
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 March 2021
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.644804
This article is part of the Research TopicIntraoperative fluorescence imaging and diagnosis in central and peripheral nervous system tumors: established applications and future perspectivesView all 14 articles
 Georgios A. Maragkos1*†
Georgios A. Maragkos1*† Alexander J. Schüpper1*†
Alexander J. Schüpper1*† Nikita Lakomkin1
Nikita Lakomkin1 Panagiotis Sideras2
Panagiotis Sideras2 Gabrielle Price1
Gabrielle Price1 Rebecca Baron1
Rebecca Baron1 Travis Hamilton3
Travis Hamilton3 Sameah Haider3
Sameah Haider3 Ian Y. Lee3
Ian Y. Lee3 Constantinos G. Hadjipanayis1,4
Constantinos G. Hadjipanayis1,4 Adam M. Robin3
Adam M. Robin3Background: Fluorescence-guided surgery (FGS) using 5-aminolevulic acid (5-ALA) is a widely used strategy for delineating tumor tissue from surrounding brain intraoperatively during high-grade glioma (HGG) resection. 5-ALA reaches peak plasma levels ~4 h after oral administration and is currently approved by the FDA for use 2–4 h prior to induction to anesthesia.
Objective: To demonstrate that there is adequate intraoperative fluorescence in cases undergoing surgery more than 4 h after 5-ALA administration and compare survival and radiological recurrence to previous data.
Methods: Retrospective analysis of HGG patients undergoing FGS more than 4 h after 5-ALA administration was performed at two institutions. Clinical, operative, and radiographic pre- and post-operative characteristics are presented.
Results: Sixteen patients were identified, 6 of them female (37.5%), with mean (SD) age of 59.3 ± 11.5 years. Preoperative mean modified Rankin score (mRS) was 2 ± 1. All patients were dosed with 20 mg/kg 5-ALA the morning of surgery. Mean time to anesthesia induction was 425 ± 334 min. All cases had adequate intraoperative fluorescence. Eloquent cortex was involved in 12 cases (75%), and 13 cases (81.3%) had residual contrast enhancement on postoperative MRI. Mean progression-free survival was 5 ± 3 months. In the study period, 6 patients died (37.5%), mean mRS was 2.3 ± 1.3, Karnofsky score 71.9 ± 22.1, and NIHSS 3.9 ± 2.4.
Conclusion: Here we demonstrate that 5-ALA-guided HGG resection can be performed safely more than 4 h after administration, with clinical results largely similar to previous reports. Relaxation of timing restrictions could improve procedure workflow in busy neurosurgical centers, without additional risk to patients.
Maximal and safe resection has been established as the initial standard of care for the treatment of high-grade gliomas (HGG) (1–5). Complete resection of the contrast-enhancing tumor (CRET) has been associated with prolonged survival for patients with the most common HGG, glioblastoma (6, 7). Due to the propensity of HGGs involving eloquent regions of the brain, maximal safe resection poses intraoperative challenges for tumor surgeons (8). As a surgical adjunct, the use of fluorescence-guided surgery (FGS) provides surgeons with improved visualization of brain tumors and the infiltrative margin. The use of FGS has been well-studied in the use of HGG resection over the past 20 years and has shown to be an effective tool for resection of HGGs (9, 10).
Multiple fluorophore agents have been studied in the use of FGS for HGGs and each come with their own advantages and disadvantages (11). The most commonly studied fluorophores are 5-aminolevulinic acid (5-ALA), fluorescein and indocyanine green (ICG) (11). 5-ALA is the most widely studied agent for FGS of HGG, and is currently the only agent (Gleolan®) that is approved by the US Food and Drug Administration (FDA) for glioma surgery (9). Administered as an oral solution, 5-ALA is metabolized in the heme biosynthesis pathway to protoporphyrin (PpIX), which accumulates intracellularly in tumor cells (12), absorbing light between 375 and 440 nm and emitting violet-red fluorescence (640–710 nm) (13). 5-ALA has been previously shown in multicenter studies to be safe and effective, with minimal associated side effects (9, 14). Since the completion of the first phase III randomized controlled trial (RCT) for FGS showing improved progression-free survival (PFS) and greater overall tumor resection following FGS (9), 5-ALA has been used broadly across Europe and other countries throughout the world. However, 5-ALA (Gleolan®) only recently has been approved by the FDA in 2017 (15), and is currently being used by neurosurgeons throughout the country.
As part of the FDA approval of 5-ALA (Gleolan® NX Development Corporation) for glioma surgery, the recommended usage in the label is stated as an “oral dose of ALA HCL solution of 20 mg/kg body weight, administered 3 h (range 2–4 h) prior to induction of anesthesia (16).” These recommendations were established after the RCT led by Stummer et al. (9). The timing of 5-ALA administration was based on rodent experiments in which a fluorescence peak was observed 6 h after administration (17). Oral administration at 3 h (2–4 h) was recommended in order to permit time for anesthesia, positioning, and craniotomy prior to peak intraoperative PpIX fluorescence (9, 10). Recently, however a study by Kaneko et al. found that maximal concentrations of fluorescence intensity were observed after 7–8 h following 5-ALA administration (18), calling for later administration than established in prior studies. In this study, we aim to study a population of patients undergoing glioma surgery beyond the 4 h window (>6 h) following 5-ALA administration as described in the FDA label to determine intraoperative fluorescence, as well as clinical and radiographic outcomes of patients undergoing FGS.
Institutional Review Board approval was obtained with waiver of patient informed consent due to the retrospective nature of the study. For the purposes of this study, all patients receiving 5-ALA for resection of radiographic high-grade glioma (HGG) were screened at two separate institutions between 2017 and 2020. Patients were included if they received anesthesia induction more than 4 h following 5-ALA administration. Patients were excluded if they received anesthesia induction within 4 h of 5-ALA, or if they had non-HGG tissue upon histopathology. Patient demographic variables including age, sex, and preoperative functional status as measured by modified Rankin scale (mRS) were collected. Treatment variables included chemotherapy or radiotherapy. Operative variables including anesthesia induction time, incision time, procedure finish time, and time to extubation were collected. Outcome variables included postoperative neurological deficits, mRS scores, Karnofsky Performance Scores (KPS), National Institutes of Health Stroke Scale (NIHSS), 6-month progression-free survival (PFS), and time until death.
Volumetric analysis from preoperative and postoperative MR imaging was prospectively collected for all patients. Volumetric measurements were made using Olea Sphere (v. 2.3, Olea Medical Solutions, La Ciotat, France) at one institution and Brainlab Elements (Brainlab, Munich, Germany) at the other. Regions of contrast-enhancement were measured in preoperative scans, immediate postoperative scans, and MRIs 6 months following surgery independently by three of the authors (R.B., P.S., S.H.), blinded to the clinical characteristics of the patient cohort, one being a neuroradiology fellow at the time of assessment. One patient volume required confirmation by a senior author (A.R.) who was not previously blinded to patient characteristics.
Clinical, operative, and radiographic pre- and post-operative characteristics are presented as frequencies and percentages for categorical variables, and means and standard deviations for continuous variables. Descriptive statistics were used to analyze both categorical and continuous variables. All statistical analyses were performed on Statistical Analysis Software version 9.4 (SASv9.4 - Cary, NC). Significance for all statistical testing was determined by p < 0.05.
A total of 16 patients met the inclusion criteria (Table 1), 6 of them female (37.5%), with a mean (SD) age of 59.3 ± 11.5. Preoperative mean modified Rankin score (mRS) was 2 ± 1. All 16 patients received chemotherapy and/or radiation therapy in addition to resection for treatment of their brain tumor. Average tumor volume was 24.9 ± 24.6 cc. Fifteen (93.8%) patients' tumors were diagnosed as glioblastoma (GBM) on histopathology (one patient had anaplastic astrocytoma), 13 (86.7%) were IDH1 wild-type and 2 (13.3%) were IDH1 mutants. Nine (56.3%) tumors were primary and seven (43.7%) were recurrent HGGs (Table 2). Twelve (75%) tumors involved eloquent cortex. The mean extent of resection (EOR) was 91.5%, with 13 (81.3%) cases having residual contrast enhancement on postoperative MRI, and a mean residual volume of 1.16 ± 1.11 cc.
All patients were dosed with 20 mg/kg 5-ALA the morning of surgery. The mean time from 5-ALA administration to incision was 507 ± 344 min and time to closure was 767 ± 395, therefore the fluorescence guided surgery was performed after >6 h in all cases. The longest time between 5-ALA administration and anesthesia induction was 27 h and 46 min. This patient received 5-ALA prior to scheduled surgery but was found to have a fever, had a full workup over the ensuing day, then proceeded to have FDG surgery the next day, with adequate fluorescence intraoperatively. All cases had adequate intraoperative fluorescence. No patients in the series experienced 5-ALA-related toxicity. All sixteen patients' tumors demonstrated intraoperative fluorescence in situ (Figures 1, 2).
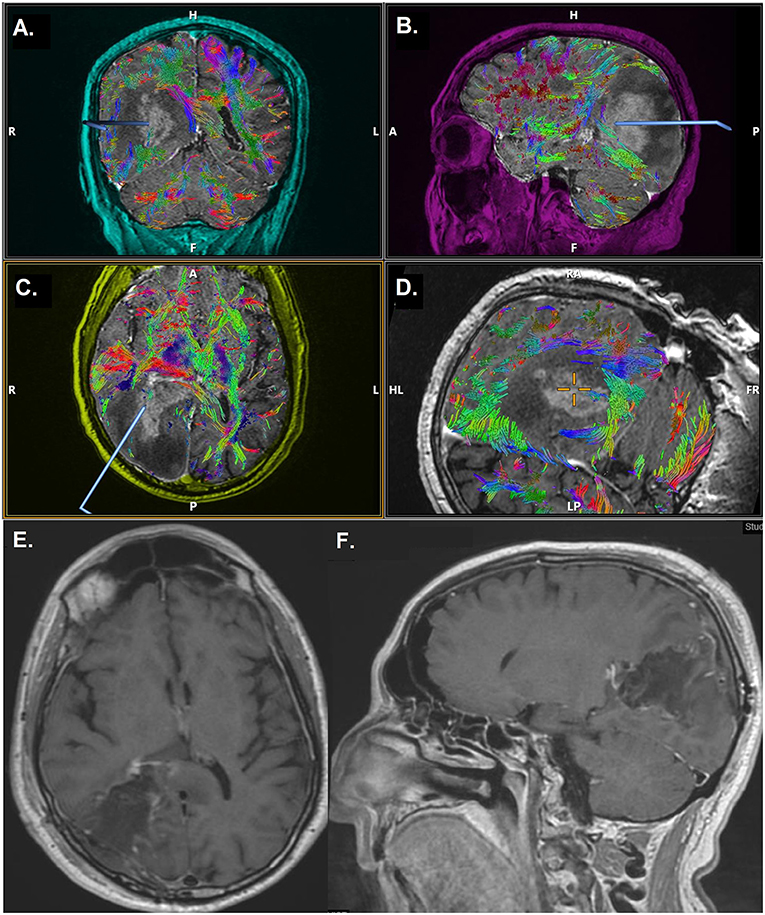
Figure 1. Case demonstration of a 69-year-old male with glioblastoma multiforme, undergoing 5-ALA FGS. Time from 5-ALA administration to anesthesia induction was 7 h and 48 min, time to incision was 9 h and 4 min and time to closure was 12 h and 14 min. (A–D) Preoperative MRI with DTI. Axial (E) and sagittal (F) postoperative MRI scan, after 5-ALA FGS, demonstrating gross total resection of the lesion. 5-ALA, 5-aminolevulinic acid; FGS, fluorescence-assisted glioma surgery; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging.
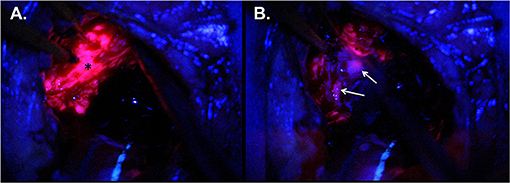
Figure 2. Intraoperative imaging for case demonstration patient. (A) Tumor bulk fluorescence after 5-ALA administration (asterisk). (B) Infiltrative margin fluorescence after 5-ALA administration (white arrows).
Five (26.7%) patients had postoperative neurological deficits, defined as decrements in the NIHSS more than one point during the initial hospitalization (Table 3). Mean progression-free survival was 5 ± 3 months. During the study period, 6 (37.5%) patients died, mean mRS is 2.3 ± 1.3, mean Karnofsky score was 71.9 ± 22.1, and mean NIHSS was 3.9 ± 2.4. Six-month progression-free survival was seen in 46.2% (6) patients. Overall survival following surgery was 6.3 ± 4.9 months (Figure 3).

Figure 3. Kaplan-Meier curve for survival after surgical excision on the present cohort. The x axis represents time from surgical excision in days, with red vertical dashed lines at the 3-, 6-, 9-, 12-, and 15-month marks. The y axis represents the percentage of patients surviving at each time point. Drop points in the curve represent patient mortality and the short vertical lines represent subject concealment from further calculations due to end of follow-up, with patients either lost to follow-up or being operated on recently from the time of analysis.
5-ALA is a well-studied intraoperative adjunct for the resection of HGGs that helps differentiate tumor from surrounding brain parenchyma and has been shown to improve gross total resection rates as well as overall patient survival (9, 19–32). Despite its widespread use, limited data exists regarding the best timing for administration of 5-ALA prior to surgery. The current guidelines set forth by the Food and Drug Administration (FDA) state that the substance should be administered “3 h (range 2–4 h) before anesthesia (16).” However, the exact fluorescence kinetics regarding PpIX accumulation within tumor tissue are currently unclear. In the current study, the safety and efficacy of 5-ALA use beyond the established time of administration was assessed. In patients administered 5-ALA over 6 h prior to the start of tumor resection, 5-ALA was not only safe to use during this time window, but also efficacious in both in diagnostic accuracy and the extent of resection.
The currently limited data regarding fluorescence kinetics of 5-ALA seem to indicate a more delayed fluorescence peak within HGGs than previously thought. In an early study utilizing an orthotopic brain tumor model, the maximum fluorescence intensity was shown to be around 6 h after administration (17). The recommended time of administration used in the summary of the product characteristics (SPC) for FDA approval (Gleolan©) was based upon animal studies showing that fluorescence peaked 6 h after administration (33). Extrapolated from these experiments, the parameter of administration of 3–4 h prior to anesthesia would allow time for operating room set up and resection in ample time for peak fluorescence during tumor resection (18). As a result, 5-ALA clinical trials, including the RCT, have utilized this parameter (9, 19–32). In the FDA New Drug Application (NDA), six clinical trials were included that used this parameter, all of which were conducted in Germany (34). Prior to this study, only one clinical trial recommended administration of 5-ALA 3–5 h prior to surgery. No studies have assessed a longer time interval.
Other studies have suggested that 5-ALA induced fluorescence may peak beyond the previously studied time window of 2–4 h. Studies measuring PpIX concentration in other parts of the human body have shown a peak in the plasma to be around 8 h (32), and in the skin to be 6.5 to 9.8 h (35). In a later study of 201 samples from 68 patients, Kaneko et al. investigated the time dependency of protoporphyrin IX (PpIX) by measuring fluorescence intensity in tumor biopsy samples at various time points during HGG surgery (18). The authors recorded the time-fluorescence curves in their ex-situ study, demonstrating that the peak intensity may be 7–8 h after 5-ALA administration, and suggested that 5-ALA be administered earlier than what is currently suggested by the FDA, specifically 4–5 h prior to anesthesia induction (18). In line with their work, we found that there was adequate intraoperative fluorescence to guide HGG resection in all our cases that were induced to anesthesia more than 4 h after imbibing 5-ALA. Our results confirm that tumor fluorescence is even present more than 24 h after oral administration in some cases.
The identification of the ideal timing of 5-ALA administration prior to surgery is of high importance for multiple reasons. In everyday practice, where operations may be postponed for various logistical reasons (staffing, cleaning of the operating room, emergent cases), it would be useful to know if any such delay could adversely affect intraoperative fluorescence and, possibly, patient outcomes. Individual institutions and surgeons should account for the time needed for exposure or mapping, and plan accordingly. For tumors known to demonstrate less fluorescence (e.g., WHO grade III gliomas), for tumor margins and in deep-seated gliomas, resection during the peak of fluorescence intensity would be ideal. Another important aspect regarding administration of 5-ALA is the timing of 5-ALA and PpIX clearance from the tumor bed. Interestingly, glioblastomas seem to maintain up to 65% of their maximal fluorescence even 10 h after 5-ALA administration (18), confirming that fluorescence clearance may be much slower than its accumulation. Kaneko et al. found that tumor type may predict clearance time of fluorescence (18), which may advocate for further investigation on establishing different time periods for administration based upon pathology. Additionally, the infiltrative tumor margin may have a later peak at around 8–9 h, further corroborating the need for more delayed administration (18, 33). However, the exact timing of fluorescence clearance from the tumor bed is not currently known and further study is warranted to determine the true extent of the 5-ALA time window in order to demonstrate in situ results regarding when fluorescence is no longer visible.
The current study did not demonstrate any concerns with patient safety, and had comparable outcomes to prior studies. Of the 16 patients included between two centers, there were no photosensitivity reactions or other 5-ALA-related toxicities noted. The average extent of resection was over 90%, defined as a gross total resection. This compares to other observational and clinical trials showing GTR rates ranging between 25 and 94% with 5-ALA (9–11). In terms of patient outcomes, patients saw an average progression-free survival (PFS) rate of 5 months, with a 6-month PFS rate of 46.2%, which is comparable to previous studies showing a 6-month PFS rate of 46% (22) and average PFS of 8.6 months (36). In our cohort, overall survival was 6.3 ± 4.9 months, which is less than prior studies on 5-ALA FGS (9–11), and may be explained by the patients in the current study. Twelve (75%) patients had tumors in eloquent cortex, which may be less amenable to maximal resection, therefore conferring a lower overall survival. Additionally, multiple patients in the cohort had surgery for recurrent GBM, which is also associated with a lower overall survival rate (37, 38). While this cohort is smaller than prior clinical trials, it did not appear that earlier administration of 5-ALA had an impact on both surgical factors and patient outcomes.
Despite the strengths of this study, there are limitations that must be addressed. The most significant limitation to the present study is the design. As a small, retrospective, single-arm study, the level of evidence is limited, and patient and clinical variables were not able to be controlled. Historical controls were used rather than a randomized control group, and therefore findings from our study may only be assessed as associations, rather than causational. Additionally, the current study was performed at two centers over a 2-year period, and therefore the results may not be representative of other centers. Intraoperative fluorescence was assessed by the primary surgeon and verified by a second surgeon, and no quantified data was obtained, therefore, there may be an observer bias as there were no controls and subjects were not blinded. To address many of these limitations, a larger, multicenter randomized controlled trial is warranted to determine the safety and efficacy of 5-ALA administration at a longer time window between administration and surgical resection.
In this preliminary case series, we demonstrate that 5-ALA FGS can be carried out safely more than 6 h after administration of the substance, with clinical results being largely similar to previous reports. The relaxation on restrictions regarding timing of surgery after 5-ALA administration could have positive implications on procedure scheduling and workflow in busy neurosurgical centers, without any additional risk to patient safety and surgical outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Mount Sinai Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
CH is a consultant for NX Development Corporation (NXDC) and Synaptive Medical. NXDC, a privately held company, markets Gleolan (5-ALA, aminolevulinic acid hydrochloride). Gleolan is an optical imaging agent approved for the visualization of malignant tissue during glioma surgery. CH is a consultant for NXDC and receives royalty payments for the sale of Gleolan. CH receives financial compensation as a consultant and lecturer for Synaptive (manufacturer of the 3D Synaptive MODUS V device). He has also received speaker fees by Carl Zeiss and Leica.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
2. Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. (2014) 32:783–90. doi: 10.1200/JCO.2013.49.3726
3. van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MCM, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. (2013) 31:344–350. doi: 10.1200/JCO.2012.43.2229
4. Wick W, Roth P, Hartmann C, Stockhammer F, Sabel MC, Wick A, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. (2016) 18:1529–37. doi: 10.1093/neuonc/now133
5. Chang S, Zhang P, Cairncross JG, Gilbert MR, Bahary JP, Dolinskas CA, et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol. (2017) 19:252–58. doi: 10.1093/neuonc/now313
6. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. (2001) 95:190–8. doi: 10.3171/jns.2001.95.2.0190
7. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. (2014) 32:774–82. doi: 10.1200/JCO.2013.51.8886
8. Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. (2012) 117:851–9. doi: 10.3171/2012.8.JNS12234
9. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. (2006) 7:392–401. doi: 10.1016/S1470-2045(06)70665-9
10. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. (2000) 93:1003–13. doi: 10.3171/jns.2000.93.6.1003
11. Senders JT, Muskens IS, Schnoor R, Karhade AV, Cote DJ, Smith TR. Agents for fluorescence-guided glioma surgery: a systematic review of preclinical and clinical results. Acta Neurochir. (2017) 159:151–67. doi: 10.1007/s00701-016-3028-5
12. Colditz MJ, Leyen K, Jeffree RL. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: theoretical, biochemical and practical aspects. J Clin Neurosci. (2012) 19:1611–6. doi: 10.1016/j.jocn.2012.03.013
13. Zehri AH, Ramey W, Georges JF, Mooney MA, Martirosyan NL, Preul MC, et al. Neurosurgical confocal endomicroscopy: a review of contrast agents, confocal systems, and future imaging modalities. Surg Neurol Int. (2014) 5:60. doi: 10.4103/2152-7806.131638
14. Teixidor P, Arraez MA, Villalba G, Garcia R, Tardáguila M, González JJ, et al. Safety and efficacy of 5-aminolevulinic acid for high grade glioma in usual clinical practice: a prospective cohort study. PLoS ONE. (2016) 11:e0149244. doi: 10.1371/journal.pone.0149244
15. Hadjipanayis CG, Stummer W. 5-ALA and FDA approval for glioma surgery. J Neurooncol. (2019) 141:479–86. doi: 10.1007/s11060-019-03098-y
16. CDC. Aminolevulinic acid hydrochloride, known as ALA HCl (Gleolan, NX Development Corp.) as an optical imaging agent indicated in patients with gliomas (2017). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/aminolevulinic-acid-hydrochloride-known-ala-hcl-gleolan-nx-development-corp-optical-imaging-agent (accessed June 3, 2020).
17. Stummer W, Stocker S, Novotny A, Heimann A, Sauer O, Kempski O, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B. (1998) 45:160–9. doi: 10.1016/S1011-1344(98)00176-6
18. Kaneko S, Suero Molina E, Ewelt C, Warneke N, Stummer W. Fluorescence-based measurement of real-time kinetics of protoporphyrin IX after 5-aminolevulinic acid administration in human in situ malignant gliomas. Neurosurgery. (2019) 85:E739–46. doi: 10.1093/neuros/nyz129
19. Pichlmeier U, Bink A, Schackert G, Stummer W, the ALA Glioma Study Group. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. (2008) 10:1025–34. doi: 10.1215/15228517-2008-052
20. Stummer W, Suero Molina E. Fluorescence imaging/agents in tumor resection. Neurosurg Clin N Am. (2017) 28:569–83. doi: 10.1016/j.nec.2017.05.009
21. Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. (2014) 74:310–9. doi: 10.1227/NEU.0000000000000267
22. Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg. (2011) 114:613–23. doi: 10.3171/2010.3.JNS097
23. Valdes PA, Fan X, Ji S, Harris BT, Paulsen KD, Roberts DW. Estimation of brain deformation for volumetric image updating in protoporphyrin IX fluorescence-guided resection. Stereotact Funct Neurosurg. (2010) 88:1–10. doi: 10.1159/000258143
24. Valdes PA, Kim A, Brantsch M, Niu C, Moses ZB, Tosteson TD, et al. delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. (2011) 13:846–56. doi: 10.1093/neuonc/nor086
25. Valdes PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. (2011) 115:11–7. doi: 10.3171/2011.2.JNS101451
26. Jaber M, Wolfer J, Ewelt C, Holling M, Hasselblatt M, Niederstadt T, et al. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery. (2016) 78:401–11. doi: 10.1227/NEU.0000000000001020
27. Johansson A, Palte G, Schnell O, Tonn JC, Herms J, Stepp H. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem Photobiol. (2010) 86:1373–8. doi: 10.1111/j.1751-1097.2010.00799.x
28. Kaneko S, Kaneko S. Fluorescence-guided resection of malignant glioma with 5-ALA. Int J Biomed Imaging. (2016) 2016:6135293. doi: 10.1155/2016/6135293
29. Lau D, Hervey-Jumper SL, Chang S, Molinaro AM, McDermott MW, Phillips JJ, et al. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg. (2016) 124:1300–9. doi: 10.3171/2015.5.JNS1577
30. Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg. (2011) 114:595–603. doi: 10.3171/2010.2.JNS091322
31. Roessler K, Becherer A, Donat M, Cejna M, Zachenhofer I. Intraoperative tissue fluorescence using 5-aminolevolinic acid (5-ALA) is more sensitive than contrast MRI or amino acid positron emission tomography ((18)F-FET PET) in glioblastoma surgery. Neurol Res. (2012) 34:314–7. doi: 10.1179/1743132811Y.0000000078
32. Stummer W, Stepp H, Wiestler OD, Pichlmeier U. Randomized, prospective double-blinded study comparing 3 different doses of 5-aminolevulinic acid for fluorescence-guided resections of malignant gliomas. Neurosurgery. (2017) 81:230–9. doi: 10.1093/neuros/nyx074
33. Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery. (2013) 72:915–20. doi: 10.1227/NEU.0b013e31828c3974
34. Ballard B. Center for Drug Evaluation and Research: Clinical Review. (2015). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208630Orig1s000MedR.pdf. (accessed June 15, 2020).
35. Rick K, Sroka R, Stepp H, Kriegmair M, Huber RM, Jacob K, et al. Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. J Photochem Photobiol B. (1997) 40:313–9. doi: 10.1016/S1011-1344(97)00076-6
36. Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. (2008) 23:361–7. doi: 10.1007/s10103-007-0494-2
37. Botros D, Dux H, Price C, Khalafallah AM, Mukherjee D. Assessing the efficacy of repeat resections in recurrent glioblastoma: a systematic review. Neurosurg Rev. (2020). doi: 10.1007/s10143-020-01331-1. [Epub ahead of print].
38. McNamara MG, Lwin Z, Jiang H, Templeton AJ, Zadeh G, Bernstein M, et al. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. (2014) 117:147–52. doi: 10.1007/s11060-014-1366-9
Keywords: fluorescence, 5-ALA, glioma, glioblastomas, brain tumors, neuro-oncology, intraoperative imaging
Citation: Maragkos GA, Schüpper AJ, Lakomkin N, Sideras P, Price G, Baron R, Hamilton T, Haider S, Lee IY, Hadjipanayis CG and Robin AM (2021) Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front. Neurol. 12:644804. doi: 10.3389/fneur.2021.644804
Received: 21 December 2020; Accepted: 08 February 2021;
Published: 09 March 2021.
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Alexander Aleksandrovich Potapov, N.N. Burdenko National Scientific and Practical Center for Neurosurgery, RussiaCopyright © 2021 Maragkos, Schüpper, Lakomkin, Sideras, Price, Baron, Hamilton, Haider, Lee, Hadjipanayis and Robin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios A. Maragkos, Z2Vvcmdpb3MubWFyYWdrb3NAbW91bnRzaW5haS5vcmc=; Alexander J. Schüpper, QWxleGFuZGVyLlNjaHVwcGVyQG1vdW50c2luYWkub3Jn
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.