
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 15 February 2021
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.648432
This article is part of the Research Topic nTMS, Connectivity and Neuromodulation in Brain Tumor Patients View all 25 articles
 Tamara Ius1*
Tamara Ius1* Teresa Somma2
Teresa Somma2 Cinzia Baiano2
Cinzia Baiano2 Ilaria Guarracino3
Ilaria Guarracino3 Giada Pauletto4
Giada Pauletto4 Annacarmen Nilo5
Annacarmen Nilo5 Marta Maieron6
Marta Maieron6 Francesca Palese7
Francesca Palese7 Miran Skrap1
Miran Skrap1 Barbara Tomasino3
Barbara Tomasino3Background: Tracking the white matter principal tracts is routinely typically included during the pre-surgery planning examinations and has revealed to limit functional resection of low-grade gliomas (LGGs) in eloquent areas.
Objective: We examined the integrity of the Superior Longitudinal Fasciculus (SLF) and Inferior Fronto-Occipital Fasciculus (IFOF), both known to be part of the language-related network in patients with LGGs involving the temporo-insular cortex. In a comparative approach, we contrasted the main quantitative fiber tracking values in the tumoral (T) and healthy (H) hemispheres to test whether or not this ratio could discriminate amongst patients with different post-operative outcomes.
Methods: Twenty-six patients with LGGs were included. We obtained quantitative fiber tracking values in the tumoral and healthy hemispheres and calculated the ratio (HIFOF–TIFOF)/HIFOF and the ratio (HSLF–TSLF)/HSLF on the number of streamlines. We analyzed how these values varied between patients with and without post-operative neurological outcomes and between patients with different post-operative Engel classes.
Results: The ratio for both IFOF and SLF significantly differed between patient with and without post-operative neurological language deficits. No associations were found between white matter structural changes and post-operative seizure outcomes.
Conclusions: Calculating the ratio on the number of streamlines and fractional anisotropy between the tumoral and the healthy hemispheres resulted to be a useful approach, which can prove to be useful during the pre-operative planning examination, as it gives a glimpse on the potential clinical outcomes in patients with LGGs involving the left temporo-insular cortex.
Low-grade gliomas (LGGs) present a surgical challenge because of the poorly defined tumoral borders and the infiltration of white matter tracts (1, 2). Maximal safe resection is the cornerstone of LGG management, in order to provide an optimal survival benefit and preserving the quality of life (3, 4). Pre-operative risk predictions based on anatomical data are insufficient in patients with language area-related LGG, mainly considering the higher inter-individual variance of functional language anatomy (2, 5). It is thus of utmost importance to develop a reliable non-invasive pre-operative method to estimate the risk of post-operative deficits in the clinical management of these patients.
Despite the inherent limitations associated with imaging reconstruction algorithms, DTI (Diffusion Tensor Imaging) has recently been included in the presurgical glioma workup to aid in the mapping of functional pathways and to prevent extensive damage associated with radical resection (3, 6). Although DTI currently represents the only way to investigate white matter in humans in vivo (7), providing a non-invasive and feasible method for evaluating changes in the main language pathways, its pre-operative predictive role to estimate the risk of post-operative deficits is poorly investigated.
The aim of our study is to use pre-operative DTI data to examine changes in the main pathways of the left temporal lobe, in patients with LGG and investigating relative differences within the Superior Longitudinal Fasciculus (SLF) and Inferior Fronto-Occipital Fasciculus (IFOF).
In a previous study (8), we performed such comparison in a group of thirty-seven patients with LGGs involving the corticospinal tract. In particular, a biomarker derived from DTI differences between healthy and tumoral hemisphere was explored. The study showed that patients who had a certain pre-operative index (<0.22), calculated by comparing the healthy and the impaired hemisphere, had a significantly lower risk of developing transient post-operative deficits (8).
In this current investigation, we applied the same analytic process on the SLF and IFOF in a consecutive series of patients with LGG in the temporo-insular cortex. In a comparative approach, we contrasted the main quantitative fiber tracking values in the tumoral (T) and healthy (H) hemispheres to test whether or not this ratio could discriminate among patients with different post-operative outcomes. In addition, as secondary endpoint, we also analyzed the potential relationships between the structural white matter (WM) changes induced by LGG infiltrative growing and post-operative seizure outcomes.
We retrospectively reviewed a consecutive series of patients operated for LGG in the left hemisphere between 2012 and 2018.
Twenty-six patients met the strict following inclusion criteria:
• Involvement of the temporo-insular cortex.
• Age ≥ 18 years.
• No previous surgery.
• No pre-operative chemo- or radiotherapy.
• At least 18 months of follow-up.
• Availability of pre-operative 3-Tesla MRI including DTI.
• Objective evaluation of pre-operative tumor volume and extent of resection (EOR) on MRI images in DICOM format based on T2-weighted MRI sequences.
• Revision of histopathological specimens by using the 2016 WHO Classification of Tumors of the Central Nervous System (9).
• All patients underwent awake surgery, brain mapping, neurophysiological monitoring and intraoperative real-time neuropsychological testing (RTNT).
Needle biopsies were excluded from the study.
Medical diaries were reviewed for history of tumor- related epilepsy, seizure frequency and ictal semiology, number and type of anti-seizure medications (ASMs).
The 2017 ILAE classification was applied to classify seizures (10). For statistical analysis, seizures were dichotomized, according to ictal semeiology, in motor (tonic, atonic, clonic, myoclonic, and hypermotor) and non-motor (sensory, autonomic, emotional, and cognitive) seizures. After surgery, seizures outcome was defined following the Engel Classification of Seizures and dichotomized as Engel Class Ia (completely seizure free) vs. all the others (11). Engel Class at 1-year follow-up was used for the analysis.
The local Ethics Committee, Comitato Etico Unico Regionale del Friuli Venezia Giulia, approved this investigation (protocol N.0036567/P/GEN/EGAS, ID study 2540). Considering the retrospective nature of the study, written consent to participate in the study was not applicable. Written informed consent was obtained for surgery.
The surgical procedures were conducted under cortical and subcortical white matter brain mapping, according to the intraoperative technique previously described. In addition to Direct Electrical Stimulation (DES), real-time neuropsychological testing (RTNT) was applied during Awake Surgery (AS) (1, 12).
All histological samples were reviewed according to the 2016 World Health Organization (WHO) classification (9). Molecular markers were evaluated as previously described (13).
MRI examination, anatomical and DTI images were performed at a 3T MR system (Achieva, Philips medical system) using an SENSE eight-element phased array head coil. The images included an high-resolution T2-weighted (TR/TE, = 2500/368.328 ms; FOV = 240 mm; 190 sagittal slices; voxel size, 1 × 1 × 1 mm) and a post-gadolinium contrast T1-weighted anatomic images (TR/TE = 8.100/3.707 ms; FOV = 240 mm; 190 sagittal slices; voxel size =1 × 1 × 1 mm) both optimized for the standard pre-operative clinical protocol adopted by the Department of Neuroradiology of the Azienda Ospedaliero Universitaria S. Maria della Misericordia, Udine.
In addition, for all patients, a single-shot echo-planar DTI sequence was acquired covering the whole brain (TR/TE = 8,800/74 ms; FOV= 224 mm; 54 contiguous axial slices; voxel size, 1.8 × 1.8 × 2.2 mm, b0 and b1000 s/mm2, 64 non-coplanar images). The gradient directions were uniformly distributed on a sphere. The total acquisition time for the entire protocol was ~20 min.
Topographic and volumetric descriptions of the tumor were obtained by retrospectively analyzing structural imaging data routinely acquired during pre-surgery investigations.
Volumes of interest (VOIs) of patients' lesions were drawn on their T2 MRI scans using MRIcron software (https://www.nitrc.org/projects/mricron). We then normalized the Region of Interests (ROIs) to the Montreal Neurological Institute (MNI) space using the “Clinical Toolbox” (https://www.nitrc.org/projects/clinicaltbx/) for SPM8 (https://www.fil.ion.ucl.ac.uk/spm/).
The MRIcron procedure was used to overlap lesion masks (VOIs) (https://www.nitrc.org/projects/mricron). The output is a percentage overlay plot showing the percentage of overlapping lesions on a color scale.
All pre- and post-operative tumor segmentations were performed manually across axial T2-weighted and post-contrast T1-weighted MRI slices.
EOR was evaluated by using 3D T2-weighted MRI axial images as follows: (pre-operative tumor volume—post-operative tumor volume)/pre-operative tumor volume) (14).
Using the FSL software (Functional Magnetic Resonance Imaging of the Brain Software Library http://www.fmrib.ox.ac.uk/fsl) DTI images were pre-processed for eddy current and head movement corrections, and a brain extraction was performed using BET tool implemented in FSL.
Those pre-processed data were later analyzed using DTI-Studio, and a three-dimensional tract reconstruction of the IFOFs was created using the FACT (Fiber Assignment by Continuous Tracking) algorithm and a multiple regions of interest (ROIs) approach. We choose a FA threshold of 0.1 and a turning angle threshold of 55° to yield biologically plausible results.
In accordance with the procedures of Wakana et al. (15), for tracking the IFOF we used two ROIs both manually drawn in a coronal plane, the first ROI included the occipital lobe, the second ROI included the entire hemisphere at the anterior part of the genu of corpus callosum. For tracking the SLF the two ROIs were located on coronal planes, the first centered at the middle of the internal capsule and the second placed at the splenium of corpus callosum. These fibers clearly don't belong to the IFOF or to the SLF and should be manually removed using “NOT.”
SLF and IFOF fiber-tracking results were visually inspected to determine whether they were anatomically accurate and, if necessary, manually corrected. The number of IFOF and SLF streamlines passing through each voxel was automatically measured by the software, defined as the total NS.
The ipsilateral tumoral and healthy contralateral SLF (TSLF and HSLF) and IFOF (TIFOF and HIFOF) were both reconstructed and assessed for each patient to define potential differences between these white matter pathways induced by the presence of the brain tumor.
The number of streamlines (NS) was calculated in accordance with Ius et al. (8).
Specifically, for each patient, we calculated the ratio of the number of streamlines (NS-index) as follows:
• SLF NS-index= (HSLF–TSLF)/ HSLF;
• IFOF NS-index= (HIFOF–TIFOF)/ HIFOF.
Patients were examined pre-operatively, immediately after surgery, 1-week after surgery and 6 months after surgery.
Patients' outcome examination includes the presence/absence of sensory-motor deficit and speech disorders.
Regarding language deficits, two categories were established:
- Transient aphasia or dysphasia: any new language deficit owing to surgery that resolved at 6 months follow-up;
- Permanent aphasia or dysphasia: any new language deficit owing to surgery that did not resolve at 6 months follow-up.
Characteristics of the study population were described using the median with interquartile range of numerical variables and the percentages of categorical variables. Wilcoxon-Mann-Whitney test and non-parametric correlation were used to explore possible associations between NS-index and post-operative outcomes (expressed as dichotomous) and continuous patient characteristics, respectively.
ANOVA analysis between subjects was performed to compare patients' and healthy subjects' NS and FA for the SLF and the IFOF.
Regarding clinical parameters, post-operative language deficit was dichotomized as 0 (no deficit) and 1 (deficit: aphasia and/or dysphasia). Similarly, post-operative seizure outcome was classified as 0 (Engel Class Ia), and 1 (Engel Classes Ib-IV).
Receiver operating characteristics (ROC) analysis with estimation of 95% confidence interval (95% CI) of the area under the curve (AUC) was performed to estimate the accuracy of the NS-index in predicting post-operative outcomes, and to determine the best cut-off value to discriminate the two Engel index groups.
A bilateral p < 0.05 was considered significant. All statistical procedures were performed using SAS software, version 9.4 (SAS, Cary, NC, USA).
The baseline demographic, pre-operative clinical, and radiological characteristics of the study population are summarized in Table 1. Seizure was the onset symptom in 24/26 cases (92.30%). In two cases the diagnosis was incidental (7.70%). In all cases, pre-operative MRI showed hypo-intense lesions on T1-weighted sequences obtained without contrast medium and hyper-intense lesions on T2-weighted sequences. Insular lobe was involved in 17 cases, while temporal lobe in 9 cases. Immediate and 1-week post-operative language deficits were recorded in 11 cases (42.31%), while 2 patients (7.70%) developed permanent language impairment at 6 months follow-up.
All the lesions were non-contrast enhancing LGGs. Pre-operative median tumor volume (cm3) calculated on T2-weighted MRI was 36.54 ± 23.73 (range 6–127).
The maximum overlap of the LGG lesion masks of patients mainly occurred in the left insula and superior temporal gyrus, temporal pole and the sagittal stratum (IFOF + ILF) (see Table 2 and Figure 1).
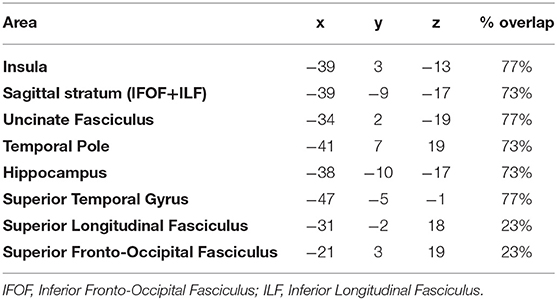
Table 2. Montreal Neurological Institute (MNI) coordinates of the areas showing higher % lesion overlap.
All patients were operated in Awake Surgery. In all cases, the resection was stopped according to DES and/or RTNT. The median EOR was 95% ± 12.24 (range 50–100).
Data regarding FA and NS-indices of SLF and IFOF, both in healthy and tumoral hemisphere, are reported in Table 3 and Figure 2.
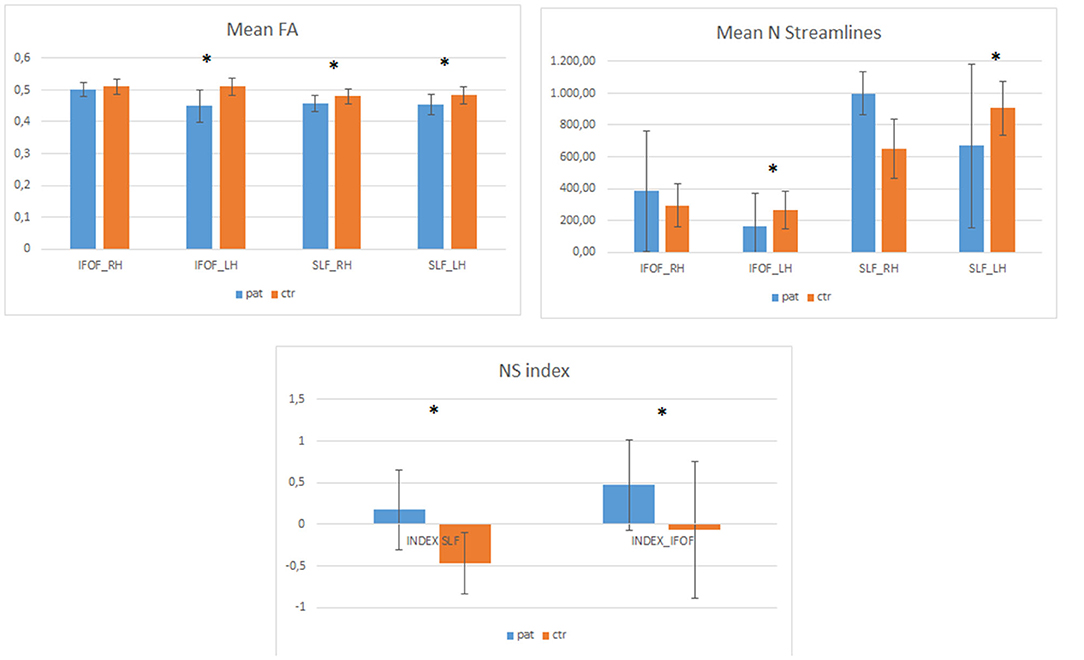
Figure 2. The Plot illustrates FA (fractional anisotropy) and the number of streamlines (NS) data, expressed as mean value and standard deviations, for SLF and IFOF both in healthy (right hemisphere = RH) and tumoral (left hemisphere = LH) hemispheres for the patients (pat) and healthy controls (ctr), respectively. Asterisks denote significant differences.
Correlation analysis between clinical-radiological data and NS- indices (SLF NS-index; IFOF NS-index) are displayed in Table 4.
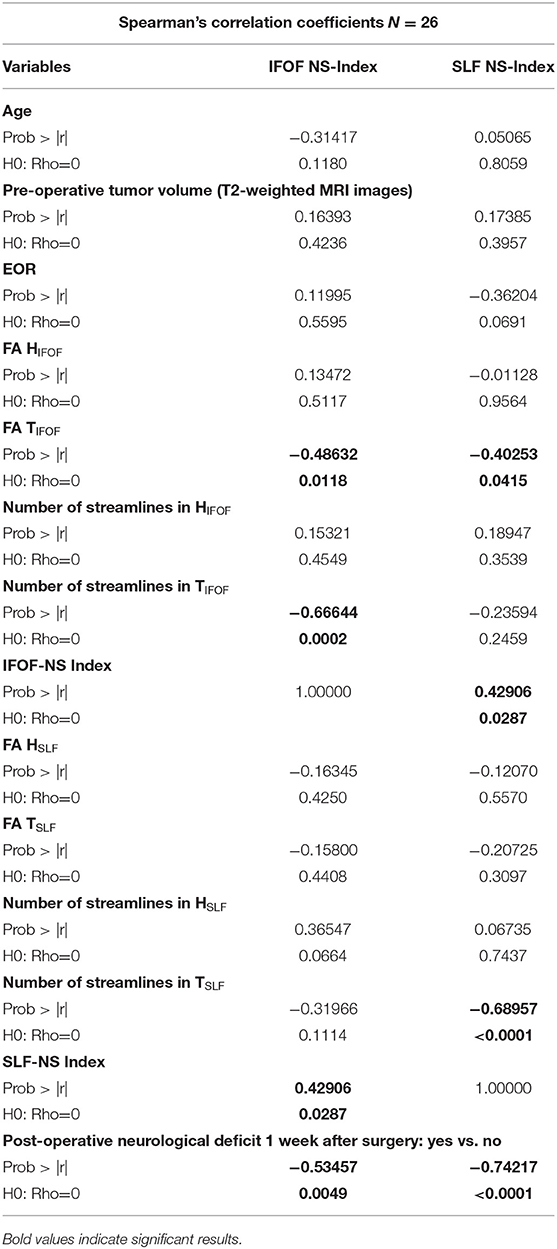
Table 4. Correlation analysis between clinical-radiological variables and NS-indices (Spearman's Correlation).
In detailed, there was a significant correlation between neurological outcome and both pre-operative SLF NS-index (p = 0.009, IC 95% = 0.001–0.136) and pre-operative IFOF NS-index (p = 0.042, IC 95% = 0.001–0.854). In addition, pre-operative SLF NS-index and pre-operative IFOF NS-index appear directly correlated to each other (p = 0.028; rs = 0.429).
We also compared patients' data with healthy controls (N = 25, all right handed, 13 F and 12 M, median age 36). The NS index was significantly different between patients and controls both for the IFOF [F (1,49) = 7.64, p = 0.008] and the SLF [F (1,49) = 29.01, p = 0.001]. Healthy controls data indicate that while the SLF is left lateralized (see Figure 2), the IFOF is almost bilateral. In addition, we found that patients had significantly lower number of streamlines for the left IFOF [F (1,49) =4.59, p = 0.037] and the left SLF [F (1,49) = 4.77, p = 0.034] when compared to controls (see Figure 2), while for the right IFOF [F(1,49) = 1.23, p = 0.2, n.s.] and the right SLF [F(1,49) = 1.6, p = 0.209, n.s.] they did not significantly differ (Figure 3).
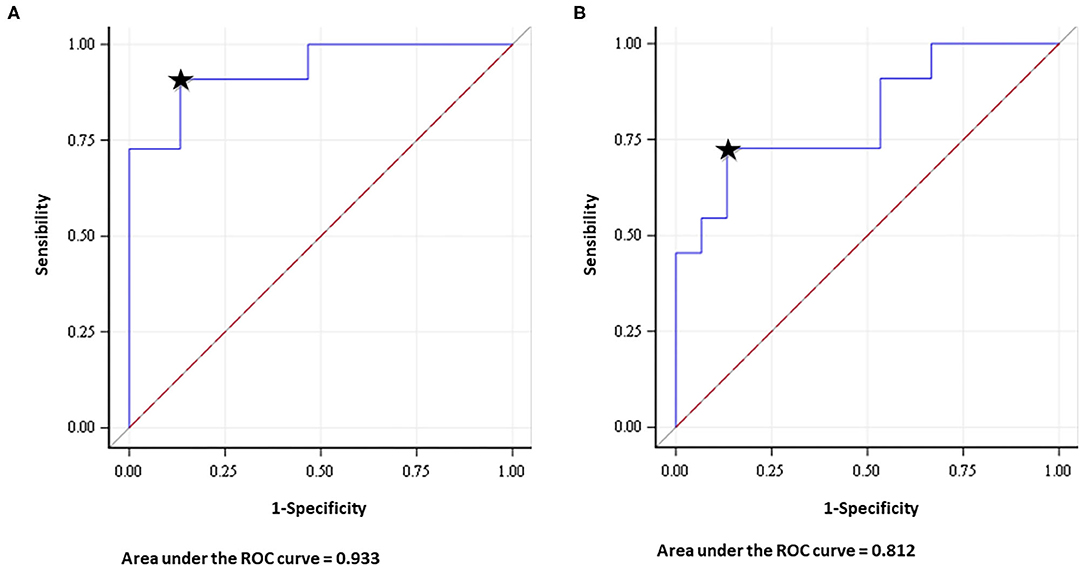
Figure 3. Receiver operating characteristic (ROC) analysis showed that the area under the curve of the NS (number of streamlines) index was 0.933 (CI 95% 0.836–1) and 0.812 (CI 95% 0.635–0.988) for SLF (A) and IFOF (B), respectively. Predictive accuracy was 86.67% for SLF and 72.42% for IFOF (It is represented by the closest point to the top left corner of the graph, indicated by the black star).
In patients affected from temporo-insular LGG in dominant hemisphere, NS-index was significantly different between patients with post-operative impairment both for SLF (p = 0.002) and IFOF (p = 0.008).
In patients with post-operative deficits the median value of SLF and IFOF NS-index was 0.622 and 0.786, respectively. Otherwise in those patients with normal post-operative surgical outcome the median value of SLF and IFOF NS-index was 0.072 and 0.587, respectively.
The optimal cut-off value for the pre-operative IFOF NS-index was 0.675. It was the point with the highest sensitivity (0.727) and specificity (0.866), with a resulting area under the curve of 0.812 (CI 95% 0.635–0.988) and a predictive accuracy of 72.42%.
For the pre-operative SLF NS-index, the cut-off value of 0.248 corresponded to the point with the highest sensitivity (0.909) and specificity (0.866), with a resulting area under the curve of 0.933 (CI 95% 0.836–1) and a predictive accuracy of 86.67% (Figure 3).
Figures 4, 5 summarized two exemplificative conditions based on different values of SLF and IFOF NS-index.
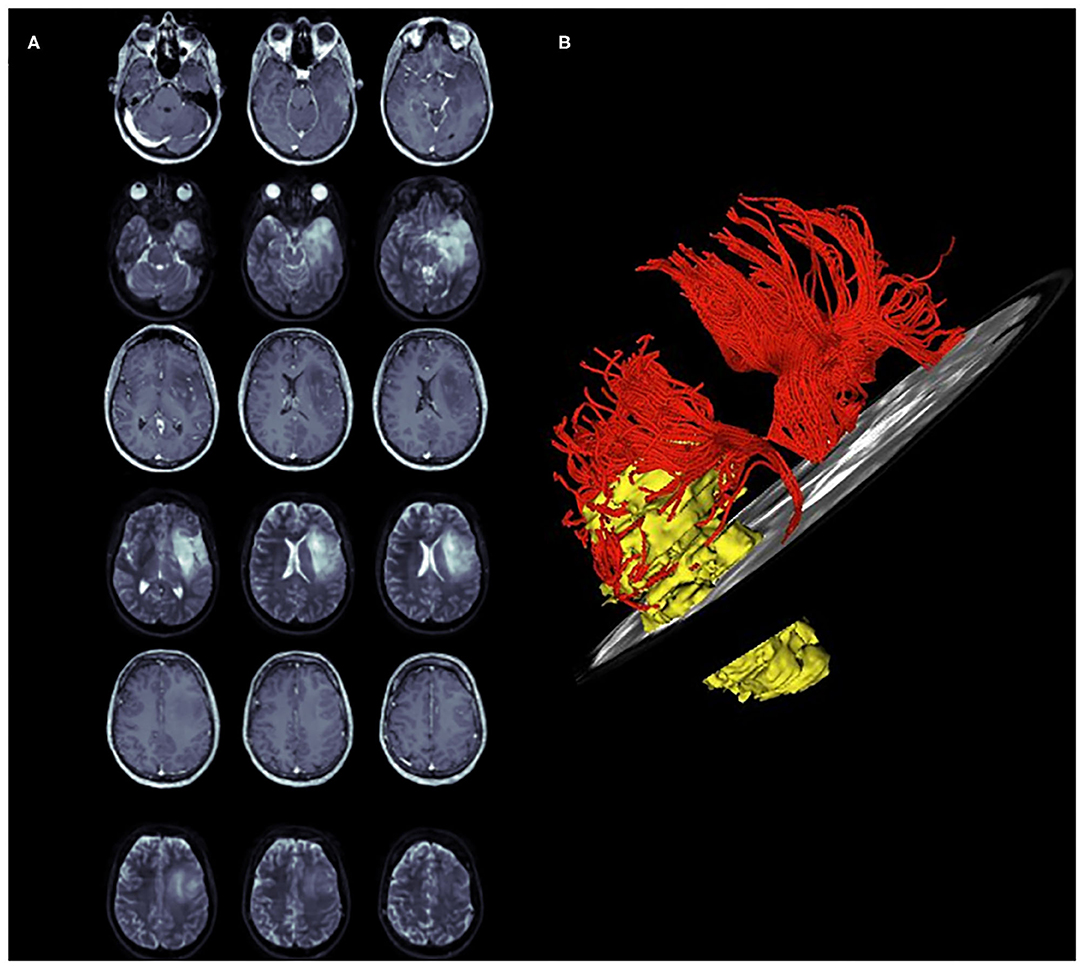
Figure 4. A case of left temporo-insular low-grade glioma (LGG). The pre-operative tumor volume computed on T1-weigted MRI and on T2-weighted MRI was 36 cm3 and 42 cm2, respectively (axial slices A). (B) represents a 3D neurological view of the tumor, TSLF and HSLF. The pre-operative SLF NS-index was 0.601.
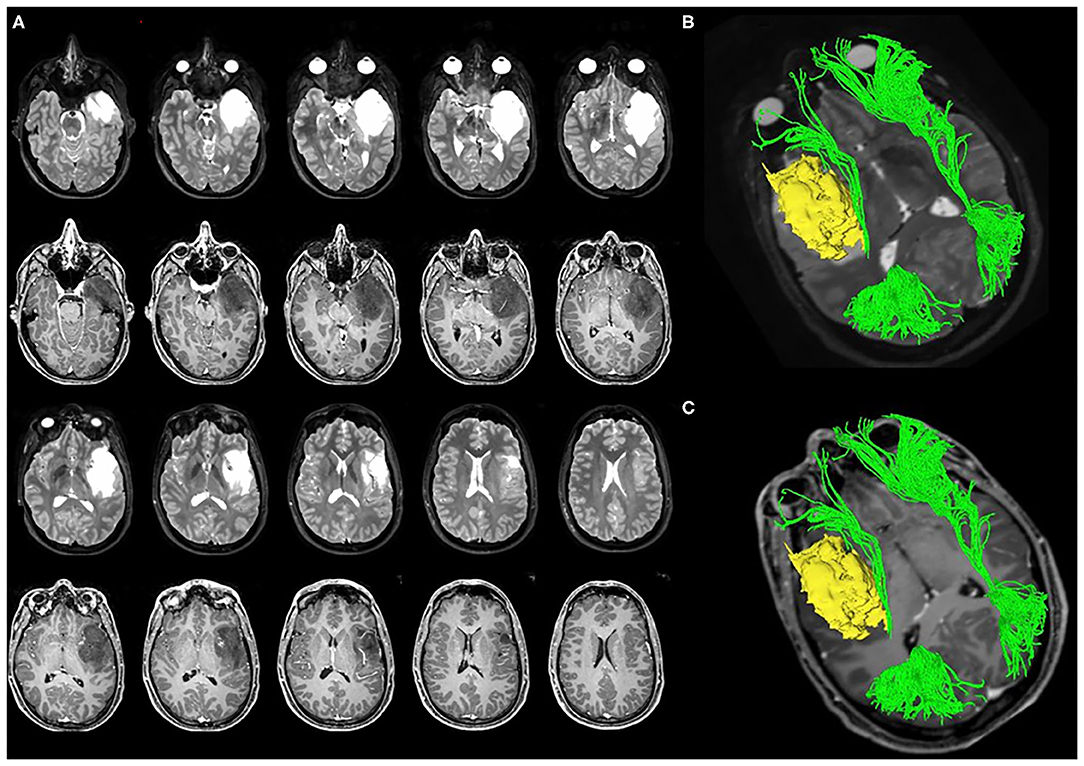
Figure 5. A case of left temporo-insular low-grade glioma (LGG). The pre-operative tumor volume computed on both T1-weigted MRI and on T2-weighted MRI was 38 cm3 (axial slices A). The tumor involves the Fronto-Occipital Longitudinal Fasciculus. (B,C) Show the 3D reconstruction of the tumor and IFOF. The pre-operative IFOF NS-index was 0.611.
The potential relationships between the structural white matter (WM) changes induced by LGG infiltrative growing and post-operative seizure outcome were also evaluated. No correlation has been highlighted between the FA, NS-indices and seizure outcome (p = 0.06 and p = 0.91, respectively for IFOF and SLF NS-indices).
A positive correlation between the pre-operative tumoral volume on T2 weighted MRI and both FA TIFOF (r = 0.670; p = 0.002) and FA TSLF (r = 0.457; p = 0.018).
In closing, we found a positive correlation between EOR and post-operatory status (r = 0.39535, p = 0.0456).
Low-grade gliomas generally affect young patients, and in almost all cases (from 70 to 90%), seizure is the onset symptom. The key point in management is based on maximal safe resection. The main technical difficulty relies on the infiltrative pattern of these tumors, especially at subcortical levels.
The cohort of patients included in this study was characterized by a relatively young age (median 35.5 years) and intermediate lesion volumes (median 36 cm3), which were almost completely resected reaching a high EOR (median 95%). It is therefore fundamental, especially given the young age and long-life expectancy, to develop new diagnostic strategies both for surgical planning and pre-operative estimation of surgical risks.
The substantial variability of the language network organization is recognized especially in neurosurgical literature (16, 17) and represents only a superficial part of a more complex and variable networks (14, 18–21). According to the “connectionist model,” there is a wide individual variability amongst both cortical areas and subcortical white matter. It is also well-documented that the brain is able to reorganize itself in pathological condition, especially in the clinical setting of slow growing tumors as LGG (20, 22–25).
In clinical practice, it would be of utmost importance to estimate the impact of surgery on the post-surgical outcomes, and to understand the potential functional recovery in response to an infiltrating slow growing tumor by means of objective measures (2, 26, 27). Given tumor-related plastic reshaping and reallocation of function, individual data are needed for patient counseling and risk assessment prior to surgery. Conventional MRI techniques provide purely anatomic information, resulting to be insufficient in patients with language area-related LGGs, mainly considering the higher inter-individual variance of functional language anatomy (2, 5, 7, 8, 21, 28–30). The DTI-based tractography has gradually become a well-established clinical tool with different applications, which include assessment of the subcortical pre-operative anatomy, characterization of epileptic networks and study of the connectomes (2, 21, 23, 28, 30–36).
The superior longitudinal fasciculus white matter complex involves transmission of speech, forming the dorsal pathway within the dual-stream model of language processing (37–39). The IFOF is mainly involved in language, specifically in lexical and semantic processing (40). With respect to naming abilities, a study involving 99 patients with glioma showed that noun naming deficits depended on damage to parts of the sagittal stratum (including the inferior fronto-occipital fasciculus and the inferior longitudinal fasciculus), in addition to cortical lesions (41). Moreover, the IFOF, together with the inferior longitudinal fasciculus, is the neuroanatomical correlate of the ventral reading route (42–45). Concerning reading, we reported that surface dyslexia could be due to impaired ventral/lexical route (as evidenced by a fractional anisotropy decrease along the inferior fronto-occipital fasciculus) in patients with glioma (46). The role of the IFOF in reading and in surface dyslexia, and of the SLF in phonological dyslexia was confirmed on a larger group study in which pre-, intra- and post-surgery reading data were presented (47).
In this investigation, we used quantitative DTI analysis in patients with LGGs involving the SLF and IFOF to estimate the potential predictive role of this technique in terms of functional post-operative outcomes. Two different white matter parameters were considered: FA and NS.
Current literature has widely documented that the FA value is lower in patients affected by glioma in comparison to FA values in healthy subjects. Our investigation confirmed these overall results. Furthermore, we found that FA values in TSLF and TIFOF were smaller than those in HSLF and HIFOF, confirming the slow infiltrative growing of LGGs (48–51).
All patients were without neurological impairment at diagnosis, despite the tumoral infiltration of white matter, supporting the brain capability to reorganize itself in pathological condition (22–24). The comparison of tumoral patient data with healthy controls highlighted that the significant effects found on the NS index, both for the IFOF and the SLF, indicate a decreased white matter representation in the pathological left hemisphere. For the right hemisphere, the differences between patients and controls were not significant, however, the mean number of streamline and standard deviations are suggestive of higher values for patients, thus implying possible plasticity related reshaping of the right hemisphere. The integrity of the IFOF and SLF, known to be part of the language-related network, were then investigated. When comparing the DTI differences between the H and T hemispheres, two indices were elaborated (pre-operative SLF NS-index and pre-operative IFOF NS-index) and correlated to post-operative outcomes. Our study found that patients with pre-operative indices close to 0 (that implies a similar number of streamlines in both hemispheres) generally had less probability of developing post-operative neurological impairment. Patients with pre-operative indices close to 1 (that means a low number of streamlines in tumoral hemisphere), in contrast, were more likely to develop post-operative deficits. An SFL NS-index <0.248, and an IFOF NS-index <0.675 have been associated with better post-operative clinical outcomes. These results are consistent with a previous study based on DTI analysis of cortico-spinal tract in patients with premotor LGGs (8), confirming that the NS analysis of the main subcortical pathways is a valid and promising approach to pre-operatively estimate the risk of post-operative deficits.
A positive correlation was found between pre-operative SLF NS-index and pre-operative IFOF NS-index, suggesting that lesions infiltrated both, which is also consistent with the MRI structural data analyses. Lesions were shown to be mainly localized in the insula and superior temporal lobes, which are areas crossed by both the IFOF in their inferior portion and the SLF in the upper portion.
The correlations between the pre-operative tumoral volume on T2 weighted MRI and both FA TIFOF and FA TSLF suggest that higher pre-operative tumor volume may induce a higher white matter infiltration probability.
In closing, we found a positive correlation between EOR and post-operatory status, thus suggesting that maximizing the resection implies assuming the risk of observing a transient immediate post-surgery decrease. In the study, we also investigated the potential relationships between the structural changes of white matter tracts, induced by LGG infiltrative growing, and post-operative seizure outcomes. No correlation was found between the pre-operative SLF NS-index, pre-operative IFOF NS-index and post-operative seizure outcomes. These findings could be based on the possibility that the epileptogenic foci were located outside of the SLF and IFOF in our sample. It is therefore possible that concerning the cases of seizure-free patients within our cohort, the epileptogenic foci could have likely been removed with the tumor and peritumoral cortex. With regards to the patients showing poor post-operative seizure control, the failure or the impossibility of removing the epileptogenic zone, and the presence of a possible secondary epileptic focus need to be considered. In both cases, IFOF and SLF infiltration may not have been relevant. Epileptogenic processes in tumor-related epilepsy (TRE) are multifactorial and not fully understood (52). Increased evidence suggests that, in up to two-thirds of patients with glioma, seizures arise from the peritumoral cortex, due to induced changes in neurotransmitters, environment and electrical properties of these regions (53, 54). The likelihood of seizure control is thus related to the possibility of resection of the epileptogenic zone (EZ), nested in the peritumoral tissue. For this reason, the extent of resection in now recognized as one of the strongest prognostic factors for post-operative seizure control (1, 55, 56). We acknowledge that our sample is small to draw definitive considerations. Moreover, aphasic seizures were poorly represented. Regarding WM tracts, the clinical challenge, in primary brain tumor epilepsy, is represented not only by the determination of the EZ, but also by the identification of the underlying epileptic network, which requires a complex neurophysiological presurgical work-up.
There are several limitations of our study. The most important is the retrospective nature of the investigation and the small simple size. Concerning the DTI methodology, different tracking approaches exist, although there is no consensus yet on the most suitable one. It would be interest to verify these preliminary results by using other DTI approaches.
In addition, this approach is only applicable to LGGs due to their specific biologically determined growth pattern (57). The elaboration of NS-indices based on numbers of streamlines in both hemispheres, strengthens the results, limiting the bias due to the individual variability. Moreover, an extensive neuropsychological assessment has not been included in this study, which has, however, been considered in our ongoing future studies currently underway. Despite the inherent limitation of DTI analysis, which include data acquisition, bio-mathematical models, user dependency, and software programs (58–60), it still represents the only way to investigate white matter in humans in vivo (7). Overall, we recognize that these preliminary results require a further validation increasing the study population and planning future prospective studies.
Future longitudinal studies, based on comparison between structural WM in healthy and tumoral hemisphere should be developed to address the strongest predictive measures of surgical risk for lesion infiltrating the functional subcortical pathways. The integration of this approach with an extensive pre- and post-operative neuropsychological assessment might provide insights into compensatory mechanisms for language deficits on the level of white matter plasticity, in patients with brain tumors. It could be of clinical importance to assess the ability of the proposed indices in identifying the risk of permanent deficits in a larger cohort of patients.
These preliminary results highlight the potential role of pre-operative DTI in assessing the risk of post-operative transient language impairment in patients undergoing surgical resection of LGGs involving the AF and IFOF in dominant hemisphere. This analytical pre-operative approach may represent a feasible predictive tool for patient counseling and risk assessment prior to surgery and pave the way to standardize approaches in glioma surgical management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the local Ethics Committee, Comitato Etico Unico Regionale del Friuli Venezia Giulia, approved this investigation (protocol N.0036567/P/GEN/EGAS, ID study 2540). Written informed consent was not provided because considering the retrospective nature of the study, written consent to participate in the study was not applicable. Written informed consent was obtained for surgery.
TI and BT: conception and design. IG, CB, and AN: acquisition of data. FP: formal analysis. FP and MM: software. TI, BT, TS, GP, and MS: supervision. TI, BT, and MS: validation. TI, BT, and GP: writing—original draft and review and editing. All authors have read and agreed to the published version of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the support by the staff of the Medical Imaging Centre (Department of Neuroradiology of the Azienda Ospedaliero Universitaria S. Maria della Misericordia, Udine). We would like to thank Mark Zeppieri MD, Ph.D. for the English revision of the manuscript.
AF, arcuate fasciculus; AS, awake surgery; ASM, anti-seizure medication; AUC, area under the curve; DES, direct electrical stimulation; DTI, diffusion tensor imaging; EOR, extent of resection; EZ, epileptogenic zone; FA, fractional anisotropy; FACT, fiber assignment by continuous tracking; FSL, Functional Magnetic Resonance Imaging of the Brain Software Libreary; HSLF, healthy SLF; HIFOF, healthy IFOF; IFOF, inferior fronto-occipital fasciculus; ILAE, International League Against Epilepsy; LGG, low-grade glioma; MNI, Montreal Neurological Institute; MRI, magnetic resonance imaging; NS, number of streamlines; ROC, receiver operating characteristics; ROIs, region of interests; RTNT, real time neuropsychological testing; SLF, superior longitudinal fasciculus; TSLF, tumoral SLF; TIFOF, tumoral IFOF; TRE, tumor-related epilepsy; VOIs, volumes of interests; WHO, World Health Organization; WM, white matter.
1. Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients. J Neurosurg. (2012) 117:1039–52. doi: 10.3171/2012.8.JNS12393
2. Mato D, Velasquez C, Gómez E, Marco de Lucas E, Martino J. Predicting the extent of resection in low-grade glioma by using intratumoral tractography to detect eloquent fascicles within the tumor. Neurosurgery. (2020) 88:E190–202. doi: 10.1093/neuros/nyaa463
3. Rudà R, Angileri FF, Ius T, Silvani A, Sarubbo S, Solari A, et al. SINch neuro-oncology section, AINO and SIN neuro-oncology section. Italian consensus and recommendations on diagnosis and treatment of low-grade gliomas. An intersociety (SINch/AINO/SIN) document. J Neurosurg Sci. (2020) 64:313–34. doi: 10.23736/S0390-5616.20.04982-6
4. Lombardi G, Barresi V, Castellano A, Tabouret E, Pasqualetti F, Salvalaggio A, et al. Clinical management of diffuse low-grade gliomas. Cancers. (2020) 12:3008. doi: 10.3390/cancers12103008
5. Sollmann N, Zhang H, Fratini A, Wildschuetz N, Ille S, Schröder A, et al. Risk assessment by presurgical tractography using navigated TMS maps in patients with highly motor- or language-eloquent brain tumors. Cancers. (2020) 12:1264. doi: 10.3390/cancers12051264
6. Mazzucchi E, La Rocca G, Ius T, Sabatino G, Della Pepa GM. Multimodality imaging techniques to assist surgery in low-grade gliomas. World Neurosurg. (2020) 133:423–5. doi: 10.1016/j.wneu.2019.10.120
7. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. (2008) 44:1105–32. doi: 10.1016/j.cortex.2008.05.004
8. Ius T, Turella L, Pauletto G, Isola M, Maieron M, Sciacca G, et al. Quantitative diffusion tensor imaging analysis of low-grade gliomas: from preclinical application to patient care. World Neurosurg. (2017) 97:333–43. doi: 10.1016/j.wneu.2016.10.006
9. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenese WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
10. Fisher RS, Cross JE, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Seizure: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
11. Engel J Jr, Burchfiel J, Ebersole J, Gates J, Gotman J, Homan R, et al. Long-term monitoring for epilepsy. report of an IFCN committee. Electroencephalogr Clin Neurophysiol. (1993) 87:437–58. doi: 10.1016/0013-4694(93)90158-R
12. Skrap M, Marin D, Ius T, Fabbro F, Tomasino B. Brain mapping: a novel intraoperative neuropsychological approach. J Neurosurg. (2016) 125:877–87. doi: 10.3171/2015.10.JNS15740
13. Cesselli D, Ius T, Isola M, Del Ben F, Da Col G, Bulfoni M, et al. Application of an artificial intelligence algorithm to prognostically stratify grade II gliomas. Cancers. (2019) 12:50. doi: 10.3390/cancers12010050
14. Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage. (2011) 56:992–1000. doi: 10.1016/j.neuroimage.2011.03.022
15. Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. (2007) 36:630–44. doi: 10.1016/j.neuroimage.2007.02.049
16. Chang EF, Breshears JD, Raygor KP, Lau D, Molinaro AM, Berger MS. Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. J Neurosurg. (2017) 126:114–21. doi: 10.3171/2015.10.JNS151087
17. Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. (1989) 71:316–26. doi: 10.3171/jns.1989.71.3.0316
18. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. (2008) 358:18–27. doi: 10.1056/NEJMoa067819
19. Conner AK, Briggs RG, Sali G, Rahimi M, Baker CM, Burks JD, et al. A connectomic atlas of the human cerebrum-chapter 13: tractographic description of the inferior fronto-occipital fasciculus. Oper Neurosurg. (2018) 15(Suppl. 1):S436–43. doi: 10.1093/ons/opy267
20. Brown DA, Hanalioglu S, Chaichana K, Duffau H. Transcorticosubcortical approach for left posterior mediobasal temporal region gliomas: a case series and anatomic review of relevant white matter tracts. World Neurosurg. (2020) 139:e737–47. doi: 10.1016/j.wneu.2020.04.147
21. Incekara F, Satoer D, Visch-Brink E, Vincent A, Smits M. Changes in language white matter tract microarchitecture associated with cognitive deficits in patients with presumed low-grade glioma. J Neurosurg. (2018) 8:1–9. doi: 10.3171/2017.12.JNS171681
22. Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. (2011) 54:49–59. doi: 10.1016/j.neuroimage.2010.07.055
23. Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Balbi S, Granieri E, et al. Towards a functional atlas of human white matter. Hum Brain Mapp. (2015) 36:3117–36. doi: 10.1002/hbm.22832
24. Cargnelutti E, Ius T, Skrap M, Tomasino B. What do we know about pre- and postoperative plasticity in patients with glioma? A review of neuroimaging and intraoperative mapping studies. Neuroimage Clin. (2020) 28:102435. doi: 10.1016/j.nicl.2020.102435
25. Altieri R, Melcarne A, Junemann C, Zeppa P, Zenga F, Garbossa D, et al. Inferior Fronto-Occipital fascicle anatomy in brain tumor surgeries: from anatomy lab to surgical theater. J Clin Neurosci. (2019) 68:290–4. doi: 10.1016/j.jocn.2019.07.039
26. Bizzi A, Nava S, Ferrè F, Castelli G, Aquino D, Ciaraffa F, et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex. (2012) 48:255–72. doi: 10.1016/j.cortex.2011.11.015
27. Li FY, Liu HY, Zhang J, Sun ZH, Zhang JS, Sun GC, et al. Identification of risk factors for poor language outcome in surgical resection of glioma involving the arcuate fasciculus: an observational study. Neural Regen Res. (2021) 16:333–7. doi: 10.4103/1673-5374.290901
28. Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. (2002) 17:77–94. doi: 10.1006/nimg.2002.1136
29. Vassal F, Pommier B, Sontheimer A, Lemaire JJ. Inter-individual variations and hemispheric asymmetries in structural connectivity patterns of the inferior fronto-occipital fascicle: a diffusion tensor imaging tractography study. Surg Radiol Anat. (2018) 40:129–37. doi: 10.1007/s00276-017-1966-0
30. Wu Y, Sun D, Wang Y, Wang Y. Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Front Neuroanat. (2016) 10:88. doi: 10.3389/fnana.2016.00088
31. Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. (2004) 25:356–69.
32. Luat AF, Chugani HT. Molecular and diffusion tensor imaging of epileptic networks. Epilepsia. (2008) 49:15–22. doi: 10.1111/j.1528-1167.2008.01506.x
33. Tuncer MS, Salvati LF, Grittner U, Hardt J, Schilling R, Bährend I, et al. Towards a tractography-based risk stratification model for language area associated gliomas. NeuroImage Clin. (2020) 29:102541. doi: 10.1016/j.nicl.2020.102541
34. Sanvito F, Caverzasi E, Riva M, Jordan KM, Blasi V, Scifo P, et al. fMRI-targeted high-angular resolution diffusion MR tractography to identify functional language tracts in healthy controls and glioma patients. Front Neurosci. (2020) 14:225. doi: 10.3389/fnins.2020.00225
35. Panesar SS, Yeh FC, Deibert CP, Fernandes-Cabral D, Rowthu V, Celtikci P, et al. A diffusion spectrum imaging-based tractographic study into the anatomical subdivision and cortical connectivity of the ventral external capsule: uncinate and inferior fronto-occipital fascicles. Neuroradiology. (2017) 59:971–87. doi: 10.1007/s00234-017-1874-3
36. Zhao Y, Chen X, Wang F, Sun G, Wang Y, Song Z, et al. Integration of diffusion tensor-based arcuate fasciculus fibre navigation and intraoperative MRI into glioma surgery. J Clin Neurosci. (2012) 19:255–61. doi: 10.1016/j.jocn.2011.03.041
37. Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Barbareschi M, Dallabona M, et al. Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp. (2016) 37:3858–72. doi: 10.1002/hbm.23281
38. Shinoura N, Suzuki Y, Tsukada M, Yoshida M, Yamada R, Tabei Y, et al. Deficits in the left inferior longitudinal fasciculus results in impairments in object naming. Neurocase. (2010) 16:135–9. doi: 10.1080/13554790903329174
39. Davtian M, Ulmer JL, Mueller WM, Gaggl W, Mulane MP, Krouwer HG. The superior longitudinal fasciculus and speech arrest. J Comput Assist Tomogr. (2008) 32:410–4. doi: 10.1097/RCT.0b013e318157c5ff
40. Moritz-Gasser S, Herbet G, Duffau H. Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: a brain electrostimulation study. Neuropsychologia. (2013) 51:1814–22. doi: 10.1016/j.neuropsychologia.2013.06.007
41. Tomasino B, Tronchin G, Marin D, Maieron M, Fabbro F, Cubelli R, et al. Noun–verb naming dissociation in neurosurgical patients. Aphasiology. (2019) 33:1418–40. doi: 10.1080/02687038.2018.1542658
42. Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, et al. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. (2008) 44:962–74. doi: 10.1016/j.cortex.2008.05.003
43. Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. (2007) 30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645
44. Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of ortographic, phonological and speech processing. Brain. (2012) 135(Pt 3):935–48. doi: 10.1093/brain/awr363
45. Maldonado IL, Moritz-Gasser S, Duffau H. Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct. (2011) 216:263–74. doi: 10.1007/s00429-011-0309-x
46. Tomasino B, Marin D, Maieron M, D'Agostini S, Fabbro F, Skrap M, et al. Double-letter processing in surface dyslexia and dysgraphia following a left temporal lesion: a multimodal neuroimaging study. Cortex. (2015) 73:112–30. doi: 10.1016/j.cortex.2015.08.010
47. Tomasino B, Ius T, Skrap M, Luzzatti C. Phonological and surface dyslexia in individuals with brain tumors: performance pre-, intra-, immediately post-surgery and at follow-up. Hum Brain Mapp. (2020) 41:5015–31. doi: 10.1002/hbm.25176
48. Miloushev VZ, Chow DS, Filippi CG. Meta-analysis of diffusion metrics for the prediction of tumor grade in gliomas. AJNR Am J Neuroradiol. (2015) 36:302–8. doi: 10.3174/ajnr.A4097
49. D'Souza S, Ormond DR, Costabile J, Thompson JA. Fiber-tract localized diffusion coefficients highlight patterns of white matter disruption induced by proximity to glioma. PLoS ONE. (2019) 14:e0225323. doi: 10.1371/journal.pone.0225323
50. Liu D, Liu Y, Hu X, Hu G, Yang K, Xiao C, et al. Alterations of white matter integrity associated with cognitive deficits in patients with glioma. Brain Behav. (2020) 10:e01639. doi: 10.1002/brb3.1639
51. Campanella M, Ius T, Skrap M, Fadiga L. Alterations in fiber pathways reveal brain tumor typology: a diffusion tractography study. PeerJ. (2014) 2:e497. doi: 10.7717/peerj.497
52. Ius T, Pauletto G, Tomasino B, Maieron M, Budai R, Isola M, et al. Predictors of postoperative seizure outcome in low grade glioma: from volumetric analysis to molecular stratification. Cancer. (2020) 12:397. doi: 10.3390/cancers12020397
53. Kohling R, Senner V, Paulus W, Speckmann EJ. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotrasplants. Neurobiol Dis. (2006) 22:64–75. doi: 10.1016/j.nbd.2005.10.001
54. de Groot M, Reijneveld JC, Aronica E, Heimans JJ. Epilepsy in patients with a brain tumor: focal epilepsy requires focused treatment. Brain. (2012) 135(Pt 4):1002–16. doi: 10.1093/brain/awr310
55. Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. (2014) 37:389–404. doi: 10.1007/s10143-014-0527-9
56. Chen DY, Chen CC, Crawford JR, Wang SG. Tumor-related epilepsy: epidemiology, pathogenesis and management. J Neurooncol. (2018) 139:13–21. doi: 10.1007/s11060-018-2862-0
57. Mandonnet E, Capelle L, Duffau H. Extension of paralimbic low grade gliomas: toward an anatomical classification based on white matter invasion patterns. J Neurooncol. (2006) 78:179–85. doi: 10.1007/s11060-005-9084-y
58. Feigl GC, Hiergeist W, Fellner C, Schebesch KM, Doenitz C, Finkenzeller T, et al. Magnetic resonance imaging diffusion tensor tractography: evaluation of anatomic accuracy of different fiber tracking software packages. World Neurosurg. (2014) 81:144–50. doi: 10.1016/j.wneu.2013.01.004
59. Campbell JSW, Pike GB. Potential and limitations of diffusion MRI tractography for the study of language. Brain Lang. (2014) 131:65–73. doi: 10.1016/j.bandl.2013.06.007
Keywords: diffusion tensor imaging analysis, intraoperative electric stimulation, low-grade gliomas, inferior fronto-occipital fasciculus, arcuate fasciculus
Citation: Ius T, Somma T, Baiano C, Guarracino I, Pauletto G, Nilo A, Maieron M, Palese F, Skrap M and Tomasino B (2021) Risk Assessment by Pre-surgical Tractography in Left Hemisphere Low-Grade Gliomas. Front. Neurol. 12:648432. doi: 10.3389/fneur.2021.648432
Received: 31 December 2020; Accepted: 25 January 2021;
Published: 15 February 2021.
Edited by:
Giovanni Raffa, University of Messina, ItalyReviewed by:
Antonino Scibilia, Institut hospitalo-universitaire de Strasbourg, FranceCopyright © 2021 Ius, Somma, Baiano, Guarracino, Pauletto, Nilo, Maieron, Palese, Skrap and Tomasino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara Ius, dGFtYXJhLml1c0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.