- Department of Neuroimaging and Interventional Radiology, National Institute of Mental Health and Neurosciences, Bangalore, India
Purpose: Hybrid whole-body magnetic resonance/positron emission tomography (MR/PET) systems are new diagnostic tools enabling the simultaneous acquisition of morphologic and multiparametric functional data, which allow for a diversified characterization of oncological diseases. This study aimed to compare the diagnostic ability of MRI with the diffusion-weighted image (DWI), and simultaneous integrated positron emission tomography MR/PET to detect malignant lesions and elucidate the utility and limitations of these imaging modalities in preoperative and postoperative follow up in cancer patients.
Material and Methods: A total of 45 patients undergoing simultaneous MR/PET for CNS ICSOL in our institution between January 2016 and July 2020 were considered in this study. Post-processing was done in Siemens syngo software to generate a b2000 image. This image was then inverted to grayscale and compared with the NAC image of PET.
Results: The lesion-based sensitivity, specificity, positive predictive value, and negative predictive value for DWI were 92.3, 83.3, 97.3, and 62.5%, respectively (at 95% CI and p was 0.000). The lesion-based sensitivity, specificity, positive predictive value, and negative predictive value for PET were 97.4, 71.4, 94.9, and 83.3%, respectively (at 95% CI and p was 0.000). The lesion-based sensitivity and specificity of DWI were comparable with those of PET.
Conclusions: Although DWI and FDG-PET reflect different tissue properties, there is an association between the measures of both methods in CNS tumors probably because of the coupling of cellularity with tumor metabolism as seen on FDG and other PET tracers. Our study shows that DWI acts as a surrogate biomarker for FDG PET and other tracers in tumors. The method of DWI image generation is simple, radiation-free, and cost-effective in a clinical setup. The simultaneous DWI-PET study provides evidence and confirms the role of DWI in surveillance imaging of tumors.
Introduction
The hybrid whole-body magnetic resonance positron emission tomography (MRPET) system is a new diagnostic tool. It enables the simultaneous acquisition of morphologic and multiple functional data and thus a diversified characterization of oncological diseases (1, 2).
Our study focuses on the use of simultaneous MR/PET. The temporal correlation of MRI and PET in a single sitting is possible with MR/PET and various MRI parameters and PET tracers can be compared with high spatial and temporal resolution. MRI is a multiparametric imaging technique and when combined with simultaneous PET is better than PET computed tomography.
Many MR/PET studies correlate with multiple advanced imaging parameters like diffusion-weighted imaging (DWI) and Perfusion weighted imaging (PWI) studies on the grading of gliomas, enabling the prediction of recurrence. It is possible to differentiate recurrence vs. radiation necrosis and the false negative in each modality is known (3–6). PET also has many tracers, each with its specificity and sensitivity (7–10).
Each modality has inherent limitations, for instance, false-positive uptake of FDG PET is due to the immediate effects of cyberknife and gamma knife, and post-radiotherapy related inflammatory changes (11). The resolution of PET at best is 4–6 mm (12). Many small lesions in the brain may be missed at this resolution in a post-operative case. MRI parameters on the other hand, such as PWI, MRS, contrast enhancement patterns have specificity and sensitivity and since these are ROI-based measurements, inherent flaws exist (12, 13). MRS and PWI can help grade tumors but since they use large voxel sizes, they have low specificity (14). Contrast enhancement pattern, which represents blood-brain barrier imaging, helps to correlate, but pseudoprogression and pseudo regression are known entities (15).
Though many DWI techniques have been discussed, no study had access to simultaneous MR/PET, especially in a neuro-oncology setup. However, the clinical benefits of simultaneous MR/PET imaging need to be balanced against the relatively high cost and availability of such an approach.
This paper discusses DWI as a potential MRI biomarker for FDG PET and other tracers. Moreover, this marker is simple, radiation-free, and cost-effective, making clinical translations more straightforward. This study compares DWI with PET images and aims to establish the sensitivity specificity and pitfalls in neurooncology.
We hypothesize that DWI and PET will have a good correlation as both show signal changes and uptake is based on cellularity. Though this paper mainly focuses on the role of MR/PET in cases of glioblastoma, we also briefly describe our experience with other histotypes of CNS tumors and also with other PET tracers as well.
Materials and Methods
Approval was obtained from our institutional ethics committee. Patient consent was waived for this retrospective observational analysis of anonymized data. The study considered all patients with intra-cranial space-occupying-lesions (ICSOL) (all grades of gliomas and various other histotypes) evaluated with MR/PET in our institution between January 2016 and July 2020. A total of 45 cases with simultaneous MR/PET imaging are included in the study. All patients had histologically proven malignancies or suspected malignancies. Whole-brain PET images along with multiplanar and multi-sequence MR images were acquired in 3-D mode after i.v. injection of 18F-FDG using a simultaneous Siemens mMR Biograph PET/MR scanner. In a few cases different tracers like C-11methionine, F-18 choline was used as part of a clinical protocol.
This study aimed to compare the diagnostic ability of MRI with a diffusion-weighted image (DWI), and simultaneous integrated positron emission tomography MR/PET to detect malignant lesions and elucidate the utility and limitations of these imaging modalities in preoperative and postoperative follow up in cancer patients.
DWI Image Acquisition
A routine multiparametric MRI was conducted including T2, SWI, and fluid-attenuated inversion recovery (FLAIR) sequences, axial and 3D T1-weighted, and GdT1w-MRI.
DWI was acquired in the axial plane before injection of contrast material for all patients by fast echo-planar T2*-weighted gradient echo sequence and used for generating ADC maps. The sequence parameters were: b-values 0 and 1,000 s/mm2,TR = 3,600 ms, TE = 81 ms, FOV = 23 cm, matrix = 128 × 128, voxel size-0.9 × 0.9 × 4 mm, 27 slices of 4 mm thickness 4 mm without spacing, NEX- 1.
Image Interpretation
Two radiologists interpreted the MR/PET images in a blinded fashion. The lesion-specific signal intensity on DWI and ADC is compared with metabolism on PET images. Histopathology, all imaging findings, and follow-up scans served as a standard of reference. The DWI images were post-processed using Siemens Heathineers syngo software (version 05.01.0000.0061). The MR basics module in the software package has options for calculating the high b value of trace images and hence we generated images at b2000 to suppress the T2 shine-through the effects of gliosis and edema and also at high value there was good background suppression and increased conspicuity of recurrent foci. For ease of visual correlation, in this study, we aimed to obtain a PET-like image from DWI by inverting the b2000 trace image to grayscale, which has the potential to be an excellent biomarker in the neuro-oncology workup.
Statistical Analysis
Data were collated offline on Microsoft Excel version 15.0.4 and statistical analysis was conducted with an online interactive statistics page using a 2 × 2 consistency table. Lesion-based sensitivity, specificity, negative predictive value, positive predictive value were calculated for DWI and PET modalities. The Yates corrected P-value < 0.005 was considered significant.
Results
Anonymized MR/PET imaging data obtained in 45 patients with ICSOL were also used in the analysis and examined glioma of all grades (n = 35) and other histotypes (n = 10, including ependymoma, craniopharyngioma, meningioma, lymphoma, atypical rhabdoid tumor) with FDG tracer (n = 31), and other PET tracers such as C-11methionine (n = 8) and F-18 choline (n = 3).
Lesion-based sensitivity, specificity, positive predictive value and negative predictive value for DWI were 92.3, 83.3, 97.3, and 62.5% respectively (at 95% CI and p < 0.05). The lesion-based sensitivity, specificity, positive predictive value, and negative predictive value for PET were 97.4, 71.4, 94.9, and 83.3% respectively (at 95% CI and p is < 0.05). These lesion-based sensitivity, specificity of DWI were comparable with those of PET. The DWI showed significantly higher specificity than PET.
The salient observations noticed on visual analysis will also be discussed underneath as:
a) our experience with Glioma: high b value diffusion as a surrogate marker for FDG PET in the diagnosis of recurrence in brain tumors (Table 1).
b) Our experience with CNS tumors of other histotypes including the whole body work up (Table 2).
c) Our experience with other tracers (Table 2).
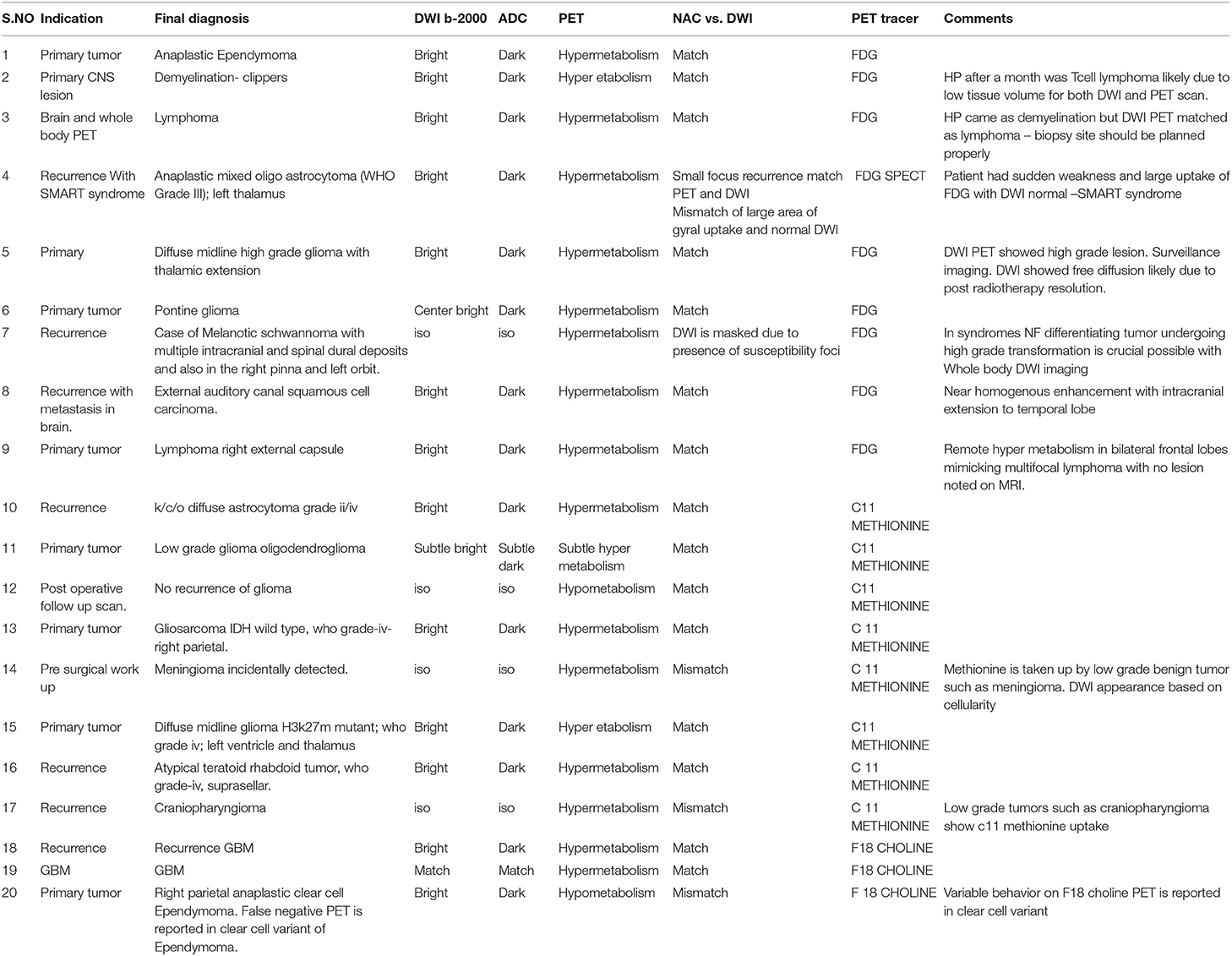
Table 2. Brief summary of other CNS histotypes cases evaluated with MRI FDG-PET and other PET tracers.
Using MRPET in Glioma to Differentiate Recurrence From Radiation Necrosis
A total of 25 post-operative patients with brain tumors were studied with preoperative histopathology and FDG MR/PET between 2016 and 2020.
Out of 25, 10 were Grade III and 8 were Grade IV tumors. A detailed histopathology report of glioma was not available in seven cases, as they were referred cases.
Among the 10 cases with Grade III, there were four who had IDH mutation, four were of p53 type and five were positive for ATRX. Among the eight cases with Grade IV tumor, two had IDH mutation, three were p53 subtype and one had ATRX mutation. The grade was based on a mitotic index. Of Grade IV cases, seven were of GBM tissue subtype and one was pilocytic astrocytoma subtype. In Grade III, six cases were of Oligodendroglioma subtype, one was of mixed Oligo astrocytoma subtype, and thee of anaplastic astrocytoma subtype.
MR/PET was done in 25 cases of glioma and reported as recurrence (n = 20) (Figure 1) and radiation necrosis (n = 5) (Figure 2) based on imaging findings. On follow-up, histopathology was available in 16 cases in the recurrence group. Recurrence on PET was noted in 20 cases with radiation necrosis in five cases. In Grade IV, five had a recurrence and among Grade III, seven had a recurrence, and three had necrosis on PET. We used PET as a reference to standardize DWI in differentiating necrosis vs. recurrence. When B2000 DWI and PET findings were compared, very similar imaging findings were noted among both modalities concerning recurrence and the radiation necrosis group complementing each other.
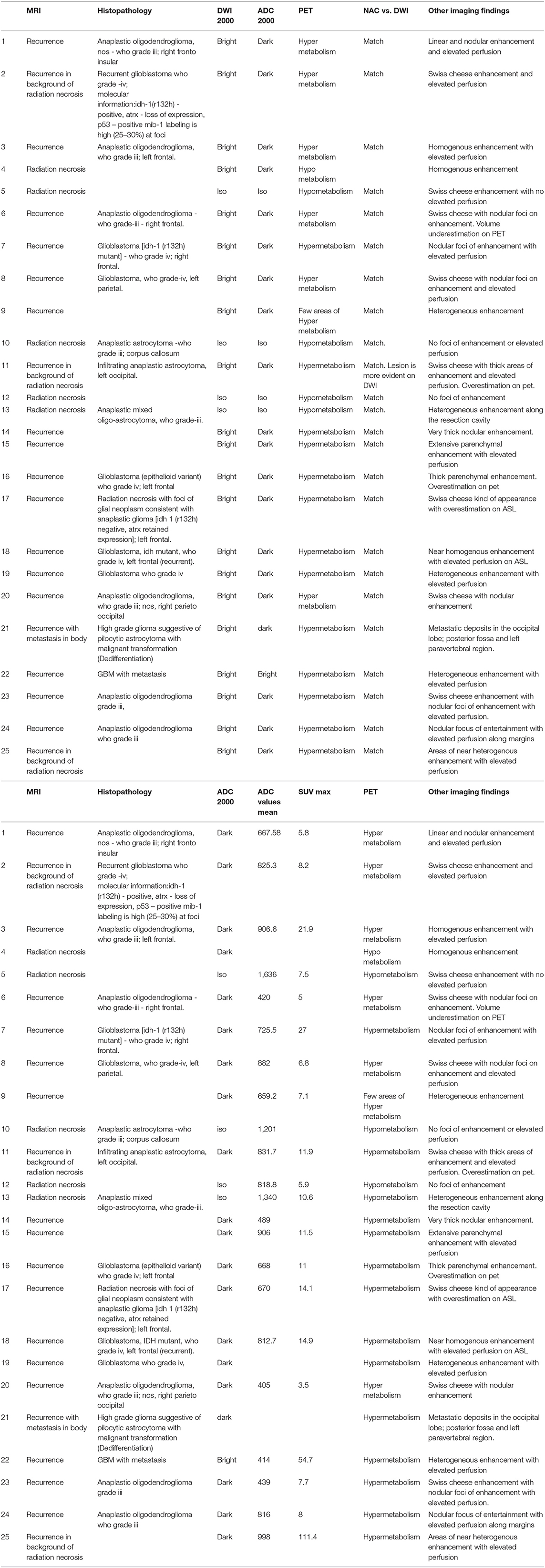
Figure 1. A 43 year old male postoperative case of Glioblastoma IDH mutant in the left frontal lobe, 5 year follow up MRI FDG PET axial images T2-FLAIR (A) post-contrast T1 (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) showed a T2 heterogeneous hypointense lesion with Swiss cheese and nodular enhancement and elevated perfusion. The lesion shows DWI restriction with hypermetabolism on PET. There is a match on the inverted DWI image and the NAC PET image. Suggestive of recurrence.
Figure 2. A 24 year old female postoperative, post-RT case of Glioma left frontal lobe, 3 year follow up MRI FDG PET axial images T2-FLAIR (A) post-contrast T1 (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) show a postoperative cavity in the left frontal lobe with Swiss cheese-like enhancing lesion posterior to postoperative either no elevated perfusion. Neither DWI restriction nor elevated metabolism on PET is seen, which is suggestive of radiation necrosis.
On comparing PET -DWI for tumor volume, overestimation or underestimation of tumor volume was noted as compared to structural imaging. Since the intensity of PET is based on the uptake, tumors with high SUV can have a spillover effect with overestimation of tumor volumes in three cases and tumors with low SUV and below an absolute standard can appear smaller as noted in one case as compared to the DWI image. DWI is based on cellularity and FDG on metabolic uptake and hence this disparity in volume (1). In MRI of CNS tumors with the heterogeneous landscape, noting the volume of the tumor, cellularity on DWI, and ADC and SUV uptake measurements are important for prognosis and treatment response, which again highlights the need for multiparametric imaging.
On CEMRI, recurrence post-radiotherapy was considered when there was a nodular enhancement in the background of the Swiss cheese pattern of enhancement. On the correlation between DWI, PET, and CEMRI, false-positive recurrence was noted in two cases CEMRI but DWI and PET were suggestive of necrosis. Similarly, four cases showed false negative for recurrence on CE MRI when compared with DWI -PET.
PWI data was available in 22 cases. On post-processing ROI was placed on the enhancing portion on CEMRI.14 cases showed elevated perfusion values consistent with recurrence and the remaining eight cases did not show any elevated perfusion that was suggestive of necrosis. When the correlation between DWI, PET, and PWI was done, recurrence was missed in 4 cases i.e., false negative on PWI. This pitfall might be due to wrong ROI placement based on the standard enhancing portion on CEMRI rather than planning on either DWI or PET. Non-enhancing recurrence foci can be missed by using CEMRI if used as criteria to identify tumor foci (2).
There was a case of pseudoprogression correctly identified by DWI and FDG PET helping in differentiating thick nodular enhancement in that case is due to radiation necrosis with no recurrence. Though CEMRI and PWI techniques showed thick nodular enhancement with no abnormal perfusion.
In two cases simultaneous ASL was performed as part of the protocol. ASL, DWI, and PET volumes matched in both cases, and ASL which is a perfusion marker has good potential in tumor imaging. ASL is known to differentiate oligodendroglioma from astrocytoma irrespective of tumor grade and enhancement pattern and may aid in the pathological molecular typing of gliomas (3).
Pilocytic astrocytoma with metastases is very rare. There was a histopathologically proven case and MR/PET corroborated this, proving metastatic spread. Whole-body DWI correlated well with FDG PET (Figure 3) (case 21).
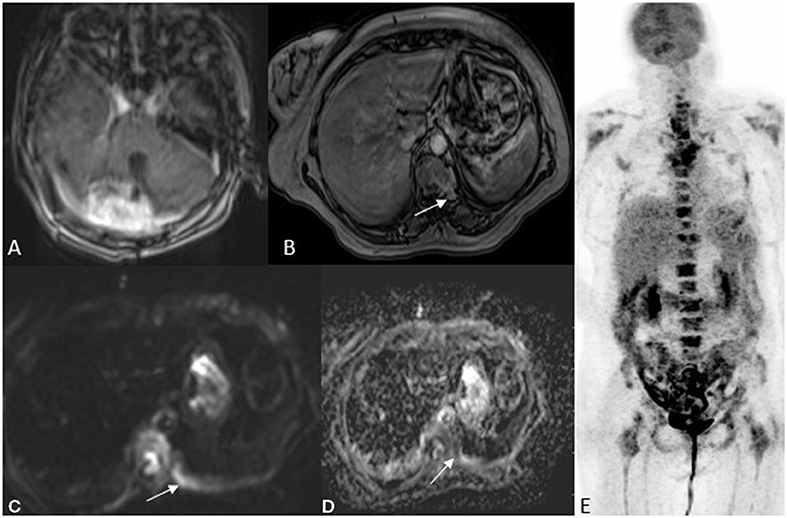
Figure 3. A 52 year old female with imbalance, MRI FDG PET axial brain images T1 post-contrast (A) image showed an enhancing lesion in the right cerebellum. Whole-body MRI with fast sequences at axial hepatic sections (B) VIBE fat sat post-contrast (C) DWI b800 (D) ADC and NAC PET (E) showing an area of metastases to the vertebral body as an area of restricted diffusion and post-contrast enhancement. HPE was suggestive of Pilocytic astrocytoma with metastasis.
There was a case of multiple intracranial lesions referred to as metastases. A whole-body MRI PET revealed it as a primary CNS tumor with metastases and both PET and DWI matched in the diagnosis (case 22).
MRPET in Other CNS Tumor Histotypes
Cases denote serial numbers in Table 2. We had a case of anaplastic Ependymoma (Figure 4), DWI, and FDG PET correlated well to confirm recurrence and rule out radiation necrosis though contrast enhancement patterns that appeared like radiation necrosis (Case 1).
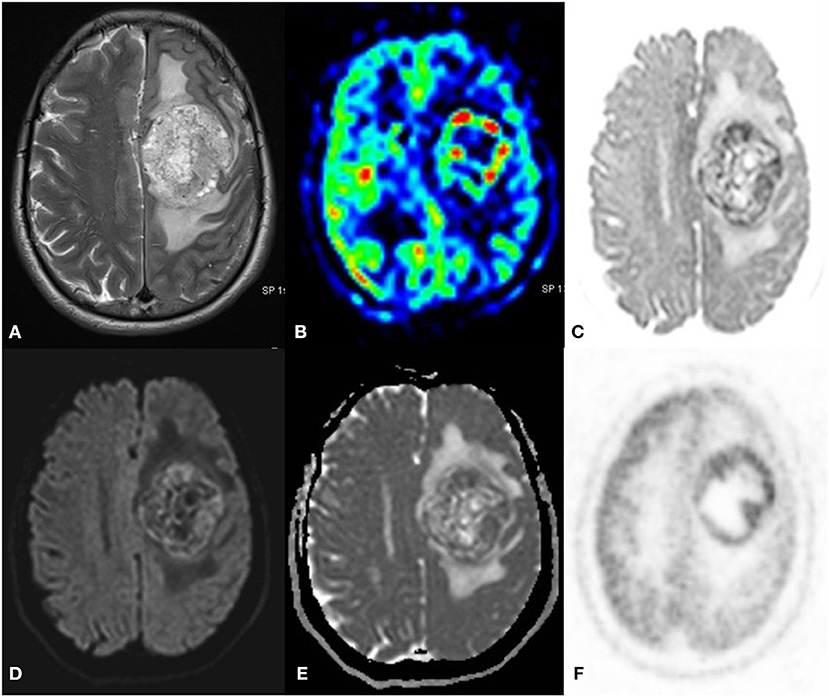
Figure 4. A 55 year old male with a progressive right-side weakness for one and a half month, MRI FDG PET axial images T2 (A) ASL (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) showed a well-defined lobulated T2 heterogeneous iso- hyperintense with increased perfusion on ASL. The lesion shows DWI restriction with hypermetabolism on PET. There is a match on the inverted DWI image and the NAC PET image. HPE is suggestive of Anaplastic Ependymoma.
There were 2 MR/PET cases of brain stem lesions diagnosed as demyelination on MR/PET and lymphoma in another, which on histopathology turned out to be T cell lymphoma and demyelination, respectively (Case 2, 3).
In another case, there was a large area of uptake on FDG mimicking recurrence but DWI, which showed only a small focus of restriction and due to DWI and FDG PET mismatch. PET with other tracers was done and DWI correlated well with F18 choline helped in planning the biopsy site. The patient had a sudden onset of transient weakness before FDG was injected and hence was diagnosed with SMART syndrome (stroke-like migraine attacks after radiation therapy) (case 4) (Figure 5).
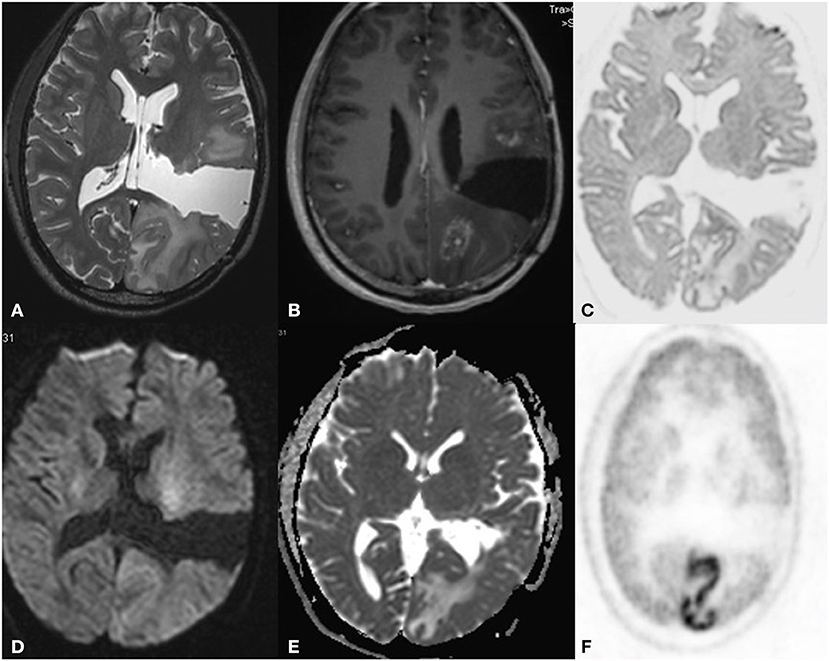
Figure 5. A 32 year old male postoperative, post-radiotherapy case of Anaplastic mixed oligoastrocytoma left thalamus, 3 year follow up MRI FDG PET axial images T2W (A) post-contrast T1 (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) show a postoperative cavity in the left parietal region. Anterior and posterior to this there is a heterogeneously enhancing lesion with elevated perfusion. The lesion shows DWI restriction with hypermetabolism on PET. This is suggestive of recurrence, but there is a large area of gyral enhancement due to SMART Syndrome. There is a false positive uptake on PET.
There was a case of midline glioma H3K27 mutant, with extension into the thalamus and cerebellar peduncles DWI and FDG PET correlated well, post-radiotherapy follow up, DWI showed free diffusion suggesting resolution of the lesion (Case 5).
Another case with MRI T1 hyperintensity and CT hyperattenuating lesion in the right cerebellum and brachium pontis, suggested melanoma and it proved to be melanotic schwannoma. MR PET whole body was asked to rule out metastatic spread or any syndromic association (NF syndrome) before planning excision. There was no diffusion restriction. The enhancement pattern was confounded by the precontrast T1 hyperintensity. The FDG pet showed uptake as expected in schwannomas.
Whole-body MRI PET confirmed the SOL to be primary with no associated syndrome or metastatic spread, as the primary or secondary status could not be commented on histopathology. PET behavior was similar to any schwannoma and showed uptake. The T1 image identified it as a melanotic variant and DWI labeled it as low grade (case 7). This case highlights the importance of multimodality and whole-body imaging.
There was a case of lymphoma in the right external capsule, DWI- FDG correlated well, but there was remote hypermetabolism in bilateral frontal lobes mimicking multifocal lymphoma with no lesion noted on MRI (Case 9).
Our Experience With Other Tracers
Tracers like F18 /C11 choline, C11 methionine, and Ammonia (NH3) PET were assessed.
DWI correlated well with FDG tracers like C11 methionine in cases of glioblastoma (Case 10–13) and F18 choline (Case 18–19) (Figure 6).
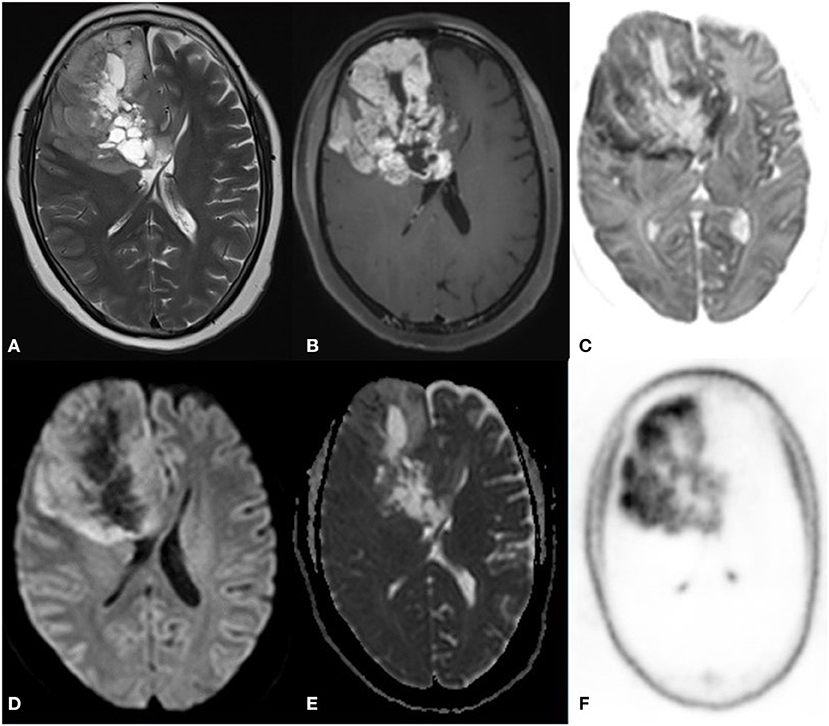
Figure 6. A 52 year old female with a headache for 1 year with recent onset of a seizure. MRI PET (F18 CHOLINE) axial images T2W (A) post-contrast TW (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) shows Ill-defined infiltrative heterogeneous lesion in the right frontal lobe with invasion to corpus callosum showing intense heterogeneous enhancement. The lesion shows DWI restriction with hypermetabolism on PET. There is a match on the inverted DWI image and the NAC PET image, which is suggestive of Glioblastoma.
Methionine had better specific uptake in small foci of recurrence as compared to DWI, especially in low-grade glioma (Case 10).
There was a case of recurrence of glioma with an incidental meningioma (Figure 7), both recurrence and meningioma were picked by C11 methionine PET but DWI helped to differentiate the two. This case of meningioma showed no DWI restriction and had post-contrast brilliant enhancement. PET showed false-positive uptake irrespective of the grade of the tumor (Case 14).
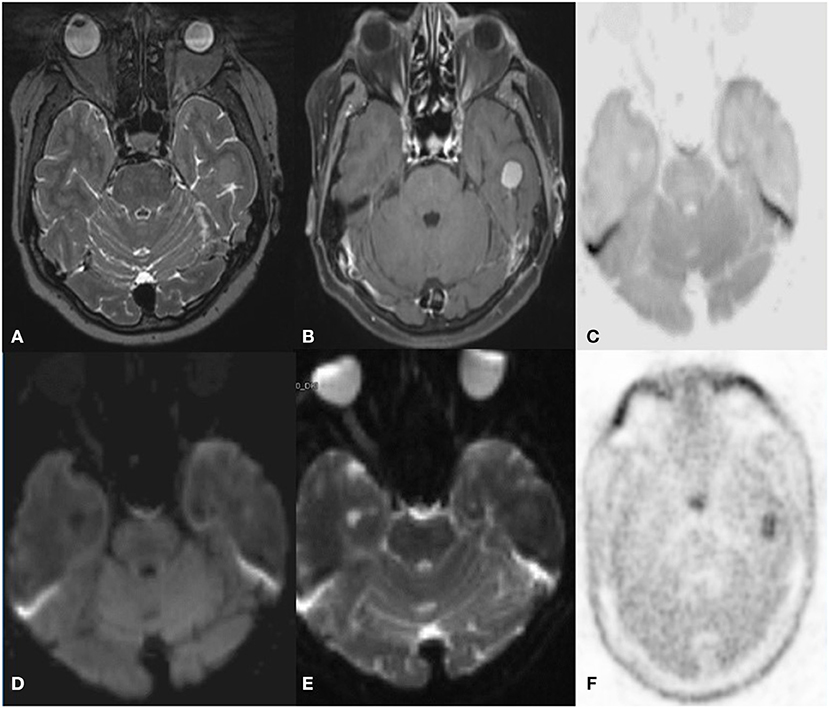
Figure 7. A 59 year old female with Glioma, incidentally detected a lesion in follow up MRI C-11 Methionine PET axial images T2W (A) post-contrast T1 (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) shows a broad-based T2 hypointense intensely enhancing in the left inferior temporal convexity. The lesion shows no DWI restriction with hypermetabolism on PET. S/O meningioma. False-positive uptake of meningioma by methionine.
There were cases of midline glioma in which both C11methionine PET and DWI correlated well. However, the intensity of uptake was more in methionine than DWI (Case 15).
Similar findings were noted in high-grade glioma recurrence in which DWI and methionine uptake was restricted to the tumor area, unlike FDG which has another non-specific uptake in the cortex, like in the case of SMART syndrome.
In the case of the recurrent atypical teratoid rhabdoid tumor (Figure 8), DWI and C11methionine PET correlated well to confirm recurrence (Case 16). In another case of recurrent craniopharyngioma, DWI showed no restriction but uptake is seen in C11 methionine PET (Case 17).
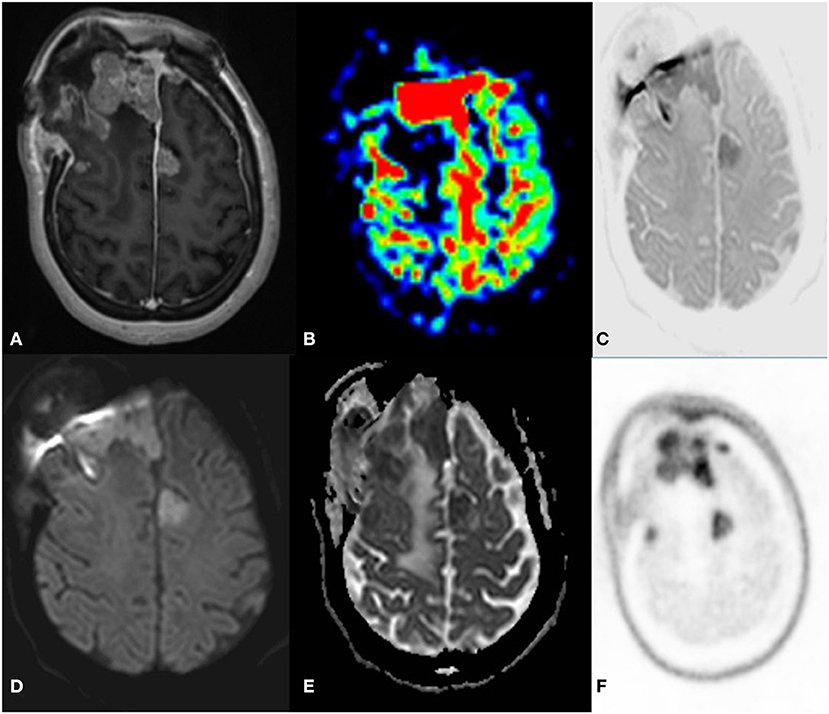
Figure 8. A 33 year old female postoperative, post-radiotherapy case of Embryonal tumor compatible (atypical teratoid rhabdoid tumor) 1 year follow up MRI C-11 Methionine PET axial images post-contrast T1 (A) ASL (B) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) showed Multiple enhancing dural based lesions are seen in bilateral frontal convexity and anterior falx with elevated perfusion. The lesion shows DWI restriction with hypermetabolism on PET. There is a match on the inverted DWI image and the NAC PET image, which is suggestive of recurrence.
We had the case of a clear cell variant of Ependymoma (Figure 9), evaluated with MR F18 choline PET. MRI DWI showed small foci of recurrence in the background of radiation necrosis. PET showed faint to no uptake as it was a clear cell variant and F18 choline has variable behavior in this subtype (4) (Case 20).
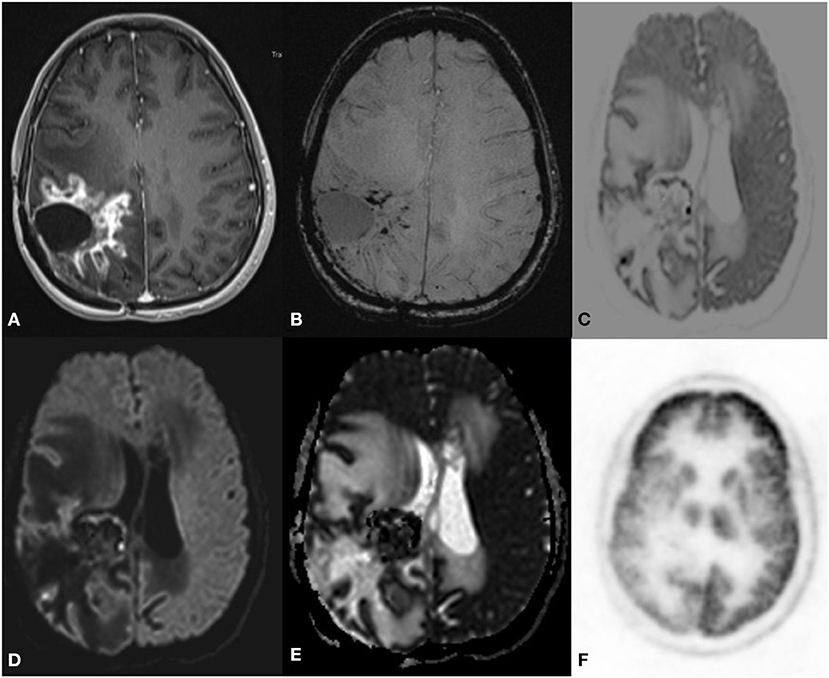
Figure 9. A 34 year old female postoperative case of anaplastic clear cell Ependymoma, 7 year follow up MRI F-18 choline PET axial images SWI (B) postcontrast T1 (A) Inverted DWI (C) DWI b2000 (D) ADC (E) NAC PET (F) showed a Swiss cheese and nodular enhancement lesion in the right parietal region. The lesion shows DWI restriction with hypermetabolism on PET. There is a match on the inverted DWI image and the NAC PET image, which is suggestive of recurrence.
In all cases where there was DWI and PET, the correlation grayscale inverted b2000 image corresponded with the NAC image of PET, which has the potential to be an excellent biomarker in neuro-oncology workup.
Discussion
This study evaluated the simultaneous acquisition of MR-DWI and PET data in the patients with suspected ICSOL, mainly focusing on gliomas and also other histotypes.
In lesion based sensitivity, the specificity of DWI was comparable with those of PET and in agreement with earlier published studies (5, 6).
In our study, we used the trace image as it nullifies T2 shine-through the effects of gliosis, and vasogenic edema, which is commonly associated with the tumor and postoperative and post-radiotherapy cases, and only tissue with increased cellularity is seen as bright on the trace image. A high b value (b2000) was generated to further decrease the background of the T2 effects and increase the contrast noise ratio. Synthetic high b value generation helps save time on the scanner and gives similar images, allowing retrospective analysis. We inverted this b2000 trace image to make it look like a PET AC corrected image. The ADC image does not have a good PET-like appearance and background parenchyma suppression is suboptimal to pick small foci.
This DWI-PET study suggests that DWI can be planned as a surrogate marker and saves time, which helps in surveillance imaging. Both DWI and PET have a resolution of 3–4 mm thick sections. In the case of tumor, DWI highlights cellularity and is immune from blood-brain barrier dysfunction and helpful in differentiating recurrence from enhancing radiation necrosis and in diagnosing non-enhancing recurrence (7). PWI and MRS have a good correlation but, since it is ROI-based, if the location of placement is wrong it can be misleading.
DWI and PET have a good one-on-one correlation (8). A combined DWI -PET increases true positive by nullifying the false positive and negative of both MRI and PET such as bleed, air/bone, the parenchyma interface artifacts of DWI, and the non-specific uptake of FDG (9).
Glioma is graded as 1–4 based on the cellularity/mitotic index. FDG PET has low sensitivity for grades 1 and 2, which is very similar to DWI (11). Excellent correlation was noted with FDG and DWI for tumor margin delineation in the background of radiation necrosis as compared to other MRI parameters (11). Tracers such as methionine and choline are better for low-grade tumors and recurrence of the low-grade tumor as compared to DWI–FDG PET in combination with conventional MRI for characterizing the lesion further into a meningioma or a glioma etc.
The intensity of uptake and DWI grayscale matches with FDG but not with other tracers. Tumor volume was better delineated with DWI and methionine as compared to FDG and GHA wherein lesions with increased SUV often had exaggerated volumes and a spill-over effect. The FDG intensity reflects the metabolic behavior and can help plan the intensity of radiotherapy and prognosticate whereas MRI can help define borders (12).
This paper highlights the role of a high b value DWI weighted image trace image with grayscale inversion, which is excellent for achieving a PET-like image. Since a close correlation between DWI and FDG has been established, developing high b value DWI as a biomarker can help in treatment response assessment in close intervals without risk of contrast, radiation, and cost.
Our experience of DWI–PET with other histotypes of brain tumor and tracers highlighted the need to know false positive and negative rates associated with FDG and other tracers and interpret PET along with MRI parameters (13) such as DWI, ASL, PWI, MRS, SWI and CEMRI for further characterization and diagnosis. False-positive uptake in meningioma (9) was noted with free diffusion and brilliant enhancement. This type of MR correlation is very important in syndromes such as Neurofibromatosis wherein both glioma and meningioma can coexist and identify malignant transformation. Similarly, brain tuberculoma can show raised FDG uptake and can mimic tumors with high SUV (14) but MRI DWI can show free diffusion and aids in differentiating them (15).
Simultaneous MR/PET of the brain and whole body helps confirm whether a lesion is a primary CNS lesion rather than metastases/systemic lymphoma. Overall hybrid imaging has many advantages such as increasing the diagnostic accuracy to demarcate viable tumor margin, planning therapy, and patient management and treatment response (16). Knowledge of the advantages and pitfalls of each modality is required. CT MRI provides excellent cross-sectional imaging with MRI having a resolution of up to 1 mm as compared to PET, which has a resolution of 4 mm. Structural MRI has excellent spatial resolution and is well-suited to differentiate tumors from edema, hemorrhage other features such as raised ICP. Advanced MRI such as ASL, DWI, PWI, SWI, and MRS also provide blood flow cellularity and metabolic information (10).
PET tracers give information on subcellular processes based on the radiopharmaceutical used such as glucose/amino acid metabolism and so on. Though tumor biology is associated with tracers to map increased glucose consumption, increased expression of amino acid transporters, increased proliferation rate, increased membrane biosynthesis, increased perfusion, and hypoxia are available and a commonly used tracer is glucose consumption imaging. FDG reflects increased tumor glucose metabolism, glycolysis via the GLUT receptors, and apoptosis rate /mitotic index in tumors. Some low-grade tumors with increased expression of GLUT receptors such as pilocytic astrocytoma and neuroma etc. can show high uptake of FDG. In these cases, MRI DWI can help differentiate benign tumors from high-grade lesions (17).
Amino acid PET imaging such as ammonia, methionine, FET, FLT, FDOPA deals with part of tumor biology related to histopathology with the Ki-67 index, proliferating cell nuclear antigen, and microvessel density (18).
A knowledge of biological distribution of different tracers in brain is required before interpretation for example ammonia is transported by process of diffusion and others are carrier mediated and dependent on BBB damage. Areas which lack BBB such as choroid plexus and pituitary gland can show uptake (F-thymidine and F choline) (19). Choice of tracers for brain tumor in these location should be planned accordingly. FLT may have lower specificity for low grade tumors as compared to methionine PET.
Methionine has a better background suppression as compared to choline and thymidine and is preferred in tumor imaging. Normal distribution of this tracer should be known and non tumor conditions such as demyelination or abscess can also show methionine uptake (20).
F DOPA is another tracer but can be taken up by other sol such as meningioma etc. Overall since FDG, methionine and FDOPA uptake is affected by body metabolism and knowledge of patient preparation is required such as controlled sugars, seizure free, low protein diet, off dopaminergic drugs for the above tracers (21).
In our series we don't have experience with FET, FLT or FDOPA for tumour and its correlation with DWI or MRPWI. Hypoxia imaging with FMISO helps demarcate tumor margins and angiogenesis. Perfusion imaging with radio water reflects angiogenesis that correlates with VEGF and antigen ki6 on histopathology which make tumor more aggressive and resistant to radiotherapy (22). Molecular imaging with hybrid technology expands the scope of tracers and imaging in vivo. Newer chemistry derivatives can be used either with Gadolinium particles, Nano particles for MRI or radiotracers for PET or fluorescein for optical imaging. It is an exciting field for in vivo imaging (16).
This paper has some limitations. First, the limited sample size and study design involved visual interpretation rather than voxel-wise correlation by fusing DWI and PET images because of quick analysis, reproducibility, and non-time consumption. Rather than highlighting the differences between DWI and PET, this paper says that both are complementary. The difference between the image and the representative histopathological specimen limited the proof of concept.
The preliminary results of this study need to be confirmed in a larger patient population. Further studies are planned to extend parameter inclusion and quantification. The main strength of this paper is that this study involves a broader spectrum of CNS ICSOL imaged with multiple types of PET tracers and all cases have been followed up to establish the diagnosis.
Conclusion
The role of imaging is not about characterizing the lesion but trying to extract virtual histopathology-like features in vivo and, additionally, keep pace with newer modes of tumor treatment regimens that target different pathways of a tumor. This approach has led to the development of multimodality advanced hybrid imaging. Although DWI and FDG-PET reflect different tissue properties, there may very well be an association between the measures of both methods, most probably because of increased cellularity and the glucose metabolism of FDG-avid CNS lesions. Presently PET is an adjunct to MRI in neurooncology. DWI helps pick the lesion, calculate tumor volume, and predict appropriate early post-treatment tumor response. MRI is a good tool when multi-time point imaging is required for the diagnosis of a tumor, for planning therapy, post-treatment response, and further surveillance. DWI acts as a surrogate to FDG PET and has promising potentials for clinical translation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee Board, NIMHANS. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SM contributed to the original concept, image analysis, the manuscript draft, and clinical MRI reporting. SV and SJ undertook data collection and manuscript drafting. AK contributed to manuscript overview and inputs. PK contributed for production of PET tracers at the institute cyclotron. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We extend thanks to the team working at MR/PET center NIMHANS.
References
1. Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current methods to define metabolic tumour volume in positron emission tomography: which one is better? Nucl Med Mol Imaging. (2018) 52:5–15. doi: 10.1007/s13139-017-0493-6
2. Eichberg DG, Di L, Morell AA, Shah AH, Semonche AM, Chin CN, et al. Incidence of high grade gliomas presenting as radiographically non-enhancing lesions: experience in 111 surgically treated non-enhancing gliomas with tissue diagnosis. J Neurooncol. (2020) 147:671–9. doi: 10.1007/s11060-020-03474-z
3. Wang N, Xie S, Liu H, Chen G, Zhang W. Arterial spin labeling for glioma grade discrimination: correlations with IDH1 genotype and 1p/19q status. Transl Oncol. (2019) 12:749. doi: 10.1016/j.tranon.2019.02.013
4. Chang JM, Lee HJ, Goo JM, Lee H-Y, Lee JJ, Chung J-K, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. (2006) 7:57. doi: 10.3348/kjr.2006.7.1.57
5. Stopa BM, Juhász C, Mittal S. Comparison of amino acid PET to advanced and emerging MRI techniques for neurooncology imaging: a systematic review of the recent studies. Mol Imaging. (2021) 2021:e8874078. doi: 10.1155/2021/8874078
6. Suzawa N, Maeda M, Matsushima N, Murashima S, Kobayashi S, Takeda K. Relationship between FDG uptake and apparent diffusion coefficients in solitary brain tumours: preliminary experience. J Nucl Med. (2009) 50:1234. doi: 10.1016/s1052-5149(02)00033-3
7. Valentini MC, Mellai M, Annovazzi L, Melcarne A, Denysenko T, Cassoni P, et al. Comparison among conventional and advanced MRI, 18F-FDG PET/CT, phenotype and genotype in glioblastoma. Oncotarget. (2017) 8:91636. doi: 10.18632/oncotarget.21482
8. Ishii S, Miyajima M, Nambu T, Watanabe H, Hakozaki M, Suenaga H, et al. Comparison of diagnostic performance between whole-body MRI with DWI and PET/MRI. J Nucl Med. (2018) 59:1372.
9. Valotassiou V, Leondi A, Angelidis G, Psimadas D, Georgoulias P. SPECT and PET imaging of meningiomas. Sci World J. (2012) 2012:412580. doi: 10.1100/2012/412580
10. Kao H-W, Chiang S-W, Chung H-W, Tsai FY, Chen C-Y. Advanced MR imaging of gliomas: an update. BioMed Res Int. (2013) 213:970586. doi: 10.1155/2013/970586
11. Marner L, Henriksen OM, Lundemann M, Larsen VA, Law I. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging. (2017) 5:135. doi: 10.1007/s40336-016-0213-8
12. Moreau A, Febvey O, Mognetti T, Frappaz D, Kryza D. Contribution of different positron emission tomography tracers in glioma management: focus on glioblastoma. Front Oncol. (2019) 9:1134. doi: 10.3389/fonc.2019.01134
13. Fink JR, Muzi M, Peck M, Krohn KA. Multimodality brain tumour imaging: MR imaging, PET, and PET/MR imaging. J Nucl Med. (2015) 56:1554–61. doi: 10.2967/jnumed.113.131516
14. Hutomo F, Yudistiro R, Mulyanto ID, Budiawan H. False positive finding from malignancy-like lesions on FDG PET/CT: case report of tuberculosis patients. BMC Med Imaging. (2020) 20:26. doi: 10.1186/s12880-020-00427-w
15. Peng J, Ouyang Y, Fang WD, Luo TY, Li YM, Lv FJ, et al. Differentiation of intracranial tuberculomas and high grade gliomas using proton MR spectroscopy and diffusion MR imaging. Eur J Radiol. (2012) 81:4057–63. doi: 10.1016/j.ejrad.2012.06.005
16. Cal-Gonzalez J, Rausch I, Shiyam Sundar LK, Lassen ML, Muzik O, Moser E, et al. Hybrid imaging: instrumentation and data processing. Front Phys. (2018) 6:47. doi: 10.3389/fphy.2018.00047
17. Rosenkrantz AB, Friedman K, Chandarana H, Melsaether A, Moy L, Ding Y-S, et al. Current status of hybrid PET/MRI in oncologic imaging. Am J Roentgenol. (2015) 206:162–72. doi: 10.2214/AJR.15.14968
18. Surov A, Meyer HJ, Wienke A. Associations between PET parameters and expression of Ki-67 in breast cancer. Transl Oncol. (2019) 12:375. doi: 10.1016/j.tranon.2018.11.005
19. Miyake K, Shinomiya A, Okada M, Hatakeyama T, Kawai N, Tamiya T. Usefulness of FDG, MET and FLT-PET studies for the management of human gliomas. J Biomed Biotechnol. (2012) 2012:205818. doi: 10.1155/2012/205818
20. Qi Y, Liu X, Li J, Yao H, Yuan S. Fluorine-18 labeled amino acids for tumour PET/CT imaging. Oncotarget. (2017) 8:60581. doi: 10.18632/oncotarget.19943
21. Jacob MJ, Pandit AG, Jora C, Mudalsha R, Sharma A, Pathak HC. Comparative study of 18 F-DOPA, 13 N-Ammonia and F18-FDG PET/CT in primary brain tumours. Indian J Nuclear Med. (2011) 26:139. doi: 10.4103/0972-3919.103996
Keywords: PET-MR, CNS tumors, FDG-PET, high value diffusion weighted imaging, glioma
Citation: Mangalore S, Vankayalapati S, Jabeen S, Kumar Gupta A and Kumar P (2021) Can High b Value Diffusion Be a Surrogate Marker for PET—A MR/PET Study in Neurooncology Set Up. Front. Neurol. 12:627247. doi: 10.3389/fneur.2021.627247
Received: 08 November 2020; Accepted: 24 May 2021;
Published: 24 September 2021.
Edited by:
Sanjeev Chawla, University of Pennsylvania, United StatesReviewed by:
Kavindra Nath, University of Pennsylvania, United StatesPradeep Kumar Gupta, University of Pennsylvania, United States
Copyright © 2021 Mangalore, Vankayalapati, Jabeen, Kumar Gupta and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandhya Mangalore, ZHJzYW5keW1AZ21haWwuY29t
 Sandhya Mangalore
Sandhya Mangalore Sriharish Vankayalapati
Sriharish Vankayalapati Shumyla Jabeen
Shumyla Jabeen Pardeep Kumar
Pardeep Kumar