
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 10 February 2021
Sec. Multiple Sclerosis and Neuroimmunology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.635753
 Thomas Langerak1
Thomas Langerak1 Irene van Rooij1
Irene van Rooij1 Laura Doornekamp1
Laura Doornekamp1 Felicity Chandler1
Felicity Chandler1 Mark Baptista2
Mark Baptista2 Harvey Yang3
Harvey Yang3 Marion P. G. Koopmans1
Marion P. G. Koopmans1 Corine H. GeurtsvanKessel1
Corine H. GeurtsvanKessel1 Bart C. Jacobs4,5
Bart C. Jacobs4,5 Barry Rockx1
Barry Rockx1 Kirsten Adriani1,6
Kirsten Adriani1,6 Eric C. M. van Gorp1*
Eric C. M. van Gorp1*Guillain-Barré syndrome (GBS) is associated with various types of preceding infections including Campylobacter jejuni and cytomegalovirus, but there is also an association with arthropod borne viruses (arboviruses), such as Zika virus, that are endemic in tropical regions. Here we present the clinical characteristics of 12 GBS patients from Suriname that were hospitalized between the beginning of 2016 and half 2018. Extensive diagnostic testing was performed for pathogens that are commonly associated with GBS, but also for arboviruses, in order to identify the preceding infection that might have led to GBS. With this extensive testing algorithm, we could identify a recent infection in six patients of which four of them had evidence of a recent Zika virus or dengue virus infection. These results suggest that arboviruses, specifically Zika virus but possibly also dengue virus, might be important causative agents of GBS in Suriname. Furthermore, we found that more accessibility of intravenous immunoglobulins or plasma exchange could improve the treatment of GBS in Suriname.
Guillain-Barré syndrome (GBS) is an immune-mediated polyradiculoneuropathy, characterized by a rapidly progressive symmetrical limb weakness and decreased or absent deep tendon reflexes (1). GBS can be a life-threatening disease because of respiratory and autonomic failure, and has an estimated mortality of 3–7% (2). The exact pathogenesis of GBS is unknown, but it is thought that preceding infections or vaccinations may trigger the production of autoantibodies to components of peripheral nerves due to molecular mimicry, leading to peripheral nerve injury (1). There are multiple clinical variants and electrophysiological GBS subtypes, such as acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN) and Miller Fisher syndrome (1). Diagnosis of GBS is based on clinical characteristics but can be supported by investigation of cerebrospinal fluid (CSF) and nerve conduction studies (3). Diagnosis and classification of GBS can be challenging because of the heterogeneity of the syndrome and the extensive differential diagnosis. Proven effective treatment of GBS are intravenous immunoglobulins and plasma exchange (1, 3).
Multiple pathogens are associated with GBS such as Campylobacter jejuni (C. jejuni), Epstein-Barr virus (EBV), cytomegalovirus (CMV), Mycoplasma pneumoniae (M. pneumoniae) and hepatitis E virus (HEV) (4). The type of preceding infection is related to the clinical presentation and course of GBS, and the variety of preceding infections contributes to the diversity in clinical variants and prognosis. Besides the above mentioned pathogens that are associated with GBS, during the 2015–2016 Zika virus (ZIKV) outbreak in the Americas, it became clear that this virus is also associated with GBS (5, 6). More recently, an association between the newly emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and GBS was suggested (7, 8).
Diagnosis of the preceding infection that might have triggered GBS can be difficult. Firstly, direct pathogen detection, for example, with polymerase chain reaction (PCR), is quite often not possible when GBS is diagnosed because of the time lag of 1–4 weeks between the initial infection and onset of GBS symptoms. Furthermore, diagnosis of preceding infections of GBS with serology can be challenging due to cross-reactivity of antibodies between certain pathogens and reactivation of non-related antibodies during infection.
Here we describe 12 patients from Suriname with GBS who were recruited in a prospective observational study taking place from February 2016 to June 2018. Suriname is a country in South-America which has, due to the tropical climate, a relative high burden of arthropod borne viruses (arboviruses) like ZIKV and dengue virus (DENV) (9, 10). The aim of this study was to describe the clinical presentation, treatment and outcomes of GBS patients in Suriname and to diagnose the preceding infections that lead to GBS in these patients. We were specifically interested in the role of arboviruses as potential preceding infections that can lead to GBS. The results of this study may result in more knowledge about the clinical presentation and the etiology of preceding infections of GBS in Suriname which could help to improve diagnostic preparedness and treatment of GBS in Suriname, and possibly other countries in South America.
Patients with suspected GBS were recruited for this prospective study as soon as possible after clinical GBS suspicion. Participants were recruited in all three hospitals in Paramaribo, Suriname, from February 2016 until July 2018. The case definitions of the Brighton Collaboration were used to determine the level of diagnostic certainty of GBS ranging from 1 (most certainty for GBS diagnosis) to 4 (least certainty for GBS diagnosis) (2). If during admission another diagnosis than GBS was made, or of if insufficient clinical information was available to verify the GBS diagnosis, these participants were excluded, as were participants from whom no blood was collected. One participant was initially suspected of a paraparetic form of GBS with paralysis of the legs and normal strength of the arms. This diagnosis was changed during admission to transverse myelitis because of a sensory level at Th5 and CSF pleiocytosis of 206 cell/μl and no further suspicion of GBS. Even though concomitant GBS and transverse myelitis can occur, this patient did not meet the Brighton diagnostic criteria for GBS and was therefore excluded from this study (11–13). Three patients (patient 1, 2, and 6) were previously described in a smaller case series on possible ZIKV associated GBS in Suriname (14).
Serum and plasma were collected at enrolment in this study and 10–14 days later, or at the day of hospital discharge if this was before day 10. Neurological examination was performed by one of the researchers every seven days until hospital discharge. Additional information regarding medical history, (onset of) symptoms, and antecedent events like infections or vaccinations was collected with a questionnaire. Data from nerve conduction studies and analysis of cerebrospinal fluid were, when available, collected from all participants.
This study protocol was approved by the ethical board of the Ministry of Health in Suriname. Informed consent was obtained from all patients and in case the patient was younger than 16 years old, from their parents or representatives. The study was carried out in accordance with the Declaration of Helsinki.
Serum samples were tested for presence of antibodies against pathogens commonly associated with GBS; C. jejuni, EBV, CMV, HEV and M. pneumoniae. Furthermore, serological tests were performed to diagnose (recent) infections with the arboviruses ZIKV, DENV and chikungunya (CHIKV). All the serological tests -except serology for C. jejuni- were performed at the department of Viroscience at Erasmus MC, Rotterdam, the Netherlands.
Presence of IgM antibodies against ZIKV and DENV and IgM and IgG antibodies against HEV and M. pneumoniae were assessed with use of commercial ELISA kits (ZIKV and DENV; Euroimmun, HEV; Wantai Biological, M. pneumoniae; Serion Diagnostics). EBV and CMV serology was performed using a chemiluminescent immunoassay (DiaSorin LIAISON®). Chikungunya antibodies were detected with an indirect immunofluorescence test (Euroimmun). All these assays were performed according to the manufacturer's instructions. In case of a positive IgM response for EBV or CMV, additional testing, detection of Epstein-Barr virus Nuclear Antigen (EBNA) antibodies and a CMV avidity test respectively, was performed to determine if this was likely to be a primary infection or not. C. jejuni serology was performed with an indirect IgG ELISA and antibody class capture ELISAs for IgM and IgA antibodies at the Department of Medical Microbiology, Reinier de Graaf Gasthuis, Delft, the Netherlands.
Neutralizing antibodies against ZIKV and DENV-2 [used as a representation of total DENV immunity (15)], were determined with an in-house micro-neutralization test (VNT) as previously described (9). Sera were tested in triplicates and the geometric mean of the highest final serum dilution was reported as titer. For both ZIKV and DENV-2, the cut-off of a positive VNT was a final serum dilution >1:32.
For ZIKV diagnosis, a reverse transcriptase polymerase chain reaction (RT-PCR) was performed on plasma or, when available, urine using the primer/probe set described by Lanciotti et al. (16).
For the interpretation of the serological and molecular tests performed in these patients, a distinction was made between confirmed recent infections, probable recent infections and possible recent infections. A recent infection was considered confirmed if one serum or urine sample was RT-PCR positive, or if IgM antibodies against the specific pathogens were present and there was a more than four-fold increase of IgG- or neutralization titer in paired samples. Presence of IgM antibodies with an increasing IgG- or neutralization titer in paired samples was considered a probable recent infection. Presence of IgM but with no increase in IgG or neutralization titer in paired samples was considered a possible recent infection. When paired blood samples were not available, presence of IgM in a single blood sample was considered a possible recent infection. Specifically for CMV, presence of anti-CMV IgM in combination with a rising IgG titer with low avidity was considered a confirmed recent CMV infection. The combination of CMV IgM with IgG with high avidity was considered as reactivation of CMV antibodies and not a recent infection (17). Presence of IgM against EBV in combination with IgG against EBV nuclear antigen (EBNA) was considered a non-recent infection (18).
In total, 27 patients with suspected GBS were enrolled in this study. Of these 27 patients, 15 were excluded because during admission another diagnosis than GBS was made or because the availability of clinical data or blood samples was insufficient, illustrated by the flowchart in Figure 1A. The number of GBS patients recruited per quarter of a year is displayed in Figure 1B. The highest peak of GBS patients, in the first quarter of 2016, coincided with the peak of the ZIKV outbreak in Suriname and South America in the first months of 2016 (19, 20).
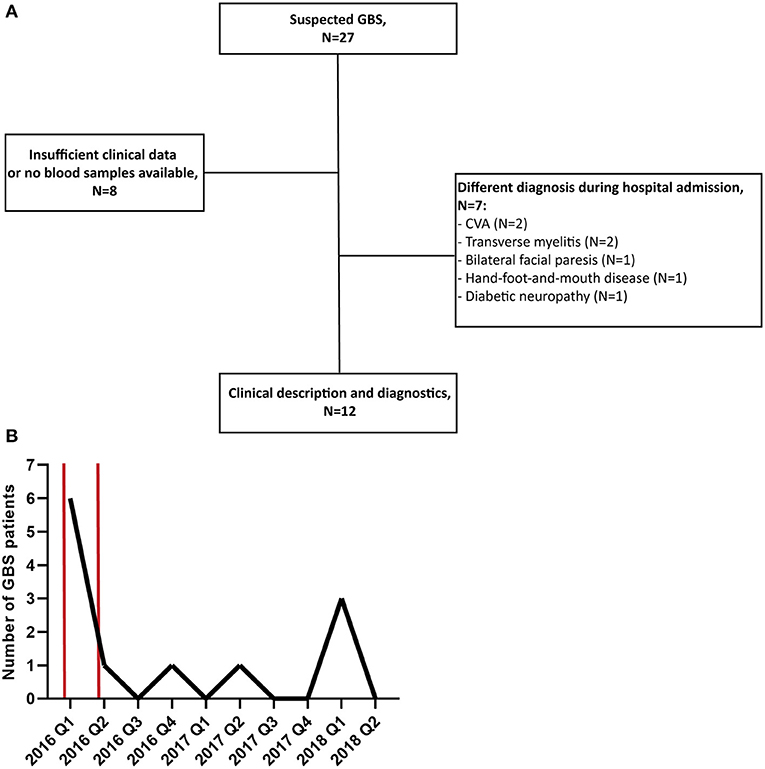
Figure 1. (A) Flowchart of patient recruitment and exclusion. (B) Number of GBS patients recruited in this study per quarter of a year (Q). The time period of the peak of the ZIKV outbreak in Suriname is marked with two red bars. CVA, cerebrovascular accident.
Seven out of 12 patients (58.3%) had a pure motor form of GBS (without sensory deficits, Table 1). Cranial nerves were affected in five patients (41.7%), and a facial palsy was present in four of these patients (80.0%). At nadir, six patients (50.0%) were confined to bed or chair of which three patients (50.0%) required assisted mechanical ventilation. Five patients (41.7%) received treatment with intravenous immunoglobulins (IVIg) while the other seven patients (58.3%) only received supportive care. None of the patients were treated with plasma exchange. Eight out of 12 patients (66.7%) underwent nerve conduction studies and electromyography during admission of which five (62.5%) showed a demyelinating type of GBS, one (12.5%) showed an axonal GBS type, one (12.5%) was classified as equivocal and in one (12.5%) the potentials were absent. Brighton criteria 1 (highest level of diagnostic certainty) for GBS diagnosis was met in six patients (50.0%), level 2 in five patients (41.7%) and level 3 in one patient (8.3%). Median hospital stay was 14 days (IQR 11–34 days).
Nine patients (75%) reported symptoms of a possible preceding infection and the average time between these symptoms and the first neurological symptoms was 9 days (range 5–22 days). Most of the patients (N = 5, 41.6%) reported non-specific, flu-like symptoms such as fever and myalgia. Two patients (16.7%) reported symptoms of a gastrointestinal infection while two other patients reported symptoms associated with an arbovirus infection, such as conjunctivitis and skin rash. The interpretation of the diagnostic test results are shown in Table 1 and the results of the diagnostic tests are shown in Table 2. In three patients (25%) we could confirm the preceding infection based on the criteria described in the methods. Of these three, patient 2 had a confirmed recent infection with ZIKV, patient 5 had a confirmed recent infection with DENV and patient 8 had a confirmed recent infection with CMV. Two patients (16.7%) had a probable recent infection, one with M. pneumoniae (patient 9) and one with ZIKV (patient 3) although a recent DENV infection could not be ruled out in this last patient. In one patient (8.3%, patient 11) we found evidence of a possible recent infection with M. pneumoniae and/or C. jejuni. Finally, in six patients (50.0%) we could not find sufficient evidence of a recent infection based on our diagnostic criteria. Four of these six patients (patient 1, 6, 10, and 12) did have non-specific symptoms of an infection, such as fever and myalgia, prior to the start of GBS symptoms. None of the patients reported to have recently received a vaccination.
In this study, we recruited patients as soon as the treating neurologist suspected GBS. In a relatively large amount of the patients, another diagnosis than GBS was subsequently made by the treating physician and these patients were excluded from this study. This demonstrates that diagnosing GBS, especially in low- and middle income countries with sometimes limited diagnostic facilities, can be challenging and that other conditions need to be ruled out before the diagnosis of GBS can be made. Because of the limited sample size of this study it is difficult to draw conclusions from the clinical data of these patients. In general, the diversity in clinical presentation of the described patients corresponds with what is known about GBS (1). One observation that can be made is that only five GBS patients were treated with IVIg while 11 patients were unable to walk or wheel chair bound and had an indication for treatment (1, 3). This undertreatment is explained by the limited availability of IVIg or plasma exchange facilities in Suriname.
We performed extensive diagnostic testing for preceding infections that are commonly associated with GBS and arboviruses that are endemic in Suriname and are possibly associated with GBS. From 9 out of 12 patients we could collect paired serum samples during hospital admission. As a result, we found indications of a preceding infection in 6 of the 12 GBS patients (50.0%). Four of these six patients (66.7%) in which we could diagnose a recent infection had evidence of a recent ZIKV or DENV infection. It is possible that this relatively high percentage of possible ZIKV associated GBS cases is because recruitment of patients for this study started during the peak of the ZIKV outbreak in Suriname at the beginning of 2016. As is indicated in Figure 1A, during this period there was a peak in GBS patients that were recruited for this study. From the results of the serological tests in Table 2, it can be concluded that it is difficult to distinguish a recent ZIKV infected from a recent DENV infection based on serology because of cross-reactivity of anti-flavivirus antibodies. In this study we used neutralization assays for DENV-2 and ZIKV serology which is the gold standard for flavivirus serology and has shown to give less cross-reactivity than, for example, ELISA tests (9, 15, 21). Another diagnostic challenge is that asymptomatic reactivation of CMV and EBV can occur during acute infections with other pathogens which can lead to the presence of anti-CMV or EBV IgM antibodies (17, 22). In order to differentiate between a primary CMV or EBV infection or reactivation of these viruses, it is possible to measure the avidity of IgG antibodies against CMV, which are low after a recent infection, and to test for presence EBNA IgG antibodies against EBV which are not present after recent EBV infection (17, 18).
Interestingly, in patient 5 we found IgM antibodies against DENV and a more than six-fold increase in the neutralizing antibody titer in paired serum samples taken nine days apart from each other. No IgM or neutralizing antibodies against ZIKV were detected. Based on these results and the fact that this 12 year old patient did not recently receive a yellow fever vaccination, he was classified as having a confirmed recent DENV infection. The patient presented with pain in the backside of both legs, bilateral foot drop and difficulty walking. During physical examination, symmetrical areflexia and pain in the lower extremities was found, the Lasègue sign was positive and no neck stiffness was observed. The CSF did not contain white blood cells or elevated albumin levels and the CSF culture was negative. An EMG was not performed in this patient. DENV has previously sporadically been associated with GBS and other neurological complications (23–25). It might be worthwhile to study this possible association in more detail with specific attention to the clinical presentation of suspected GBS or a GBS-like syndrome caused by DENV.
Besides ZIKV and DENV, we also found evidence of preceding infections with pathogens that are commonly associated with GBS; CMV, M. pneumoniae and C. jejuni. In two patients (9 and 11) we found evidence of a possible recent infection with both M. pneumoniae and C. jejuni. For C. jejuni, IgM was only positive in the first collected sample from both patients, this can indicate that the possible C. jejuni infection was not very recent. However, it has been shown that IgM antibodies against C. jejuni can be short lived or even absent, especially in asymptomatic infections (26). A recent M. pneumoniae infection was probable in patient 9 because of the presence of anti M. pneumoniae IgM antibodies and a rise in the IgG titer. In patient 11, a recent infection with M. pneumoniae was less likely since, even though IgM antibodies were present in the first sample, no kinetics were observed in the anti M. pneumoniae IgG titers. We did not find evidence of recent infections with HEV, EBV or CHIKV in any of these patients. In patient 1 and 6 we could not make a diagnosis of a preceding infection based on the diagnostic criteria described above. However, both patients already had a high titer of neutralizing antibodies against ZIKV early in the ZIKV outbreak (20). This could be indicative of a recent ZIKV infection since ZIKV did not circulate in Suriname before the end of 2015 and both patients reported to have had symptoms that are associated with arboviral infection such as myalgia and arthralgia. However, since it has been shown that after a recent DENV infection, cross-neutralization of ZIKV can occur in some cases, it is also possible that these two patients had a recent DENV infection (15, 21).
A strength of this study is that we performed extensive and state of the art, diagnostic testing to try to identify preceding infection that might have triggered GBS. Because of this extensive testing we were able to demonstrate the challenges that arise with the interpretation of serological diagnostic results. A limitation is that the small sample size of this study and the period of recruitment of the patients (partially during the ZIKV outbreak) does not allow us to generalize the results found in this study with respect to amongst others the incidence of GBS in Suriname and the exact contribution of the different pathogens in causing GBS. Furthermore, because of insufficient clinical data, we had to exclude a relatively large amount of study participants.
In conclusion, we found that—apart from infections with pathogens that are commonly associated with GBS—infections with ZIKV and possibly DENV might play an important role in causing GBS in Suriname. Furthermore, more accessibility to IVIg or plasma exchange could improve the treatment of GBS in Suriname.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the ethical board of the Ministry of Health in Suriname. Written informed consent to participate in this study was provided by the participant or the participants' legal guardian/next of kin.
TL, LD, EG, and HY designed the study. TL, IR, LD, HY, and MB recruited the patients and collected the data. TL and FC performed the virus neutralization tests. CG, TL, MK, and BR interpreted the serological data. BJ and KA interpreted the clinical data. IR and TL wrote the draft manuscript. All authors participated in writing the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the study participants and study personnel.
1. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. (2016) 388:717–27. doi: 10.1016/S0140-6736(16)00339-1
2. Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. (2014) 137(Pt 1):33–43. doi: 10.1093/brain/awt285
3. Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
4. Jacobs BC, Rothbarth PH, van der Meche FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barre syndrome: a case-control study. Neurology. (1998) 51:1110–5. doi: 10.1212/WNL.51.4.1110
5. Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. (2016) 387:1531–9. doi: 10.1016/S0140-6736(16)00562-6
6. GeurtsvanKessel CH, Islam Z, Islam MB, Kamga S, Papri N, van de Vijver D, et al. Zika virus and Guillain-Barre syndrome in Bangladesh. Ann Clin Transl Neurol. (2018) 5:606–15. doi: 10.1002/acn3.556
7. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. (2020) 382:2574–6. doi: 10.1056/NEJMc2009191
8. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
9. Langerak T, Brinkman T, Mumtaz N, Arron G, Hermelijn S, Baldewsingh G, et al. Zika virus seroprevalence in urban and rural areas of Suriname, 2017. J Infect Dis. (2019) 220:28–31. doi: 10.1093/infdis/jiz063
10. Oei W, Lieshout-Krikke RW, Kretzschmar ME, Zaaijer HL, Coutinho RA, Eersel M, et al. Estimating the risk of dengue transmission from Dutch blood donors travelling to Suriname and the Dutch Caribbean. Vox Sang. (2016) 110:301–9. doi: 10.1111/vox.12370
11. Howell KB, Wanigasinghe J, Leventer RJ, Ryan MM. Concomitant transverse myelitis and acute motor axonal neuropathy in an adolescent. Pediatr Neurol. (2007) 37:378–81. doi: 10.1016/j.pediatrneurol.2007.05.020
12. Lin JJ, Hsia SH, Wu CT, Wang HS, Lin KL, Lyu RK. Risk factors and outcomes of Guillain-Barre syndrome with acute myelitis. Pediatr Neurol. (2011) 44:110–6. doi: 10.1016/j.pediatrneurol.2010.08.013
13. Mancera-Paez O, Roman GC, Pardo-Turriago R, Rodriguez Y, Anaya JM. Concurrent Guillain-Barre syndrome, transverse myelitis and encephalitis post-Zika: a case report and review of the pathogenic role of multiple arboviral immunity. J Neurol Sci. (2018) 395:47–53. doi: 10.1016/j.jns.2018.09.028
14. Langerak T, Yang H, Baptista M, Doornekamp L, Kerkman T, Codrington J, et al. Zika virus infection and guillain-barre syndrome in three patients from Suriname. Front Neurol. (2016) 7:233. doi: 10.3389/fneur.2016.00233
15. Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, Puerta-Guardo H, Jadi R, et al. Longitudinal analysis of antibody cross-neutralization following zika virus and dengue virus infection in Asia and the Americas. J Infect Dis. (2018) 218:536–45. doi: 10.1093/infdis/jiy164
16. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. (2008) 14:1232–9. doi: 10.3201/eid1408.080287
17. Prince HE, Lape-Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol. (2014) 21:1377–84. doi: 10.1128/CVI.00487-14
18. Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, et al. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci USA. (1987) 84:570–4. doi: 10.1073/pnas.84.2.570
19. Regional Zika Epidemiological Update (Americas) August 25, 2017: Pan American Health Organization/World Health Organization (2017). Available online at: https://www.paho.org/hq/index.php?option=com_content&view=article&id=11599&Itemid=41691&lang=en (accessed August 25, 2017).
20. Codrington J, Roosblad J, Baidjoe A, Holband N, Adde A, Kazanji M, et al. Zika virus outbreak in Suriname, a report based on laboratory surveillance data. PLOS Currents Outbreaks. (2018). doi: 10.1371/currents.outbreaks.ff0f6190d5431c2a2e824255eaeaf339
21. Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, et al. Lack of durable cross-neutralizing antibodies against zika virus from dengue virus infection. Emerg Infect Dis. (2017) 23:773–81. doi: 10.3201/eid2305.161630
22. Macsween KF, Crawford DH. Epstein-Barr virus-recent advances. Lancet Infect Dis. (2003) 3:131–40. doi: 10.1016/S1473-3099(03)00543-7
23. Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain-Barre syndrome: a case report and review of the literature. J Med Case Rep. (2018) 12:137. doi: 10.1186/s13256-018-1626-y
24. Verma R, Sharma P, Garg RK, Atam V, Singh MK, Mehrotra HS. Neurological complications of dengue fever: experience from a tertiary center of north India. Ann Indian Acad Neurol. (2011) 14:272–8. doi: 10.4103/0972-2327.91946
25. Santos NQ, Azoubel AC, Lopes AA, Costa G, Bacellar A. Guillain-Barre syndrome in the course of dengue: case report. Arq Neuropsiquiatr. (2004) 62:144–6. doi: 10.1590/S0004-282X2004000100025
Keywords: Guillain-Barré syndrome, Suriname, Zika virus, dengue virus, arthropod borne viruses
Citation: Langerak T, van Rooij I, Doornekamp L, Chandler F, Baptista M, Yang H, Koopmans MPG, GeurtsvanKessel CH, Jacobs BC, Rockx B, Adriani K and van Gorp ECM (2021) Guillain-Barré Syndrome in Suriname; Clinical Presentation and Identification of Preceding Infections. Front. Neurol. 12:635753. doi: 10.3389/fneur.2021.635753
Received: 30 November 2020; Accepted: 20 January 2021;
Published: 10 February 2021.
Edited by:
Serge Nataf, Université Claude Bernard Lyon 1, FranceReviewed by:
Florian Deisenhammer, Innsbruck Medical University, AustriaCopyright © 2021 Langerak, van Rooij, Doornekamp, Chandler, Baptista, Yang, Koopmans, GeurtsvanKessel, Jacobs, Rockx, Adriani and van Gorp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric C. M. van Gorp, ZS52YW5nb3JwQGVyYXNtdXNtYy5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.