
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 04 March 2021
Sec. Applied Neuroimaging
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.644198
This article is part of the Research TopicnTMS, Connectivity and Neuromodulation in Brain Tumor PatientsView all 25 articles
 Giuseppe Emmanuele Umana1*
Giuseppe Emmanuele Umana1* Gianluca Scalia2
Gianluca Scalia2 Francesca Graziano2,3
Francesca Graziano2,3 Rosario Maugeri3
Rosario Maugeri3 Nicola Alberio1
Nicola Alberio1 Fabio Barone1
Fabio Barone1 Antonio Crea1,4
Antonio Crea1,4 Saverio Fagone1
Saverio Fagone1 Giuseppe Roberto Giammalva3
Giuseppe Roberto Giammalva3 Lara Brunasso3
Lara Brunasso3 Roberta Costanzo3
Roberta Costanzo3 Federica Paolini3
Federica Paolini3 Rosa Maria Gerardi3
Rosa Maria Gerardi3 Silvana Tumbiolo5
Silvana Tumbiolo5 Salvatore Cicero1
Salvatore Cicero1 Giovanni Federico Nicoletti2
Giovanni Federico Nicoletti2 Domenico Gerardo Iacopino3
Domenico Gerardo Iacopino3Background: The surgical strategy for brain glioma has changed, shifting from tumor debulking to a more careful tumor dissection with the aim of a gross-total resection, extended beyond the contrast-enhancement MRI, including the hyperintensity on FLAIR MR images and defined as supratotal resection. It is possible to pursue this goal thanks to the refinement of several technological tools for pre and intraoperative planning including intraoperative neurophysiological monitoring (IONM), cortico-subcortical mapping, functional MRI (fMRI), navigated transcranial magnetic stimulation (nTMS), intraoperative CT or MRI (iCT, iMR), and intraoperative contrast-enhanced ultrasound. This systematic review provides an overview of the state of the art techniques in the application of nTMS and nTMS-based DTI-FT during brain tumor surgery.
Materials and Methods: A systematic literature review was performed according to the PRISMA statement. The authors searched the PubMed and Scopus databases until July 2020 for published articles with the following Mesh terms: (Brain surgery OR surgery OR craniotomy) AND (brain mapping OR functional planning) AND (TMS OR transcranial magnetic stimulation OR rTMS OR repetitive transcranial stimulation). We only included studies regarding motor mapping in craniotomy for brain tumors, which reported data about CTS sparing.
Results: A total of 335 published studies were identified through the PubMed and Scopus databases. After a detailed examination of these studies, 325 were excluded from our review because of a lack of data object in this search. TMS reported an accuracy range of 0.4–14.8 mm between the APB hotspot (n1/4 8) in nTMS and DES from the DES spot; nTMS influenced the surgical indications in 34.3–68.5%.
Conclusion: We found that nTMS can be defined as a safe and non-invasive technique and in association with DES, fMRI, and IONM, improves brain mapping and the extent of resection favoring a better postoperative outcome.
The surgical strategy for brain glioma has changed dramatically throughout the years, shifting from tumor debunking with subtotal resection to a more careful tumor dissection with the aim of a gross-total resection (GTR) while sparing neurologic functions. This more aggressive strategy was demonstrated to increase survival, the actual goal of glioma surgery, and has been extended beyond the contrast-enhancement MRI, including the hyperintensity on FLAIR MR images and defined as supratotal resection (SpTR). It is possible to pursue this goal thanks to the refinement of several technological tools for pre and intraoperative planning including intraoperative neurophysiological monitoring (IONM), cortico-subcortical mapping, functional MRI (fMRI), navigated transcranial magnetic stimulation (nTMS), intraoperative CT or MRI (iCT, iMR), and intraoperative contrast-enhanced ultrasound (CEUS) (1–6). These methods not only allow more detailed preoperative planning but are effective in the evaluation of motor pathways integrity and are a valuable tool to guide tumor resection. It has been reported that, cortically, the closer the distance between the tumor and motor cortex, the greater the risk of new motor deficit, as demonstrated by lesion to activation distance (LAD) assessment in fMRI (1, 7–9). Similarly, at the subcortical stage, usually the proximity of the tumor to the corticospinal tract (CST) is related to a higher risk of motor deficits, but a great variability has also been reported (10–12). Moreover, repeated subcortical stimulation and its intensity modulation present a positive correlation for the detection of the CST (13, 14). The reliability of preoperative tractography is well-demonstrated to be consistent with subcortical stimulation for the CST location, in about 95% of cases (15), providing a marked improvement in the tractography data, which is not surgeon-dependent and has a strong clinical correlation allowing for reliable subcortical mapping associated with diffusion tensor imaging fiber-tracking (DTI FT) (16–19). This association has only been reported twice in literature, stating that it offers patient-specific analysis of the risk of deficit for lesions sited in eloquent areas, which can be avoided when keeping 8 mm from the CTS (15, 19). This systematic review provides an overview of the state of the art techniques in the application of nTMS and nTMS-based DTI-FT during brain tumor surgery.
A systematic literature review was performed according to the PRISMA statement and related checklists. The authors searched the PubMed and Scopus databases until July 2020 for published articles with the following Mesh terms: (Brain surgery OR surgery OR craniotomy) AND (brain mapping OR functional planning) AND (TMS OR transcranial magnetic stimulation OR rTMS OR repetitive transcranial stimulation); a language restriction to English only papers was also applied. All included studies were meticulously reviewed and scrutinized for their study design, methodology, and patient characteristics. We only included 10 studies regarding motor mapping in craniotomy for brain tumors, which reported data about CTS sparing (Figure 1). Data for all patients were recorded when available, including accuracy, GTR, STR, permanent deficits, change of strategy, and intraoperative tools used (Table 1).
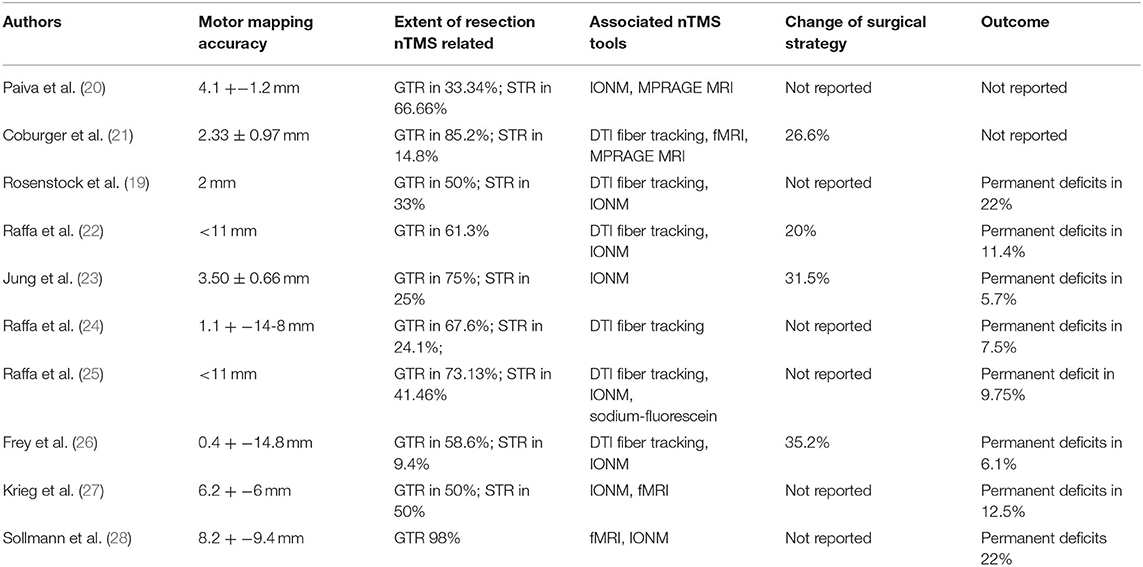
Table 1. Summary of the systematic review including authors, motor mapping accuracy, extent of resection nTMS related, associated nTMS tools, eventual change of surgical strategy and outcome.
A linear regression analysis was performed using Excel software. R2 is the coefficient of determination. We compared estimated and actual y-values, and ranges in value from 0 to 1. If it was 1, there was perfect correlation in the sample—there was no difference between the estimated y-value and the actual y-value. At the other extreme, if the coefficient of determination was 0, the regression equation was not helpful in predicting a y-value. f is the F statistic, or the F-observed value. We used the F statistic to determine whether the observed relationship between the dependent and independent variables occurred by chance (slope +− fault slope, intercepts +− fault intercepts, r2, f).
A total of 335 published studies were identified through the PubMed and Scopus databases. After a detailed examination of these studies, 325 were excluded from our review because of a lack of data object in this search, or did not report accurate data.
All the fits showed a low r2 value, while F was high. Linear multiple regression analysis showed that there was no correlation from the extracted data among the variables plotted in the graphs (Figure 2). Accuracy reported rate ranged from 0.4 to 14.8 mm; GTR range was 33–98%, and STR range 9.4–66.6%. The associated nTMS tools used included DTI fiber tracking, fMRI, MPRAGE MRI, IONM, and sodium-fluorescein. IONM was used in 8 out of 10 studies suggesting that this was considered the most reliable tool, followed by DTI fiber tracking (6 out of 10), fMRI (4 out of 10), and sodium fluorescence as the emerging tool (1 out of 10).
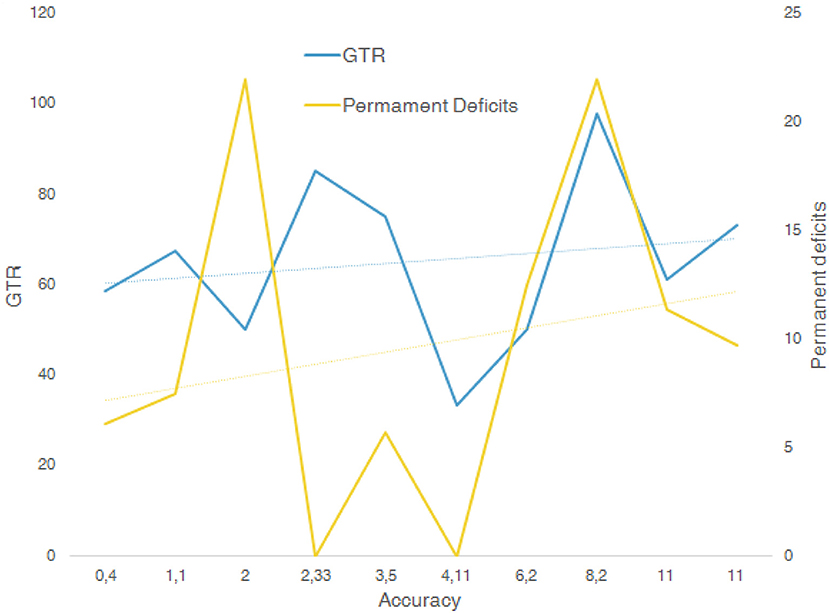
Figure 2. Multiple linear regression analysis between variables GTR, accuracy, and permanent deficits.
The gold standard for functional assessment and surgical planning is represented by DES associated with IONM (29–32). With the aim to improve risk stratification of motor eloquent area detection in the preoperative phase, other techniques have been introduced. Function MRI is a valuable tool, which helps to obtain visuo-spatial data of motor and language functions, which can be merged with the anatomic multiplanar MRI study in navigation planning (22, 33–35). fMRI offers 59–100% sensitivity, with 0–97% specificity, which although a drawback offers a great variability operator dependent of language mapping, while tractography representation does not offer functional data (36–39). TMS mapping is not a novelty by itself, introduced in 1985 (40), it has been reported to be a valuable tool in risk stratification and the mapping of motor and language areas for surgical planning (41–50). Of notice, nTMS is used directly by neurosurgeons, in the context of the neurosurgical department and it is independent of neuroradiological availability, helping its routine use in the setting of surgical planning.
An important TMS parameter is stimulation focality, which corresponds to the cortical area where the TMS' electric field strength reaches half the maximum value (51, 52). The smaller this area is, the better the focally and accuracy. Thielscher and Kammer (52) reported that the variability map size documented among patients can related to different coil–cortex distances and cortex radii. The focally of a coil can be quantitatively estimated by the electric field on a hemisphere representing the brain cortex radius r = 8 cm. Furthermore, Thielscher and Kammer reported that the variability in map dimension among different patients is related to two parameters: coil–cortex distances and cortex radii. Thus, the variability documented in our research can be related mainly to operator-dependent variables, rather than technical TMS characteristics, confirming the reliability and the utility of nTMS in a multimodal motor mapping setting. In literature, it has been reported that TMS displayed an accuracy range of 0.4–14.8 mm between the APB hotspot (n1/4 8) in nTMS and DES from the DES spot (19, 23–28, 48, 52–57). These data endorse the reliability of nTMS in motor mapping, representing a useful tool in multimodal brain mapping. An important point is the reduction of the surgical time: nTMS plays an important role in the guidance of the intraoperative stimulation, saving time during cortical mapping. Moreover, the preoperative cortical mapping related to nTMS reduces the need of large cortical exposure, thus reducing the craniotomy size and again the surgical time related to the craniotomy opening/closing step.
nTMS reliability has been proven to be very strong and can influence the surgical indication to change from no surgery/biopsy to craniotomy removal in 34.3–68.5% of cases (23, 24, 26, 58–60). As already reported in the previous paragraph, the size of the craniotomy is reduced and thus the surgical strategy is modified according to the nTMS mapping, which allows professionals to plan for the location of the motor cortex, guiding the “no-look” positioning of the strip electrode, without direct visualization of the cortical motor cortex. Moreover, if brain mapping shows the absence of an eloquent area at the level of the anatomic cortical landmark, it allows surgeons to conduct the surgical removal through the cortex in otherwise considered functional areas. Jung et al. (23) reported a transopercular approach guided by the negative correspondence between the anatomic area and the language mapping of the nTMS, likewise another patient in which nTMS documented the absence of motor function at the level of the premised primary motor cortex in a patient affected by cavernoma, modifying the clinical management from no survey to indication of craniotomy.
In literature, several authors documented the positive influence of nTMS on surgical planning and postoperative outcome, with a significant role in risk stratification (26, 27, 31, 45, 61, 62). Interestingly, and apparently in contrast to these data, some authors reported more postoperative neurological deficits, with delayed recovery. An interpretation of this finding could be that more deficits are relative to a more aggressive surgical strategy encouraged by the combined use of DES and nTMS in eloquent areas (26). Even if in the literature there are several reports about sodium fluorescence (63, 64), it is not possible to provide statistically significant data as it is an emerging tool, reported only in 1 out of 10 of the selected paper in this review.
About the role of nTMS and its effect on the extent of surgical resection (ESR), there are no univocal reports. Despite the fact that some authors (23) did not find a direct relation between nTMS and ESR, others documented a greater ESR in surgical series in which nTMS was associated with DES and IONM, and a longer progression-free survival (26, 27, 45, 65–67). These different findings could be related to the novelty of this technique and thus to the learning curve. Of course, a better understanding and a systematic analysis of data is required through randomized multicentric studies.
From the analysis of the present systematic review, we found that nTMS can be defined as a safe and non-invasive technique, which when associated with DES, fMRI, and IONM improves brain mapping and the extent of resection with a better postoperative outcome. Of notice, the reliability of nTMS has been documented to modify the surgical strategy for oncologic patients.
The original contributions generated for this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
GU, GS, FG, and RM: conception and design of study and analysis and/or interpretation of data. GU and GS: acquisition of data. GU, GS, FG, RM, NA, FB, AC, SF, GG, LB, RC, FP, and RG: drafting the manuscript. ST, SC, GN, and DI: revising the manuscript critically for important intellectual content. GU, GS, FG, RM, NA, FB, AC, SF, GG, LB, RC, FP, RG, ST, SC, GN, and DI: approval of the version of the manuscript to be published. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
fMRI, Functional MRI; nTMS, Navigated transcranial magnetic stimulation; IONM, Intraoperative monitoring; SpTR, Supratotal resection; GTR, Gross-total resection; CST, Corticospinal tract; EOR, Extent of resection; CEUS, Contrast-enhanced ultrasound; DES, Direct electrical stimulation.
1. Almenawer SA, Badhiwala JH, Alhazzani W, Greenspoon J, Farrokhyar F, Yarascavitch B, et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol. (2015) 17:868–81. doi: 10.1093/neuonc/nou349
2. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta- analysis. JAMA Oncol. (2016) 2:1460–9. doi: 10.1001/jamaoncol.2016.1373
3. Duffau H, Mandonnet E. The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien). (2013) 155:951–7. doi: 10.1007/978-1-4471-2213-5
4. Lee CH, Kim DG, Kim JW, Han JH, Kim YH, Park CK, et al. The role of surgical resection in the management of brain metastasis: a 17-year longitudinal study. Acta Neurochir (Wien). (2013) 155:389–97. doi: 10.1007/s00701-013-1619-y
5. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, We-ingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. (2009) 65:463–70. doi: 10.1227/01.NEU.0000349763.42238.E9
6. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. (2008) 62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf
7. Håberg A, Kvistad KA, Unsgård G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. (2004) 54:902–15. doi: 10.1227/01.NEU.0000114510.05922.F8
8. Krishnan R, Raabe A, Hattingen E, Szelenyi A, Yahya H, Hermann E, et al. Functional magnetic resonance imagingintegrated neuronavigation: correlation between lesion-to- motor cortex distance and outcome. Neurosurgery. (2004) 55:904–15. doi: 10.1227/01.NEU.0000137331.35014.5C
9. Wood JM, Kundu B, Utter A, Gallagher TA, Voss J, Nair VA, et al. Impact of brain tumor location on morbidity and mortality: a retrospective functional MR imaging study. AJNR Am J Neuroradiol. (2011) 32:1420–5. doi: 10.3174/ajnr.A2679
10. Bailey PD, Zacà D, Basha MM, Agarwal S, Gujar SK, Sair HI, et al. Presurgical fMRI and DTI for the prediction of perioperative motor and language deficits in primary or metastatic brain lesions. J Neuroimaging. (2015) 25:776–84. doi: 10.1111/jon.12273
11. Nimsky C, Ganslandt O, Merhof D, Sorensen AG, Fahlbusch R. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage. (2006) 30:1219–29. doi: 10.1016/j.neuroimage.2005.11.001
12. Ulmer JL, Salvan CV, Mueller WM, Krouwer HG, Stroe GO, Aralasmak A, et al. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: implications for preoperative risk assessments and postoperative outcomes. Technol Cancer Res Treat. (2004) 3:567–76. doi: 10.1177/153303460400300606
13. Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neuro Surg. (2014) 120:1015–24. doi: 10.3171/2014.1.JNS13909
14. Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg. (2013) 118:287–96. doi: 10.3171/2012.10.JNS12895
15. Sollmann N, Wildschuetz N, Kelm A, Conway N, Moser T, Bulubas L, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J Neurosurg. (2018) 128:800–10. doi: 10.3171/2016.11.JNS162322
16. Conti A, Raffa G, Granata F, Rizzo V, Germanò A, Tomasello F. Navigated transcranial magnetic stimulation for “somatotopic” tractography of the corticospinal tract. Neurosurgery. (2014) 10:542–54. doi: 10.1227/NEU.0000000000000502
17. Frey D, Strack V, Wiener E, Jussen D, Vajkoczy P, Picht T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized frac- tional anisotropy values. Neuroimage. (2012) 62:1600–9. doi: 10.1016/j.neuroimage.2012.05.059
18. Krieg SM, Buchmann NH, Gempt J, Shiban E, Meyer B, Ringel F. Diffusion tensor imaging fiber tracking using navi- gated brain stimulation—a feasibility study. Acta Neurochir (Wien). (2012) 154:555–63. doi: 10.1007/s00701-011-1255-3
19. Rosenstock T, Grittner U, Acker G, Schwarzer V, Kulchytska N, Vajkoczy P, et al. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J Neurosurg. (2017) 126:1227–37. doi: 10.3171/2016.4.JNS152896
20. Paiva WS, Fonoff ET, Marcolin MA, Cabrera HN, Teixeira MJ. Cortical mapping with navigated transcranial magnetic stimulation in low-grade glioma surgery. Neuropsychiatr Dis Treat. (2012) 8:197–201. doi: 10.2147/NDT.S30151
21. Coburger J, Musahl C, Henkes H, Horvath-Rizea D, Bittl M, Weissbach C, et al. Comparison of navigated transcranial magnetic stimulation and functional magnetic resonance imaging for preoperative mapping in rolandic tumor surgery. Neurosurg Rev. (2013) 36:65–75. doi: 10.1007/s10143-012-0413-2
22. Raffa G, Conti A, Scibilia A, Sindorio C, Quattropani MC, Visocchi M, et al. Functional reconstruction of motor and language pathways based on navigated transcranial magnetic stimulation and DTI fiber tracking for the preoperative planning of low grade glioma surgery: a new tool for preservation and restoration of eloquent networks. Acta Neurochir Suppl. (2017) 124:251–61. doi: 10.1007/978-3-319-39546-3_37
23. Jung J, Lavrador JP, Patel S, Giamouriadis A, Lam J, Bhangoo R, et al. First United Kingdom experience of navigated transcranial magnetic stimulation in preoperative mapping of brain tumors. World Neurosurg. (2019) 122:e1578–87. doi: 10.1016/j.wneu.2018.11.114
24. Raffa G, Quattropani MC, Germanò A. When imaging meets neurophysiology: the value of navigated transcranial magnetic stimulation for preoperative neurophysiological mapping prior to brain tumor surgery. Neurosurg Focus. (2019) 47:E10. doi: 10.3171/2019.9.FOCUS19640
25. Raffa G, Scibilia A, Conti A, Cardali SM, Rizzo V, Terranova C, et al. Multimodal surgical treatment of high-grade gliomas in the motor area: the impact of the combination of navigated transcranial magnetic stimulation and fluorescein-guided resection. World Neurosurg. (2019) 128:e378–90. doi: 10.1016/j.wneu.2019.04.158
26. Frey D, Schilt S, Strack V, Zdunczyk A, Rösler J, Niraula B, et al. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. (2014) 16:1365–72. doi: 10.1093/neuonc/nou110
27. Krieg SM, Sabih J, Bulubasova L, Obermueller T, Negwer C, Janssen I, et al. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro Oncol. (2014) 16:1274–82. doi: 10.1093/neuonc/nou007
28. Sollmann N, Zhang H, Fratini A, Wildschuetz N, Ille S, Schröder A, et al. Risk assessment by presurgical tractography using navigated TMS maps in patients with highly motor- or language-eloquent brain tumors. Cancers (Basel). (2020) 12:1264. doi: 10.3390/cancers12051264
29. Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg. (1989) 71:316–26. doi: 10.3171/jns.1989.71.3.0316
30. Sollmann N, Kelm A, Ille S, Schröder A, Zimmer C, Ringel F, et al. Setup presentation and clinical outcome analysis of treating highly language-eloquent gliomas via preoperative navigated transcranial magnetic stimulation and tractography. Neurosurg Focus. (2018) 44:E2. doi: 10.3171/2018.3.FOCUS1838
31. Raffa G, Picht T, Scibilia A, Rösler J, Rein J, Conti A, et al. Surgical treatment of meningiomas located in the rolandic area: the role of navigated transcranial magnetic stimulation for preoperative planning, surgical strategy, and prediction of arachnoidal cleavage and motor outcome. J Neurosurg. (2019) 14:1–12. doi: 10.3171/2019.3.JNS183411
32. Rizzo V, Terranova C, Conti A, Germanò A, Alafaci C, Raffa G, et al. Preoperative functional mapping for rolandic brain tumor surgery. Neurosci Lett. (2014) 583:136–41. doi: 10.1016/j.neulet.2014.09.017
33. Tie Y, Rigolo L, Norton IH, Huang RY, Wu W, Orringer D, et al. Defining lan- guage networks from resting-state fMRI for surgical planning—a feasibility study. Hum Brain Mapp. (2014) 35:1018–30. doi: 10.1002/hbm.22231
34. Kallioniemi E, Pitkänen M, Könönen M, Vanninen R, Julkunen P. Localization of cortical primary motor area of the hand using navigated transcranial magnetic stimulation, BOLD and arterial spin labeling fMRI. J Neurosci Methods. (2016) 273:138–48. doi: 10.1016/j.jneumeth.2016.09.002
35. Umana GE, Raudino G, Alberio N, Inserra F, Giovinazzo G, Fricia M, et al. Slit-like hypertensive hydrocephalus: report of a late, complex, and multifactorial complication in an oncologic patient. Surg Neurol Int. (2020) 11:219. doi: 10.25259/SNI_145_2020
36. Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. (2010) 66:113–20. doi: 10.1227/01.NEU.0000360392.15450.C9
37. Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. (2011) 1:169–83. doi: 10.1089/brain.2011.0033
38. Dell'Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. (2012) 25:375–83. doi: 10.1097/WCO.0b013e328355d544
39. Tomasi SO, Umana GE, Scalia G, Rubio-Rodriguez RL, Cappai PF, Capone C, et al. Importance of veins for neurosurgery as landmarks against brain shifting phenomenon: an anatomical and 3D-MPRAGE MR reconstruction of superficial cortical veins. Front Neuroanat. (2020) 14:596167. doi: 10.3389/fnana.2020.596167
40. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. (1985) 1:1106–7. doi: 10.1016/S0140-6736(85)92413-4
41. Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, et al. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. (2012) 117:354–62. doi: 10.3171/2012.5.JNS112124
42. Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, et al. Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage. (2013) 82:260–72. doi: 10.1016/j.neuroimage.2013.05.018
43. Sollmann N, Hauck T, Obermüller T, Hapfelmeier A, Meyer B, Ringel F, et al. Inter- and intraobserver variability in motor mapping of the hotspot for the abductor policis brevis muscle. BMC Neurosci. (2013) 14:94. doi: 10.1186/1471-2202-14-94
44. Lefaucheur JP, Picht T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin. (2016) 46:125–33. doi: 10.1016/j.neucli.2016.05.001
45. Picht T, Schulz J, Hanna M, Schmidt S, Suess O, Vajkoczy P. Assessment of the influence of navigated transcranial magnetic stimulation on surgical planning for tumors in or near the motor cortex. Neurosurgery. (2012) 70:1248–56. doi: 10.1227/NEU.0b013e318243881e
46. Picht T, Frey D, Thieme S, Kliesch S, Vajkoczy P. Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: a controlled observational study. J Neurooncol. (2016) 126:535–43. doi: 10.1007/s11060-015-1993-9
47. Raffa G, Picht T, Angileri FF, Youssef M, Conti A, Esposito F, et al. Surgery of malignant motor-eloquent gliomas guided by sodium-fluorescein and navigated transcranial magnetic stimulation: a novel technique to increase the maximal safe resection. J Neurosurg Sci. (2019) 63:670–8. doi: 10.23736/S0390-5616.19.04710-6
48. Raffa G, Conti A, Scibilia A, Cardali SM, Esposito F, Angileri FF, et al. The impact of diffusion tensor imaging fiber tracking of the corticospinal tract based on navigated transcranial magnetic stimulation on surgery of motor-eloquent brain lesions. Neurosurgery. (2018) 83:768–82. doi: 10.1093/neuros/nyx554
49. Umana GE, Alberio N, Amico P, Lavecchia AM, Fagone S, Fricia M, et al. Giant cystic brain metastasis from ovarian papillary serous adenocarcinoma: case report and review of the literature. Interdiscip Neurosurg. (2020) 20:100668. doi: 10.1016/j.inat.2020.100668
50. Umana GE, Scalia G, Spitaleri A, Alberio N, Fricia M, Tomasi SO, et al. Use of gelatin-thrombin hemostatic matrix for control of ruptured cerebral aneurysm. Cent Eur Neurosurg. (2020). doi: 10.1055/s-0040-1720986
51. Roth BJ, Saypol JM, Hallett M, Cohen LG. A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Muscle Nerve. (1990) 13:734–41. doi: 10.1002/mus.880130812
52. Thielscher A, Kammer T. Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin Neurophysiol. (2004) 115:1697–708. doi: 10.1016/j.clinph.2004.02.019
53. Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, et al. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. (2011) 69:581–8. doi: 10.1227/NEU.0b013e3182181b89
54. Takahashi S, Vajkoczy P, Picht T. Navigated transcranial magnetic stimulation for mapping the motor cortex in patients with rolandic brain tumors. Neurosurg Focus. (2013) 34:E3. doi: 10.3171/2013.1.FOCUS133
55. Raffa G, Scibilia A, Germanò A, Conti A. “nTMS-Based DTI Fiber Tracking of Motor Pathways. In: Krieg SM, editor. Navigated Transcranial Magnetic Stimulation in Neurosurgery. Cham: Springer International Publishing (2017). p. 97–114. doi: 10.1007/978-3-319-54918-7_6
56. La Torre D, Maugeri R, Angileri FF, Pezzino G, Conti A, Cardali SM, et al. Human leukocyte antigen frequency in human high-grade gliomas: a case-control study in Sicily. Neurosurgery. (2009) 64:1082–8. doi: 10.1227/01.NEU.0000345946.35786.92
57. Maugeri R, Schiera G, Liegro Di CM, Fricano A, Iacopino DG, Di Liegro I. Aquaporins and Brain Tumors. Int J Mol Sci. (2016) 17:1029. doi: 10.3390/ijms17071029
58. Raffa G, Scibilia A, Conti A, Ricciardo G, Rizzo V, Morelli A, et al. The role of navigated transcranial magnetic stimulation for surgery of motor-eloquent brain tumors: a systematic review and meta-analysis. Clin Neurol Neurosurg. (2019) 180:7–17. doi: 10.1016/j.clineuro.2019.03.003
59. Giammalva GR, Iacopino DG, Azzarello G, Gaggiotti C, Graziano F, Gulì C, et al. End-of-life care in high-grade glioma patients. The palliative and supportive perspective. Brain Sci. (2018) 8:125. doi: 10.3390/brainsci8070125
60. Pino MA, Imperato A, Musca I, Maugeri R, Giammalva GR, Costantino G, et al. New hope in brain glioma surgery: the role of intraoperative ultrasound. A review. Brain Sci. (2018) 8:202. doi: 10.3390/brainsci8110202
61. Barone F, Alberio N, Iacopino DG, Giammalva GR, D'Arrigo C, Tagnese W, et al. Brain mapping as helpful tool in brain glioma surgical treatment-toward the ”perfect surgery“? Brain Sci. (2018) 8:192. doi: 10.3390/brainsci8110192
62. Graziano F, Bavisotto CC, Gammazza AM, Rappa F, de Macario EC, Macario AJL, et al. Chaperonology: the third eye on brain gliomas. Brain Sci. (2018) 8:110. doi: 10.3390/brainsci8060110
63. Maugeri R, Villa A, Pino M, Imperato A, Giammalva GR, Costantino G, et al. With a little help from my friends: the role of intraoperative fluorescent dyes in the surgical management of high-grade gliomas. Brain Sci. (2018) 8:31. doi: 10.3390/brainsci8020031
64. Francaviglia N, Iacopino DG, Costantino G, Villa A, Impallaria P, Meli F, et al. Fluorescein for resection of high-grade gliomas: a safety study control in a single center and review of the literature. Surg Neurol Int. (2017) 8:145. doi: 10.4103/sni.sni_89_17
65. Caruso Bavisotto C, Graziano F, Rappa F, Marino Gammazza A, Logozzi M, Fais S, et al. Exosomal chaperones and miRNAs in gliomagenesis: state-of-art and theranostics perspectives. Int J Mol Sci. (2018) 19:2626. doi: 10.3390/ijms19092626
66. Iacopino DG, Gagliardo C, Giugno A, Giammalva GR, Napoli A, Maugeri R, et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson's disease. Neurosurg Focus. (2018) 44:E7. doi: 10.3171/2017.11.FOCUS17614
67. Francaviglia N, Maugeri R, Odierna Contino A, Meli F, Fiorenza V, Costantino G, et al. Skull bone defects reconstruction with custom-made titanium graft shaped with electron beam melting technology: preliminary experience in a series of ten patients. Acta Neurochirurgica Supplementum. (2017) 124:137–41. doi: 10.1007/978-3-319-39546-3_21
Keywords: NTMs, motor mapping, surgical planning, glioma, craniotomy, tractography
Citation: Umana GE, Scalia G, Graziano F, Maugeri R, Alberio N, Barone F, Crea A, Fagone S, Giammalva GR, Brunasso L, Costanzo R, Paolini F, Gerardi RM, Tumbiolo S, Cicero S, Federico Nicoletti G and Iacopino DG (2021) Navigated Transcranial Magnetic Stimulation Motor Mapping Usefulness in the Surgical Management of Patients Affected by Brain Tumors in Eloquent Areas: A Systematic Review and Meta-Analysis. Front. Neurol. 12:644198. doi: 10.3389/fneur.2021.644198
Received: 20 December 2020; Accepted: 08 February 2021;
Published: 04 March 2021.
Edited by:
Giovanni Raffa, University of Messina, ItalyReviewed by:
Antonino Scibilia, Universitaire de Strasbourg, FranceCopyright © 2021 Umana, Scalia, Graziano, Maugeri, Alberio, Barone, Crea, Fagone, Giammalva, Brunasso, Costanzo, Paolini, Gerardi, Tumbiolo, Cicero, Federico Nicoletti and Iacopino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Emmanuele Umana, dW1hbmEubmNoQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.