- 1ProMedica Neurosciences Center, Toledo, OH, United States
- 2Department of Neurology, College of Medicine and Life Sciences, University of Toledo, Toledo, OH, United States
- 3Department of Neurology, Antelope Valley Hospital, Lancaster, CA, United States
Background: Neurointerventional procedures in acute ischemic stroke often require immediate antiplatelet therapy in the cases of acute stenting and occasionally re-occluding vessels. Intravenous cangrelor is a P2Y12 receptor antagonist with short onset and quick offset. The study objective was to evaluate the safety and efficacy of intravenous cangrelor in patients with acute ischemic stroke requiring urgent antiplatelet effect.
Methods: Patients who received intravenous cangrelor intra-procedurally during acute ischemic stroke treatment were identified from a prospectively collected database. Cangrelor was administered as a bolus of 15 mcg/kg, followed by an infusion rate of 2 mcg/kg/min. A historical control group consisting of anterior circulation tandem occlusions was used to compare to patients with similar lesions who received intravenous cangrelor. Outcomes of interest included in-stent thrombosis, thromboembolic complications, intracranial hemorrhage, and functional outcomes at 90 days.
Results: Twelve patients received intravenous cangrelor for acute ischemic stroke between October 2018 and April 2020 at a comprehensive stroke center. Eleven patients had intra or extracranial stenting performed, which included two posterior circulation lesions. No cases of symptomatic intracranial hemorrhage were reported. At 90 day follow-up, two patients had died and 10 had a good functional outcome. Patients with anterior circulation tandem occlusions who received cangrelor and those who received dual antiplatelets orally had similar radiographic and clinical outcomes.
Conclusion: Low dose intravenous cangrelor is similar in safety and efficacy to oral antiplatelets in acute ischemic stroke in a small case series. Larger prospective studies on the efficacy, safety, and effect on procedure times of intravenous cangrelor in neurointervention are warranted.
Introduction
Mechanical thrombectomy for acute ischemic stroke often requires immediate antiplatelet therapy when underlying intracranial atherosclerotic disease is encountered, and this frequently needs to be weighed against the risk of bleeding. Current American Heart Association guidelines allow for consideration of antiplatelet therapy within 24 h of IV tPA administration when it provides substantial benefit or withholding such treatment is known to cause substantial risk (Level IA) (1). Administration of antiplatelet therapy for acute intracranial or extracranial stenting is often done with incomplete information such as final infarct volume, previous systemic bleeding complications, medication intolerances, and potential need for decompressive cranial surgery. Furthermore, to avoid the risk of aspiration due to oral administration of antiplatelet medications, emergent placement of a nasogastric tube before or during mechanical thrombectomy is frequently required, often after administration of intravenous tPA. Clopidogrel is a commonly used antiplatelet therapy in acute ischemic stroke, has a peak onset of ~2 h after a loading dose, may be affected by underlying co-morbidities, may interact with other medications, and has an unpredictable antiplatelet effect in patients with CYP genetic polymorphisms (2).
Intravenous cangrelor is a potent, reversible, non-thienopyridine ADP analog that provides adequate platelet inhibition within 3–6 min and has a short half-life. It does not require hepatic activation, can be infused through a peripheral intravenous line, and its antiplatelet activity can be accurately assessed by the VerifyNow PRU assay (3). Given the predictable pharmacokinetics in patients with a wide variety of underlying co-morbidities, it provides an important solution for acute neurointerventions. Randomized clinical trials in interventional cardiology have found that intravenous cangrelor may be a useful and cost-effective substitute for currently used oral antiplatelets (4–6). Given the differences in patient characteristics, clinical outcomes, and treatment considerations from cardiac patients, we sought to determine the safety and efficacy of using low dose cangrelor infusions in patients with acute ischemic stroke.
Materials and Methods
After institutional board review approval, we reviewed consecutive patients in our prospectively collected acute ischemic stroke database for patients over 18 years who presented between October 2018 and April 2020 and received intravenous cangrelor within 6 h of hospital admission and underwent mechanical thrombectomy. We compared this group with consecutive patients from the same database who presented with anterior circulation tandem occlusions based on digital subtraction imaging who underwent mechanical thrombectomy in the previous 4 years (October 2014–October 2019) prior to the availability of intravenous cangrelor at our comprehensive stroke center. Exclusion criteria included patients who were already on dual antiplatelet therapy.
Baseline demographics, time metrics, neurointerventional procedural details, and follow-up neuroimaging were abstracted from our database. We retrospectively evaluated ischemic core volume on post-procedural MRI scans (or CT brain if not available) using the ABC/2 method (7). Follow-up mRS scores were obtained by in-person visits with a stroke physician at 90 days.
The primary study outcome was intracranial hemorrhage within 24 h of starting cangrelor infusion. Secondary outcomes included discharge disposition, disability at 90 days as assessed by the modified Rankin scale, worsening hemorrhage within 36 h of cangrelor infusion or any hemorrhage requiring discontinuation of intravenous cangrelor, and recurrent transient ischemic attack or ischemic stroke within 30 days.
Treatment and Cangrelor Administration Protocol
Patients were selected for mechanical thrombectomy based on CT angiogram if they presented within 6 h of the last known normal, and CT perfusion if they presented between 6 and 24 h of the last known normal.
Patients requiring acute antiplatelet therapy regardless of intravenous tPA infusion were administered intravenous cangrelor in the neuroangiography suite immediately prior to stenting. Carotid artery stenting was always performed after recanalization of the intracranial occlusion. A bolus of 15 mcg/kg was administered over 2 min, followed by an infusion rate of 2 mcg/kg/min. We selected a 15 mcg/kg bolus followed by 2 mcg/kg/min dosing schedule for cangrelor given that it provides similar amounts of platelet aggregation and time to recovery of platelet function as the higher dose (8). Decision regarding acute stenting was left to the discretion of the neurointerventionalists. When acute stenting was required, cangrelor bolus was completed prior to stent placement. Assessment of P2Y12 levels was performed at the discretion of the treating provider. Patients were maintained on intravenous cangrelor for 12–24 h and transitioned to dual oral antiplatelet therapy after post-thrombectomy neuroimaging. Aspirin was administered prior to discontinuation of intravenous cangrelor. For patients who were unable to swallow, oral antiplatelets were administered through a nasogastric tube. If patients were transitioned to oral ticagrelor, this was administered immediately prior to cangrelor infusion discontinuation. If clopidogrel was chosen as the second antiplatelet, it was administered 15 min after discontinuing the intravenous cangrelor infusion. Choice of oral antiplatelet transition from cangrelor was left to the discretion of the treating neurointerventionalist.
Statistical Analysis
Collected data were exported from Microsoft Excel into statistical software “R: A language and environment for statistical computing; EZR version 1.32” (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Continuous and categorical variables are presented as mean (standard deviation), median (interquartile range), and percentages where appropriate. Continuous variables were analyzed with the Student t-test or Mann-Whitney test where appropriate, and categorical data were analyzed using the Fisher exact test. A p-value of ≤ 0.05 was considered significant. All analyses were two-tailed and the level of significance was set at 0.05.
Results
Between October 2018 and April 2020, 12 patients received intravenous cangrelor during neurointerventional procedures in the setting of acute ischemic stroke (see Table 1). There were five (41.7%) women, mean age of the cohort was 67.9 ± 12, and median ASPECT/pc-ASPECT score was 9.5 (IQR 9–10). Median NIHSS on presentation was 15 (13–21), seven (58.3%) patients received intravenous tPA, and all patients achieved successful reperfusion (TICI 2b or greater) (Table 2 describes individual characteristic & treatment variables).
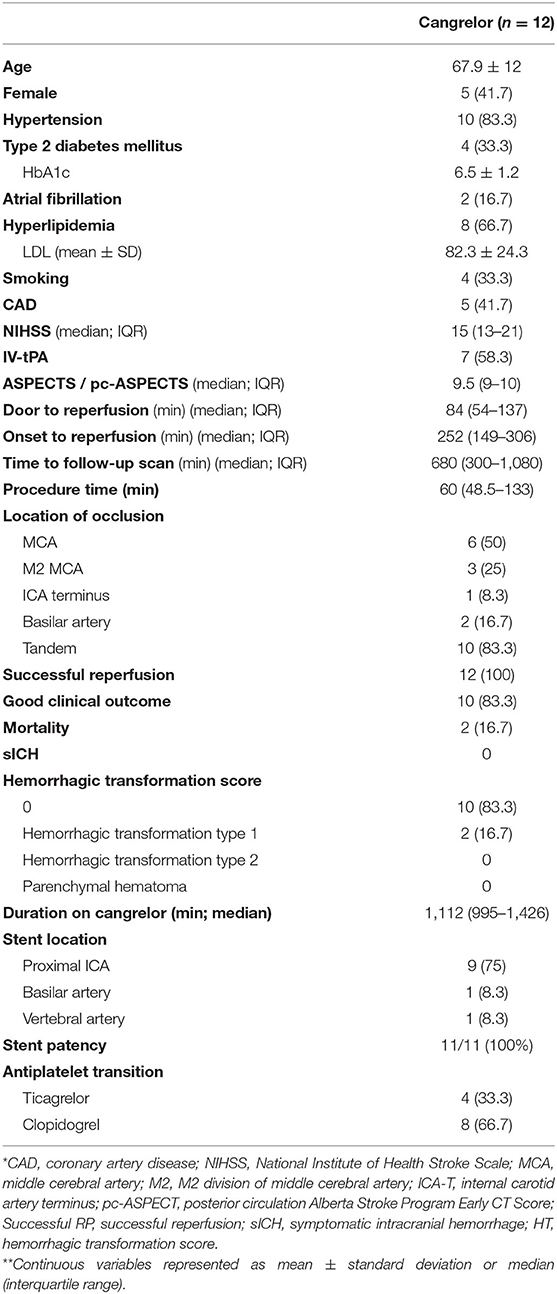
Table 1. Baseline demographics and treatment variables for patients who received intravenous cangrelor for acute ischemic stroke.
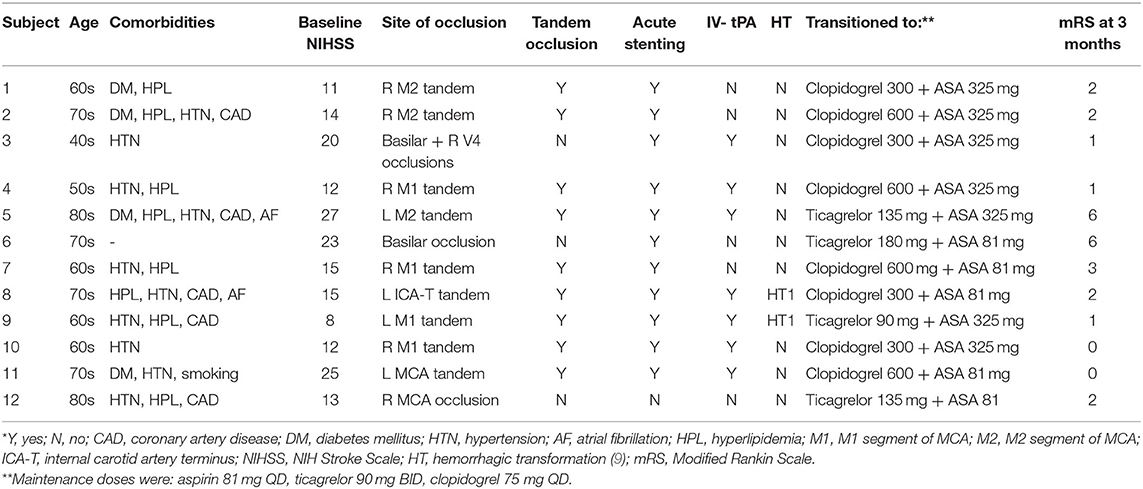
Table 2. Baseline characteristics and treatment variables of patients receiving intravenous cangrelor for acute ischemic stroke.
Of the cohort, nine patients had tandem anterior circulation occlusions, two had basilar occlusions, and one patient had an MCA occlusion secondary to severe stenosis. Eleven of the 12 patients had a single intra or extra cranial stent placed immediately after thrombectomy. One patient with severe MCA stenosis had re-occlusion after initial mechanical thrombectomy. Intravenous cangrelor was started prior to the subsequent thrombectomy on this patient, and did not require intracranial stenting.
Patients were kept on intravenous cangrelor for an average of 18.5 h (IQR 995–1,426 min) after which they were transitioned to oral aspirin and ticagrelor (4; 33%) or clopidogrel (8; 67%). P2Y12 levels were assessed in four patients; one while on cangrelor infusion, two after transition to clopidogrel and aspirin, and one after transitioning to ticagrelor and aspirin. Their respective P2Y12 reaction unit levels were 31, 271, 124, and 77. All patients had a repeat neuroimaging within 24 h and seven patients had a third scan after. There were two cases of HT1 per the Heidelberg Bleeding Classification scoring system (9) as assessed on CT brain images, but no other cases of intracranial hemorrhage. Neither patient had worsening intracranial hemorrhage on follow-up scans. There were no cases of in-stent thrombosis, parent vessel thrombosis, or distal thromboembolic events.
Of the studied patients, three (25%) were discharged to home, six (50%) to in-patient rehabilitation, and three (25%) to a skilled nursing facility. Two patients in the cohort died, one of whom had a pulmonary embolism at a skilled nursing facility and another who entered hospice care after discharge at a skilled nursing facility. At 90 day follow-up, all remaining 10 patients had a good functional outcome with a modified Rankin score of ≤ 2 or return to baseline functional status and no recurrent TIA or ischemic stroke (see Supplementary Table 1 for mRS breakdown).
Comparison of Anterior Circulation Tandem Occlusions With and Without Cangrelor
Prior to the availability of intravenous cangrelor, anterior circulation tandem occlusions were loaded with aspirin (325 mg) and clopidogrel (600 mg) acutely via a nasogastric tube peri-procedurally (no-cangrelor group). Patients with anterior circulation tandem occlusions who received intravenous cangrelor for acute proximal internal carotid artery stenting (n = 9) had similar baseline demographics and NIHSS scores to those in the no-cangrelor (dual antiplatelet) group (n = 23) (See Table 3). However, the cangrelor group had a higher median ASPECT score (10 vs. 8; p = 0.008). Although not significant, cangrelor tandem occlusions had a lower number of ICA terminus occlusions (11 vs. 26%; p = 0.40) and shorter procedure times (51 vs. 82 min; p = 0.79). Successful reperfusion was comparable between the two groups (100 vs. 96%; p = 1) as was a good functional outcome (89 vs. 61%; p = 0.21) and mortality (11 vs. 22%; p = 0.65). Three patients in the dual antiplatelet group suffered symptomatic ICH and none occurred in the cangrelor group (p = 0.54).
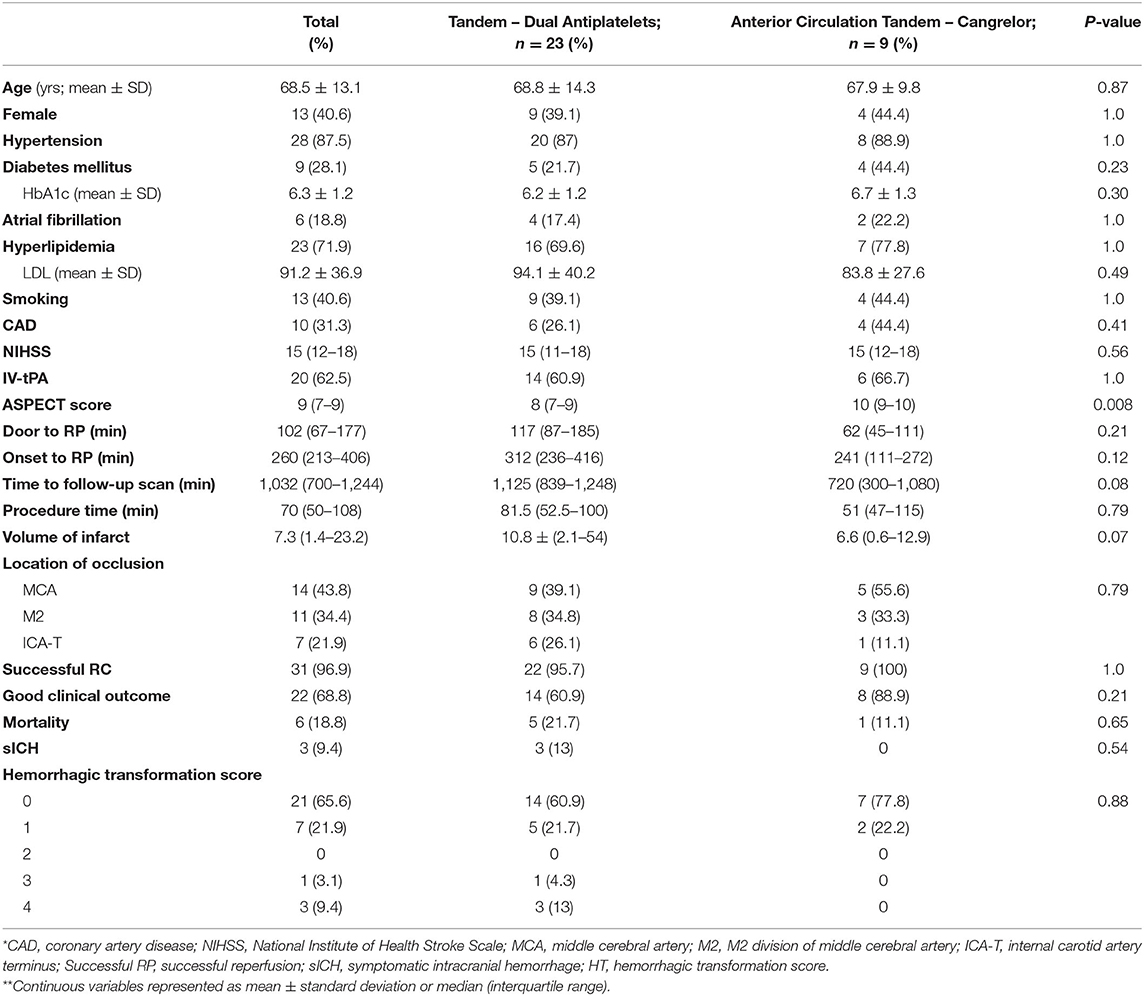
Table 3. Anterior circulation tandem occlusions treated with acute administration of oral antiplatelets compared with those who were treated acutely with intravenous cangrelor.
Discussion
This single center study evaluated 12 patients who received intravenous cangrelor in acute ischemic stroke and found no cases of symptomatic intracranial hemorrhage or in-stent thrombosis. When compared to historical controls with similar occlusions, use of intravenous cangrelor for tandem occlusions found similar rates of good clinical outcome, procedure time, and symptomatic ICH. This study, and others, indicate that acute use of intravenous cangrelor in acute ischemic stroke may be safe and effective and requires further evaluation.
Safety and efficacy of cangrelor has been assessed in patients with cardiac disease. In the CHAMPION PCI study, 8,716 patients with acute coronary syndrome were randomized to receive intravenous cangrelor for a minimum of 2 h followed by transition to clopidogrel, or a clopidogrel 600 mg load at the beginning of the procedure (4). The study was terminated prematurely with 98.6% of planned enrollment completed after an interim analysis revealed that the study was unlikely to show superiority of cangrelor over clopidogrel. Primary endpoints of death, myocardial infarction, revascularization, and in-stent thrombosis were similar between the two groups. Additionally, cangrelor and clopidogrel had similar rates of major bleeding. Following this study, the double-blind placebo-controlled CHAMPION PHOENIX trial compared cangrelor infusion to clopidogrel 300–600 mg load at the start or end of PCI in patients with stable angina, NSTEMI, or STEMI (5). Cangrelor reduced the primary composite end point of death, MI, ischemia-driven revascularization, and death in this study (4.7 vs. 5.9%; CI 0.66–0.93, p = 0.005). Intravenous infusion of cangrelor achieved lower rates of intra-procedural (0.6 vs. 1.0%; CI 0.42–0.99, p = 0.04), 30-d stent-thrombosis (0.8 vs. 1.4%; CI 0.43–0.9; p = 0.01), and procedural complications (3.4 vs. 4.5%; 0.61–0.9; p = 0.002) with similar rates of minor and major bleeding. Finally, the BRIDGE study evaluated intravenous cangrelor in a randomized multicenter trial of 210 patients with ACS or post-coronary stenting who were awaiting CABG surgery (10). Compared to placebo, cangrelor infusion at lower titrated doses based on P2Y12 Reaction Units (PRUs) for 2–7 d resulted in higher rates of platelet inhibition. Incidence of bleeding and ischemic events were low in both groups, likely secondary to the lack of power to assess for a difference between the two groups.
Use of cangrelor from cardiology cannot be extrapolated for use in ischemic stroke cases. Patients with acute ischemic stroke, particularly after intravenous tPA or those with low ASPECT scores are at higher risk of intracranial hemorrhage. Furthermore, AIS patients are often not able to follow commands and approximately one fourth of all AIS patients have dysphagia, limiting the use of oral antiplatelets (11). These patients require placement of uncomfortable nasogastric tubes in the neuroangiography suite which often requires sedation in patients not under general anesthesia. Lastly, at the time of acute antiplatelet therapy in AIS, the volume of core infarct is unknown and may create challenges in patients who require early decompressive hemicraniectomy.
Evidence for early stenting for AIS due to tandem occlusions is limited and has not been studied in prospective randomized trials. A meta-analysis of tandem occlusions revealed similar rates of good clinical outcome, procedural complications, and intracranial hemorrhage in patients with stenting or angioplasty of the carotid lesion (12). However, acute stenting treats the symptomatic vessel provoking thrombus and reduces risk of early re-occlusion. Balloon angioplasty alone is not the standard of care for carotid stenosis and may increase the risk of vessel dissection and re-stenosis. Sub-analysis of the STRATIS registry revealed that patients who underwent acute stenting of extracranial carotid stenosis had a higher rate of good clinical outcomes with similar safety to patients not undergoing stenting (13). All nine patients with tandem occlusions had a good functional outcome in our case series, likely a reflection of the short onset to reperfusion times (mean 245 ± 176 min). On the other hand, early stenting with irreversible oral dual antiplatelet therapy may result in poor clinical outcomes if there is any hemorrhagic transformation.
Acute stenting may also be used as a rescue technique after failed mechanical thrombectomy or for severe intracranial stenosis. Although current evidence indicates that stenting for intracranial atherosclerotic disease should be performed at least 7 d after stroke, high grade stenosis with intraprocedural re-development of superimposed thrombus is a subset of AIS patients who require acute stenting. Two of our patients required acute intracranial stents for severely stenotic, re-occluding posterior circulation strokes. Furthermore, use of intravenous antiplatelet therapy may prevent re-development of superimposed thrombus and allow for delayed intracranial stenting as noted in one of our study patients with severe stenosis of the MCA M1 division.
Intravenous cangrelor competitively inhibits the P2Y12 receptor with high affinity and provides a rapid onset of ADP-induced platelet inhibition in the acute stroke setting. Cangrelor does not have any clinically significant drug interactions, does not require activation via hepatic metabolism, and is not affected by renal function, making it an effective weight-based, dose-dependent medication in stroke patients in whom complete medical history may not be available. Its linear pharmacokinetics allows for a dependable amount of platelet inhibition and rapid dephosphorylation by nucleosides allow for rapid inactivation. Lastly, platelet-induced aggregation to the agonist ADP can be tested using VerifyNow Platelet Reaction Units (PRU). Optimal PRU ranges are ambiguous but patients can be assessed to determine their hypo (>240) or hyper-responder (<60) status (14).
While most studies evaluated intravenous cangrelor infusion for short periods, the BRIDGE trial allowed cangrelor infusion for up to 7 d (10). Most patients in our cohort were transitioned to oral antiplatelets within 24 h. Cangrelor is a non-thienopyridine adenosine triphosphate analog and can competitively block binding to the ADP receptor by the unstable clopidogrel metabolite. Hence, oral clopidogrel was administered at the completion of the cangrelor infusion similar to the protocol used in the CHAMPION PHOENIX study (5). Alternatively, the non-competitive ADP analog ticagrelor has a longer half-life and no significant interaction with cangrelor (15). When ticagrelor was used, it was administered approximately half an hour prior to cangrelor infusion cessation. Similarly, aspirin, an irreversible cyclooxygenase 1 and 2 inhibitor, can be administered at any time during the cangrelor infusion as it does not bind to ADP receptors.
Other intravenous antiplatelet medications used in interventional cardiology have limited studies in neurointervention. Glycoprotein 2b/3a inhibitors function downstream of ADP analogs and inhibit the final pathway of platelet aggregation by preventing fibrinogen binding to platelets and cross-linking to other platelets. While early clinical trials showed benefit of these agents in reducing cardiovascular events, use of these agents also resulted in more bleeding (16). Use of potent dual antiplatelet regimens, second generation stent technologies, and other advances in interventional cardiology has limited the use of Gp 2b/3a inhibitors to patients with a large thrombus burden or as a bail out therapy in high risk PCI interventions (17).
Given the increased risk of bleeding with Gp2b/3a inhibitors, use of these agents has been limited in acute ischemic stroke studies. Abciximab has been shown to be associated with significant increases in symptomatic intracranial hemorrhage without any improvement in long-term functional outcomes (18). However, half-dose abciximab bolus without infusion for acute stenting in stroke was shown to be safe in 99 patients (19). Eptifibatide in conjunction with intravenous tPA has been shown to be safe in a small clinical trials of 27 patients (20). A recent study in 29 patients who underwent acute carotid stenting for tandem occlusions found that eptifibatide was associated with low rates of symptomatic intracranial hemorrhage (21). Similarly, tirofiban has been shown to be safe in acute ischemic stroke (22) and in emergent carotid stenting (23). However, Gp2b/3a inhibitors are associated with spontaneous pulmonary hemorrhage in up to 1% of patients which are likely under-reported and can be fatal in up to 50% of cases (24–26). Lastly, Gp2b/3a inhibitors may affect PRU results which can make interpretation of antiplatelet activity challenging in the early stroke period (27).
Intravenous cangrelor provides a useful additional tool for managing acute ischemic strokes. Preliminary studies of the use of cangrelor for stenting in acute ischemic stroke has shown it be a promising and feasible alternative to oral antiplatelet therapies (see Table 4). In a study by Linfante et al., full cardiac dosing of cangrelor was used in five ischemic stroke patients and resulted in gastrointestinal bleeding in one patient (33). A French study with a similar cangrelor dosing regimen resulted in one case of symptomatic intracranial hemorrhage, two cases of asymptomatic intracranial hemorrhage, and one retroperitoneal bleed amongst 12 stroke patients (31). The largest case series published from Italy found no cases of peri-procedural in-stent thrombosis in 38 AIS patients requiring acute stenting and three cases of delayed in-stent thrombosis when cangrelor was transitioned to an antiplatelet other than ticagrelor. In this study, Cervo et al. also used the full cardiac dose of cangrelor and had four patients who developed basal ganglia intracranial hemorrhages requiring cangrelor discontinuation, although it was deemed safe to continue in two patients with post-procedure asymptomatic intracranial hemorrhage (30). When half the cardiac dose was used, similar to our study, all seven patients with acute ischemic stroke had adequate (PRU < 200) antiplatelet activity and no evidence of intracranial hemorrhage or in-stent thrombosis (29). Lastly, a study by Entezami et al. treated 21 patients with ischemic stroke or symptomatic critical stenosis (32). A cangrelor bolus of 5 mcg/kg was followed by a maintenance infusion of 0.75 or 1 mcg/kg/min. Patients in this study had cangrelor infusion rates adjusted based on post-procedure PRU levels. One patient required up-titration and two required a lower dose of cangrelor. While none of the ischemic stroke patients had in-stent thrombosis, 2 out of 37 patients in the study did. Our preliminary experience, combined with previous clinical studies, suggest that a half cardiac dose of cangrelor is safe and efficacious (8). Due to its pharmacokinetics, intravenous cangrelor allows neurointerventionalists and neurosurgeons to strictly control antiplatelet activity in the acute stroke setting and allows for rapid changes in management according to a patient's clinical status.
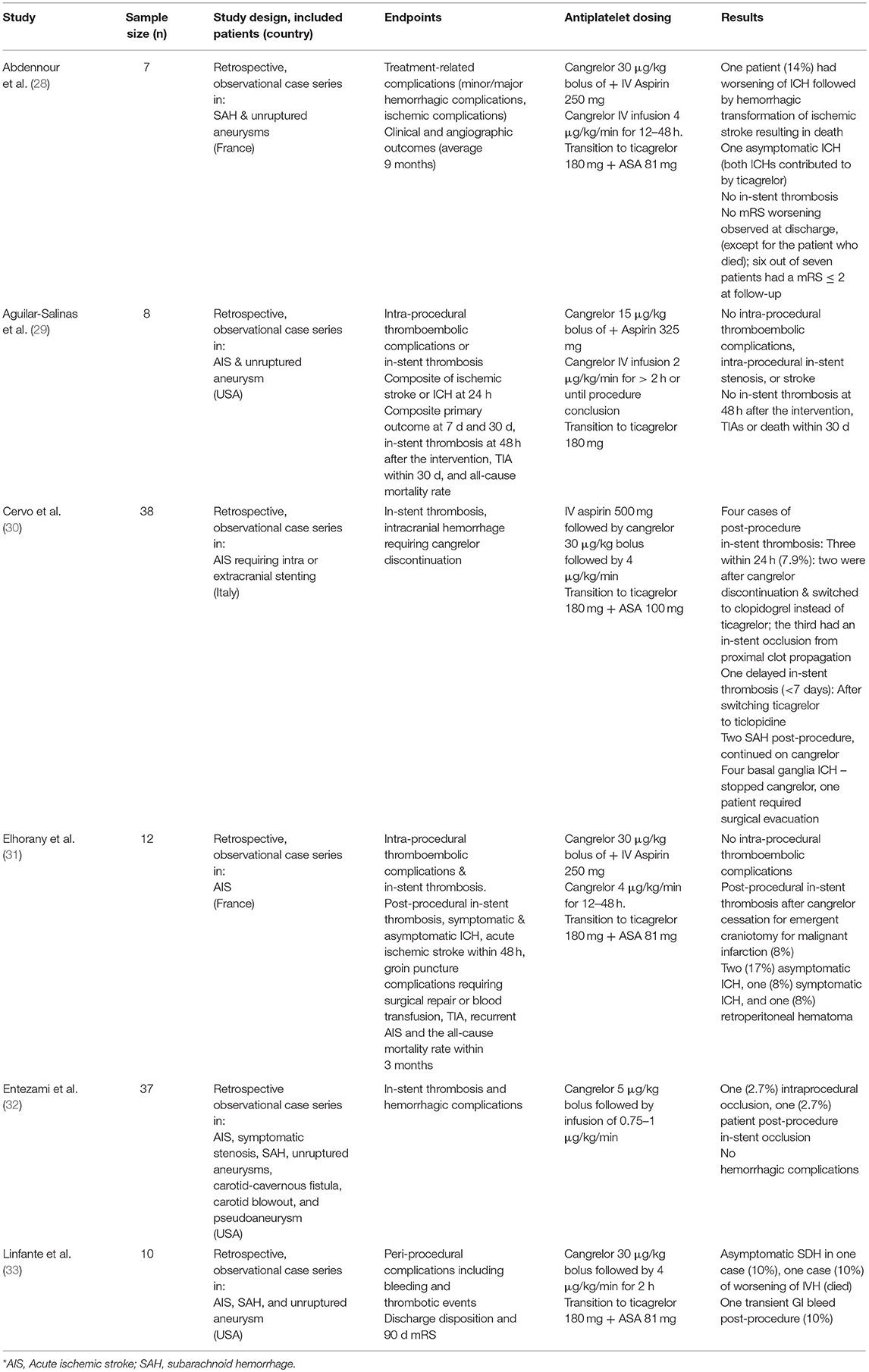
Table 4. Summary of recently published studies evaluating intravenous cangrelor for cerebrovascular diseases.
While our study did not evaluate the cost implications of a prolonged cangrelor infusion, sub-analysis of the CHAMPION PHOENIX study revealed that the increased cost of cangrelor may be offset by a reduction in intra-procedural thrombotic events during PCI resulting in lower major adverse cardiac events, mortality, procedural and hospitalization costs, and shorter hospital stays (6). Rates of major and minor bleeding were similar between cangrelor and clopidogrel in this study, but needs further study in the ischemic stroke population who are prone to intracranial hemorrhage. Ultimately, larger studies are needed to determine the cost-effectiveness of intravenous cangrelor in AIS patients.
Limitations
This study has multiple limitations. This is an underpowered single-center study and results may not be generalizable to other centers. Furthermore, patients included in the study had a range of intracranial occlusions. While we compared anterior circulation tandem occlusions with and without cangrelor, this study did not provide sufficient power to detect large differences between the therapies. Lastly, antiplatelet effect was not assessed by P2Y12 levels, different stents were used during neurointerventional procedures, and patients were transitioned off cangrelor to different oral antiplatelet therapies at different doses.
Conclusion
Intravenous cangrelor is a promising medication for patients with acute ischemic stroke who require an immediate antiplatelet effect. Larger prospective studies on the efficacy, safety, and effect on procedure times of intravenous cangrelor in neurointervention are warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Promedica Neurosciences Insitutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HS contributed to the conception of the work, data collection, data analysis and interpretation, and drafting the article. GD contributed to the data collection and drafting of the article. SZ contributed to the conception of the work and critical revision of the article. JS contributed to the data collection of the article. RB contributed to the critical revision of the article. MJ contributed to the conception of the work, data interpretation, critical revision of the article, and final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.636682/full#supplementary-material
References
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Colley R, Yan B. Genetic determinations of variable responsiveness to clopidogrel and implications for neurointerventional procedures. Interv Neurol. (2012) 1:22–30. doi: 10.1159/000338359
3. Angiolillo DJ, Schneider DJ, Bhatt DL, French WJ, Price MJ, Saucedo JF, et al. Pharmacodynamic effects of cangrelor and clopidogrel: The platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (champion) trials. J Thromb Thrombolysis. (2012) 34:44–55. doi: 10.1007/s11239-012-0737-3
4. Harrington RA, Stone GW, McNulty S, White HD, Lincoff AM, Gibson CM, et al. Platelet inhibition with cangrelor in patients undergoing pci. N Engl J Med. (2009) 361:2318–29. doi: 10.1056/NEJMoa0908628
5. Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. Effect of platelet inhibition with cangrelor during pci on ischemic events. N Engl J Med. (2013) 368:1303–13. doi: 10.1056/NEJMoa1300815
6. Tamez H, Genereux P, Yeh RW, Amin AP, Fan W, White HD, et al. Cost implications of intraprocedural thrombotic events and bleeding in percutaneous coronary intervention: results from the champion phoenix economics study. Catheter Cardiovasc Interv. (2018) 92:E348–55. doi: 10.1002/ccd.27638
7. Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. Abc/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. (2009) 72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329
8. Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral p2y12 receptor antagonist. J Clin Pharmacol. (2010) 50:27–35. doi: 10.1177/0091270009344986
9. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
10. Angiolillo DJ, Firstenberg MS, Price MJ, Tummala PE, Hutyra M, Welsby IJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA. (2012) 307:265–74. doi: 10.1001/jama.2011.2002
11. Al-Khaled M, Matthis C, Binder A, Mudter J, Schattschneider J, Pulkowski U, et al. Dysphagia in patients with acute ischemic stroke: early dysphagia screening may reduce stroke-related pneumonia and improve stroke outcomes. Cerebrovasc Dis. (2016) 42:81–9. doi: 10.1159/000445299
12. Wilson MP, Murad MH, Krings T, Pereira VM, O'Kelly C, Rempel J, et al. Management of tandem occlusions in acute ischemic stroke - intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J Neurointerv Surg. (2018) 10:721–8. doi: 10.1136/neurintsurg-2017-013707
13. Jadhav AP, Zaidat OO, Liebeskind DS, Yavagal DR, Haussen DC, Hellinger FR, et al. Emergent management of tandem lesions in acute ischemic stroke. Stroke. (2019) 50:428–33. doi: 10.1161/STROKEAHA.118.021893
14. Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, et al. Last-recorded p2y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. AJNR Am J Neuroradiol. (2014) 35:128–35. doi: 10.3174/ajnr.A3621
15. Ravnefjord A, Weilitz J, Emanuelsson BM, van Giezen JJ. Evaluation of ticagrelor pharmacodynamic interactions with reversibly binding or non-reversibly binding p2y(12) antagonists in an ex-vivo canine model. Thromb Res. (2012) 130:622–8. doi: 10.1016/j.thromres.2012.07.021
16. Safley DM, Venkitachalam L, Kennedy KF, Cohen DJ. Impact of glycoprotein iib/iiia inhibition in contemporary percutaneous coronary intervention for acute coronary syndromes: insights from the national cardiovascular data registry. JACC Cardiovasc Interv. (2015) 8:1574–82. doi: 10.1016/j.jcin.2015.04.031
17. Capodanno D, Milluzzo RP, Angiolillo DJ. Intravenous antiplatelet therapies (glycoprotein iib/iiia receptor inhibitors and cangrelor) in percutaneous coronary intervention: from pharmacology to indications for clinical use. Ther Adv Cardiovasc Dis. (2019) 13:1753944719893274. doi: 10.1177/1753944719893274
18. Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein iib-iiia inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev. (2014) 2014:CD005208. doi: 10.1002/14651858.CD005208.pub3
19. Delgado F, Oteros R, Jimenez-Gomez E, Bravo Rey I, Bautista MD, Valverde Moyano R. Half bolus dose of intravenous abciximab is safe and effective in the setting of acute stroke endovascular treatment. J Neurointerv Surg. (2019) 11:147–52. doi: 10.1136/neurintsurg-2018-014163
20. Adeoye O, Sucharew H, Khoury J, Vagal A, Schmit PA, Ewing I, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke. (2015) 46:2529–33. doi: 10.1161/STROKEAHA.115.010260
21. Osteraas ND, Crowley RW, Panos N, Dafer RM. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J Stroke Cerebrovasc Dis. (2020) 29:105021. doi: 10.1016/j.jstrokecerebrovasdis.2020.105021
22. Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Rother J, et al. Safety of tirofiban in acute ischemic stroke: the satis trial. Stroke. (2011) 42:2388–92. doi: 10.1161/STROKEAHA.110.599662
23. Gruber P, Hlavica M, Berberat J, Victor Ineichen B, Diepers M, Nedeltchev K, et al. Acute administration of tirofiban versus aspirin in emergent carotid artery stenting. Interv Neuroradiol. (2019) 25:219–24. doi: 10.1177/1591019918808777
24. Ali A, Hashem M, Rosman HS, Kazmouz G, Gardin JM, Schrieber TL. Use of platelet glycoprotein iib/iiia inhibitors and spontaneous pulmonary hemorrhage. J Invasive Cardiol. (2003) 15:186–8.
25. Dangas G, Iakovou I. Pulmonary hemorrhage during gp iib/iiia inhibitor therapy: an uncommon but life-threatening (and under-recognized) complication. J Invasive Cardiol. (2003) 15:189–90.
26. Guo J, Xu M, Xi Y. Tirofiban-induced diffuse alveolar hemorrhage: after primary angioplasty. Tex Heart Inst J. (2012) 39:99–103.
27. Verifynow reference guide (2015). Available online at: https://pbrainmd.files.wordpress.com/2016/04/verifynow-reference-guide.pdf (accessed August 2, 2020).
28. Abdennour L, Sourour N, Drir M, Premat K, Shotar E, Taylor G, et al. Preliminary experience with cangrelor for endovascular treatment of challenging intracranial aneurysms. Clin Neuroradiol. (2020) 30:453–61. doi: 10.1007/s00062-019-00811-2
29. Aguilar-Salinas P, Agnoletto GJ, Brasiliense LBC, Santos R, Granja MF, Gonsales D, et al. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: preliminary experience in a single-center pilot study. J Neurointerv Surg. (2019) 11:347–51. doi: 10.1136/neurintsurg-2018-014396
30. Cervo A, Ferrari F, Barchetti G, Quilici L, Piano M, Boccardi E, et al. Use of cangrelor in cervical and intracranial stenting for the treatment of acute ischemic stroke: a “real life” single-center experience. AJNR Am J Neuroradiol. (2020) 41:2094–9. doi: 10.3174/ajnr.A6785
31. Elhorany M, Lenck S, Degos V, Sourour NA, Frasca Polara G, Shotar E, et al. Cangrelor and stenting in acute ischemic stroke: monocentric case series. Clin Neuroradiol. (2020). doi: 10.1007/s00062-020-00907-0. [Epub ahead of print].
32. Entezami P, Holden DN, Boulos AS, Paul AR, Field NC, Nourollahzadeh E, et al. Cangrelor dose titration using platelet function testing during cerebrovascular stent placement. Interv Neuroradiol. (2020) 2020:1591019920936923. doi: 10.1177/1591019920936923
Keywords: thrombectomy, ischemic stroke, cangrelor, antiplatelet therapy, stent, tandem occlusion
Citation: Salahuddin H, Dawod G, Zaidi SF, Shawver J, Burgess R and Jumaa MA (2021) Safety of Low Dose Intravenous Cangrelor in Acute Ischemic Stroke: A Case Series. Front. Neurol. 12:636682. doi: 10.3389/fneur.2021.636682
Received: 01 December 2020; Accepted: 19 April 2021;
Published: 04 June 2021.
Edited by:
Diogo C. Haussen, Emory University, United StatesReviewed by:
Mikayel Grigoryan, Glendale Adventist Medical Center, United StatesNirav Bhatt, Emory University, United States
Copyright © 2021 Salahuddin, Dawod, Zaidi, Shawver, Burgess and Jumaa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mouhammad A. Jumaa, TW91aGFtbWFkLmp1bWFhQHV0b2xlZG8uZWR1
 Hisham Salahuddin
Hisham Salahuddin Giana Dawod
Giana Dawod Syed F. Zaidi
Syed F. Zaidi Julie Shawver2
Julie Shawver2 Mouhammad A. Jumaa
Mouhammad A. Jumaa