
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 February 2021
Sec. Endovascular and Interventional Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.642877
This article is part of the Research Topic Ischemic Stroke Management: From Symptom Onset to Successful Reperfusion and Beyond View all 60 articles
 Adrien Guenego1
Adrien Guenego1 Robert Fahed2
Robert Fahed2 Eric S. Sussman1
Eric S. Sussman1 Matthew Leipzig1
Matthew Leipzig1 Gregory W. Albers3
Gregory W. Albers3 Blake W. Martin1
Blake W. Martin1 David G. Marcellus1
David G. Marcellus1 Gabriella Kuraitis1
Gabriella Kuraitis1 Michael P. Marks1
Michael P. Marks1 Maarten G. Lansberg3
Maarten G. Lansberg3 Max Wintermark1
Max Wintermark1 Jeremy J. Heit1*
Jeremy J. Heit1*Objectives: The susceptibility-vessel-sign (SVS) allows thrombus visualization, length estimation and composition, and it may impact reperfusion during mechanical thrombectomy (MT). SVS can also describe thrombus shape in the occluded artery: in the straight M1-segment (S-shaped), or in an angulated/traversing a bifurcation segment (A-shaped). We determined whether SVS clot shape influenced reperfusion and outcomes after MT for proximal middle-cerebral-artery (M1) occlusions.
Methods: Between May 2015 and March 2018, consecutive patients who underwent MT at one comprehensive stroke center and who had a baseline MRI with a T2* sequence were included. Clinical, procedural and radiographic data, including clot shape on SVS [angulated/bifurcation (A-SVS) vs. straight (S-SVS)] and length were assessed. Primary outcome was successful reperfusion (TICI 2b-3). Secondary outcome were MT complication rates, MT reperfusion time, and clinical outcome at 90-days. Predictors of outcome were assessed with univariate and multivariate analyses.
Results: A total of 62 patients were included. 56% (35/62) had an A-SVS. Clots were significantly longer in the A-SVS group (19 mm vs. 8 mm p = 0.0002). Groups were otherwise well-matched with regard to baseline characteristics. There was a significantly lower rate of successful reperfusion in the A-SVS cohort (83%) compared to the S-SVS cohort (96%) in multivariable analysis [OR 0.04 (95% CI, 0.002–0.58), p = 0.02]. There was no significant difference in long term clinical outcome between groups.
Conclusion: Clot shape as determined on T2* imaging, in patients presenting with M1 occlusion appears to be a predictor of successful reperfusion after MT. Angulated and bifurcating clots are associated with poorer rates of successful reperfusion.
Adrien Guenego, MD and Matthew Leipzig, BS conducted all the statistical analyses.
Mechanical thrombectomy (MT) is an effective treatment for acute ischemic stroke patients (AIS due to large vessel occlusion (LVO). Rapid and successful reperfusion, defined as thrombolysis in cerebral infarction (TICI) 2b-3, increases the likelihood of a favorable outcome (1, 2). Nevertheless, MT does not result in successful reperfusion in up to 29% of patients (1), and biomarkers that identify patients at risk of failed reperfusion failure are needed.
Clot composition, length, and shape may impact MT success, and imaging predictors of clot response to MT may lead to tailored MT techniques, such as stent-retriever or contact-aspiration, to maximize the likelihood of successful treatment (3, 4). Magnetic resonance imaging (MRI) often demonstrates the thrombus on T2* gradient-echo sequence (GRE) as a region of intravascular hypointense signal abnormality, which is termed the susceptibility vessel sign (SVS). SVS has been used as a measure of clot length to predict response to intravenous thrombolysis (5), to detect small distal occlusions (6), to assess multiplicity of intracranial thrombus fragments (7), and even predict clot composition or stroke etiology (8–13). However, whether SVS depiction of clot shape and extension into vessel branches impacts the likelihood of successful reperfusion has not been investigated. Thrombus that involves a bifurcation or accentuated angle may be more prone to fragmentation and may be more difficult to remove (14).
We hypothesized that SVS may be used to visualize the extent of the clot within the middle cerebral artery branches at the point of vessel occlusion and to determine whether the clot is located in a straight branch (S-SVS) or in an angulated/traversing a bifurcation segment (A-SVS). We determined SVS clot shape, branch occlusion patterns, and the impact of these factors on successful reperfusion and favorable clinical outcomes after thrombectomy for proximal middle cerebral artery occlusions.
The study protocol was approved by the institutional review board (IRB) and complied with the Health Insurance Portability and Accountability Act (HIPAA). Patient informed consent was waived by our review board for this single center retrospective analysis of anonymized data acquired prospectively. Adherence to the STROBE criteria (15) was enforced.
We performed a retrospective cohort study of consecutive patients who underwent MT treatment for acute ischemic stroke at our comprehensive stroke center between May 2015 and March 2018. Patient inclusion criteria were: (1) pre-MT brain magnetic resonance imaging (MRI) that included an axial T2* sequence [gradient-echo (GRE)], diffusion-weighted imaging (DWI) and perfusion weighted-imaging (PWI) that was free of motion degradation or significant artifact, and (2) middle cerebral artery occlusion (M1 or both M1 and M2). Basilar and internal carotid occlusions were excluded to obtain homogeneous groups and avoid the impact of posterior circulation strokes on the overall outcome.
Clinical and stroke treatment data were determined from a prospectively maintained stroke database and from the electronic medical records. Stroke severity was assessed by the National Institute of Health Stroke Scale (NIHSS) at the time of MT triage. All thrombectomies were performed according to the standard departmental protocols under general anesthesia, using combined stent-retriever (diameter of 6 mm) and contact aspiration with a 6F intermediate catheter, balloon-guided catheters were not used at that time, there were five different attendings.
All imaging was performed on either a 1.5T GE Signa or 3.0T GE MR750 MRI scanner using standard departmental protocols and approach, using an 8 channel GE HR brain coil (GE Healthcare, Milwaukee, Wisconsin). T2* gradient-echo axial sequences were performed as: TR 650.0 ms, TE 15.0 ms, slice was 5 mm, slice gap of 0.0 mm, FOV of 24.0 × 24.0 cm.
SVS was assessed on GRE sequences and was defined as “presence of a hypointensity within the proximal middle cerebral artery, in which the diameter of the hypointense signal within the vessel exceeded the contralateral vessel diameter” (16). SVS length was measured in millimeters. A-SVS was defined as SVS that involved an angulated M1–M2 segment or in a MCA bifurcation. Clots entirely within a straight M1-segment and without any significant extension into an angulated M2 branch were defined as S-SVS (Figure 1). Patients without SVS were excluded as we couldn't evaluate the clot shape.
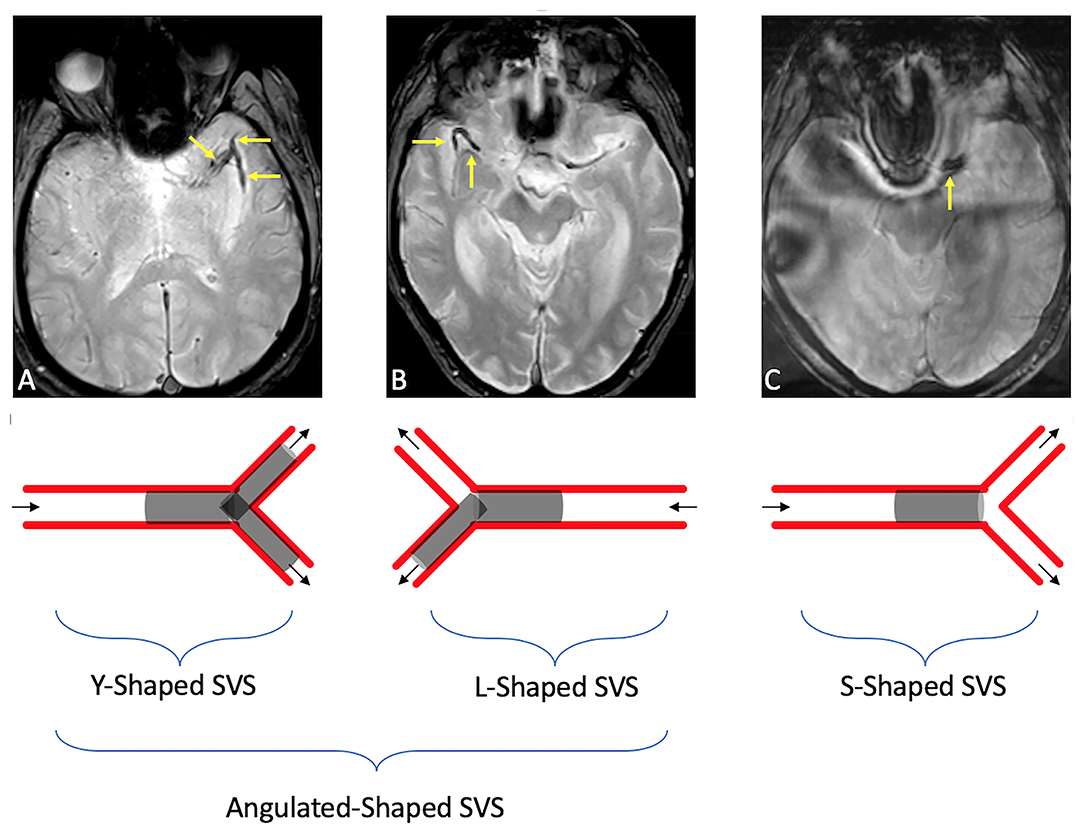
Figure 1. Illustrations of SVS shape in an angulated (A-shaped) branch [either in an MCA bifurcation [a], or an angulated M1–M2 [b]] or in a straight [S-shaped [c]] branch. (A) Susceptibility vessel sign in a M1 segment seen as a linear hypo-intensity branch, dividing into two clots within the M2 middle cerebral artery branches. (B) Susceptibility vessel sign in a M1 segment seen as a horizontal hypo-intensity in the M1 branch, continuing with a 90° angle in the M1/M2 branch. (C) Susceptibility vessel sign in a M1 segment seen as a linear/straight hypo-intensity in the M1 branch (I).
MR perfusion-weighted imaging (PWI) data were processed by an automated program (RAPID, iSchemaView, Menlo Park, CA). The ischemic core was defined as the volume of tissue with an apparent diffusion coefficient <620 s/mm2. The penumbra was defined as the volume of tissue with a Time-to-maximum (TMax) delay of >6 s. Mismatch volume (17) was assessed as the difference between the ischemic core and the penumbra, and the mismatch ratio (18) was calculated as the ratio between the TMax >6 s lesion volume and the core volume. The hypoperfusion intensity ratio (HIR) was used as a measure of tissue collaterals and was calculated as the volumetric ratio of tissue with a TMax >10 s divided by TMax >6 s (18, 19).
All images were anonymized and blindly analyzed by two neurointerventionalists (A.G. and E.S.S. with 5 and 6 years of experience, respectively). Thrombus length was evaluated from measurements between the proximal and distal extent of the SVS on T2* MR sequences, when the thrombus extended into different branches of the middle cerebral artery, the maximal thrombus length as it extended into one branch vessel was calculated rather than summation of length within all of the involved branches.
Interpretation disagreements were resolved by consensus reading, which was supervised by a third neurointerventionalist (J.J.H. with 10 years of experience).
Primary outcome was the difference in rate of successful reperfusion (defined as TICI ≥2b) after MT.
Secondary outcomes were MT complication rates, MT reperfusion time (minutes), and clinical outcomes. Early clinical outcomes were assessed using NIHSS on day 1 post-MT (POD1), NIHSS shift between baseline and POD1, and discharge NIHSS. Long term clinical outcomes were assessed using the modified Rankin Scale (mRS) 90-days after MT; excellent clinical outcome was defined as mRS 0–1, good clinical outcome was defined as mRS 0–2, and poor outcome was defined as mRS 3–6.
Recorded MT complications included emboli to a new vascular territory, arterial perforation, or symptomatic hemorrhage, which was defined as a parenchymal hematoma (PH1 or PH2) with associated worsening from the baseline NIHSS of at least four points.
Nominal variables were first summarized using frequency descriptive analysis then compared using Fisher's exact test. Continuous variables were summarized using median, quartiles and interquartile range, then tested on univariate analysis using the Mann-Whitney test. Normality of the variables was tested by the Shapiro-Wilk test. Statistical significance was set at the p = 0.05 level.
Logistic regression models were designed to assess the association of SVS clot-shape with successful reperfusion (TICI ≥2b). To adjust for baseline and MT potential confounders, a multivariate binary logistic regression analysis was conducted.
Factors with a significant association (P < 0.10) in the univariate analysis (clot length) were included in the multivariable model and factors associated with patient's outcome in the literature were forced into. Results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs) using S-SVS as reference group.
Other logistic regression models were subsequently designed to assess the association of SVS clot-shape with favorable clinical outcome (mRS 0–2), excellent clinical outcome (mRS 0–1), and mortality (mRS 6).
Initial agreement between the two interventional neuroradiologists was measured using Kappa of Cohen, then disagreements were resolved by consensus reading.
All statistical analyses were performed with XLSTAT (Addinsoft, New York City, NY).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Sixty-two patients met inclusion criteria (Figure 2). Patients were dichotomized into S-SVS (27 patients; 44%) and A-SVS (35 patients; 56%) groups; reader agreement of SVS classification was substantial (Cohen's Kappa 0.711) (20).
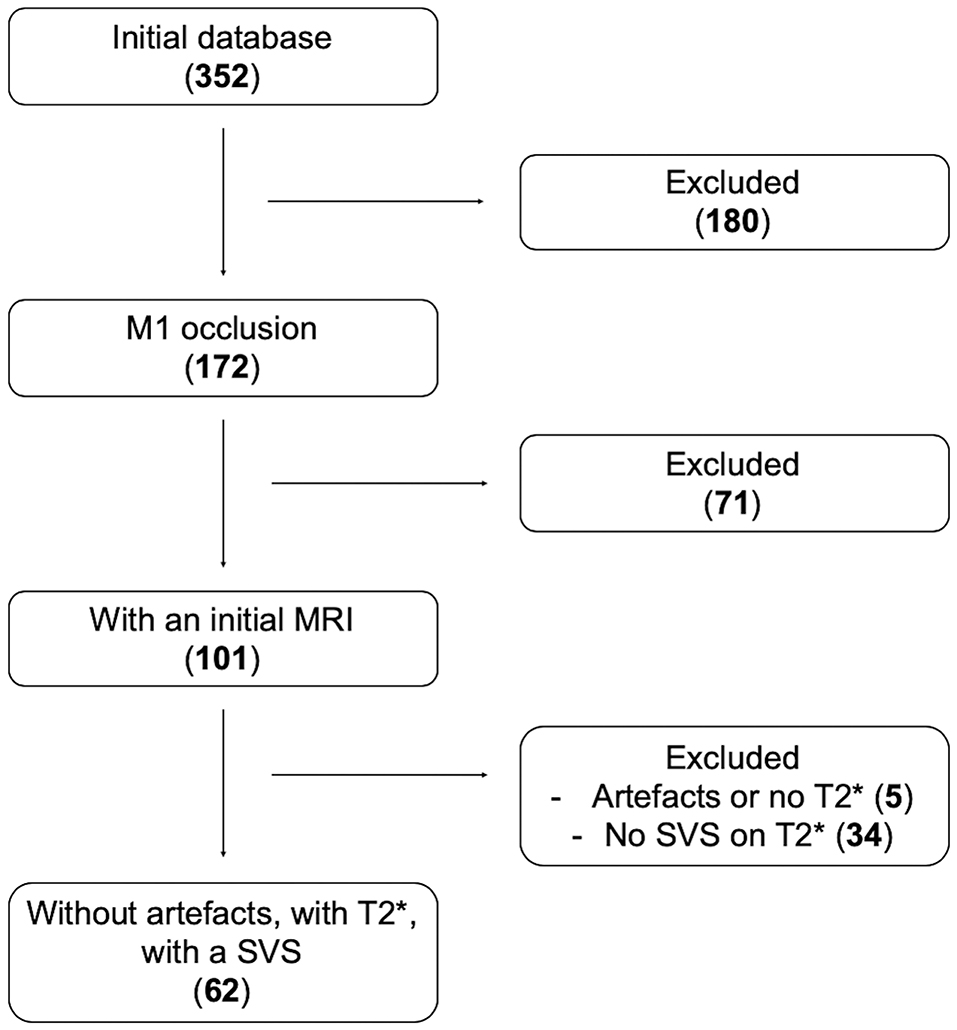
Figure 2. Flow-chart. Number of patients screened then included according to our inclusion criteria: Initial database 352 patients; among them 172 had a proximal MCA (M1) occlusion; 101 of them were screened by MRI, and 71 were screened by CT scan and excluded; 62 patients had A SVS on their GRE imaging.
There were no differences between S-SVS and A-SVS with respect to patient age [72 (IQR, 63–79) vs. 67 (IQR, 55–76), p = 0.960], female sex (52 vs. 54%, p = 1.000), baseline NIHSS [14 (IQR, 10–20) vs. 17 (IQR, 13–22), p = 0.12], or frequency of left sided occlusions (71 vs. 54%, p = 0.19), respectively (Table 1). A-SVS clots were significantly longer compared to S-SVS clots [19 mm (IQR, 15–24) vs. 8 mm (IQR, 10–15), p = 0.0002] (Table 2). There were no other significant differences between the two groups with regard to neuroimaging variables, which included core infarction volume, penumbra volume, and HIR collateral robustness (Table 2). Likewise, MT procedural outcomes were similar between S-SVS and A-SVS groups (Table 3). There were no differences in time from groin puncture to reperfusion [36 min (IQR, 25–62) vs. 44 min (IQR, 23–77), p = 0.70] or MT complication rates (4 vs. 11%, p = 0.27).
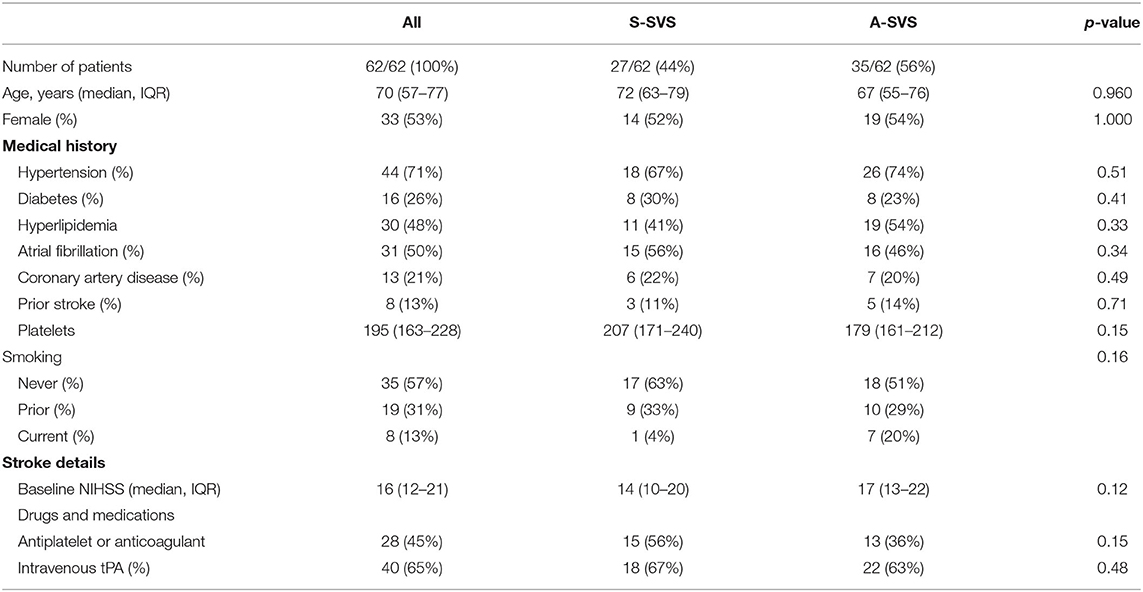
Table 1. Baseline characteristics for patients with an A-shaped SVS compared to those with an S-shaped SVS (S-SVS).
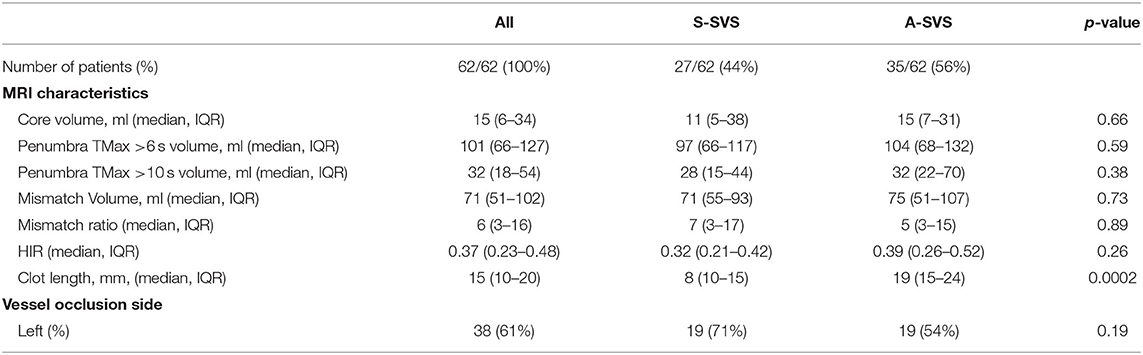
Table 2. Imaging characteristics for patients with an A-shaped SVS compared to those with an S-shaped SVS.
All other univariate analysis are described in Table 3.
In the multivariable binary logistic regression model (AUC = 0.893), A-SVS was an independent negative predictor of successful reperfusion [OR 0.04 (95% CI, 0.002–0.58), p = 0.02, Table 4].
There was no impact of A-SVS clot shape in the multivariable binary logistic regression models on good clinical outcome [OR 0.72 (95% CI, 0.21–2.46), p = 0.59], excellent clinical outcome [OR 0.35 (95% CI, 0.09–1.46), p = 0.15], or mortality [OR 3.22 (95% CI, 0.67–15.49), p = 0.14], respectively (Table 4).
In this study, we found that thrombus morphology measured by SVS-shape influences the likelihood of successful reperfusion after MT. However, SVS morphology did not affect the likelihood of achieving a favorable clinical outcome. Our findings that SVS thrombus morphology is a biomarker of reperfusion have important implications for MT.
Prior studies have used SVS identified on gradient-echo imaging (T2*) (21) to detect (5), localize (22), and measure clot length (5, 22) without contrast administration. Whether SVS is a predictor of reperfusion after MT remains controversial (9, 23). Our findings support the hypothesis that SVS is a biomarker of reperfusion when thrombus morphology is considered. S-SVS are linear clots that do not extend into arterial branch vessels, and clots with this morphology were likely to undergo complete reperfusion compared to angulated clots and clots that extend into branch vessels (A-SVS). Whether a prospective change to MT technique results in higher rates of reperfusion of A-SVS clots requires further study. While clot morphology will not impact the decision to perform a thrombectomy procedure, we hypothesize that it could impact technical strategy, and the routine use of balloon guide sheaths, longer stentretrievers, or even double stentretriever techniques (placement of two devices into two branch points involved with a A-SVS clot) may increase the likelihood of complete reperfusion of A-SVS clots (24, 25).
Patients selection for MT depends on a fast identification of a LVO and on the evaluation of early ischemic changes, and computed-tomography (CT) and magnetic-resonance-imaging (MRI) are recommended for patient evaluation (26). However, MRI is superior to CT for detection of acute ischemia (27), is associated with better outcomes after thrombectomy treatment (28), whereas CT has been associated with an increased risk of futile reperfusion (29). While MRI duration is often reported to be longer in patient's screening for MT (30), MRI did not delay MT (30) nor impact patient's functional outcome in recent studies (30). Use of MRI in AIS screening may then depend on local protocols and optimal institutional workflows.
In contrast to few prior studies that evaluated the importance of clot length on reperfusion (22, 31) our study focused on clot morphology only. In contrast to intravenous thrombolysis stroke treatment, the impact of clot length on successful reperfusion after MT remains controversial (32, 33) and requires further study.
Successful reperfusion has been correlated to clinical outcome in multiple studies (34, 35) and, therefore, one would expect S-SVS to be correlated with a greater likelihood of a favorable clinical outcome. In our study, S-SVS patients had a lower NIHSS the day after thrombectomy and at discharge, but this early recovery did not translate to better outcomes at 90 days. We hypothesize that our study is under powered to detect an outcome difference between S-SVS and A-SVS patients.
Our study is limited by its retrospective observational and single center design, which may introduce bias. The relatively small sample size and missing clinical outcomes in 7/62 patients may also introduce bias in our secondary outcome analysis, our findings need to be confirmed in a larger prospective study. The use of GRE MRI to identify SVS and SVS morphology rather than volumetric susceptibility or CT techniques as well as the exclusion of patients without SVS may limit the generalizability of our findings.
SVS clot morphology appears to be an independent predictor of successful reperfusion after MT in our cohort.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Stanford Medical Center Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AG, RF, ES, ML, GA, BM, DM, GK, MM, ML, MW, and JH participated to study design, data collection, data analysis, and writing. All authors contributed to the article and approved the submitted version.
GA reports equity and consulting for iSchemaView and consulting from Medtronic. MM reports Ownership Interest in ThrombX Medical. JH reports Consultant or Advisory Board for Medtronic, Inc., MicroVention, Inc., and iSchemaView.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.642877/full#supplementary-material
AIS, Acute Ischemic Stroke; A-SVS/S-SVS, patients with an angulated or bifurcation-shaped (A-shaped) Susceptibility Vessel Sign/patients with a straight (S-shaped) Susceptibility Vessel Sign; M1, Proximal Middle Cerebral Artery; mRS, modified Rankin Scale; MT, Mechanical Thrombectomy; NIHSS, National Institute of Health Stroke Scale; POD1, Post-Operative Day 1 post-MT; SVS, Susceptibility Vessel Sign; TICI, Thrombolysis In Cerebral Infarction; TMax, Time to Maximum (sec); CT, Computed Tomography; HIR, Hypoperfusion Intensity Ratio; ICA, Internal Carotid Artery; ICA T, Internal Carotid Artery Termination; IQR, Inter-Quartile Range; LVO, Large Vessel Occlusion; MCA-M1, Proximal Middle Cerebral Artery; MRI, Magnetic Resonance Imaging; tPA, thrombolysis Plasminogen Activator.
1. Goyal M, Menon BK, Van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Marks MP, Heit JJ, Lansberg MG, Kemp S, Christensen S, Derdeyn CP, et al. Endovascular treatment in the DEFUSE 3 study. Stroke. (2018) 49:2000–3. doi: 10.1161/STROKEAHA.118.022147
3. Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Song D, et al. Importance of truncal-type occlusion in stentriever-based thrombectomy for acute stroke. Neurology. (2016) 87:1542–50. doi: 10.1212/WNL.0000000000003202
4. Consoli A, Rosi A, Coskun O, Nappini S, Di Maria F, Renieri L, et al. Thrombectomy for M1-middle cerebral artery occlusion: angiographic aspect of the arterial occlusion and recanalization: a preliminary observation. Stroke. (2018) 49:1286–9. doi: 10.1161/STROKEAHA.117.018987
5. Naggara O, Raymond J, Domingo Ayllon M, Al-Shareef F, Touze E, Chenoufi M, et al. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS ONE. (2013) 8:e76727. doi: 10.1371/journal.pone.0076727
6. Park MG, Oh SJ, Baik SK, Jung DS, Park KP. Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. J Stroke. (2016) 18:73–9. doi: 10.5853/jos.2015.01417
7. Gratz PP, Schroth G, Gralla J, Mattle HP, Fischer U, Jung S, et al. Whole-brain susceptibility-weighted thrombus imaging in stroke: fragmented thrombi predict worse outcome. AJNR Am J Neuroradiol. (2015) 36:1277–82. doi: 10.3174/ajnr.A4275
8. Bourcier R, Detraz L, Serfaty JM, Delasalle BG, Mirza M, Derraz I, et al. MRI interscanner agreement of the association between the susceptibility vessel sign and histologic composition of thrombi. J Neuroimaging. (2017) 27:577–82. doi: 10.1111/jon.12464
9. Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke. (2017) 48:1554–9. doi: 10.1161/STROKEAHA.116.016217
10. Zhang R, Zhou Y, Liu C, Zhang M, Yan S, Liebeskind DS, et al. Overestimation of susceptibility vessel sign: a predictive marker of stroke cause. Stroke. (2017) 48:1993–6. doi: 10.1161/STROKEAHA.117.016727
11. Bourcier R, Derraz I, Delasalle B, Beaumont M, Soize S, Legrand L, et al. Susceptibility vessel sign and cardioembolic etiology in the THRACE trial. Clin Neuroradiol. (2018). doi: 10.1007/s00062-018-0699-8
12. Bourcier R, Derraz I, Bracard S, Oppenheim C, Naggara O, Investigators T. Two-layered susceptibility vessel sign and high overestimation ratio on MRI are predictive of cardioembolic stroke. AJNR Am J Neuroradiol. (2019) 40:65–7. doi: 10.3174/ajnr.A5865
13. Bourcier R, Pautre R, Mirza M, Castets C, Darcourt J, Labreuche J, et al. MRI quantitative T2* mapping to predict dominant composition of in vitro thrombus. AJNR Am J Neuroradiol. (2019) 40:59–64. doi: 10.3174/ajnr.A5938
14. Kaesmacher J, Boeckh-Behrens T, Simon S, Maegerlein C, Kleine JF, Zimmer C, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol. (2017) 38:991–8. doi: 10.3174/ajnr.A5105
15. Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
16. Rovira A, Orellana P, Alvarez-Sabin J, Arenillas JF, Aymerich X, Grive E, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. (2004) 232:466–73. doi: 10.1148/radiol.2322030273
17. Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. (2009) 40:469–75. doi: 10.1161/STROKEAHA.108.526954
18. Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. (2014) 45:1018–23. doi: 10.1161/STROKEAHA.113.003857
19. Guenego A, Mlynash M, Christensen S, Kemp S, Heit JJ, Lansberg MG, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol. (2018) 84:616–20. doi: 10.1002/ana.25320
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33:159–74. doi: 10.2307/2529310
21. Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. (2015) 22:967–72. doi: 10.1111/ene.12693
22. Derraz I, Bourcier R, Soudant M, Soize S, Hassen WB, Hossu G, et al. Does clot burden score on baseline T2*-MRI impact clinical outcome in acute ischemic stroke treated with mechanical thrombectomy? J Stroke. (2019) 21:91–100. doi: 10.5853/jos.2018.01921
23. Darcourt J, Withayasuk P, Vukasinovic I, Michelozzi C, Bellanger G, Guenego A, et al. Predictive value of susceptibility vessel sign for arterial recanalization and clinical improvement in ischemic stroke. Stroke. (2019). doi: 10.1161/STROKEAHA.118.022912
24. Klisch J, Sychra V, Strasilla C, Taschner CA, Reinhard M, Urbach H, et al. Double solitaire mechanical thrombectomy in acute stroke: effective rescue strategy for refractory artery occlusions? AJNR Am J Neuroradiol. (2015) 36:552–6. doi: 10.3174/ajnr.A4133
25. Asadi H, Brennan P, Martin A, Looby S, O'hare A, Thornton J. double stent-retriever technique in endovascular treatment of middle cerebral artery saddle embolus. J Stroke Cerebrovasc Dis. (2016) 25e:9–11. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.005
26. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
27. Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. (2007) 369:293–8. doi: 10.1016/S0140-6736(07)60151-2
28. Campbell BCV, Majoie C, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. (2019) 18:46–55.
29. Meinel TR, Kaesmacher J, Mosimann PJ, Seiffge D, Jung S, Mordasini P, et al. Association of initial imaging modality and futile recanalization after thrombectomy. Neurology. (2020). doi: 10.1212/WNL.0000000000010614
30. Provost C, Soudant M, Legrand L, Ben Hassen W, Xie Y, Soize S, et al. magnetic resonance imaging or computed tomography before treatment in acute ischemic stroke. Stroke. (2019) 50:659–64. doi: 10.1161/STROKEAHA.118.023882
31. Baek JH, Yoo J, Song D, Kim YD, Nam HS, Kim BM, et al. Predictive value of thrombus volume for recanalization in stent retriever thrombectomy. Sci Rep. (2017) 7:15938. doi: 10.1038/s41598-017-16274-9
32. Weisstanner C, Gratz PP, Schroth G, Verma RK, Kochl A, Jung S, et al. Thrombus imaging in acute stroke: correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol. (2014) 24:1735–41. doi: 10.1007/s00330-014-3200-3
33. Seker F, Pfaff J, Wolf M, Schonenberger S, Nagel S, Herweh C, et al. Impact of thrombus length on recanalization and clinical outcome following mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. (2017) 9:937–9. doi: 10.1136/neurintsurg-2016-012591
34. Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg. (2017) 9:117–21. doi: 10.1136/neurintsurg-2015-012218
Keywords: stroke, thrombectomy, endovascular recanalization, magnetic resonance imaging, clot
Citation: Guenego A, Fahed R, Sussman ES, Leipzig M, Albers GW, Martin BW, Marcellus DG, Kuraitis G, Marks MP, Lansberg MG, Wintermark M and Heit JJ (2021) Impact of Clot Shape on Successful M1 Endovascular Reperfusion. Front. Neurol. 12:642877. doi: 10.3389/fneur.2021.642877
Received: 16 December 2020; Accepted: 08 January 2021;
Published: 01 February 2021.
Edited by:
Johanna Ospel, University Hospital of Basel, SwitzerlandReviewed by:
Alex Brehm, University Hospital of Basel, SwitzerlandCopyright © 2021 Guenego, Fahed, Sussman, Leipzig, Albers, Martin, Marcellus, Kuraitis, Marks, Lansberg, Wintermark and Heit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy J. Heit, amhlaXRAc3RhbmZvcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.