
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Neurol. , 09 February 2021
Sec. Neuro-Otology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.635359
This article is part of the Research Topic Neuroimmunology of the Inner Ear View all 21 articles
Editorial on the Research Topic
Neuroimmunology of the Inner Ear
Although the term was first officially used in 1982 (1), neuroimmunology is now a mature field that has gained immense traction in the past decade. Thanks to novel technological advances, the cellular and molecular mechanisms that mediate the crosstalk between the immune and nervous systems are increasingly appreciated in both physiological and pathological states (1).
Similar to the brain, the inner ear has long been considered an “isolated” system devoted to auditory and vestibular signal processing and protected by a blood-labyrinth barrier (BLB) (2–5), and the early neuroimmunology of the inner ear was mainly focused on autoimmunity (6, 7), and on the role of macrophages in cochlear damage (8, 9). In parallel to the brain, awareness about non-neural cells and molecules affecting inner ear functions has been steadily growing1, and neuro-immunological studies of the inner ear face multiple challenges, including an overwhelming number of cellular and molecular interactions, which will require a systems biology approach to grasp their full functionality. In addition, the inner ear poses unique difficulties due to its tight bone encasing and complex fluid regulation.
Like most organs, including the brain, the inner ear immune cells are dominated by several populations of macrophages [reviewed in (10, 11)], which largely contribute to both inflammatory/phagocytic and regenerative/protective responses. However, several questions are still open, such as:
• What are the signals exchanged between immune cells and inner ear cells in healthy and pathological settings?
• How much communication is there between the inner ear and surrounding tissues and fluids?
• What is the neuroimmune role of the endolymphatic sac?
• What are the roles, nature, and location of several immune cell populations and subpopulations, e.g., mast cells (12), lymphocytes (13), or other leukocytes (14)?
• How are local and systemic immune responses regulated—and especially dysregulated- in various kinds of damage (e.g., infection, noise trauma, and ototoxicity)?
• How do neuroimmune interactions translate in the modulation of inner ear functions?
The articles collected in this Research Topic reflect this increasingly diverse field in several main threads, broadly divided into immune cell characterization, responses to diseases or damage, biomarkers, and immune molecular targets (Figure 1). Both human and animal studies are included in this Special Topic. The system's complexity at both cellular and functional levels requires the integration of invasive approaches only possible in animals and testing of functions and markers that may be incompletely overlapping between humans and other animals. Table 1 summarizes the main points that were contributed by human and animal studies within this Research Topic.

Figure 1. Neuroimmunology of the inner ear. Visual abstract of this Research Topic: each paper within this Topic explores molecules, cells and/or functions related to inner ear neuroimmune interactions. HL, hearing loss; ES, endolymphatic sac; SCC, semicircular canals. Created with Biorender.com.
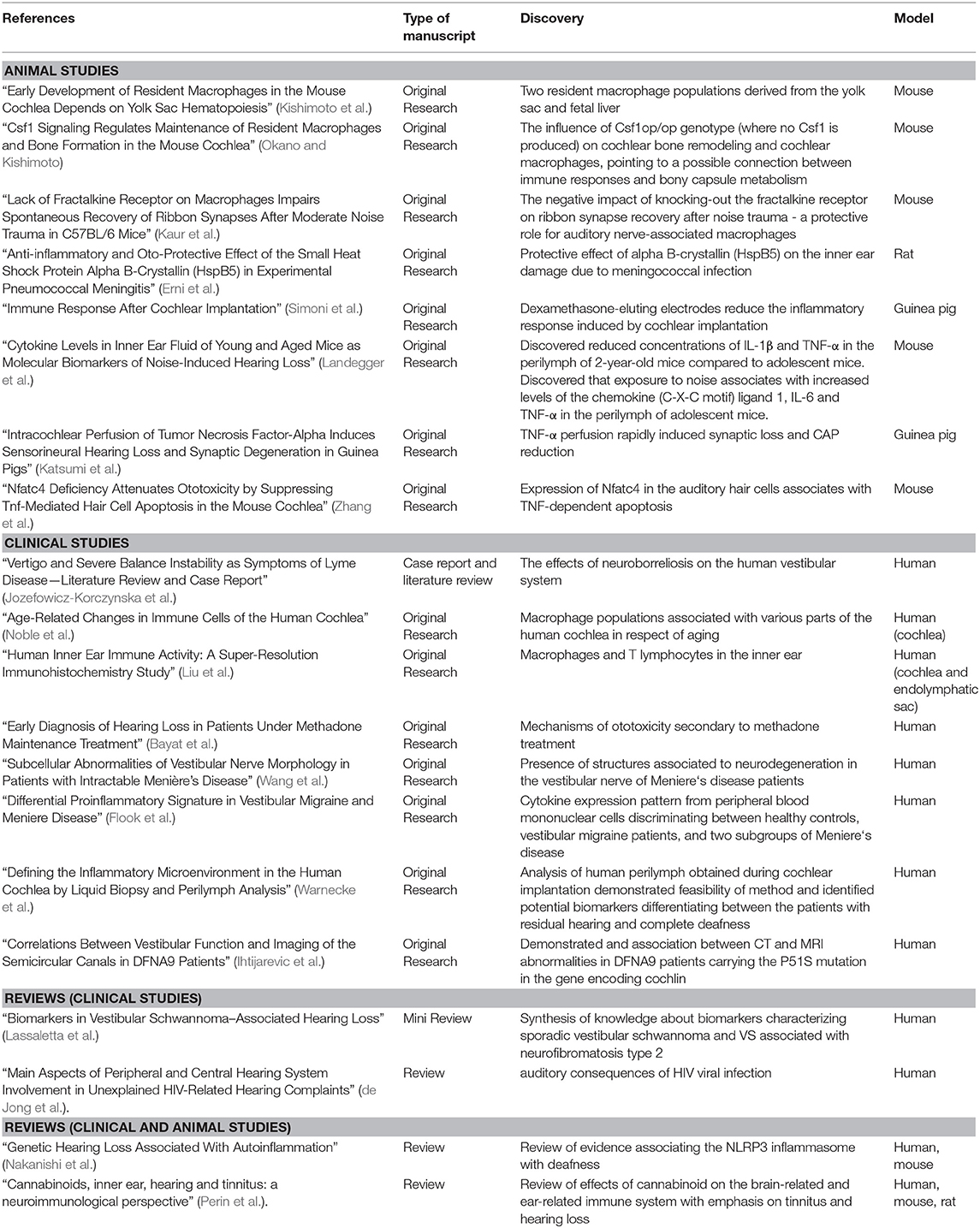
Table 1. A summary of data published in the Research Topic “Neuroimmunology of the Inner Ear” based on the type of manuscript and the research model used.
Five papers published in this Research Topic focus on the description of the immune cells of the inner ear, with a predominance of studies regarding cochlear macrophages, consistent with their role as leading players both by number and function, as reviewed in (10, 11).
The paper “Early Development of Resident Macrophages in the Mouse Cochlea Depends on Yolk Sac Hematopoiesis” describes the presence of two resident macrophage populations in the mouse cochlea, derived respectively from the yolk sac and fetal liver (Kishimoto et al.). These populations are different in their Csf1-dependence and final cochlear localization. The origin of macrophages may be reflected in their various shapes in the adult cochlea, as seen in human samples in the following two papers. The first, “Age-Related Changes in Immune Cells of the Human Cochlea,” describes macrophage populations associated with various parts of the human cochlea and the aging-dependent changes occurring in these cells (Noble et al.). Similarly, the paper “Human Inner Ear Immune Activity: A Super-Resolution Immunohistochemistry Study,” describes immune cells (mainly macrophages and to a lesser extent T lymphocytes) associated with the human cochlea and endolymphatic sac (Liu et al.). Both papers observed an association between cell shapes and their localization in the inner ear. Finally, two groups studied macrophage-related gene knock-out effects on the inner ear function and anatomy using the mouse model. “Csf1 Signaling Regulates Maintenance of Resident Macrophages and Bone Formation in the Mouse Cochlea” underlines the impact of the Csf1op/op genotype (where no Csf1 is produced) on cochlear bone remodeling and cochlear macrophages, therefore pointing to a possible connection between immune responses and bony capsule metabolism (Okano and Kishimoto). On the other hand, the paper “Lack of Fractalkine Receptor on Macrophages Impairs Spontaneous Recovery of Ribbon Synapses After Moderate Noise Trauma in C57BL/6 Mice” demonstrates the negative impact of knocking-out the fractalkine receptor on ribbon synapse recovery after noise trauma, suggesting a protective role for auditory nerve-associated macrophages (Kaur et al.).
Eight other papers in this Research Topic studied immune responses in the inner ear in a pathological context, three of which (two in humans and one in a rat model) focus on infectious diseases. Passive barriers strongly protect the inner ear, but pathogens may enter it through its connections with CSF and middle ear, plus vascular and neural routes additionally available for viruses (15). Moreover, even systemic responses to pathogens may affect the ear indirectly, due to immune crossreactivity (as suggested after viral or fungal infection, reviewed in (16) or BLB impairment opening the way to ototoxic factors into the inner ear (17–19).
The review “Main Aspects of Peripheral and Central Hearing System Involvement in Unexplained HIV-Related Hearing Complaints” presents an interesting study on the auditory consequences of HIV viral infection (which show similarities to age-induced hearing impairment), an aspect not well-understood and of enormous importance (de Jong et al.). The paper “Vertigo and Severe Balance Instability as Symptoms of Lyme Disease—Literature Review and Case Report” describes the effects of neuroborreliosis, which can target the 8th nerve, on the human vestibular system from a clinical point of view (Jozefowicz-Korczynska et al.). The work described in “Anti-inflammatory and Oto-Protective Effect of the Small Heat Shock Protein Alpha B-Crystallin (HspB5) in Experimental Pneumococcal Meningitis” demonstrates in a rat model that inner ear damage due to meningococcal infection goes together with an increase of proinflammatory cytokines in CSF, and rise in the numbers of cochlear neutrophils and macrophages (Erni et al.). The cytokine (but not the cellular) response could be reduced by intracisternal injection with the small heat shock protein alpha B-crystallin (HspB5), which also ameliorated hearing loss.
Besides infections, the inner ear can be affected by exposure to stress factors (including noise, drugs, and surgery), aging, genetic defects, or pathologies of unclear etiology, such as Menière's disease (MD). Many of these settings are accompanied by inflammation—a classical response to damage that is beneficial per se but may become detrimental if dysregulated, further damaging the inner ear [reviewed in (20, 21)]. Moreover, inflammation related to invasive inner ear intervention, such as cochlear implantation (CI), may lead to fibrosis and bone neoformation (22, 23), which degrade residual hearing. An effective otoprotective strategy in CI is the use of dexamethasone-eluting electrodes (24–26). However, understanding the otoprotective mechanisms of dexamethasone in CI is difficult since steroids can block all inflammatory response phases (20). Interestingly, CI influences the composition of macrophages subsets in animals (27, 28) and humans (29, 30). In the animal model, where the entire inflammatory process can be followed, macrophages have been found in the inflammatory, cytotoxic phenotype (27), and in the reparative phenotype, which is associated to matrix deposition and remodeling, and therefore to fibrosis (28). Moreover, a protective macrophage subpopulation has been observed in rodents' spiral ganglion (31) and suggested to exist in humans (29). The foreign-body responses may also induce the macrophages to form giant multinucleated cells with osteoblast-like properties (32). Finally, CI may also recruit B and T lymphocytes (33). The manuscript “Immune Response After Cochlear Implantation” shows that dexamethasone-eluting electrodes reduce both the cellular and cytokine signature of acute inflammatory response and fibrosis associated with implantation in a guinea pig, a well-established animal model for CI (Simoni et al.).
The paper “Genetic Hearing Loss Associated With Autoinflammation” describes deafness correlated with mutations affecting the NLRP3 inflammasome and other genes that influence macrophages (Nakanishi et al.). Inflammation of strial blood vessels was suggested, among other possible causes, in ototoxicity secondary to methadone treatment in humans in the paper “Early Diagnosis of Hearing Loss in Patients Under Methadone Maintenance Treatment” by Bayat et al..
Finally, two papers focus on the immune-inflammatory response in Menière's disease. As of today, MD diagnosis and monitoring are based on clinical symptoms, and no selective biomarker is available (34, 35). Endolymphatic hydrops has been long associated (although not exclusively) with MD, and endolymph-producing and reabsorbing structures in the ear are being targeted in treatment options [reviewed in (34, 35)]. Also, recent studies show a breakdown of the human utricular BLB in MD due to increased vesicular transport in endothelial cells and pericytes (36). Therefore, perilymph production appears to be affected, as well. In fact, a diagnostic tool based on gadolinium-enhanced MRI is being considered for MD (37, 38).
MD etiology is still debated and includes a combination of genetic, immune, inflammatory, environmental, hemodynamic, and hormonal factors (39). Immune-inflammatory responses seem however central, given that several genes linked to MD belong to immune or inflammation pathways (40), and that a significant percentage of patients with MD is also affected by other autoimmune diseases (41), or displays enhanced and anomalous inflammatory responses (42). Moreover, the endolymphatic sac, which is involved in endolymph reabsorption and displays morphological changes in MD (43), is the primary immune structure of the inner ear (44), and inner ear vasculature permeability is strongly affected by inflammation (45). Finally, MD immune-inflammatory model explains the effectiveness of steroid treatment (46). However, as for other immune diseases, treatment of subjects non-responsive to steroids requires personalized approaches that depend on the particular pathway being deranged (40).
The report “Subcellular Abnormalities of Vestibular Nerve Morphology in Patients with Intractable Menière's Disease” found the presence of structures associated to neurodegeneration (corpora amylacea, lipofuscin, microglia) in the vestibular nerve of MD patients (Wang et al.). On the other hand, the paper “Differential Proinflammatory Signature in Vestibular Migraine and Meniere Disease” describes a cytokine subset whose expression level in peripheral blood mononuclear cells can discriminate between healthy controls, vestibular migraine patients, and two subgroups of MD patients, thus yielding the basis for a blood test helping MD diagnosis (Flook et al.).
Two papers focused on a hot spot in auditory research: biomarkers for various types of inner ear diseases. “Cytokine Levels in Inner Ear Fluid of Young and Aged Mice as Molecular Biomarkers of Noise-Induced Hearing Loss” approaches the connection between noise-induced and age-related hearing loss using the mouse model (Landegger et al.). The second paper, “Biomarkers in Vestibular Schwannoma–Associated Hearing Loss,” reviews the proteins and genes that could potentially be included in the clinical evaluation panel of vestibular schwannoma (Lassaletta et al.).
The paper “Defining the Inflammatory Microenvironment in the Human Cochlea by Liquid Biopsy and Perilymph Analysis” studied human perilymph from patients assigned for CI, observing the expression of a panel of immune-related and growth factor-related proteins (Warnecke et al.). Finally, in the paper “Correlations Between Vestibular Function and Imaging of the Semicircular Canals in DFNA9 Patients,” MRI was used to visualize abnormalities of the semicircular canals that were attributed to cochlin deposits and fibrosis in the light of functional deficiencies (Ihtijarevic et al.).
Regarding molecular targets for neuroimmune signaling in the ear, most papers in this Research Topic confirmed an essential role for TNF signaling [see discussion in (47)]. Two papers focused on that issue. “Intracochlear Perfusion of Tumor Necrosis Factor-Alpha Induces Sensorineural Hearing Loss and Synaptic Degeneration in Guinea Pigs” found that TNF-α perfusion rapidly induced synaptic loss and CAP reduction, which resembled primary cochlear neuropathy (Katsumi et al.), whereas “Nfatc4 Deficiency Attenuates Ototoxicity by Suppressing Tnf-Mediated Hair Cell Apoptosis in the Mouse Cochlea” describes the expression of Nfatc4 in the auditory hair cells being linked to TNF-dependent apoptosis (Zhang et al.).
The last paper, “Cannabinoids, inner ear, hearing and tinnitus: a neuroimmunological perspective” reviews cannabinoid effects on the immune system and their possible roles in tinnitus and hearing loss, for which only neuronal effects have been considered so far (Perin et al.).
By exploring several consolidated or novel mechanisms and effects of neuroimmune interactions in the inner ear, this Research Topic yields a broad perspective on possible innovative therapeutic and diagnostic approaches to audiovestibular diseases and contributes to increasing visibility of this fascinating subject. We hope that in the next decade, the current research will elucidate biochemical pathways connecting immune responses in sensory circuits to functional changes at the cellular and systems levels. Further exploration of the inner ear's neuro-immuno-sensory axis might impact future therapy and monitoring of some otological diseases.
PP and AS drafted the manuscript. PP drew the figure. PP, FM, IV-N, and AS revised and approved the final version of this manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^PUBMED search with the query “(inner ear OR cochlea OR vestibular) AND (neuroimmune OR neuroimmunology)” only retrieves 14 results, but this reflects the still sparse use of “neuroimmune”, since the related queries “(inner ear OR cochlea OR vestibular) AND (immune OR macrophage OR cytokine)” and “(inner ear OR cochlea OR vestibular) AND (inflammatory OR inflammation)” retrieve 1,734 and 2,043 results, respectively.
1. Nutma E, Willison H, Martino G, Amor S. Neuroimmunology - the past, present and future. Clin Exp Immunol. (2019) 197:278–93. doi: 10.1111/cei.13279
2. Jahnke K. The blood-perilymph barrier. Arch Otorhinolaryngol. (1980) 228:29–34. doi: 10.1007/BF00455891
3. Juhn SK, Rybak LP, Fowlks WL. Transport characteristics of the blood–perilymph barrier. Am J Otolaryngol. (1982) 3:392–6. doi: 10.1016/S0196-0709(82)80016-1
4. Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci USA. (2007) 104:6229–34. doi: 10.1073/pnas.0605108104
5. Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res. (2016) 338:52–63. doi: 10.1016/j.heares.2016.01.010
6. Quick CA. Antigenic causes of hearing loss. Otolaryngol Clin North Am. (1975) 8:385–94. doi: 10.1016/S0030-6665(20)32776-6
7. Tomiyama S, Harris JP. The endolymphatic sac: its importance in inner ear immune responses. Laryngoscope. (1986) 96:685–91. doi: 10.1288/00005537-198606000-00018
8. Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. (2005) 489:180–94. doi: 10.1002/cne.20619
9. Sato E, Shick HE, Ransohoff RM, Hirose K. Repopulation of cochlear macrophages in murine hematopoietic progenitor cell chimeras: the role of CX3CR1. J Comp Neurol. (2008) 506:930–42. doi: 10.1002/cne.21583
10. Hirose K, Rutherford MA, Warchol ME. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res. (2017) 352:70–81. doi: 10.1016/j.heares.2017.04.006
11. Warchol ME. Interactions between macrophages and the sensory cells of the inner ear. Cold Spring Harb Perspect Med. 9:a033555. doi: 10.1101/cshperspect.a033555
12. Szczepek AJ, Dudnik T, Karayay B, Sergeeva V, Olze H, Smorodchenko A. Mast cells in the auditory periphery of rodents. Brain Sci. (2020) 10:697. doi: 10.3390/brainsci10100697
13. Rai V, Wood MB, Feng H, Schabla NM, Tu S, Zuo J. The immune response after noise damage in the cochlea is characterized by a heterogeneous mix of adaptive and innate immune cells. Sci Rep. (2020) 10:15167. doi: 10.1038/s41598-020-72181-6
14. Hu BH, Zhang C, Frye MD. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res. (2018) 362:14–24. doi: 10.1016/j.heares.2017.12.009
15. Dewyer NA, Kiringoda R, Mckenna MJ. Inner Ear Infections (Labyrinthitis). In: Durand M, Deschler D, editors. Infections of the Ears, Nose, Throat, and Sinuses. Cham: Springer (2018). p. 79–88. doi: 10.1007/978-3-319-74835-1_7
16. Buki B, Junger H, Zhang Y, Lundberg YW. The price of immune responses and the role of vitamin D in the inner ear. Otol Neurotol. (2019) 40:701–9. doi: 10.1097/MAO.0000000000002258
17. Oh GS, Kim HJ, Choi JH, Shen A, Kim CH, Kim SJ, et al. Activation of lipopolysaccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J Immunol. (2011) 186:1140–50. doi: 10.4049/jimmunol.1002183
18. Hirose K, Li SZ, Ohlemiller KK, Ransohoff RM. Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. J Assoc Res Otolaryngol. (2014) 15:555–70. doi: 10.1007/s10162-014-0458-8
19. Koo JW, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, et al. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med. (2015) 7:298ra118. doi: 10.1126/scitranslmed.aac5546
20. Kalinec GM, Lomberk G, Urrutia RA, Kalinec F. Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise- and age-related hearing loss. Front Cell Neurosci. (2017) 11:192. doi: 10.3389/fncel.2017.00192
21. Wood MB, Zuo J. The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front Cell Neurosci. (2017) 11:106. doi: 10.3389/fncel.2017.00106
22. Fayad JN, Makarem AO, Linthicum FHJr. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3D reconstruction. Otolaryngol Head Neck Surg. (2009) 141:247–52. doi: 10.1016/j.otohns.2009.03.031
23. Jia H, Wang J, Francois F, Uziel A, Puel JL, Venail F. Molecular and cellular mechanisms of loss of residual hearing after cochlear implantation. Ann Otol Rhinol Laryngol. (2013) 122:33–9. doi: 10.1177/000348941312200107
24. Astolfi L, Simoni E, Giarbini N, Giordano P, Pannella M, Hatzopoulos S, et al. Cochlear implant and inflammation reaction: Safety study of a new steroid-eluting electrode. Hear Res. (2016) 336:44–52. doi: 10.1016/j.heares.2016.04.005
25. Bas E, Bohorquez J, Goncalves S, Perez E, Dinh CT, Garnham C, et al. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res. (2016) 337:12–24. doi: 10.1016/j.heares.2016.02.003
26. Wilk M, Hessler R, Mugridge K, Jolly C, Fehr M, Lenarz T, et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS ONE. (2016) 11:e0147552. doi: 10.1371/journal.pone.0147552
27. Bas E, Gupta C, Van De Water TR. A novel organ of corti explant model for the study of cochlear implantation trauma. Anat Rec. (2012) 295:1944–56. doi: 10.1002/ar.22585
28. Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, et al. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. (2015) 9:303. doi: 10.3389/fncel.2015.00303
29. Okayasu T, O'Malley JT, Nadol JB Jr. Density of macrophages immunostained with anti-iba1 antibody in the vestibular endorgans after cochlear implantation in the human. Otol Neurotol. (2019) 40:e774–81. doi: 10.1097/MAO.0000000000002313
30. Noonan KY, Lopez IA, Ishiyama G, Ishiyama A. Immune response of macrophage population to cochlear implantation: cochlea immune cells. Otol Neurotol. (2020) 41:1288–95. doi: 10.1097/MAO.0000000000002764
31. Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, et al. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci. (2015) 35:15050–61. doi: 10.1523/JNEUROSCI.2325-15.2015
32. Barbeck M, Booms P, Unger R, Hoffmann V, Sader R, Kirkpatrick CJ, et al. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J Biomed Mater Res A. (2017) 105:1105–11. doi: 10.1002/jbm.a.36006
33. Nadol JB, O'Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. (2014) 318:11–17. doi: 10.1016/j.heares.2014.09.007
34. Magnan J, Ozgirgin ON, Trabalzini F, Lacour M, Escamez AL, Magnusson M, et al. European position statement on diagnosis, and treatment of Meniere's disease. J Int Adv Otol. (2018) 14:317–21. doi: 10.5152/iao.2018.140818
35. Basura GJ, Adams ME, Monfared A, Schwartz SR, Antonelli PJ, Burkard R, et al. Clinical Practice guideline: Meniere's disease. Otolaryngol Head Neck Surg. (2020) 162:S1–55. doi: 10.1177/0194599820909438
36. Ishiyama G, Lopez IA, Ishiyama P, Vinters HV, Ishiyama A. The blood labyrinthine barrier in the human normal and Meniere's disease macula utricle. Sci Rep. (2017) 7:253. doi: 10.1038/s41598-017-00330-5
37. Nakashima T, Naganawa S, Sugiura M, Teranishi M, Sone M, Hayashi H, et al. Visualization of endolymphatic hydrops in patients with Meniere's disease. Laryngoscope. (2007) 117:415–20. doi: 10.1097/MLG.0b013e31802c300c
38. Van Steekelenburg JM, Van Weijnen A, De Pont LMH, Vijlbrief OD, Bommeljé CC, Koopman JP, et al. Value of endolymphatic hydrops and perilymph signal intensity in suspected Ménière disease. AJNR Am J Neuroradiol. (2020) 41:529–34. doi: 10.3174/ajnr.A6410
39. Oberman BS, Patel VA, Cureoglu S, Isildak H. The aetiopathologies of Meniere's disease: a contemporary review. Acta Otorhinolaryngol Ital. (2017) 37:250–63. doi: 10.14639/0392-100X-793
40. Lopez-Escamez JA, Batuecas-Caletrio A, Bisdorff A. Towards personalized medicine in Meniere's disease. F1000Res. (2018) 7:F1000 Faculty Rev-1295. doi: 10.12688/f1000research.14417.1
41. Greco A, Gallo A, Fusconi M, Marinelli C, Macri G, De Vincentiis M. Meniere's disease might be an autoimmune condition? Autoimmun Rev. (2012) 11:731–8. doi: 10.1016/j.autrev.2012.01.004
42. Frejo L, Gallego-Martinez A, Requena T, Martin-Sanz E, Amor-Dorado JC, Soto-Varela A, et al. Proinflammatory cytokines and response to molds in mononuclear cells of patients with Meniere disease. Sci Rep. (2018) 8:5974. doi: 10.1038/s41598-018-23911-4
43. Bachinger D, Luu NN, Kempfle JS, Barber S, Zurrer D, Lee DJ, et al. Vestibular aqueduct morphology correlates with endolymphatic sac pathologies in meniere's disease-a correlative histology and computed tomography study. Otol Neurotol. (2019) 40:e548–55. doi: 10.1097/MAO.0000000000002198
44. Kampfe Nordstrom C, Danckwardt-Lilliestrom N, Laurell G, Liu W, Rask-Andersen H. The human endolymphatic sac and inner ear immunity: macrophage interaction and molecular expression. Front Immunol. (2018) 9:3181. doi: 10.3389/fimmu.2018.03181
45. Trune DR, Nguyen-Huynh A. Vascular pathophysiology in hearing disorders. Semin Hear. (2012) 33:242–50. doi: 10.1055/s-0032-1315723
46. Hao W, Yu H, Li H. Effects of intratympanic gentamicin and intratympanic glucocorticoids in Meniere's disease: a network meta-analysis. J Neurol. (2021). doi: 10.1007/s00415-020-10320-9. [Epub ahead of print].
Keywords: inner ear, immunology, neuroimmunology, balance, hearing, disorders
Citation: Perin P, Marino F, Varela-Nieto I and Szczepek AJ (2021) Editorial: Neuroimmunology of the Inner Ear. Front. Neurol. 12:635359. doi: 10.3389/fneur.2021.635359
Received: 30 November 2020; Accepted: 07 January 2021;
Published: 09 February 2021.
Edited by:
Michael Strupp, Ludwig Maximilian University of Munich, GermanyReviewed by:
Göran Frans Emanuel Laurell, Uppsala University, SwedenCopyright © 2021 Perin, Marino, Varela-Nieto and Szczepek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Perin, pperin@unipv.it; Agnieszka J. Szczepek, agnes.szczepek@charite.de
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.