmore RM, Ganti L. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev. (2014) 1:CD005346. doi: 10.1002/14651858.CD005346.pub4
17. Godoy DA, Pinero GR, Svampa S, Papa F, Di Napoli M. Hyperglycemia and short-term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. (2008) 9:217–29. doi: 10.1007/s12028-008-9063-1
18. Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. (2006) 34:850–6. doi: 10.1097/01.CCM.0000201875.12245.6F
19. Bilotta F, Spinelli A, Giovannini F, Doronzio A, Delfini R, Rosa G. The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol. (2007) 19:156–60. doi: 10.1097/ANA.0b013e3180338e69
20. Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. (2007) 6:397–406. doi: 10.1016/S1474-4422(07)70080-7
21. Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. (2006) 26:230–52. doi: 10.1038/sj.jcbfm.9600183
22. Mechanick JI. Metabolic mechanisms of stress hyperglycemia. J Parenter Enteral Nutr. (2006) 30:157–63. doi: 10.1177/0148607106030002157
23. Kernan WN. Screening for diabetes after stroke and transient ischemic attack. Cerebrovasc Dis. (2013) 36:290–1. doi: 10.1159/000355142
24. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl. 1):S13–27. doi: 10.2337/dc18-S002
25. Zhang G, Wu F, Xu Y, Feng J, Cai Z, Xu B, et al. Prestroke glycemic status is associated with the functional outcome in spontaneous intracerebral hemorrhage. Neurol Sci. (2015) 36:927–34. doi: 10.1007/s10072-014-2057-1
26. Rocco A, Heuschmann PU, Schellinger PD, Kohrmann M, Diedler J, Sykora M, et al. Glycosylated hemoglobin A1 predicts risk for symptomatic hemorrhage after thrombolysis for acute stroke. Stroke. (2013) 44:2134–8. doi: 10.1161/STROKEAHA.111.675918
27. Wang G, Zhang J. Hematoma expansion: clinical and molecular predictors and corresponding pharmacological treatment. Curr Drug Targets. (2017) 18:1367–76. doi: 10.2174/1389450117666160712092224
28. Kang K, Lu J, Ju Y, Wang W, Shen Y, Wang A, et al. Association of pre- and post-stroke glycemic status with clinical outcome in spontaneous intracerebral hemorrhage. Sci Rep. (2019) 9:19054. doi: 10.1038/s41598-019-55610-z
29. Wang P. What clinical laboratorians should do in response to extremely low hemoglobin A1c results. Lab Med. (2017) 48:89–92. doi: 10.1093/labmed/lmw050
30. Aggarwal V, Schneider AL, Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. (2012) 35:2055–60. doi: 10.2337/dc11-2531
31. Jalloh I, Carpenter KL, Helmy A, Carpenter TA, Menon DK, Hutchinson PJ. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab Brain Dis. (2015) 30:615–32. doi: 10.1007/s11011-014-9628-y
33. Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. (2008) 36:3233–8. doi: 10.1097/CCM.0b013e31818f4026
34. Meierhans R, Bechir M, Ludwig S, Sommerfeld J, Brandi G, Haberthur C, et al. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care. (2010) 14:R13. doi: 10.1186/cc8869
35. Vespa P, McArthur DL, Stein N, Huang SC, Shao W, Filippou M, et al. Tight glycemic control increases metabolic distress in traumatic brain injury: a randomized controlled within-subjects trial. Crit Care Med. (2012) 40:1923–9. doi: 10.1097/CCM.0b013e31824e0fcc
36. Jalloh I, Helmy A, Shannon RJ, Gallagher CN, Menon DK, Carpenter KL, et al. Lactate uptake by the injured human brain: evidence from an arteriovenous gradient and cerebral microdialysis study. J Neurotrauma. (2013) 30:2031–7. doi: 10.1089/neu.2013.2947
37. Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. (2011) 134:484–94. doi: 10.1093/brain/awq353
38. Lahousen T, Hegenbarth K, Ille R, Lipp RW, Krause R, Little RR, et al. Determination of glycated hemoglobin in patients with advanced liver disease. World J Gastroenterol. (2004) 10:2284–6. doi: 10.3748/wjg.v10.i15.2284
39. Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med. (2019) 51:333–44. doi: 10.1080/07853890.2019.1693056
40. Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. (2008) 358:2545–59. doi: 10.1056/NEJMoa0802743
41. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. (2009) 360:129–39. doi: 10.1056/NEJMoa0808431
42. Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. doi: 10.1056/NEJMoa0802987
43. Saremi A, Bahn GD, Reaven PD, Veterans Affairs Diabetes T. A link between hypoglycemia and progression of atherosclerosis in the veterans affairs diabetes trial (VADT). Diabetes Care. (2016) 39:448–54. doi: 10.2337/dc15-2107
44. Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC, Jr., Peterson K, et al. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. (2012) 35:409–14. doi: 10.2337/dc11-0996
45. Diabetes C, Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. (1993) 329:977–86. doi: 10.1056/NEJM199309303291401
46. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. (2009) 52:17–30. doi: 10.1007/s00125-008-1157-y
47. Fritsche A, Stefan N, Haring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med. (2001) 134:729–36. doi: 10.7326/0003-4819-134-9_Part_1-200105010-00009
48. Forti P, Maioli F, Nativio V, Maestri L, Coveri M, Zoli M. Association of prestroke glycemic status with stroke mortality. BMJ Open Diabetes Res Care. (2020) 8:e000957. doi: 10.1136/bmjdrc-2019-000957
49. Kamouchi M, Matsuki T, Hata J, Kuwashiro T, Ago T, Sambongi Y, et al. Prestroke glycemic control is associated with the functional outcome in acute ischemic stroke the fukuoka stroke registry. Stroke. (2011) 42:2788–94. doi: 10.1161/STROKEAHA.111.617415
50. Zhang X, Jing J, Zheng H, Jia Q, Zhao X, Liu L, et al. Prognosis of intracerebral hemorrhage with newly diagnosed diabetes mellitus according to hemoglobin A1c criteria. J Stroke Cerebrovasc Dis. (2018) 27:1127–33. doi: 10.1016/j.jstrokecerebrovasdis.2017.11.019
51. Liu SY, Cao WF, Wu LF, Xiang ZB, Liu SM, Liu HY, et al. Effect of glycated hemoglobin index and mean arterial pressure on acute ischemic stroke prognosis after intravenous thrombolysis with recombinant tissue plasminogen activator. Medicine (Baltimore). (2018) 97:e13216. doi: 10.1097/MD.0000000000013216
52. Saliba W, Barnett-Griness O, Gronich N, Molad J, Naftali J, Rennert G, et al. Association of diabetes and glycated hemoglobin with the risk of intracerebral hemorrhage: a population-based cohort study. Diabetes Care. (2019) 42:682–8. doi: 10.2337/dc18-2472
53. Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, Bogousslavsky J, Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology. (2004) 62:1558–62. doi: 10.1212/01.WNL.0000123252.55688.05
54. Liebkind R, Gordin D, Strbian D, Meretoja A, Thorn LM, Hagg-Holmberg S, et al. Diabetes and intracerebral hemorrhage: baseline characteristics and mortality. Eur J Neurol. (2018) 25:825–32. doi: 10.1111/ene.13603
Keywords: HbA1c, glucose, diabetes, intracerebral hemorrhage, mortality
Citation: Lu P, Cui L, Wang Y, Kang K, Gu H, Li Z, Liu L, Wang Y and Zhao X (2021) Relationship Between Glycosylated Hemoglobin and Short-Term Mortality of Spontaneous Intracerebral Hemorrhage. Front. Neurol. 12:648907. doi: 10.3389/fneur.2021.648907
Received: 02 January 2021; Accepted: 15 March 2021;
Published: 16 April 2021.
Edited by:
Robert G. Kowalski, University of Colorado, United StatesReviewed by:
Anna Helene Balabanski, The Alfred Hospital, AustraliaOzge Altintas Kadirhan, Kirklareli University, Turkey
Copyright © 2021 Lu, Cui, Wang, Kang, Gu, Li, Liu, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingquan Zhao, enhxQHZpcC4xNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ping Lu
Ping Lu Lingyun Cui
Lingyun Cui Yu Wang
Yu Wang Kaijiang Kang
Kaijiang Kang Hongqiu Gu
Hongqiu Gu Zixiao Li
Zixiao Li Liping Liu
Liping Liu Yilong Wang
Yilong Wang Xingquan Zhao
Xingquan Zhao