- 1Multiple Sclerosis Center, Binaghi Hospital, Azienda Tutela della Salute (ATS) Sardegna, Cagliari, Italy
- 2Multiple Sclerosis Center, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 3Radiology Unit, Binaghi Hospital, Azienda Tutela della Salute (ATS) Sardegna, Cagliari, Italy
Background: Cognitive impairment (CI) is common in people with multiple sclerosis (pwMS). The assessment of CI is based on neuropsychological tests and accurate anamnesis, involving the patients and caregivers (CG). This study aimed to assess the complex interplay between self-perception of CI, objective CI and the brain atrophy of MS patients, also exploring the possible differences with CI evaluated by caregivers.
Methods: Relapsing pwMS were enrolled in this study. Subjects underwent neuropsychological examination using the Brief Cognitive Assessment for Multiple Sclerosis (BICAMS) and evaluation of self-reported cognitive status using the patient-version of the Multiple Sclerosis Neuropsychological Questionnaire (p-MSNQ). Depression and anxiety were also evaluated using the Back Depression Inventory-version II (BDI-II) and Zung Anxiety Scale. Brain MRI images were acquired and brain volumes estimated. For each patient that was enrolled, we spoke to a caregiver and collected their perception of the patient's CI using the MSNQ- Caregiver version.
Results: Ninety-five MS subjects with their caregivers were enrolled. CI was detected in 51 (53.7%) patients. We found a significant correlation (p < 0.001) between BICAMS T scores and lower whole brain (Rho = 0.51), gray matter (Rho = 0.54), cortical gray matter (Rho = 0.51) volumes and lower p-MSNQ (Rho = 0.31), and cg-MSNQ (Rho = 0.41) scores. Multivariate logistic regression showed that p-MSNQ is related to a patient's anxiety to evaluate by Zung Score (p < 0.001) while cg-MSNQ to patient's brain volume (p = 0.01).
Conclusion: Our data confirm that neuropsychological evaluation results are related to the perception of CI and brain volume measures and highlight the importance of the caregiver's perception for cognitive assessment of pwMS.
Introduction
Cognitive dysfunctions are frequent and represent a major concern for people living with multiple sclerosis (pwMS). Several studies estimated that the prevalence of cognitive impairment (CI) among pwMS ranges between 40 and 70%, occurring in subjects with different clinical course and MS features, early as in more advanced stages of the disease (1). In the last few years, growing attention has been paid to the evaluation of CI in MS, also because of the impact of this invisible but heavy symptom on several aspects of patients' lives. For this reason, numerous neuropsychological assessments have been proposed, including rapid screen tools principally useful in a clinical setting and self-reported questionnaires aimed to evaluate the perception of patients' cognitive functioning (2). The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) is used in clinical settings, due to its rapidity of administration and the evaluation of principle cognitive domains affected by MS (3–5). The Multiple Sclerosis Neuropsychological Questionnaire (MSNQ), including a patient and caregiver (CG) version, has emerged as the most used tool worldwide for evaluating the perception of patients' CI (6). The relationship between the objective and perceived CI is notoriously extremely complicated and is potentially influenced by MS-related structural brain damage (7–9) as well as several others factors (10, 11) among which are also mood disorders (7, 8). Based on these considerations, this study aims to evaluate the complex interplay between CI of pwMS and the perception of cognitive functioning reported by patients and their CG, also exploring the possible relationships with brain volume measurements.
Methods
Participants
Patient Recruitment
Consecutive relapsing remitting pwMS were enrolled at the Multiple Sclerosis Center of Binaghi Hospital, ATS Sardegna. Exclusion criteria were: (i) exposure to corticosteroid or occurrence of clinical relapse in the previous 30 days; (ii) change in disease modifying therapy in the previous 6 months; (iii) presence of other chronic comorbidities; (iv) use of drugs or substances with a psychotropic effect; (v) contraindications to underwent MRI; (vi) presence of a physical disability that did not allow the neuropsychological evaluation (i.e., blindness).
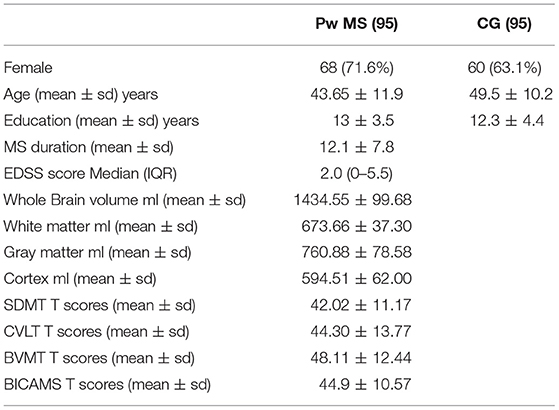
All included MS patients underwent a clinical, neuropsychological, and brain MRI examination in the same week. Demographics and clinical MS features [gender, age, education, disease duration, and level of disability, assessed by Expanded Disability Status Scale (EDSS) score] (12) were also collected.
Caregiver Recruitment
For each enrolled patient, a caregiver was included. Caregivers were classified based on the relationship with the patients. Thus, the CG version of MSNQ (13) was administrated to the participants to capture their views on the patient's cognitive functioning. Informed consent was obtained from all participants (pwMS and CG) included in the study, which was approved by the local ethics committee.
Neuropsychological Assessment
The cognitive functions of the included patients were evaluated using the Italian version of the BICAMS battery (5) with implemented normative values for the Italian population and corrections for sex, age, and years of education (14). The BICAMS battery includes the Symbol Digit Modalities Test (SDMT) for evaluating the information processing speed, the California Verbal Learning Test (CVLT-II) for evaluating verbal learning and memory, and the Brief Visual Memory Test (BVMT) for evaluating visual learning and memory (5). In our study, according to the Italian validation process of the BICAMS battery, we included the total number of correct responses in 90 seconds for SDMT, the total number of words recalled over five learning trials (Total Learning, TL) for CVLT-II, and total recall score across the three trials.
According to the authors' definition, each test was classified as altered if the T Score was below 35 points. Thus, the self-perception of the CI of the patients was evaluated using the p-version of MSNQ (13).
The T score of any BICAMS tests was reported for each included patient, the mean T score of all BICAMS tests and the sum of BICAMS tests scored below 35 T score (number of altered tests). Finally, depression and anxiety were evaluated using the BDI-II and the Zung Anxiety Scale (15, 16).
MRI Acquisition
Brain MRIs were acquired using a Magnetom Avanto Scanner (Siemens, Enlargen) at 1.5 T. The MRI protocol included the following sequence: 3D T1-Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE): echo time (TE): 2.37 ms; repetition time (TR): 1,730 ms; inversion time (TI): 1,050 ms; field of view (FOV): 244 mm; voxel size: 1 × 1 × 1 mm, (176 contiguous slices). A dual-echo, turbo spin-echo sequence (repetition time/echo time 1/echo time 2 5 2,075/30/90 ms, 256 3256 matrix, one signal average, 250-mm field of view, 50 contiguous 3-mm slices) yielding proton density–weighted and T2-weighted images oriented to exactly match the MPRAGE image acquisition. Brain parenchyma volumes were measured on T1W gradient echo images using the cross-sectional version of SIENA (structural image evaluation using normalization of atrophy) software, SIENAX (part of FSL 4.0: http://www.fmrib.ox.ac.uk/fsl/), and a previously described method to estimate the overall brain volume, normalized for head size. MRI analysis allowed us to obtain normalized whole brain volume (WB), normalized gray matter volume (GM), normalized white matter volume (WM), and normalized cortical gray matter volume (cGM). T1 hypo-intense lesion refilling was performed as previously described (17, 18). The radiologist was blinded to the results of the cognitive and neurological evaluation.
Statistical Analysis
All statistical analyses were performed using SPSS for Mac version 20.0 (SPSS Inc., Chicago. IL, USA). First, descriptive analysis was performed. Next, we used the Shapiro-Wilks and Kolmogorov-Smirnov for testing the normality of variables. Based on normal distribution evaluation, we used a parametric or non-parametric test to evaluate the correlation between the variables evaluated. the relationship of BICAMS Tests Results with brain volumes was assessed by Pearson or Spearman test. Analogously, the relationship of p-MSNQ and cg-MSNQ scores with BICAMS Tests Results and brain measurements were evaluated. Thus, regression analyses were performed to evaluate which factors influence p-MSNQ and cg-MSNQ scores, included in each model as dependent variable, also controlling for BDI-II and Zung Anxiety scores. Moreover, we performed a collinearity diagnostic test regarding the linear regression. For all assays, the statistical significance was set at P < 0.05.
The results were filtered using the Benjamini-Hochberg procedure for FDR correction (FDR < 0.05). The test of the collinearity of variables also included multivariate linear regression analysis.
Results
The sample included 95 MS relapsing remitting patients (68/95; 71.6% female). Mean values for age and disease duration were, respectively, 43.65 (SD: 11.9) and 12.1 (SD: 7.8) years, while the median EDSS score was 2.0 (IQR: 0–5.5). For each MS patient, a caregiver was included. Of these, 62 were partners (65.2%), and 33 family caregivers (34.8%). Table 1 shows the demographic and clinical features of participants included in the study. CI, defined by at least one impaired test at the BICAMS assessment, was relieved in 51 (53.7%) of patients.
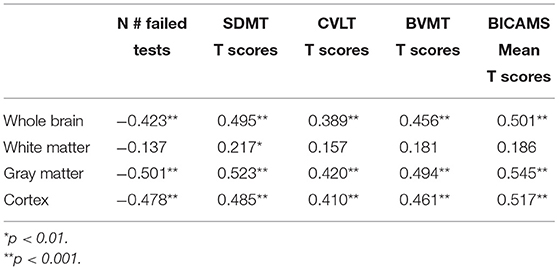
We found a significant correlation of mean BICAMS T scores with measurements of WB (Rho = 0.50), GM (Rho = 0.545), and cGM (Rho = 0.517), (p < 0.001), as shown in Table 2.
As shown in Table 3, the relationship of mean BICAMS T scores with p-MSNQ (Rho = 0.31 p < 0.01) and cg-MSNQ (Rho = 0.41; p < 0.001) is also observed. In addition, the perception of CG, as indicated by cg-MSNQ score, inversely correlates with WB (Rho = −0.495), GM (Rho = −0.554) and cGM (Rho = −0.563) volumes. No significant correlation was found between the patient's point of view, indicated as p-MSNQ scores, and brain volume measurements (Table 3).

Table 3. Correlations of p-MSNQ and cg-MSNQ scores with BICAMS results and brain volume measurements.
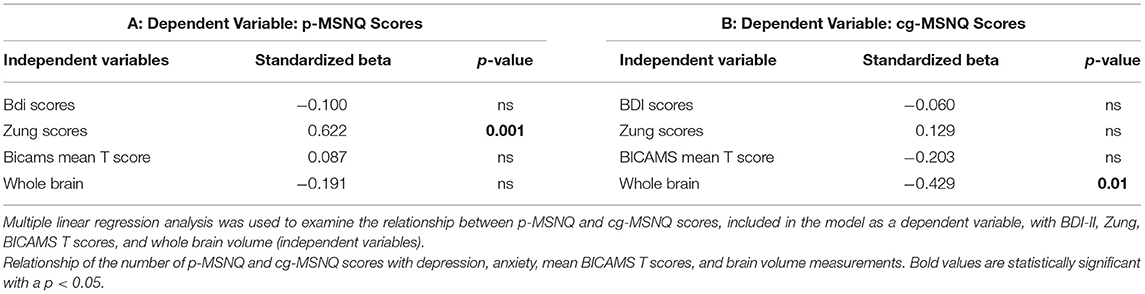
A multivariate linear regression model was also performed. First, we included as dependent variable p-MSNQ founding a significant association of p-MSNQ scores with anxiety evaluated by Zung scores (P = 0.001) also controlling for BDI results, mean of BICAMS T scores, and brain volume (Table 4A). Moreover, we performed another analysis including the cg-MSNQ score as a dependent variable, highlighting a relationship with the patients' lower brain volume (p = 0.01) with no significant relationship with depression, anxiety, and BICAMS results (Table 4B). The variance inflation factor (VIF) values and the condition index results were not indicative of collinearity for variables included in multivariate linear regression analysis.
Discussion
Our results confirmed the universally recognized role of MRI analysis as principal biomarkers of cognitive functions in MS (19). The present study also found a strong correlation between the volumes of the whole brain, gray matter, and cortical volume with the results of cognitive tests.
As observed in other neurological diseases, MRI measurements are not enough to fully explain cognitive deficits in MS (20). In recent decades several studies have aimed to investigate how other factors play a role (21). Among these factors, cognitive reserve, several demographic, clinical, mood disorders, and social variables could act as moderators (22, 23). However, brain volume measures showed a strong and significant relationship with all cognitive functions evaluated and the global cognitive status of MS patients.
The other aim of our study was to explore the reliability perception of cognitive impairment in Multiple Sclerosis. The data show that caregiver perception is more strongly correlated to the objective cognitive performance of people with MS than their self-judgment. In other neurological pathologies such as neurodegenerative diseases, it is a common observation that the cognitive ability self-perception of the patient is less accurate than caregiver perception (24–26).
As previously described, cognition self-judgment is often more conditioned by mood disorders such as depression and anxiety than by objective cognitive deficit (27). A severe mood disorder could interfere with both anamnestic interview and neuropsychological evaluation (28), complicating the estimation of cognitive functions and leading to overestimation of the impairment of cognitive abilities. As in our cohort, the perception of cognitive functioning reported by patients appeared to be related to anxiety in a model controlled for brain volume and the results of neuropsychological assessment (28).
Several other previous studies have evaluated the reliability of cognitive function self-judgment compared to caregiver evaluation and relationship with a mood disorder. O'Brien et al. found that p-MSNQ correlated with depression as assessed by BDI, while cg-MSNQ was independent from mood disorders, but was correlated with cognitive impairment as assessed by an extended neuropsychological battery (29). Another previous study indicated that in MS patients, after controlling for demographic variables, anxiety was a significant predictor of p-MSNQ scores, while the patients' point of view did not correlate with the results of neuropsychological examination (30). A recent study, conducted on the Danish MS population confirmed that the p-MSNQ version measures these items more than the cognitive abilities of the patients (31). These previous studies are in line with our results which confirm that the patient's self-assessment of their cognitive functions is related more to the characteristics of their mood than to objective evaluation.
Interestingly, the relationship between caregiver perception of a patient's cognition and patients' brain volume emerged as an unexpected result of our study. The perception of CI reported by the caregivers shows a strong correlation with patient brain volume measures, whole brain, and gray matter, while there is no correlation between p-MSNQ and brain atrophy. In the multivariate analysis, the cg-MSNQ scores were also related to patients' brain volume, even after controlling for depression BDI-II scores, anxiety Zung scores, and neuropsychological test results. As previously described (28), the caregiver's evaluation of the patient's cognitive functions is based on multiple issues such as skills in daily life, detailed knowledge of the premorbid level of cognitive skills, and the social context of the patient. Consequently, our data support the hypothesis that the perception of the caregiver is related to the effective cognitive functioning of the patient as documented by the strong correlation with the brain volume confirmed also in the multivariate analysis. Thus, caregiver evaluation of cognitive functioning in MS emerges as related to brain volume as an indication of structural damage. The absence of a correlation between patient self-evaluation and brain volume measure could be explained by processes such as the influence of mood disorders, especially anxiety, on self-evaluation and a lack of insight about impairment in patients with severe brain atrophy.
Recently, several studies on metacognition have also contributed to the understanding of the complex interplay that regulates the perception of cognitive disorders in MS (32). These findings are in line with our results and point to the role of mood disorders in self-perception of cognitive impairment in people with MS. Our study also adds the significant relationship between the caregiver's point of view, cognitive measures and brain volume as the main biomarker of cognitive impairment.
Our study shows several limitations. First, the limited number of pwMS included in the research could influence the application of the results. Second, the MRI biomarkers included only the brain volume measurements while also other radiological features are associated with CI in MS as white matter total lesion load that was not included in the present study. Furthermore, even if using appropriate statistical tests, given the limited size of the sample, it is not possible to exclude errors due to the association between the evaluated measures.
Conclusions
In conclusion, our study confirmed the well-known importance of MRI volumetric measurements as biomarkers of CI in MS based on the relationship with cognitive results. Furthermore, the caregiver's point of view appears to be stronger related to neuroradiological biomarkers of cognitive deficit and neuropsychological assessment test results rather than patient self-evaluation.
This data suggests the importance of including the caregivers' judgment in the anamnestic evaluation of pwMS undergoing neuropsychological assessment. Further studies are needed to better evaluate what tools to use in a clinical setting to capture both MS patients' and caregivers' perceptions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Cagliari. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GF and LL participated in the design of the study and drafted the manuscript. ECa, MA, MF, and AC carried out the neuropsychological evaluation and performed the statistical analysis and drafted the manuscript. JF and GC revised the manuscript for important intellectual content and performed the statistical analysis. FC and MB acquired MRI images. ECo helped draft the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Conflict of Interest
GF was an editorial board member of BMC Neurology and received a travel grant, speaker fee, and consultancy from Biogen Idec, Sanofi, Teva, Admirall, Genzyme, Merck Serono, and Novartis. LL, GC, JF, and ECo received travel grants, speaker fees, and consultancy from Biogen Idec, Sanofi, Teva, Admirall, Genzyme, Merck Serono, and Novartis. MB and FC received travel grants from Biogen Idec.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank the patients and their caregivers for their time and commitment to this research.
Abbreviations
BICAMS, Brief International Cognitive Assessment for Multiple Sclerosis; BVMT, Brief Visual Memory Test-Revised; CFs, Cognitive Functions; cgMSNQ, Multiple Sclerosis Neuropsychological Questionnaire- caregiver version; CGs, Caregivers; CI, Cognitive impairment; CVLT, California Verbal Learning Test; MS, Multiple Sclerosis; pMSNQ, Multiple Sclerosis Neuropsychological Questionnaire-patient version; SDMT, Symbol Digit Modalities Test; WB, whole brain; WM, whole white matter; GM, whole gray matter; cGM, cortical gray matter.
References
1. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. (2008) 7:1139–51. doi: 10.1016/S1474-4422(08)70259-X
2. Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. (2018) 24:1665–1680. doi: 10.1177/1352458518803785
3. Corfield F, Langdon D. A systematic review and meta-analysis of the brief cognitive assessment for multiple sclerosis (BICAMS). Neurol Ther. (2018) 7:287–306. doi: 10.1007/s40120-018-0102-3
4. Langdon D. Cognitive assessment in MS. Neurodegener Dis Manag. (2015) 5:43–5. doi: 10.2217/nmt.15.62
5. Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler. (2012) 18:891–8. doi: 10.1177/1352458511431076
6. Benedict RH, Zivadinov R. Reliability and validity of neuropsychological screening and assessment strategies in MS. J Neurol. (2007) 254: II22–5. doi: 10.1007/s00415-007-2007-4
7. Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. (2015) 14:302–17. doi: 10.1016/S1474-4422(14)70250-9
8. Zivadinov R, Jakimovski D, Gandhi S, Ahmed R, Dwyer MG, Horakova D, et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother. (2016) 16:777–93. doi: 10.1080/14737175.2016.1181543
9. van Munster CE, Jonkman LE, Weinstein HC, Uitdehaag BM, Geurts JJ. Gray matter damage in multiple sclerosis: impact on clinical symptoms. Neuroscience. (2015) 303:446–61. doi: 10.1016/j.neuroscience.2015.07.006
10. Hu M, Muhlert N, Robertson N, Winter M. Perceived fatigue and cognitive performance change in multiple sclerosis: uncovering predictors beyond baseline fatigue. Mult Scler Relat Disord. (2019) 32:46–53. doi: 10.1016/j.msard.2019.04.011
11. Fenu G, Fronza M, Lorefice L, Arru M, Coghe G, Frau J, et al. Performance in daily activities, cognitive impairment and perception in multiple sclerosis patients and their caregivers. BMC Neurol. (2018) 18:212–24. doi: 10.1186/s12883-018-1224-z
12. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/wnl.33.11.1444
13. Benedict RH, Munschauer F, Linn R, Miller C, Murphy E, Foley F, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler. (2003) 9:95–101. doi: 10.1191/1352458503ms861oa
14. Goretti B, Niccolai C, Hakiki B, Sturchio A, Falautano M, Minacapelli E, et al. The brief international cognitive assessment for multiple sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol. (2014) 14:171. doi: 10.1186/s12883-014-0171-6
15. Solaro C, Trabucco E, Signori A, Martinelli V, Radaelli M, Centonze D, et al. Depressive symptoms correlate with disability and disease course in multiple sclerosis patients: an italian multi-center study using the beck depression inventory. PLoS ONE. (2016) 11:e0160261. doi: 10.1371/journal.pone.0160261
16. Zung WW. The measurement of affects: depression and anxiety. Mod Probl Pharmacopsychiatry. (1974) 7:170–88. doi: 10.1159/000395075
17. Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. (2002) 17:479–89. doi: 10.1006/nimg.2002.1040
18. Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. (2012) 33:2062–71. doi: 10.1002/hbm.21344
19. Rocca MA, Battaglini M, Benedict RH, De Stefano N, Geurts JJ, Henry RG, et al. Brain MRI atrophy quantification in MS: from methods to clinical application. Neurology. (2017) 88:403–413. doi: 10.1212/WNL.0000000000003542
20. Johnen A, Schiffler P, Landmeyer NC, Tenberge JG, Riepl E, Wiendl H, et al. Resolving the cognitive clinico-radiological paradox -Microstructural degeneration of fronto-striatal-thalamic loops in early active multiple sclerosis. Cortex. (2019) 121:239–52. doi: 10.1016/j.cortex.2019.08.022
21. Fenu G, Lorefice L, Arru M, Sechi V, Loi L, Contu F, et al. Cognition in multiple sclerosis: between cognitive reserve and brain volume. J Neurol Sci. (2018) 386:19–22. doi: 10.1016/j.jns.2018.01.011
22. Oreja-Guevara C, Ayuso Blanco T, Brieva Ruiz L, Hernández Pérez MÁ, Meca-Lallana V, Ramió-Torrentà L. Cognitive dysfunctions and assessments in multiple sclerosis. Front Neurol. (2019) 10:581. doi: 10.3389/fneur.2019.00581
23. Brochet B, Ruet A. Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol. (2019) 10:261. doi: 10.3389/fneur.2019.00261
24. National Academies of Sciences Engineering and Medicine. Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward. Washington, DC: The National Academies Press (2021). doi: 10.17226/26026
25. Zhang Q, Aldridge GM, Narayanan NS, Anderson SW, Uc EY. Approach to cognitive impairment in Parkinson's disease. Neurotherapeutics. (2020) 17:1495–10. doi: 10.1007/s13311-020-00963-x
26. Oppo V, Serra G, Fenu G, Murgia D, Ricciardi L, Melis M, et al. Parkinson's disease symptoms have a distinct impact on caregivers' and patients' stress: a study assessing the consequences of the COVID-19 lockdown. Mov Disord Clin Pract. (2020) 7:865–7. doi: 10.1002/mdc3.13030
27. Nauta IM, Balk LJ, Sonder JM, Hulst HE, Uitdehaag BM, Fasotti L, et al. The clinical value of the patient-reported multiple sclerosis neuropsychological screening questionnaire. Mult Scler. (2019) 25:1543–6. doi: 10.1177/1352458518777295
28. Goretti B, Viterbo RG, Portaccio E, Niccolai C, Hakiki B, Piscolla E, et al. Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci. (2014) 35:559–63. doi: 10.1007/s10072-013-1544-0
29. O'Brien A, Gaudino-Goering E, Shawaryn M, Komaroff E, Moore NB, DeLuca J. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol. (2007) 22:933–48. doi: 10.1016/j.acn.2007.07.002
30. Akbar N, Honarmand K, Feinstein A. Self-assessment of cognition in Multiple Sclerosis: the role of personality and anxiety. Cogn Behav Neurol. (2011) 24:115–21. doi: 10.1097/WNN.0b013e31822a20ae
31. Sejbæk T, Blaabjerg M, Sprogøe P, Ravnborg M. Reliability and validity of a danish version of the multiple sclerosis neuropsychological screening questionnaire. Int J MS Care. (2018) 20:49–54. doi: 10.7224/1537-2073.2017-011
Keywords: multiple sclerosis, cognitive impairment, caregiver, brain volume, patients
Citation: Fenu G, Lorefice L, Carta E, Arru M, Carta A, Fronza M, Coghe G, Frau J, Contu F, Barracciu MA and Cocco E (2021) Brain Volume and Perception of Cognitive Impairment in People With Multiple Sclerosis and Their Caregivers. Front. Neurol. 12:636463. doi: 10.3389/fneur.2021.636463
Received: 01 December 2020; Accepted: 24 March 2021;
Published: 06 May 2021.
Edited by:
Rosa Cortese, University College London, United KingdomReviewed by:
Dejan Jakimovski, Buffalo Neuroimaging Analysis Center, United StatesNils Muhlert, The University of Manchester, United Kingdom
Copyright © 2021 Fenu, Lorefice, Carta, Arru, Carta, Fronza, Coghe, Frau, Contu, Barracciu and Cocco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Fenu, Z2l1c2VmZW51QGdtYWlsLmNvbQ==
 Giuseppe Fenu
Giuseppe Fenu Lorena Lorefice
Lorena Lorefice Elisa Carta
Elisa Carta Mauro Arru2
Mauro Arru2 Alice Carta
Alice Carta Eleonora Cocco
Eleonora Cocco