
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 25 March 2021
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.637198
This article is part of the Research TopicImaging of Neuromuscular DiseasesView all 21 articles
 Eva M. Kesenheimer1,2,3
Eva M. Kesenheimer1,2,3 Maria Janina Wendebourg1,2,3
Maria Janina Wendebourg1,2,3 Matthias Weigel1,3,4
Matthias Weigel1,3,4 Claudia Weidensteiner4,5
Claudia Weidensteiner4,5 Tanja Haas4
Tanja Haas4 Laura Richter1,2,3
Laura Richter1,2,3 Laura Sander1,2,3
Laura Sander1,2,3 Antal Horvath5
Antal Horvath5 Muhamed Barakovic3,5
Muhamed Barakovic3,5 Philippe Cattin5
Philippe Cattin5 Cristina Granziera1,3,5
Cristina Granziera1,3,5 Oliver Bieri4,5
Oliver Bieri4,5 Regina Schlaeger1,2,3*
Regina Schlaeger1,2,3*Background: MR imaging of the spinal cord (SC) gray matter (GM) at the cervical and lumbar enlargements' level may be particularly informative in lower motor neuron disorders, e. g., spinal muscular atrophy, but also in other neurodegenerative or autoimmune diseases affecting the SC. Radially sampled averaged magnetization inversion recovery acquisition (rAMIRA) is a novel approach to perform SC imaging in clinical settings with favorable contrast and is well-suited for SC GM quantitation. However, before applying rAMIRA in clinical studies, it is important to understand (i) the sources of inter-subject variability of total SC cross-sectional areas (TCA) and GM area (GMA) measurements in healthy subjects and (ii) their relation to age and sex to facilitate the detection of pathology-associated changes. In this study, we aimed to develop normalization strategies for rAMIRA-derived SC metrics using skull and spine-based metrics to reduce anatomical variability.
Methods: Sixty-one healthy subjects (age range 11–93 years, 37.7% women) were investigated with axial two-dimensional rAMIRA imaging at 3T MRI. Cervical and thoracic levels including the level of the cervical (C4/C5) and lumbar enlargements (Tmax) were examined. SC T2-weighted sagittal images and high-resolution 3D whole-brain T1-weighted images were acquired. TCA and GMAs were quantified. Anatomical variables with associations of |r| > 0.30 in univariate association with SC areas, and age and sex were used to construct normalization models using backward selection with TCAC4/C5 as outcome. The effect of the normalization was assessed by % relative standard deviation (RSD) reductions.
Results: Mean inter-individual variability and the SD of the SC area metrics were considerable: TCAC4/5: 8.1%/9.0; TCATmax: 8.9%/6.5; GMAC4/C5: 8.6%/2.2; GMATmax: 12.2%/3.8. Normalization based on sex, brain WM volume, and spinal canal area resulted in RSD reductions of 23.7% for TCAs and 12.0% for GM areas at C4/C5. Normalizations based on the area of spinal canal alone resulted in RSD reductions of 10.2% for TCAs and 9.6% for GM areas at C4/C5, respectively.
Discussion: Anatomic inter-individual variability of SC areas is substantial. This study identified effective normalization models for inter-subject variability reduction in TCA and SC GMA in healthy subjects based on rAMIRA imaging.
Substantial advances in understanding spinal muscular atrophy (SMA) etiopathogenesis have catalyzed the development of novel therapeutic strategies. With the approval of the first disease-modifying treatments for SMA, the need for biomarkers that allow reliable monitoring of the disease course and the therapeutic response in SMA patients has substantially grown. Current advances in morphometric MRI development allow gray (GM) and white matter (WM) quantification in the spinal cord (SC) (1–7), which may help in improving the in vivo characterization of motor neuron diseases or other neurodegenerative SC diseases. Imaging the cervical and lumbar enlargements could be informative, especially in lower motor neuron diseases, e.g., SMA, or lower motor neuron-predominant amyotrophic lateral sclerosis (ALS).
Radially sampled averaged magnetization inversion recovery acquisition (rAMIRA) (8, 9) is a novel magnetic resonance imaging (MRI) approach to perform SC imaging with favorable contrast in clinical settings, which is well-suited for GM/WM quantitation not only in the cervical, but also in the thoracic SC. Briefly, after an inversion recovery preparation, rAMIRA typically acquires five radially sampled images with increasing inversion times (8). The first images of the series with shorter inversion times display high gray matter to white matter contrast, while the images with longer inversion times show a bright CSF in contrast to a dark SC (8, 9). These acquired inversion images can be combined to fine-tune and even enhance the signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) (9). Due to the radial sampling scheme with a balanced steady-state free precession readout module, rAMIRA provides a low sensitivity to motion effects such as heartbeat and breathing, which is a crucial issue in imaging of the thoracic SC (8). Based on these advantages and a good in-plane resolution, rAMIRA images are well-suited for quantifying both GM area and total cross-sectional area (TCA) in the SC.
More recently, several semi-automated and automated tools (10–17) have been developed for segmentation of the SC GM and WM from MR images, including promising automated segmentation algorithms specifically developed for the AMIRA approach (7, 14). The reliability of the segmentation methods in single center studies is in general high, and some methods have been validated in multi-centric settings (18). Despite these substantial advances, the anatomic accuracy for delineation of SC GM is still judged based on manual algorithms (18), in particular in studies involving the thoracic SC.
Prior to applying morphometric SC techniques such as rAMIRA in larger clinical studies in lower motor neuron disorders, it is important to understand the sources of inter-subject variability of SC GM and WM area measurements in healthy subjects to increase both the sensitivity and specificity in detecting pathology-associated changes. Normalization aims to reduce biological, anatomical variation unrelated to the disease.
Previous studies in multiple sclerosis patients proposed to decrease anatomic inter-subject variability by applying normalization approaches based on correlations between the upper cervical total cross-sectional SC area/cervical SC volume and (i) skull size in healthy subjects (19, 20), (ii) lumbar enlargement SC area (21), or (iii) SC length (22, 23). However, results remain partly conflicting. Papinutto et al. (24) recently reported a 17.7% reduction of inter-subject variability in upper cervical total cross-sectional SC area at the intervertebral disc level C2/C3 based on a normalization approach combining SIENAX v-scale (25) and the product of the maximum axial anterior–posterior and lateral diameters of the cervical spinal canal in a cohort of healthy middle-aged adults based on phase-sensitive inversion recovery imaging.
The level C2/C3 has been the major target in multiple sclerosis imaging studies (26, 27) as it is clearly situated above the cervical SC enlargement and is therefore anatomically less variable than the levels below.
Nevertheless, the lower levels of the cervical and thoracic SC that contain the motor neurons to the arm and leg muscles are of special relevance to the study of lower motor neuron disorders (e.g., SMA) and have been less well-studied. There are only a few MRI studies assessing the SC of children and adolescents (28–30), none focusing on the SC GM. Children and adolescents are the leading target group for the recently approved SMA treatments; therefore, data on SC GM variations are needed to develop treatment monitoring methods.
The aims of this study were to assess the anatomic inter-subject variability in TCA and GM areas at several levels in the cervical and thoracic SC based on rAMIRA imaging in a cohort of healthy subjects with a broad age range including both adults as well as minors and to develop and evaluate potential normalization strategies for inter-subject variability reduction.
Sixty-one healthy subjects (range 11–93 years, mean age 46.0 years, SD 24.7, 37.7% women) including 18 minors (range 11–17 years, mean age 13.9, SD 1.9, 46.2% female) without a neurological or cognitive disease were included into the study after written informed consent was obtained. The local ethics committee approved the study.
All participants were examined with the same 3T whole-body MR system (Siemens Magnetom PRISMA, Siemens Healthineers, Erlangen, Germany) using a 64-channel head and neck coil and the built-in spine coil for reception. Axial two-dimensional rAMIRA images (8, 9) were acquired perpendicular to the SC at the intervertebral disc levels C2/C3, C3/C4, C4/C5, C5/6, T9/T10, and Tmax [level of the lumbar enlargement, which was identified by visual inspection on the corresponding sagittal and coronal T2-weighted turbo spin echo images of the spine (cf. below) by TH (>20 y of experience)]. The employed rAMIRA acquisition protocol was identical to the “optimized standard protocol version” presented in the corresponding methods paper (8). Thus, the relevant sequence parameters for rAMIRA were: field of view = 128 × 128 mm2, 512 readout samples (includes 2 × oversampling), 260 projections, isotropic in-plane resolution 0.50 × 0.50 mm2, slice thickness 8 mm, bandwidth = 310 Hz/Px, flip angle = 50 deg, signal averaging = 2. Five images with the mean inversion times TIeff = 174, 239, 304, 368, 433 ms were acquired simultaneously and later combined (cf. below and Figure 1). The sequence uses cardiac triggering to mitigate potential pulsation artifacts, which was realized with a standard infrared finger clip (simple pulse triggering). Hence, for a heart rate of 60 bpm, rAMIRA's acquisition time corresponds to 2:39 min per slice, for instance.
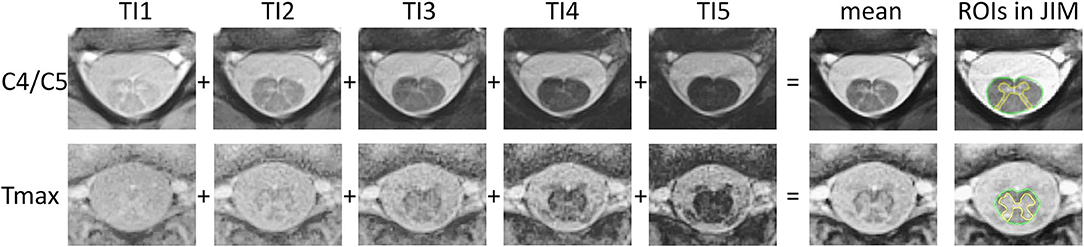
Figure 1. rAMIRA images of the spinal cord in an 11-year-old boy. From left to right: axial rAMIRA images at intervertebral disc level C4/C5 (top row) and Tmax (bottom row) acquired at five inversion times (TI), mean image (combination of the images at five TIs); and mean images with the region of interests (ROIs) spinal cord total cross-sectional area (TCA, green) and gray matter area (GMA, yellow) which were segmented using the software JIM.
Furthermore, all participants received T2-weighted turbo spin echo imaging covering the whole SC in sagittal and coronal slice orientation. Here, the most relevant sequence parameters were [1] sagittal: in-plane resolution = 0.7 × 0.7 mm2, 17 slices of thickness 3 mm, TR = 3,400 ms, TE = 102 ms; [2] coronal: in-plane resolution = 1.4 × 1.4 mm2, 17 slices of thickness 3 mm, TR = 3,500 ms, TE = 95 ms. Additionally, 3D isotropic high-resolution whole-brain T1-weighted images were acquired with the magnetization prepared rapid gradient echo (MPRAGE) sequence, using the following parameters: 1.0 mm isotropic resolution, TI = 1,100 ms, TR = 2,000 ms, TE = 2.12 ms, flip angle = 8 deg, matrix = 256 × 256 × 192.
All rAMIRA images were visually inspected for image quality [by TH (>20 y of experience), EK (1 y), MJW (3 y), and CW (>20 y)]. Only images with sufficient quality were segmented. Segmentations were not possible in 3 out of 244 images in the cervical SC and in 12 out of 122 in the thoracic SC (for details s. results section). For the quantitation of the GM area and TCA, one mean image of all five simultaneously acquired inversion images of the rAMIRA series was calculated, which shows a high gray matter to white matter contrast, as well as sufficient contrast between SC and CSF (Figure 1) (8, 9).
Total cross-sectional areas were segmented in a semi-automated way using the software JIM 7 (http://www.xinapse.com) (31). Following a previously published reliable segmentation algorithm (5, 6, 26), GM was segmented manually three times by one single rater and the mean was calculated (by MJW).
Brain T1-weighted images were investigated using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) to determine the total intracranial volume (TIV; a frequently used normalization parameter for brain volumes) (32) as well as brain GM and WM volumes.
In addition, the following parameters were determined as potential normalization factors at the levels C4/C5 and Tmax (Figure 2) (by EK):
- Maximum axial anterior–posterior and lateral spinal canal diameters, axial spinal canal area, maximum axial lateral vertebral body width on the axial two-dimensional rAMIRA images; additionally, the product of the anterior–posterior and lateral spinal canal diameter was calculated at both levels.
- Middle vertebra height C4 and T12 on the T2-weighted sagittal images.
- Anterior–posterior and lateral diameters, as well as area of the foramen magnum, and distance between Basion–Opisthion on an isotropic, high-resolution 3D whole-brain T1-weighted image. The product of the anterior–posterior and lateral diameter of the foramen magnum was calculated as well.
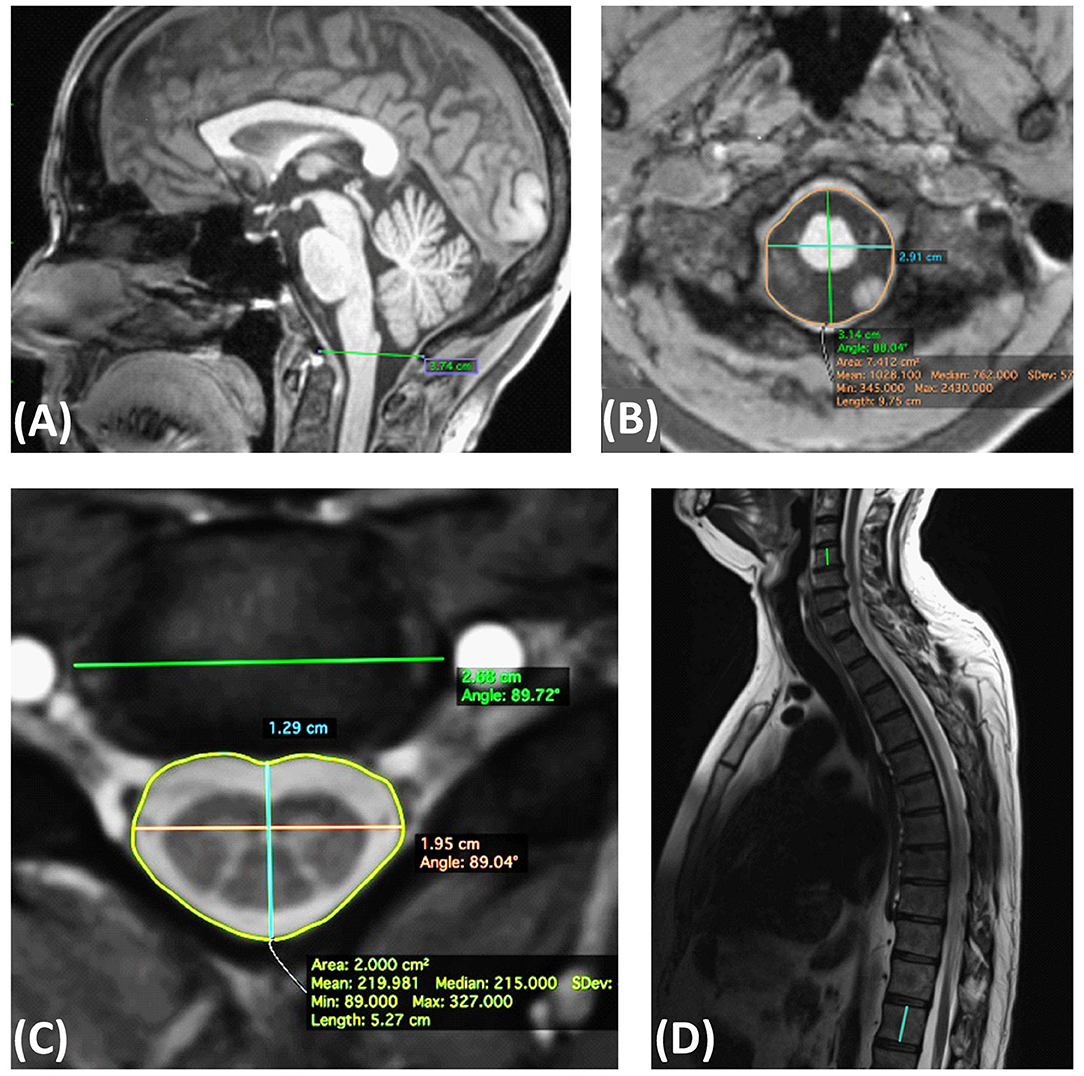
Figure 2. Definition of the skeletal metrics. (A) Sagittal T1 weighted MPRAGE: McRae line (distance between Basion–Opisthion). (B) Axial MPRAGE: anterior posterior and lateral diameters, as well as area of the foramen magnum. (C) Axial rAMIRA: Maximum axial anterior posterior and lateral spinal canal diameters, spinal canal area, maximum lateral vertebral body width. (D) Sagittal T2 weighted turbo spin echo: middle vertebra height C4 and T12.
The inter-rater reliability of the manually assessed anatomical parameters was determined in 14 datasets by two independent operators (by EK and CW) and showed excellent reliability with intra-class correlation coefficients (ICCs, two-way random, absolute agreement) (33) >0.96, as listed in the supplement (Supplementary Table 1). Segmentation was done with OsirixLite (https://www.osirix-viewer.com/).
Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 25.0, Armonk, NY: IBM Corp., and JMP pro, Version 14. SAS Institute Inc., Cary, NC, 1989-2019.
- Inter-Individual Variability Assessments
To assess the inter-individual variability of SC areas, the respective deviation from the group mean was calculated for each subject as
- Assessing the Effect of Age and Sex
Differences in rAMIRA-based SC areas between men and women were assessed using linear regression analysis co-varying for age.
Linear and quadratic fits for (a) TCA and (b) SC GM areas vs. age were assessed for the whole study group (n = 61), and the r2 of the models was reported. For practicability reasons to facilitate future analyses in specific target populations, we also assessed SC areas in three nearly equally sized sub-groups: minors (Group 1: aged <18 years, n = 18), middle-aged (Group 2: aged 18–65 years, n = 23) and elderly subjects (Group 3: aged >65 years, n = 20) using linear regression analysis with sex as additional covariate, respectively.
- Normalization Models
For the development of the normalization models, the associations of the anatomical parameters with rAMIRA-based SC areas at C4/C5 and Tmax were first assessed using Pearson correlation coefficients since all variables were normally distributed. Bonferroni correction was performed with a correction factor of 17 (p < 0.05/17) to correct for n = 17 tests. This analysis was performed using the data from all subjects (n = 61).
We then performed a backward selection procedure starting with a model containing all anatomical variables with a Pearson correlation coefficient of |r| > 0.30 (34) in univariate analysis as well as age and sex as predictor variables and TCA at the level C4/C5 as outcome parameter.
This procedure was performed (a) considering brain GM and WM volumes as potential predictors (approach suitable for studies in healthy controls and diseases known to not affect brain GM and/or WM volumes) and (b) without considering brain GM and WM volumes (approach suitable for studies in diseases known to affect these brain volumes).
The adjusted r2 of the models resulting from the backward selection was reported.
For normalization we used the approach described by Sanfilipo and Papinutto (25, 35):
with a, b, and c being the estimates (regression coefficients) obtained by the linear regression analysis for the predictor variables surviving the backward selection procedure and X, Y, Z the measured values of these variables.
The performance of the resulting normalization models was expressed a) as the % reduction of inter-individual variability of the normalized SC areas of each model in relation to the variability of the non-normalized areas and b) as the % relative standard deviation (% RSD) reductions of the predicted areas to the % RSD of the non-normalized, measured areas. The relative standard deviation (RSD) is the standard deviation divided by the mean SC area.
The normalization model with the largest relative inter-individual variability reduction was then applied to all other SC level measurements.
In analogy, we developed a normalization model for the sub-group of minors (n = 18).
The acquired rAMIRA images displayed a good quality in general. Segmentations were not possible in 1.2% of the images in the cervical SC (due to motion artifacts in minors) and in 9.8% in the thoracic SC due to image artifacts originating from flow in near-by large vessels or due to artifacts originating from cardiac and breathing motion.
The inter-individual mean relative variability for TCA and GM area, as well as the % relative SD (% RSD: defined as the SD divided by the group mean), are summarized in Table 1.

Table 1. Mean % inter-individual variability, mean, SD and % RSD (% relative SD) of spinal cord total cross-sectional areas (TCA) and gray matter areas (GMA) at the intervertebral disc levels C2/C3–C5/C6, T9/T10, and Tmax (level of the lumbar enlargement) of the whole study population.
Effects of age and sex are summarized in the supplement in Supplementary Tables 2, 3.
In brief, men showed significantly larger SC GM areas at C3/C4 (p = 0.0350) and at Tmax (p = 0.0497) than women. At all other levels, we detected no significant area differences between sexes (Supplementary Table 2).
The linear and quadratic fits for TCA and GM areas vs. age for the whole group showed in general relatively low r2 indicating low accuracy of the models: r2 (linear/quadratic fit) TCAC2/C3: 0.009/0.088; TCAC3/C4: 0.005/0.049; TCAC4/C5: 0.001/0.031; TCAC5/C6: 0.010/0.034; TCAT9/T10: 0.041/0.101; TCATmax: 0.000/0.014; GM areaC2/C3: 0.051/0.055; GM areaC3/C4: 0.000/0.017; GM areaC4/C5: 0.009/0.018; GM areaC5/C6: 0.038/0.045; and GM areaT9/T10: 0.229/0.24, GM areaTmax: 0.13/0.137.
For reasons of practicability, we also analyzed SC areas in age sub-groups of minors, middle-aged, and elderly subjects with adjustment for sex. These results are summarized in Supplementary Table 3. Maximum mean TCA values were consistently observed in the sub-group of middle-aged subjects at all levels, with TCA differences being significant between minors and middle-aged subjects at the level C2/C3 (mean difference 4.78 mm2, SE 2.34, 95% CI of the difference: 0.09–9.47, p = 0.0457), and between middle-aged and elderly subjects at the level T9/T10 (mean difference 3.30 mm2, SE 1.28, 95% CI of the difference: 0.72–5.88, p = 0.0131).
While minimum mean GM area values were consistently observed in the subgroup of elderly subjects at all levels, maximum mean GM area values were observed in the subgroup of middle-aged subjects only at the level C3/C4 (Supplementary Table 3). Significant GM area differences could be detected between middle-aged and elderly subjects at the level C2/C3 (mean difference 0.83 mm2, SE 0.41, 95% CI of the difference: 0.00–1.66, p = 0.0495) and between minors and middle-aged subjects at the level T9/T10 (mean difference 0.80 mm2, SE 0.37, 95% CI: 0.06–1.55, p = 0.0353) (Supplementary Table 3).
Among the chosen metrics, TIV, brain WM volume, anterior–posterior spinal canal diameter, and area of the spinal canal, as well as the product of the anterior–posterior and lateral spinal canal showed a correlation with TCA at the level C4/C5 with Pearson correlation coefficients of |r| > 0.30 (Table 2), with the other parameters showing lower correlation coefficients. After Bonferroni correction, none of the investigated metrics did show a significant association with SC areas at Tmax (Supplementary Table 4); thus, normalization model development was primarily focused on the cervical SC.
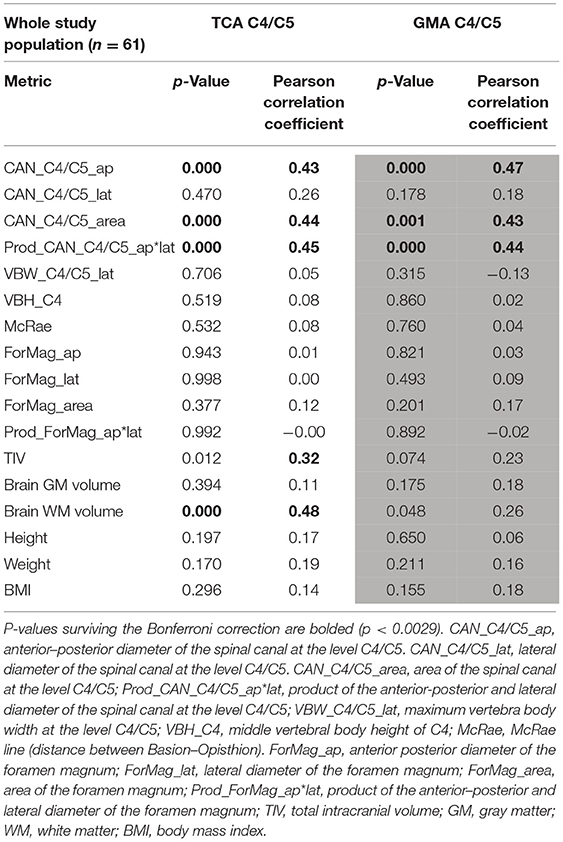
Table 2. Associations between the anatomical metrics and total cross-sectional area (TCA) and gray matter area (GMA) at the C4/C5 level using Pearson correlation coefficients.
The backward selection procedure yielded a model with sex, brain WM volume, and the area of the spinal canal at the level C4/C5 as predictor variables for TCA at C4/C5 as outcome (Model 1) (Table 3). Age was not a predictor variable.
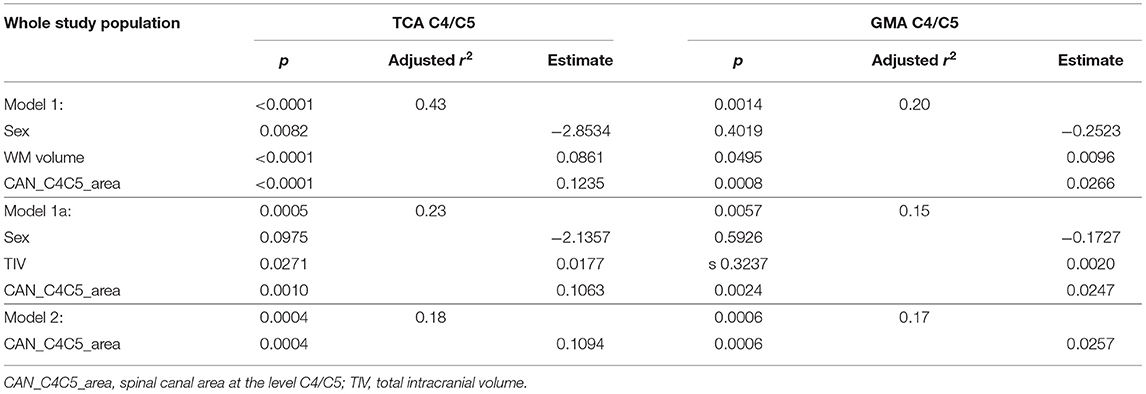
Table 3. Linear regression analysis with total cross-sectional area (TCA) at the C4/C5 level as outcome and normalization variables after backward selection; corresponding models with gray matter area (GMA) as outcome.
Normalization strategies that are based on brain WM or GM volumes can be used in healthy subjects, but are not well-suited in diseases with potential changes in WM or GM volumes caused by the underlying disease itself (e.g., gray matter pathology in ALS, SMA; or mainly white matter pathology in adrenomyeloneuropathy).
We, therefore, performed a second backward selection—without considering brain GM and WM volumes—yielding a model (in analogy to Model 1) with sex, TIV, and the area of the spinal canal at the level C4/C5 as predictors for TCA at C4/C5 as outcome, permitting variables with p < 0.1 (Model 1a). This model was further simplified using backward selection to a model only containing the area of the spinal canal at the level C4/C5 as univariate predictor (Model 2) (Table 3).
The effect of the normalization of TCA and GM areas at the level C4/C5 based on Models 1, 1a, and 2 on the mean inter-individual variability and % relative standard deviation is summarized in Table 4.
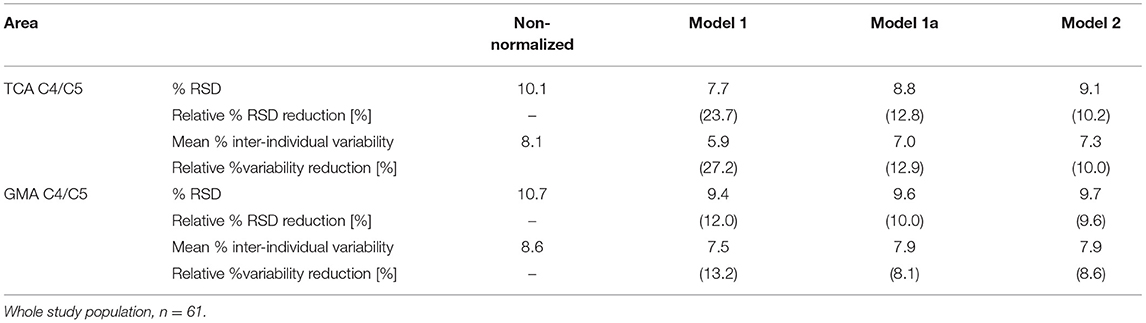
Table 4. % relative standard deviation (RSD, standard deviation divided by the mean area), relative % RSD reduction, mean % inter-individual variability [(measured area in a given subject–group mean area)/group mean area], and relative % inter-individual variability reduction with respect to the measured total cross-sectional cord area (TCA) and gray matter area (GMA) at the intervertebral disc level C4/C5 for normalizations based on Models 1, 1a, and Model 2.
Model 1 and Model 2 were then applied to all SC level measurements (Table 5, Figure 3).
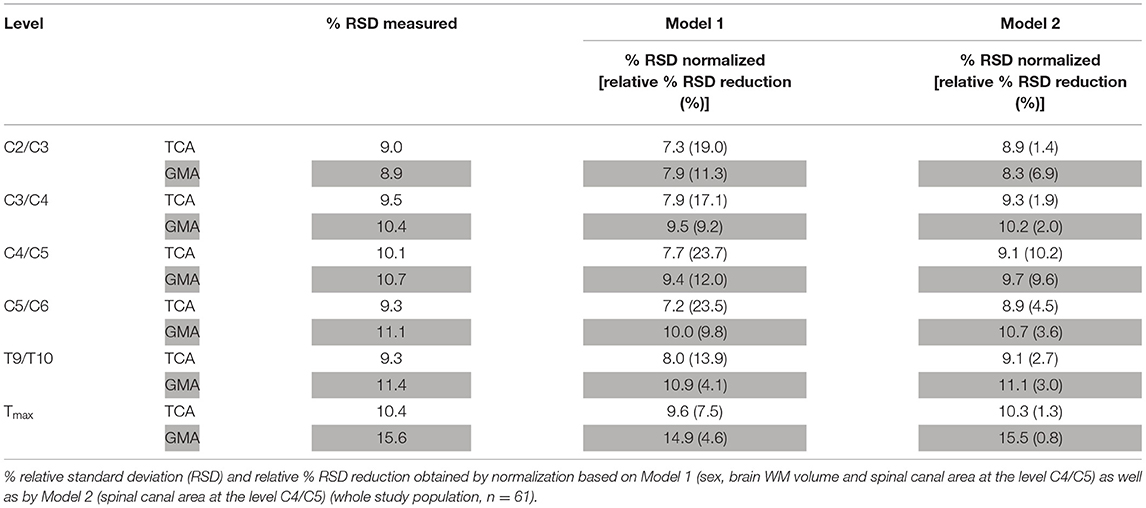
Table 5. Performance of normalization by Model 1 and Model 2 for total cross-sectional cord areas (TCA) and gray matter areas (GMA) at all other cord levels.
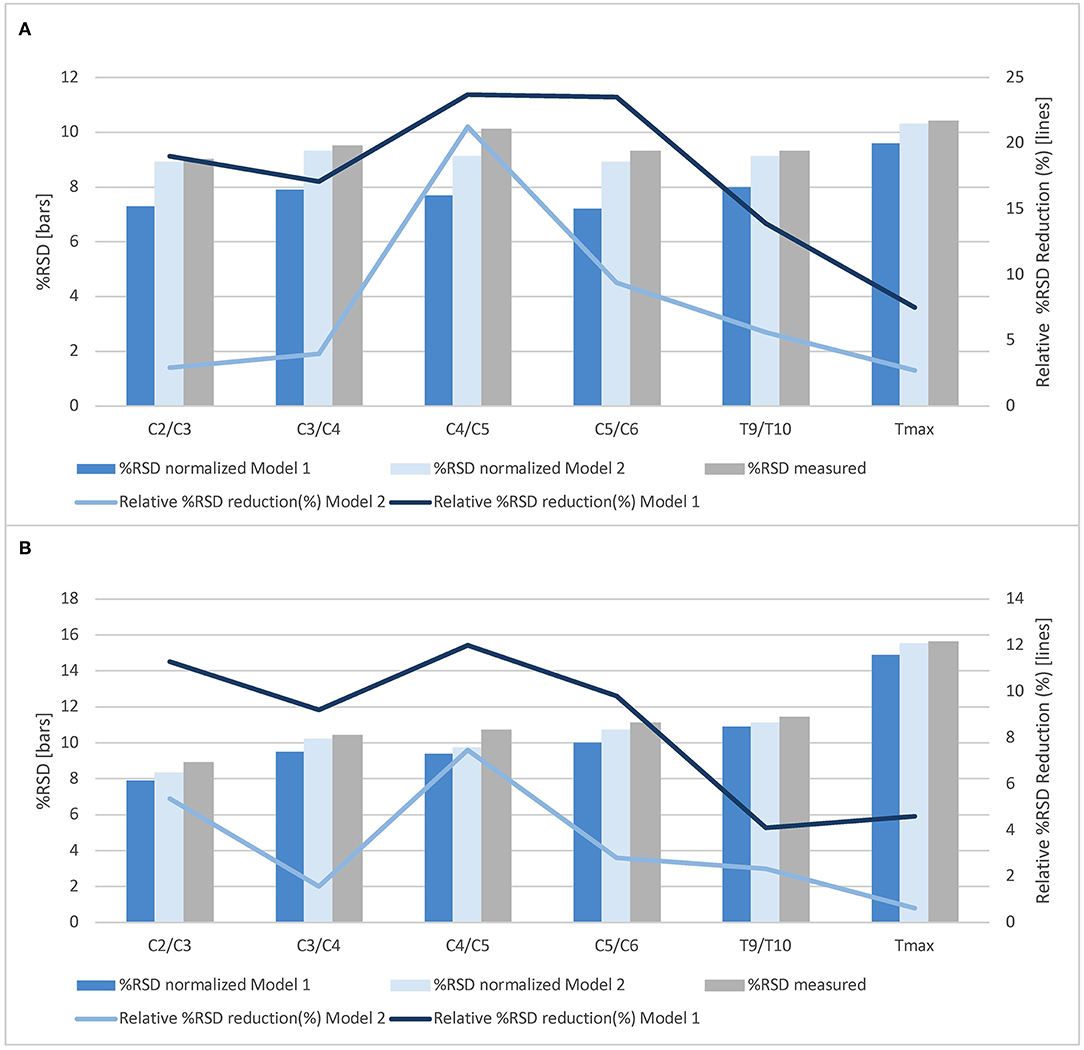
Figure 3. Normalization effects of Model 1 and Model 2 on total cross-sectional cord areas and gray matter areas at all cord levels. The % relative standard deviation (RSD, standard deviation divided by the mean area) and relative % RSD reduction obtained by normalization based on Model 1 (sex, brain white matter volume and spinal canal area at the level C4/C5) as well as by Model 2 (spinal canal area at the level C4/C5) for (A) total cross-sectional cord areas and (B) spinal cord gray matter areas (whole study population, n = 61).
In a subgroup analysis, we separately examined potential normalization variables for anatomical variability reduction in minors (n = 18) at the C4/C5 level. Supplementary Table 5 in the supplement shows the univariate associations between SC areas and potential covariates using Pearson correlation.
Backward selection (starting with a model containing all variables with a Pearson correlation coefficient of |r| > 0.30 in univariate analysis together with age and sex as predictor variables and TCA at C4/C5 as outcome parameter) resulted in a model with the area of the spinal canal at level C4/C5 and weight as predictor variables (Model 3 m) (p of the model = 0.0027, adjusted r2 = 0.48). Normalization reduced the RSD about 32.5% for TCA and 18.3% for GM area at the level C4/C5. Inter-individual variability was reduced from 8.1 to 5.7% for TCA and reduced from 9.3 to 7.1% for GM area. This normalization model was applied to all other measured SC levels (Table 6).
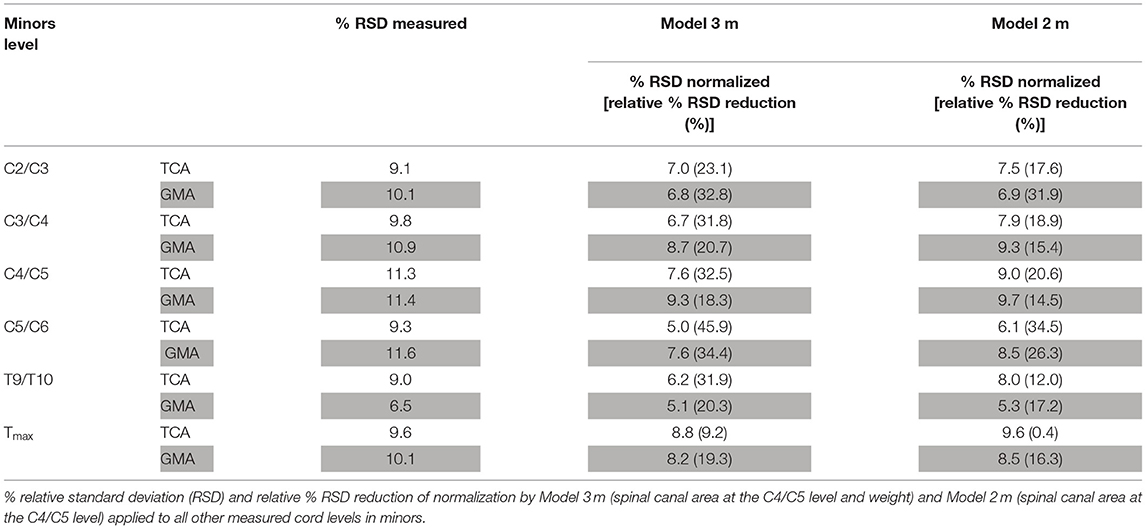
Table 6. Performance of normalization by Model 3 m and Model 2 m for total cross-sectional cord areas (TCA) and gray matter areas (GMA) at all other cord levels in minors.
For reasons of practicability, Model 3 m was further simplified to a univariate model containing the area of the spinal canal of the C4/C5 level as single predictor variable in minors (in analogy to Model 2 named Model 2 m) (p = 0.0074, adjusted r2 = 0.33). This normalization model was also applied to all other measured SC levels in minors (Table 6).
Anatomical inter-subject variations in healthy subjects are a relevant source of SC TCA and GM area variability. Our study demonstrated an inter-individual variability of TCA and SC GM areas ranging from 6 to 9% in the cervical and 9–12% in the thoracic SC, which is in line with prior reports investigating the upper cervical SC (24).
Before applying morphometric SC GM/WM imaging techniques such as the novel rAMIRA approach for atrophy assessments in clinical studies in lower motor neuron disorders, it therefore seems necessary to develop efficient strategies to reduce the inter-subject variability of SC area measurements in healthy subjects to facilitate the detection of pathology-related changes.
This study explored potential normalization strategies for variability reduction in 61 healthy subjects with a broad age range from 11 to 93 years (including a subgroup of 18 minors).
Normalization models were developed using backward selection starting with a model containing the predictor variables age and sex as well as all anatomical metrics with a Pearson correlation coefficient of |r| > 0.3 in univariate analysis with TCA at C4/C5—the level of the cervical enlargement—as outcome. The backward selection procedure yielded a model including the predictor variables sex, spinal canal area at C4/C5, and brain WM volume. Normalization of SC areas by this model reduced the % RSD of TCA at the level C4/C5 by 24%, of GM area by 12%, and also at the other cervical SC levels in the range of 17–24% (TCA) and 9–12% (GM area). Therefore, this approach seems an effective normalization strategy suited for studies in healthy subjects or in SC diseases that do not affect cerebral WM.
However, as brain WM volume is frequently altered in SC diseases either directly by a disease pathology affecting both brain and SC (e.g., in multiple sclerosis) or potentially also secondary to lower motor neuron disorders, e.g., by retrograde trans-synaptic degeneration, we also performed a second backward analysis containing only demographic and skeleton-based variables otherwise following the above mentioned selection criteria. The resulting model contained the spinal canal area as single predictor. Normalization by this model reduced the % RSD of TCA and GM area at C4/C5 by 10%, respectively. However, application of this normalization to other SC levels, particularly to the thoracic SC, showed only small effects in our cohort, with relative % RSD reductions ranging from 1 to 7%.
Age was not a significant predictor variable for SC areas in this study. During normalization model development, age consistently was eliminated in the backward selection process. We consistently observed maximum mean TCA values in the middle-aged group at all SC levels. This is in line with the observation by Papinutto et al. (24) of a TCA peak at the level C2/C3 at ~45 years of age. While at all levels minimum mean SC GM area values were observed in the subgroup of elderly subjects, maximum mean SC GM area values were observed in the subgroup of middle-aged subjects only at the level C3/C4, with equally high or higher mean SC GM area values in minors at all other levels.
Since children and adolescents are the main target group for the recently approved SMA treatments (36) and no data exists on SC GM area inter-subject variability, we conducted a sub-group analysis in minors (n = 18). Following the same variable selection procedure as described above, normalization based on the spinal canal area at the level C4/C5 and the variable body weight resulted in a reduction of % RSD of TCA values at the level C4/C5 by 32%. Analogous normalization of GM areas reduced the % RSD by 18% at the level C4/C5, and also consistently at all other cervical and thoracic levels by 19–34%. The % RSD reduction observed in TCAs by this normalization method are slightly larger than what has been described in a very recently published study in minors aged 7–17 years by normalization with the product of the anterior posterior and lateral diameter of the spinal canal at the level C2/3 and skull volume (30). This difference could be partly explained by difference in in-plane resolution of the images used for SC segmentation (0.5 vs. 1 mm2) (30) and by the chosen predictors. Effects on SC GM area were not investigated in that study (30).
The spinal canal area is relatively easy to measure and showed excellent inter-rater reliability coefficients (33). Normalization based on spinal canal area at the level C4/C5 and body weight could therefore be a promising and brain volume-independent procedure in upcoming studies involving minors with lower motor neuron diseases to reduce anatomical inter-subject variability. Whether this approach increases the sensitivity and specificity in detecting disease-related changes in motor neuron disorders or treatment effects, needs to be further investigated in subsequent studies involving patients.
Image quality of the cervical rAMIRA images was generally high permitting SC, TCA, and GM segmentation in >98% of acquired cervical SC images of the study population. Imaging the thoracic SC is in general more challenging: Factors, that can negatively impact image quality at the thoracic levels include lower coil sensitivity in this area (Figure 1, bottom row) and potential artifacts arising from heart and breathing motion, from pulsating large blood vessels, and from increased susceptibility variations. Despite these limitations, the novel rAMIRA method enabled TCA and GM area segmentation in >90% of the acquired thoracic SC images. Segmentation was not reliably possible at the level T9/T10 and Tmax in 6 out of 61 subjects, respectively, because of the upper mentioned issues. The % RSD of the SC metrics was higher at Tmax compared to the cervical levels (Table 1), which is likely due to the high inherent anatomical variability in this region. None of the investigated regional anatomical metrics did show a significant association with SC areas at Tmax. A unifying normalization concept based on normalization of SC areas at different levels, by one model based on anatomical metrics measured at one selected level, and not by several models with regional normalization metrics, was therefore chosen. Though not perfect, this approach will also permit comparison of atrophy effects between levels.
It should be mentioned that the study has the following limitations: the sample size of 61 healthy subjects, including only 18 minors, is rather small. The assumption that the investigated skeleton-derived metrics are fully disease independent might be questioned, as secondary orthopedic complications, immobilization, and also treatment effects might alter spine metrics in a way that is not easily predicted. Spine-derived metrics were assessed manually in this study but showed excellent inter-rater reliability.
In conclusion, rAMIRA imaging is a novel approach to perform SC imaging with favorable contrast in clinical settings that is well-suited for GM/WM quantitation in both the cervical and thoracic SC. Our study demonstrates that the inter-individual variability of SC area measurements in healthy subjects is relatively high but can be effectively reduced in the cervical and to a lesser degree also in the thoracic SC, particularly in minors, by a model based on spinal canal area at the level C4/C5 and weight. By reducing anatomical inter-subject variability, these approaches may facilitate the detection of pathology-related changes and the improvement of therapeutic monitoring in lower motor neuron disorders in the future.
Upon reasonable request, we will render the detailed results derived from the reported analyses available.
The studies involving human participants were reviewed and approved by Ethikkommission Nordwest- und Zentralschweiz - EKNZ. Written informed consent to participate in this study was provided by the participants or the participants' legal guardian/next of kin.
EK, MW, CW, OB, and RS conceptualized and designed the study. MW, CW, AH, PC, and OB handled the methods development. EK, JW, LR, TH, and RS collected the data. EK, JW, CW, TH, MB, LS, and RS conducted the data analysis. EK, MW, CW, and RS were in charge of the paper drafting. EK, JW, MW, CW, TH, LR, LS, AH, MB, PC, CG, OB, and RS reviewed the manuscript. OB and RS obtained the funding. All authors contributed to the article and approved the submitted version.
This study was supported by the Swiss National Science Foundation (SNSF) (Grant No 320030_156860); Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung, and Biogen Inc. The funder did not play any role in study design, data, analysis and interpretation of the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all study participants.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.637198/full#supplementary-material
1. Fradet L, Arnoux PJ, Ranjeva JP, Petit Y, Callot V. Morphometrics of the entire human spinal cord and spinal canal measured from in vivo high-resolution anatomical magnetic resonance imaging. Spine. (2014) 39:E262–9. doi: 10.1097/BRS.0000000000000125
2. Taso M, Le Troter A, Sdika M, Ranjeva JP, Guye M, Bernard M, et al. Construction of an in vivo human spinal cord atlas based on high-resolution MR images at cervical and thoracic levels: preliminary results. MAGMA. (2014) 27:257–67. doi: 10.1007/s10334-013-0403-6
3. Yiannakas MC, Kakar P, Hoy LR, Miller DH, Wheeler-Kingshott CA. The use of the lumbosacral enlargement as an intrinsic imaging biomarker: feasibility of grey matter and white matter cross-sectional area measurements using MRI at 3T. PLoS ONE. (2014) 9:e105544. doi: 10.1371/journal.pone.0105544
4. Yiannakas MC, Kearney H, Samson RS, Chard DT, Ciccarelli O, Miller DH, et al. Feasibility of grey matter and white matter segmentation of the upper cervical cord in vivo: a pilot study with application to magnetization transfer measurements. Neuroimage. (2012) 63:1054–9. doi: 10.1016/j.neuroimage.2012.07.048
5. Kearney H, Yiannakas MC, Abdel-Aziz K, Wheeler-Kingshott CA, Altmann DR, Ciccarelli O, et al. Improved MRI quantification of spinal cord atrophy in multiple sclerosis: cord atrophy in MS. J Magn Reson Imaging. (2014) 39:617–23. doi: 10.1002/jmri.24194
6. Papinutto N, Schlaeger R, Panara V, Caverzasi E, Ahn S, Johnson KJ, et al. 2D phase-sensitive inversion recovery imaging to measure in vivo spinal cord gray and white matter areas in clinically feasible acquisition times. J Magn Reson Imaging. (2015) 42:698–708. doi: 10.1002/jmri.24819
7. Tsagkas C, Horvath A, Altermatt A, Pezold S, Weigel M, Haas T, et al. Automatic spinal cord gray matter quantification: a novel approach. AJNR Am J Neuroradiol. (2019) 40:1592–600. doi: 10.3174/ajnr.A6157
8. Weigel M, Haas T, Wendebourg MJ, Schlaeger R, Bieri O. Imaging of the thoracic spinal cord using radially sampled averaged magnetization inversion recovery acquisitions. J Neurosci Methods. (2020) 343:108825. doi: 10.1016/j.jneumeth.2020.108825
9. Weigel M, Bieri O. Spinal cord imaging using averaged magnetization inversion recovery acquisitions. Magn Reson Med. (2018) 79:1870–81. doi: 10.1002/mrm.26833
10. Sabaghian S, Dehghani H, Batouli SAH, Khatibi A, Oghabian MA. Fully automatic 3D segmentation of the thoracolumbar spinal cord and the vertebral canal from T2-weighted MRI using K-means clustering algorithm. Spinal Cord. (2020) 58:811–20. doi: 10.1038/s41393-020-0429-3
11. Gros C, De Leener B, Badji A, Maranzano J, Eden D, Dupont SM, et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage. (2019) 184:901–15. doi: 10.1016/j.neuroimage.2018.09.081
12. Prados F, Cardoso MJ, Yiannakas MC, Hoy LR, Tebaldi E, Kearney H, et al. Fully automated grey and white matter spinal cord segmentation. Sci Rep. (2016) 6:36151. doi: 10.1038/srep36151
13. Datta E, Papinutto N, Schlaeger R, Zhu A, Carballido-Gamio J, Henry RG. Gray matter segmentation of the spinal cord with active contours in MR images. Neuroimage. (2017) 147:788–99. doi: 10.1016/j.neuroimage.2016.07.062
14. Horvath A, Tsagkas C, Andermatt S, Pezold S, Parmar K, Cattin P. Spinal Cord Gray Matter-White Matter Segmentation on Magnetic Resonance AMIRA Images With MD-GRU. (2018). Available online at: https://arxiv.org/pdf/1808.02408.pdf (accessed March 04, 2021).
15. Blaiotta C, Freund P, Curt A, Cardoso J, Ashburner J. A probabilistic framework to learn average shaped tissue templates and its application to spinal cord image segmentation. In: Proceedings of the 24th Annual Meeting of ISMRM. Singapore (2016). p. 1449.
16. Porisky A, Brosch T, Ljungberg E, Tang LYW, Yoo Y, De Leener B, Traboulsee A, et al. Grey matter segmentation in spinal cord MRIs via 3D convolutional encoder networks with shortcut connections. In: Cardoso MJ, Arbel T, Carneiro G, editors. Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support. Springer International Publishing (2017). p. 330–7.
17. Dupont SM, De Leener B, Taso M, Le Troter A, Nadeau S, Stikov N, et al. Fully-integrated framework for the segmentation and registration of the spinal cord white and gray matter. Neuroimage. (2017) 150:358–72. doi: 10.1016/j.neuroimage.2016.09.026
18. Prados F, Ashburner J, Blaiotta C, Brosch T, Carballido-Gamio J, Cardoso MJ, et al. Spinal cord grey matter segmentation challenge. Neuroimage. (2017) 152:312–29. doi: 10.1016/j.neuroimage.2017.03.010
19. Engl C, Schmidt P, Arsic M, Boucard CC, Biberacher V, Röttinger M, et al. Brain size and white matter content of cerebrospinal tracts determine the upper cervical cord area: evidence from structural brain MRI. Neuroradiology. (2013) 55:963–70. doi: 10.1007/s00234-013-1204-3
20. Mann RS, Constantinescu CS, Tench CR. Upper cervical spinal cord cross-sectional area in relapsing remitting multiple sclerosis: application of a new technique for measuring cross-sectional area on magnetic resonance images. J Magn Reson Imaging. (2007) 26:61–5. doi: 10.1002/jmri.20959
21. Song F, Huan Y, Yin H, Ge Y, Wei G, Chang Y, et al. Normalized upper cervical spinal cord atrophy in multiple sclerosis. J Neuroimaging. (2008) 18:320–7. doi: 10.1111/j.1552-6569.2007.00222.x
22. Healy BC, Arora A, Hayden DL, Ceccarelli A, Tauhid SS, Neema M, et al. Approaches to normalization of spinal cord volume: application to multiple sclerosis. J Neuroimaging. (2012) 22:e12–9. doi: 10.1111/j.1552-6569.2011.00629.x
23. Oh J, Seigo M, Saidha S, Sotirchos E, Zackowski K, Chen M, et al. Spinal cord normalization in multiple sclerosis. J Neuroimaging. (2014) 24:577–84. doi: 10.1111/jon.12097
24. Papinutto N, Asteggiano C, Bischof A, Gundel TJ, Caverzasi E, Stern WA, et al. Intersubject variability and normalization strategies for spinal cord total cross-sectional and gray matter areas. J Neuroimaging. (2020) 30:110–8. doi: 10.1111/jon.12666
25. Sanfilipo MP, Benedict RHB, Zivadinov R, Bakshi R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: the proportion vs. residual method. Neuroimage. (2004) 22:1732–43. doi: 10.1016/j.neuroimage.2004.03.037
26. Schlaeger R, Papinutto N, Panara V, Bevan C, Lobach IV, Bucci M, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol. (2014) 76:568–80. doi: 10.1002/ana.24241
27. Casserly C, Seyman EE, Alcaide-Leon P, Guenette M, Lyons C, Sankar S, et al. Spinal cord atrophy in multiple sclerosis: a systematic review and meta-analysis: spinal cord atrophy in multiple sclerosis: systematic review. J Neuroimaging. (2018) 28:556–86. doi: 10.1111/jon.12553
28. Isik S, Samanci MY. Analysis of MRI morphometric parameters of the pediatric cervical spine and spinal cord. J Turk Spinal Surg. (2019) 30:157–61. Available online at: http://cms.galenos.com.tr/Uploads/Article_27965/jtss-30-157-En.pdf (accessed March 02, 2021).
29. Lao L, Chen Z, Qiu G, Shen J. Whole-spine magnetic resonance imaging study in healthy Chinese adolescents. Orthop Surg. (2013) 5:164–70. doi: 10.1111/os.12046
30. Papinutto N, Cordano C, Asteggiano C, Caverzasi E, Mandelli ML, Lauricella M, et al. MRI measurement of upper cervical spinal cord cross-sectional area in children. J Neuroimaging. (2020) 30:598–602. doi: 10.1111/jon.12758
31. Horsfield MA, Sala S, Neema M, Absinta M, Bakshi A, Sormani MP, et al. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. Neuroimage. (2010) 50:446–55. doi: 10.1016/j.neuroimage.2009.12.121
32. Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. NeuroImage. (2015) 104:366–72. doi: 10.1016/j.neuroimage.2014.09.034
33. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
34. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates (1988).
35. Papinutto N, Schlaeger R, Panara V, Zhu AH, Caverzasi E, Stern WA, et al. Age, gender and normalization covariates for spinal cord gray matter and total cross-sectional areas at cervical and thoracic levels: a 2D phase sensitive inversion recovery imaging study. PLoS ONE. (2015) 10:e0118576. doi: 10.1371/journal.pone.0118576
Keywords: spinal cord gray matter imaging, MRI, normalization, inter-subject variability, minors, spinal muscular atrophy
Citation: Kesenheimer EM, Wendebourg MJ, Weigel M, Weidensteiner C, Haas T, Richter L, Sander L, Horvath A, Barakovic M, Cattin P, Granziera C, Bieri O and Schlaeger R (2021) Normalization of Spinal Cord Total Cross-Sectional and Gray Matter Areas as Quantified With Radially Sampled Averaged Magnetization Inversion Recovery Acquisitions. Front. Neurol. 12:637198. doi: 10.3389/fneur.2021.637198
Received: 02 December 2020; Accepted: 05 February 2021;
Published: 25 March 2021.
Edited by:
Anna Pichiecchio, Neurological Institute Foundation Casimiro Mondino (IRCCS), ItalyReviewed by:
Giovanni Savini, University of Milan, ItalyCopyright © 2021 Kesenheimer, Wendebourg, Weigel, Weidensteiner, Haas, Richter, Sander, Horvath, Barakovic, Cattin, Granziera, Bieri and Schlaeger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regina Schlaeger, cmVnaW5hLnNjaGxhZWdlckB1c2IuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.