- 1Department of Neurology, Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA, United States
- 2Department of Occupational Therapy and Graduate Institute of Behavioral Sciences, Chang Gung University, Taoyuan, Taiwan
- 3Healthy Aging Research Center, Chang Gung University, Taoyuan, Taiwan
- 4Laboratory of Brain Imaging and Neural Dynamics (BIND Lab), Chang Gung University, Taoyuan, Taiwan
- 5Department of Psychiatry, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 6Neuroscape, University of California, San Francisco, San Francisco, CA, United States
- 7Department of Psychiatry, University of California, San Francisco, San Francisco, CA, United States
- 8Department of Physiology, University of California, San Francisco, San Francisco, CA, United States
Background: The study aimed to evaluate the effects of transcranial direct current stimulation (tDCS) on cognition, mood disturbance, pain, and fatigue in people with multiple sclerosis (PwMS).
Methods: A literature search was performed on articles published between January 1990 and May 2020 in Pubmed, Medline, and Web of Science using the following keywords and their abbreviation in combinations: multiple sclerosis and transcranial direct current stimulation. Mean effect size (ES) and 95% confidence interval were calculated for each domain of interest.
Results: Seventeen articles with a total of 383 PwMS were included in this analysis. For cognition, a strong effect size was found for the trial administering the Symbol Digit Modalities Test (ES: 1.15), whereas trials applying the Attention Network Test showed a negative effect size of −0.49. Moderate to strong effect sizes were observed for mood disturbance (mean ES: 0.92), pain (mean ES: 0.59), and fatigue (mean ES: 0.60). Further subgroup analyses for MS-related fatigue showed that both high and low intensities of stimulation lead to nearly the same degree of favorable effects. More pronounced effects were observed in studies administering the Fatigue Severity Scale compared with studies using other fatigue measures such as the Modified Fatigue Impact Scale.
Conclusion: These results provide preliminary evidence that tDCS has a favorable effect on cognitive processing speed, mood disturbance, pain, and fatigue in MS. However, the effects on cognition and fatigue vary based on the specific assessment used.
Introduction
Multiple sclerosis (MS) is the most common non-traumatic cause of neurological disability in young adults, affecting ~1,000,000 people in the United States (1) and 2.5 million people worldwide (2). Over the disease course, a wide variety of disabling symptoms may develop, including motor and sensory disturbance, vision symptoms, cognitive impairment, mood disturbance, pain, and fatigue. These functional deficits and symptoms have a drastic impact on a patient's personal functioning, social interactions, employment, and overall quality of life. Although disease modifying therapies (DMTs) that target primarily the inflammatory immunopathology of MS can slow the development of functional disabilities (3, 4), these do not specifically alleviate symptoms such as cognitive impairment, mood disturbance, pain, and fatigue. Therefore, it is of utmost importance to develop effective and alternative approaches to symptom management.
Recently, transcranial direct current stimulation (tDCS), a form of non-invasive transcranial electrical stimulation, has been probed as a possible form of non-pharmacological intervention in several neurological and psychiatric disorders (5–7), due to its safety, portability, and potential for at-home application. tDCS modulates neuronal transmembrane potential toward hyperpolarization or depolarization by delivering weak electrical currents to the scalp, thereby altering plasticity in the stimulated brain regions (8, 9). These effects have been associated with changes in resting membrane potential, alteration of transmembrane proteins, and N-methyl-d-aspartate receptor efficiency (10, 11). Depending on whether anodal or cathodal stimulation is applied, tDCS either increases or decreases cortical excitability, respectively (12, 13), in turn affecting a wide range of behavioral measures (14, 15). Studies have reported beneficial effects of tDCS on language performance (16), learning processes (17), working memory function (18), and multitasking performance (19) in healthy adults.
Specifically in patients with MS, studies suggest that tDCS could serve as a promising tool to improve cognition (20, 21), neuropathic pain (22, 23), mood (24), and fatigue (25, 26). It has been reported that by applying daily sessions of anodal tDCS for 10 days over the dorsolateral prefrontal cortex (DLPFC) during cognitive training improved attention, information processing and executive function. Further, the improvement was sustained 6 months after last treatment (21). While studies provide intriguing evidence supporting tDCS as a therapeutic strategy for MS patients [reviewed in (27–29)], beneficial effects are not always observed. For example, in a randomized, controlled trial, 1-week tDCS application showed no measurable differences in fatigue score between stimulation and placebo interventions post stimulation (30). A study with three daily tDCS over DLPFC found no effects on mood, fatigue, or attention (22). Another study administering 10 sessions of tDCS also reported that the stimulation and control groups did not differ in standard cognitive measures after the intervention (20).
The methodological discrepancies across these trials have yielded conflicting results and therefore a lack of consensus regarding the effect of tDCS on cognitive impairment, mood disturbance, pain, and fatigue in MS. To enable more definitive conclusions regarding the potential of tDCS as a therapeutic strategy for the described MS-related domains, we performed a systematic review and meta-analysis of the available data.
Materials and Methods
Study Identification
Computerized searches were performed in PubMed, Medline, and Web of Science to identify pertinent studies. The search terms were “multiple sclerosis” / “MS” and “transcranial direct current stimulation” / “tDCS.” Manual searches of bibliographies of relevant reviews, book chapters, and original articles were also conducted. The searches were limited to human studies published from January 1990 to May 2020 and written in English. Articles were included when the following criteria were met: (1) original research article with a main goal to examine tDCS effects on at least one of the four domains of interest (i.e., cognition, mood, pain, fatigue); (2) the patients were adults with a diagnosis of MS; (3) reports of ≥5 participants receiving tDCS; (4) outcome measures were quantitatively reported; (5) the study included experimental and control conditions. We reviewed the full text of articles that appeared to be relevant.
Quality Assessments
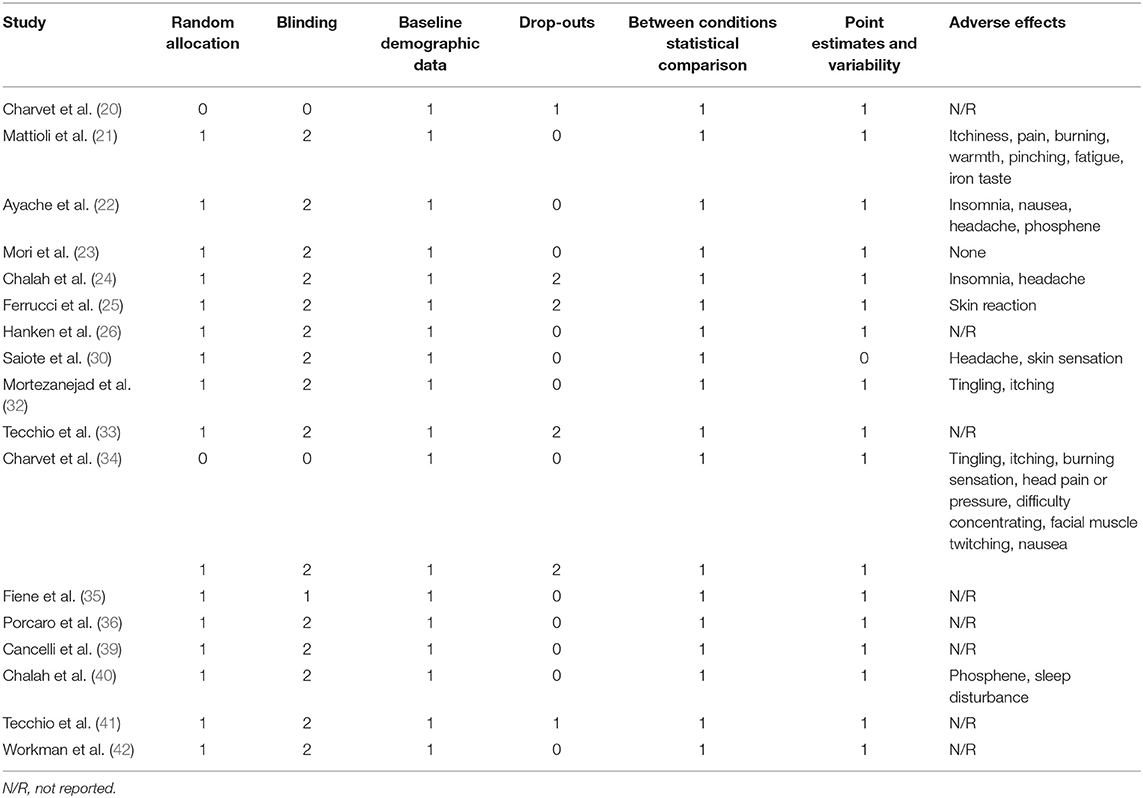
To evaluate the methodological quality of the included studies, we used a modified checklist derived from a quality screening form revised by Moher et al. (31). The quality of each study was evaluated according to the following criteria: (1) random allocation: recorded as 1 if the study pointed out that participants were randomly allocated into different groups; (2) blinding procedure: ranged from 0 to 2, where 0 represented a non-described or non-blinded procedure, and 1 and 2 indicated single-blind and double-blind procedures, respectively; (3) drop-out number: recorded as the number of participants who withdrew from the study; (4) description of baseline demographic data: recorded as 1 when provided; (5) statistical comparison between interventions: denoted as 1 if performed; (6) point estimates and measures of variability: recorded as 1 if provided; (7) adverse effects: recorded as type of the events.
Quantitative Analyses
The relevant information from each study was extracted by one author (W.-Y. H.) using a standard data recording form that included number of participants, MS subtype, mean age, mean/median Expanded Disability Status Scale (EDSS) disease severity score, mean disease duration, stimulation protocol [i.e., duration and intensity of tDCS, targeted brain region(s), method of sham stimulation], domain(s) of measures relevant to current analysis, number of dropouts, study quality (see above), outcome measures, and post-intervention mean (M) as well as standard deviation (SD) for each outcome measure in the experimental and control groups. For studies with multiple measuring points after the intervention, the post-intervention data was based on the first measurement taken after the intervention period. A wide variety of outcome measures was found across the studies, and some evaluated multiple measures. For the purposes of this meta-analysis, the measure used to assess each study was the explicitly declared primary outcome. If the primary outcome was not clearly defined, the first outcome that was reported in the results section was chosen.
For cognition and mood, one of the studies contributed more than one trial, due to different stimulation sites (24). For fatigue, four articles contributed more than one trial because they applied the stimulation over different brain regions (24, 32, 33) or employed two studies with different design (34). For pain, SD was calculated from standard error of mean (SEM) in one study (23). For fatigue outcome measures, pooled M and SD data were calculated based on subgroup M and SEM in one study (25) and estimated from a subgroup plot in another study (26). One of the studies did not report the M and SD of their outcome measures and the data were extracted from the figures (30). The SD was calculated from SEM (32, 35) and data range (36) based on the range rule of thumb (37, 38) in three of the studies. All the extracted data were carefully checked by another author (C.-H. C.) and disagreements were resolved by discussion.
The analyses were performed with Comprehensive Meta-Analysis 3.0 software (Biostat Inc, Englewood). The standardized effect sizes and 95% confidence interval (CI) were calculated to test the results of different trials. The effect sizes were calculated based on differences between the post-treatment evaluations (22, 24, 25, 32, 33, 36, 39–42), changes relative to the baseline (23), or the mean changes between pre- and post-treatments (20, 21, 26, 30, 34, 35) in the experimental and control groups, divided by the pooled SD. Because the effect sizes from each study may be influenced by the sample sizes, a weighting factor was applied to give more weight to the studies with larger samples. Finally, the mean effect sizes were obtained after combining the weighted effect size of each study. Absolute effect sizes that ranged from 0.2 to 0.49 were considered to be small (43) and a value of 0.5 is likely to be clinically meaningful (44).
The heterogeneity across effect sizes was assessed with Q-statistics (45) and the I2 index (46), which is useful for assessing consistency between trials (47). When significant heterogeneity was found by Q-statistics or when I2 > 50%, a random effects model was applied. Otherwise, a fixed effects model was used. Begg and Mazumdar rank correlation (48) was also applied to assess the publication bias. In addition, a funnel plot (49) was used to further address publication bias. In a funnel plot, the effect size is plotted against the standard error. Studies with larger sample sizes appear toward the top of the plot, and near the mean effect size, whereas studies with smaller sample sizes appeared toward the bottom of the plot, indicating more variation in these smaller studies. In the absence of publication bias, the plot may show a symmetrical distribution. Conversely, in the presence of publication bias, the funnel plot would be asymmetrical. The Trim and Fill procedure (50), a funnel plot-derived approach aimed at identifying publication bias and adjusting the results, was applied to correct for publication bias. The significance level was set at p ≤ 0.05.
Results
Evidence Base
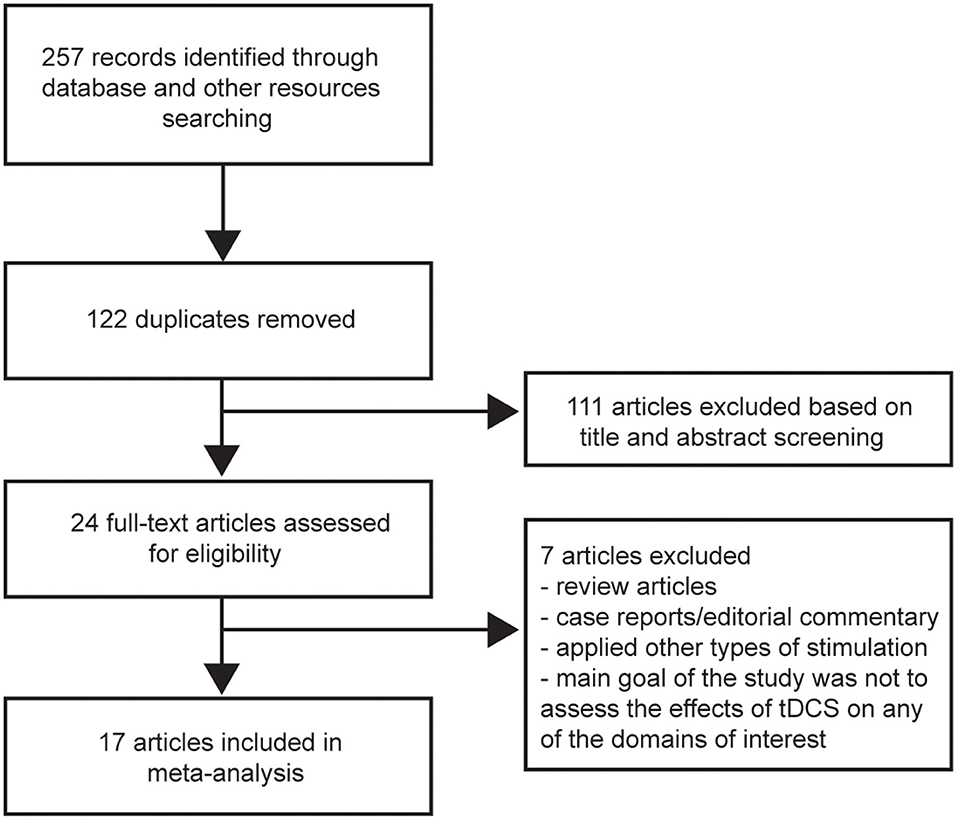
The search yielded 257 records. After duplicates were removed, 135 articles were screened based on title and abstract. Twenty-four potentially relevant articles were obtained for full-text review; 17 articles that met our inclusion criteria were then selected (20–26, 30, 32–36, 39–42). The other seven articles were excluded for the following reasons: review articles or case reports/editorial commentary, applied other types of stimulation, or the main goal of the study was not to assess the effects of tDCS on any of the domains of interest (i.e., cognition, mood, pain, fatigue) (Figure 1). Table 1 summarizes the characteristics of the studies included in our meta-analysis. A total of 383 MS patients were involved, 251 of whom had relapsing-remitting MS. Of the 17 articles, four focused on more than one domain (22, 24, 40, 42). Four studies assessed cognition (20–22, 24). Mood and pain were measured in four (22, 24, 40, 42) and three (22, 23, 42) studies, respectively. Two studies evaluated mood status before and after the intervention, with a purpose to control for mood as a potential confounding factor (23, 30). Fourteen articles evaluated fatigue (22, 24–26, 30, 32–36, 39–42).
Intervention
These studies employed different study designs. Two studies were designed as single session trials (26, 35). Ten studies applied the stimulation at an intensity lower than 2 mA (20, 25, 26, 30, 32, 33, 35, 36, 39, 41). Target stimulation regions included motor cortex (23, 25, 32, 42), dorsolateral prefrontal cortex (20–22, 24, 30, 32, 34, 35, 40), primary somatosensory cortex (33, 36, 39, 41), sensorimotor cortex (33) and parietal cortex (24, 26).
Outcome Measures
A variety of outcome measures was used in the selected articles. For cognition, Attention Network Test (22, 24), Symbol Digit Modalities Test (21) and Brief International Cognitive Assessment for MS (20) were performed. For mood, Hospital Anxiety and Depression Scale (22, 24, 40) and Beck Depression Inventory (42) were included. Pain was assessed with Visual Analog Scale (22, 23, 42). Fatigue was assessed using the Modified Fatigue Impact Scale in eight trials (30, 33, 36, 39–41, 51); other outcome measures for fatigue included Fatigue Impact Scale (25), vigilance task (26), Fatigue Severity Scale (24, 32), Patient-Reported Outcomes Measurement Information System-fatigue short form (34), simple reaction time task (35), and fatigue index (42).
Methodological Quality
Table 2 shows the quality assessment results of the included studies. Random allocation was achieved in all the studies except two trials (20, 34). Most of the studies were of double-blind (21–26, 30, 32–34, 36, 39–42) or single-blind (35) design. Baseline demographic data were described in all the studies. Six studies had drop-outs (20, 24, 25, 33, 34, 41). Statistical comparisons were completed in all the articles; however, one study did not provide point estimates and measures of variability (30). Eight studies reported adverse events. These included skin reaction, insomnia, tingling, itching, phosphene, burning sensation, head pain or pressure, difficulty concentrating, facial muscle twitching, nausea, fatigue, and iron taste (21, 22, 24, 25, 30, 32, 34, 40). One study (23) reported no adverse events.
Meta-Analysis
Table 3 summarizes the domains of measures, outcome measures, the number of participants in the post-treatment evaluations, mean and SD, and effect size of each study.
Cognition
A total of five effect sizes was obtained from four articles with 90 patients (Table 3). Since it has been demonstrated that tDCS effects on cognition are task- and cognitive domain-specific (52, 53), we divided the studies into two separate analyses based on the cognitive tasks evaluated: [Symbol Digit Modalities Test (SDMT) vs. Attention Network Test (ANT)], given that SDMT is the most widely used measure of information processing speed in MS (54, 55) and ANT is the most commonly administered task in the five trials. One study that administered the SDMT as part of the Brief International Cognitive Assessment for MS but only reported composite scores (20) was excluded from the subsequent analyses. Therefore, only four trials with a total of 46 patients were included in task-specific analyses. The analyses revealed an effect size of 1.15 (95% CI, 0.20–2.10, p = 0.01) for the trial administering the SDMT (21). Mean effect size for trials that applied ANT was −0.49 (95% CI, −0.97 to −0.02, p = 0.04) (Figure 2A). We did not find heterogeneity among the studies that applied ANT (Q = 3.42, I2 = 41.55, p = 0.18). Heterogeneity analysis was not applicable for SDMT since only one trial was included. Publication bias was not found based on rank correlation (tau = −0.30, p = 0.46) when considering all five trials investigating tDCS effects on cognition. The funnel plot resembles an inverted symmetrical funnel, which confirmed that publication bias is absent (Figure 3A).
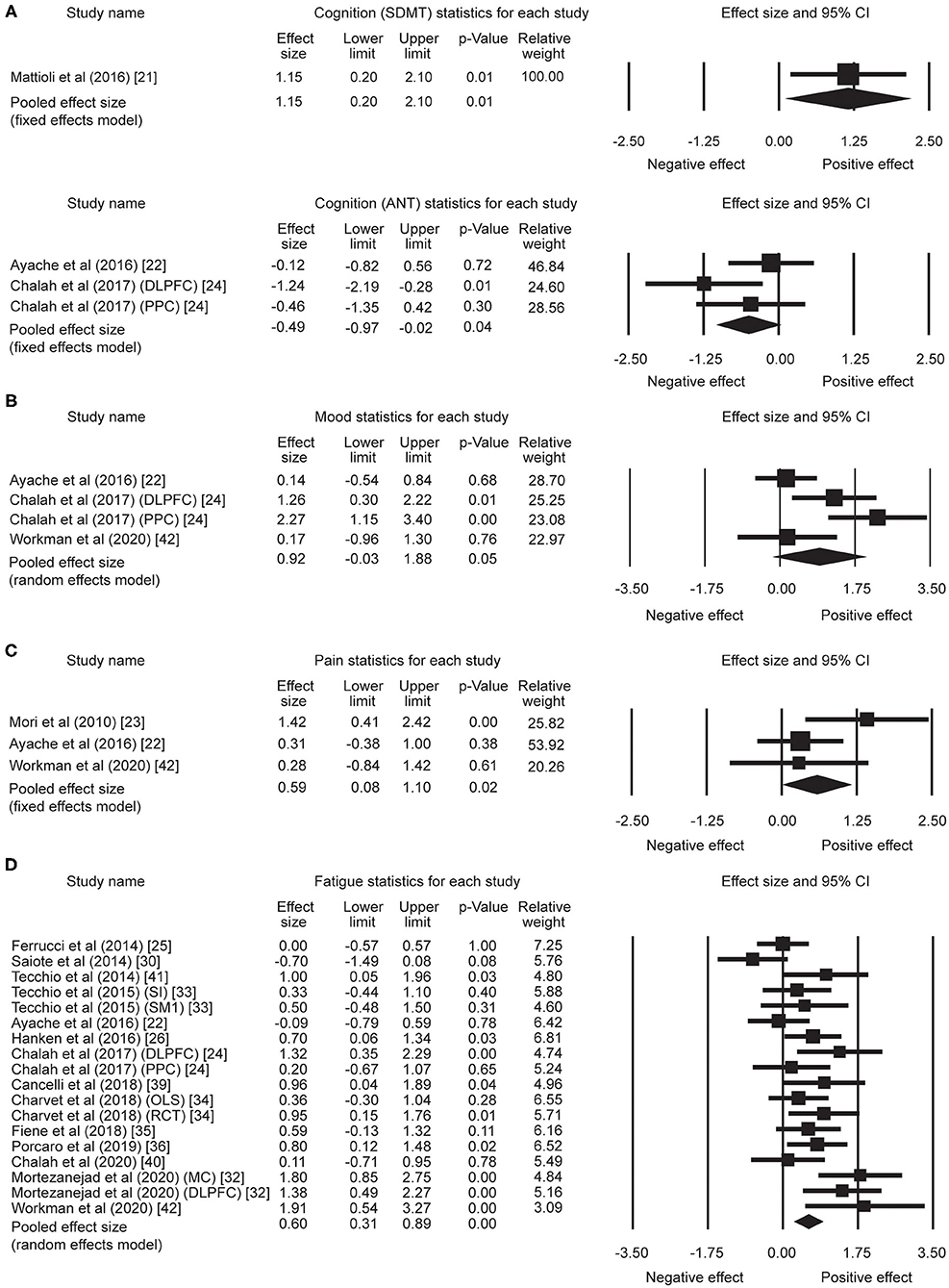
Figure 2. Statistical summary and forest plot of effect sizes for (A) cognition, (B) mood, (C) pain, and (D) fatigue outcome measures. SDMT, Symbol Digit Modalities Test; ANT, Attention Network Test; CI, confidence interval; DLPFC, dorsolateral prefrontal cortex; PPC, posterior parietal cortex; SI, whole body somatosensory areas; SM1, hand sensorimotor areas; OLS, open-label study; RCT, randomized controlled trial; MC, motor cortex.
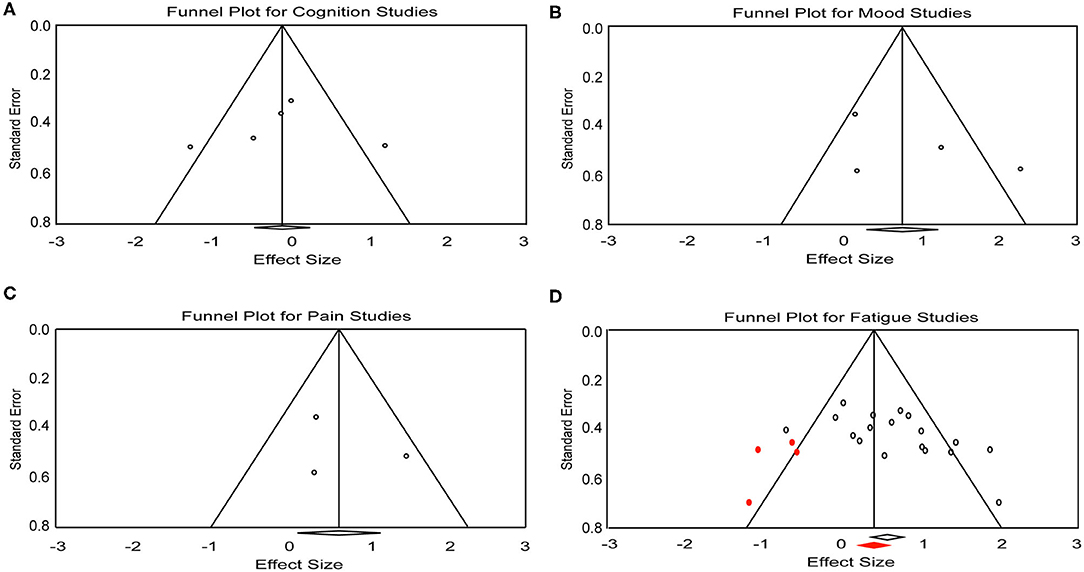
Figure 3. Funnel plot for (A) cognition, (B) mood, (C) pain, and (D) fatigue studies included in the meta-analysis. Red dots represent the imputed missing studies. Red rhombus shows the adjusted mean effect size.
Mood
Four effect sizes were obtained from three articles with a total of 32 patients for mood. A strong mean effect size of 0.92 (95% CI, −0.03–1.88, p = 0.05) (Figure 2B) was found. There was heterogeneity across the studies (Q = 12.08, I2 = 75.17, p = 0.007). The results of rank correlation (tau = 0.33, p = 0.49) and the symmetrical funnel plot (Figure 3B) indicate that publication bias did not seem to affect the validity of the overall effect size obtained by the meta-analysis of mood. Two studies evaluating mood as a control, rather than outcome variable, were not included in the meta-analysis (23, 30). Mood status was measured by Chalah et al. (40) but the effect sizes could not be determined since point estimates for the control group were not reported.
Pain
Three effect sizes were determined for pain from three articles with a total of 41 patients. A moderate mean effect size of 0.59 (95% CI, 0.08–1.10, p = 0.02) (Figure 2C) was discovered. We did not find heterogeneity among the studies (Q = 3.49, I2 = 42.82, p = 0.17). Publication bias was not found by either rank correlation (tau = 0.00, p = 1.00) or the funnel plot (Figure 3C).
Fatigue
A total of 18 effect sizes were extracted from 14 articles (with 291 patients), and the mean effect size was 0.60 (95% CI, 0.31–0.89, p < 0.001) (Figure 2D). Heterogeneity was observed across studies (Q = 38.45, I2 = 55.79, p = 0.002). Publication bias was discovered by rank correlation (tau = 0.39, p = 0.02) and an asymmetrical funnel plot showing a higher concentration of studies on one side of the mean than the other (Figure 3D). Therefore, a planned Trim and Fill procedure (50) was applied to impute missing studies. After adjusting for missing studies, a mean effect size of 0.39 was found.
Since a larger number of effect sizes (i.e., 18) was extracted for fatigue, we explored whether other variables would influence the measured effect. To achieve this, we performed subgroup analyses based on stimulation intensity (low: <2 mA vs. high: ≥2 mA) and outcome measures [Fatigue Severity Scale (FSS) vs. Modified Fatigue Impact Scale (MFIS) vs. other outcomes for fatigue] that were applied in the studies. The subgroup analysis of stimulation intensity revealed a mean effect size of 0.62 (95% CI, 0.05–1.19, p = 0.03) for six trials from five studies (22, 24, 34, 40, 42) with a “high” intensity (i.e., ≥2 mA). Mean effect size for 12 trials from 10 studies (25, 26, 30, 32–36, 39, 41) with “low” intensity (i.e., <2 mA) was 0.60 (95% CI, 0.25–0.95, p = 0.001). For the analysis of outcome measures, a mean effect size of 1.14 (95% CI, 0.68–1.60, p < 0.001) was found for FSS [four trials (24, 32)]. The mean effect sizes for MFIS [eight trials (22, 30, 33, 36, 39–41)] and other fatigue outcomes [six trials, including Fatigue Impact Scale (25), vigilance task (26), Patient-Reported Outcomes Measurement Information System-fatigue short form (34), simple reaction time task (35), and fatigue index (42)] were 0.31 (95% CI, 0.03–0.60, p = 0.03) and 0.53 (95% CI, 0.23–0.82, p < 0.001), respectively.
Discussion
The results of this meta-analysis suggest that tDCS might be helpful in improving cognition (processing speed), mood disturbance, pain, and fatigue in MS. There has been increasing interest in treatment strategies to improve cognitive impairment (56). Here, we found a strong effect size of 1.15 for the trial that administered SDMT, and a negative effect for the trials that used ANT (effect size = −0.49). The results suggest that tDCS-induced cognitive improvement is task-specific or cognitive domain-specific. However, the findings should be interpreted with caution given the small sample size. SDMT is a widely used test in MS clinical trials and mainly evaluates information processing speed and immediate visual memory recall. Since cognitive processing speed is the most commonly affected cognitive domain (57, 58), it is possible that the test is more sensitive to detect cognitive improvements, including changes induced by tDCS. It is unclear why the performance of ANT was not improved by tDCS. One possibility is that the stimulation duration might not have been optimal. For instance, in the trial that administered SDMT and showed positive effects, 10 sessions of stimulation were applied (21). However, in studies using ANT as an outcome, no more than five sessions of stimulation were employed (22, 24). Study design may also affect the results: the study administering SDMT delivered tDCS during cognitive training, whereas the studies using ANT did not pair the stimulation with cognitive tasks. Another possible explanation is that baseline cognitive performance is a critical factor in determining whether tDCS—or any cognitive intervention—enhances cognitive performance (59, 60). Since most of the studies included in this meta-analysis did not specifically recruit patients with cognitive impairment, the heterogeneity in cognitive performance across participants may have affected the results. Further investigation with more homogeneous patient populations, different stimulation protocols, and cognitive assessments is needed to draw a conclusion regarding the optimal stimulation protocol and the effect of tDCS on different dimensions of cognition.
A strong mean effect size of 0.92 was discovered for mood disturbance. Further, studies that measured pain showed a mean effect size of 0.59, which is clinically meaningful (44). Neuropathic pain is one of the most common symptoms (61) and it is thought to be a consequence of maladaptive plastic changes within the nociceptive system which alters nociceptive signal processing (62). Studies have suggested that pain decreased by tDCS may be the result of functional changes in brain structures that are critical in pathogenesis of neuropathic pain (22, 23). By acting on pain-related corticosubcortical and corticocortical pathways, tDCS modulates perception of pain and reduces chronic neuropathic pain. However, further studies are warranted to better differentiate tDCS effects on neuropathic and nociceptive pain. While the results suggested beneficial effects of tDCS on mood disturbance and pain, the findings should be viewed conservatively since the sample size is small (mood: 32 patients; pain: 41 patients).
The mean effect size for fatigue was 0.60. A subgroup analysis was conducted to explore whether stimulation intensity and outcome measures being applied would influence the measured effect for fatigue. Both high and low intensities of stimulation demonstrated moderate effect sizes (high: effect size = 0.62; low: effect size = 0.60), suggesting that high and low intensities could yield nearly the same level of favorable effects on fatigue. Interestingly, graded stimulation effects were reported previously, where a larger learning effect was observed in healthy adults when the stimulation is applied at a higher intensity (63). Given that chronic inflammatory activity (64) and central inflammation (65) are related to synaptic plasticity, it is possible that how the brain responds to the tDCS intervention is altered. In this scenario, stimulation could lead to qualitatively different outcomes in intact vs. dysfunctional neural circuits. In contrast to the findings in healthy adults, we found that both high and low stimulation intensities relieved fatigue, with a similar degree of effect. Subgroup analysis of outcome measures demonstrated a relatively higher effect size for trials using the FSS (effect size = 1.14) than those using the MFIS (effect size = 0.31) and other outcomes assessments (effect size = 0.53), indicating that the FSS may be more sensitive to detect changes in fatigue induced by tDCS. Both the FSS and MFIS are widely used in assessing fatigue, but the item contents of the two scales are different. While the FSS primarily targets physical aspects of fatigue, MFIS measures physical, cognitive and psychosocial fatigue. Since the two scales measure different aspects of fatigue (66), the observed larger effect size for trials using the FSS suggests that tDCS effects may be more beneficial to treat physical fatigue. Physical fatigue in MS is associated with a progressive disease course and greater physical disability (67). Often, the impact of physical dysfunction on daily activities can be recognized more easily than that of mental fatigue. However, it is unclear how reliably a patient can actually distinguish between physical and mental fatigue, since perceived mental or physical fatigue does not correlate with objective measures of cognitive or physical performance (68, 69). Thus, further studies in a larger population are required to better determine the most sensitive outcome measures for detecting tDCS effects on fatigue.
One important consideration for this systematic review and meta-analysis is the methodological quality of the selected studies. Most of the trials included did achieve random allocation, and reported control groups and blinding procedures. However, two studies measuring tDCS effects on fatigue provided no point estimates or measures of variability, and these data were estimated from their figures (26, 30). The influence of non-precise data on the mean effect size cannot be fully excluded. Further, possible publication bias was detected in studies for fatigue. Although a Trim and Fill procedure (50) was performed to adjust the mean effect size, the results obtained in the present meta-analysis must be viewed conservatively. Despite the funnel plot and rank correlation analyses both indicating there was no publication bias in the studies for cognition, mood and pain, bias could not be fully excluded since the small number of trials included could limit the bias detection.
While tDCS is generally thought to be safe for both healthy adults and clinical populations, and no severe adverse effects have been reported, investigators should adhere to safety guidelines (70) and conduct follow-up assessments to monitor longer-term risks and benefits. In addition to safety concerns, several crucial questions should be addressed in future studies with proper experimental design. First, it is essential to elucidate the underlying neural mechanisms of positive effects on cognition, mood, pain, and fatigue induced by the tDCS. Second, further investigation is needed for optimizing stimulation protocols and finding the most effective parameters to apply tDCS as a treatment approach for MS. Third, studies with subgroups that are varied in subtypes of MS and clinical severity are necessary to identify the subgroups of patients most likely to benefit from tDCS. Studies have demonstrated that the efficacy of non-invasive electrical stimulation is correlated with the magnitude of the electric field that reaches the targeted brain area, which highlights the importance of anatomical variability and individualizing stimulation protocols (71–73). Thus, inter-individual variability in response to tDCS should be taken into account.
Some limitations exist in the review. First, it is difficult to estimate potential confounders such as regimens and types of DMTs, disease evolution profiles and effects of medicinal products. In the studies included in the meta-analysis, mood, pain, and fatigue were mainly measured with patient-reported outcome measures, which have very little or no motor component involved. For cognition, a motor component was involved in performing the task. However, how motor function, and other factors such as spasticity and fatigue, could have influenced the cognitive performance was not explicitly discussed. Second, we may have missed relevant studies that were published in non-English languages. Third, the findings of the current study should be taken with caution given the relatively small sample size and the repeated analyses in the same domain (e.g., ANT task) with the same patient population. The fact that relapsing-remitting MS was the majority population also makes it difficult to provide information about differences in treatment response between MS subtypes. Finally, methodological variations existed between the selected studies with respect to outcome measures, patient inclusion criteria, experimental design (e.g., cross-over vs. parallel design), and tDCS protocols. For instance, in studies measuring fatigue, the number of stimulation sessions varied across trials, with a range from single session to 20 sessions. Previous studies have reported that repeated sessions of tDCS can result in cumulative effects (74, 75). Although trials applied 20 sessions of tDCS (34) did not show a larger ES (0.95) compared to trials with five or six sessions of stimulation (ES ranging from −0.7 to 1.91), the influence of heterogeneity across the studies on the effect estimation cannot be ruled out. Stimulation timing (“online” vs. “offline”) and intervals between stimulation sessions are also critical factors that may affect the observed effects. However, subgroup analyses based on these factors are not suitable given the low number of total studies included, which limited us to simply determine the different degrees of the effect generated by timing of the stimulation and stimulation intervals.
In conclusion, this meta-analysis suggests preliminary evidence of favorable effects of tDCS on cognition, mood disturbance, pain, and fatigue in MS. For cognition, tasks targeting cognitive aspects including processing speed, may be more suitable to reflect tDCS-enhanced cognitive performance. For fatigue, applying high and low intensities of stimulation generate nearly the same grade of beneficial effects, and a relatively higher effect size was noted in studies using FSS as an outcome, suggesting that it may be more sensitive in capturing tDCS-induced changes in fatigue. Further well-designed studies are necessary to determine the neural plasticity changes induced by tDCS, optimize stimulation protocol and identify the subgroups of patients who would benefit most.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
W-YH: conceptualization, methodology, data analysis, visualization, and manuscript writing. C-HC: data curation and content curation. TZ, AG, and RB: conceptualization and manuscript editing. All authors: contributed to the article and approved the submitted version.
Funding
This work was supported by National Multiple Sclerosis Society FG-1908-34831.
Conflict of Interest
RB has received research support from the National Multiple Sclerosis Society, the Hilton Foundation, the California Initiative to Advance Precision Medicine, the Sherak Foundation and Akili Interactive. RB has also received personal compensation for consulting from Alexion, Biogen, EMD Serono, Novartis, Pear Therapeutics, Roche Genentech and Sanofi Genzyme. AG is a scientific advisor for Neuroelectrics and Halo Neuroscience, companies that produces tES devices, and co-founder, shareholder, BOD member, and advisor for Akili Interactive Lab, a company that produces therapeutic video games. TZ is a scientific advisor for HUMM, a company that produces tES devices.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. (2019) 92:e1029–40. doi: 10.1212/WNL.0000000000007035
2. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. (2013) 19(2 Suppl):S15–20.
3. Birnbaum G. Current and future treatments for relapsing-remitting multiple sclerosis. Curr Opin Drug Discov Dev. (2010) 13:214–25.
4. Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis–a multifaceted adversary. Nat Rev Drug Discov. (2008) 7:909–25. doi: 10.1038/nrd2358
5. Bennabi D, Haffen E. Transcranial direct current stimulation (tDCS): a promising treatment for major depressive disorder? Brain Sci. (2018) 8:81. doi: 10.3390/brainsci8050081
6. Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke. (2017) 48:229–32. doi: 10.1161/STROKEAHA.116.014988
7. Goodwill AM, Teo WP, Morgan P, Daly RM, Kidgell DJ. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: a pilot study. Front Hum Neurosci. (2016) 10:258. doi: 10.3389/fnhum.2016.00258
8. Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. (2011) 105:1141–9. doi: 10.1152/jn.00608.2009
9. Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, et al. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci. (2007) 27:3807–12. doi: 10.1523/JNEUROSCI.5348-06.2007
10. Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. (2005) 568(Pt 2):653–63. doi: 10.1113/jphysiol.2005.088310
11. Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. (2011) 17:37–53. doi: 10.1177/1073858410386614
12. Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. (2005) 22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x
13. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. (2008) 1:206–23. doi: 10.1016/j.brs.2008.06.004
14. Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. (2012) 216:1–10. doi: 10.1007/s00221-011-2891-9
15. Kuo MF, Nitsche MA. Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci. (2012) 43:192–9. doi: 10.1177/1550059412444975
16. Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, et al. Speech facilitation by left inferior frontal cortex stimulation. Curr Biol. (2011) 21:1403–7. doi: 10.1016/j.cub.2011.07.021
17. Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. (2004) 42:113–7. doi: 10.1016/S0028-3932(03)00124-6
18. Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. (2011) 4:84–9. doi: 10.1016/j.brs.2010.06.004
19. Hsu WY, Zanto TP, Anguera JA, Lin YY, Gazzaley A. Delayed enhancement of multitasking performance: effects of anodal transcranial direct current stimulation on the prefrontal cortex. Cortex. (2015) 69:175–85. doi: 10.1016/j.cortex.2015.05.014
20. Charvet L, Shaw M, Dobbs B, Frontario A, Sherman K, Bikson M, et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation. (2018) 21:383–89. doi: 10.1111/ner.12583
21. Mattioli F, Bellomi F, Stampatori C, Capra R, Miniussi C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler. (2016) 22:222–30. doi: 10.1177/1352458515587597
22. Ayache SS, Palm U, Chalah MA, Al-Ani T, Brignol A, Abdellaoui M, et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci. (2016) 10:147. doi: 10.3389/fnins.2016.00147
23. Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. (2010) 11:436–42. doi: 10.1016/j.jpain.2009.08.011
24. Chalah MA, Riachi N, Ahdab R, Mhalla A, Abdellaoui M, Creange A, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci. (2017) 372:131–37. doi: 10.1016/j.jns.2016.11.015
25. Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, Tomasini E, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. (2014) 34:121–7. doi: 10.3233/NRE-131019
26. Hanken K, Bosse M, Mohrke K, Eling P, Kastrup A, Antal A, et al. Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Front Neurol. (2016) 7:154. doi: 10.3389/fneur.2016.00154
27. Iodice R, Manganelli F, Dubbioso R. The therapeutic use of non-invasive brain stimulation in multiple sclerosis - a review. Restor Neurol Neurosci. (2017) 35:497–509. doi: 10.3233/RNN-170735
28. Lefaucheur JP, Chalah MA, Mhalla A, Palm U, Ayache SS, Mylius V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol Clin. (2017) 47:173–84. doi: 10.1016/j.neucli.2017.03.003
29. Palm U, Ayache SS, Padberg F, Lefaucheur JP. Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimul. (2014) 7:849–54. doi: 10.1016/j.brs.2014.09.014
30. Saiote C, Goldschmidt T, Timaus C, Steenwijk MD, Opitz A, Antal A, et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor Neurol Neurosci. (2014) 32:423–36. doi: 10.3233/RNN-130372
31. Moher D, Schulz KF, Altman D, Group C. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. (2001) 285:1987–91. doi: 10.1001/jama.285.15.1987
32. Mortezanejad M, Ehsani F, Masoudian N, Zoghi M, Jaberzadeh S. Comparing the effects of multi-session anodal trans-cranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on fatigue and quality of life in patients with multiple sclerosis: a double-blind, randomized, sham-controlled trial. Clin Rehabil. (2020) 34:1103–11. doi: 10.1177/0269215520921506
33. Tecchio F, Cancelli A, Cottone C, Ferrucci R, Vergari M, Zito G, et al. Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Front Neurol. (2015) 6:141. doi: 10.3389/fneur.2015.00141
34. Charvet LE, Dobbs B, Shaw MT, Bikson M, Datta A, Krupp LB. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. (2018) 24:1760–69. doi: 10.1177/1352458517732842
35. Fiene M, Rufener KS, Kuehne M, Matzke M, Heinze HJ, Zaehle T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J Neurol. (2018) 265:607–17. doi: 10.1007/s00415-018-8754-6
36. Porcaro C, Cottone C, Cancelli A, Rossini PM, Zito G, Tecchio F. Cortical neurodynamics changes mediate the efficacy of a personalized neuromodulation against multiple sclerosis fatigue. Sci Rep. (2019) 9:18213. doi: 10.1038/s41598-019-54595-z
37. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
38. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
39. Cancelli A, Cottone C, Giordani A, Migliore S, Lupoi D, Porcaro C, et al. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult Scler. (2018) 24:1366–74. doi: 10.1177/1352458517720528
40. Chalah MA, Grigorescu C, Padberg F, Kumpfel T, Palm U, Ayache SS. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: a randomized sham-controlled study. J Neural Transm. (2020) 127:953–61. doi: 10.1007/s00702-020-02166-2
41. Tecchio F, Cancelli A, Cottone C, Zito G, Pasqualetti P, Ghazaryan A, et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol. (2014) 261:1552–8. doi: 10.1007/s00415-014-7377-9
42. Workman CD, Kamholz J, Rudroff T. Transcranial direct current stimulation (tDCS) for the treatment of a Multiple Sclerosis symptom cluster. Brain Stimul. (2020) 13:263–64. doi: 10.1016/j.brs.2019.09.012
44. Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. (2005) 58:1217–9. doi: 10.1016/j.jclinepi.2005.07.009
45. Cochran WG. The combination of estimates from different experiments. Biometrics. (1954) 10:101–29. doi: 10.2307/3001666
46. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
47. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
48. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
49. Egger M, Smith GD. Misleading meta-analysis. BMJ. (1995) 310:752–4. doi: 10.1136/bmj.310.6982.752
50. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
51. Ayache SS, Chalah MA. Stem cells therapy in multiple sclerosis - a new hope for progressive forms. J Stem Cells Regen Med. (2016) 12:49–51. doi: 10.46582/jsrm.1201007
52. Pope PA, Brenton JW, Miall RC. Task-specific facilitation of cognition by anodal transcranial direct current stimulation of the prefrontal cortex. Cereb Cortex. (2015) 25:4551–8. doi: 10.1093/cercor/bhv094
53. Zmigrod S, Zmigrod L, Hommel B. Transcranial direct current stimulation (tDCS) over the right dorsolateral prefrontal cortex affects stimulus conflict but not response conflict. Neuroscience. (2016) 322:320–5. doi: 10.1016/j.neuroscience.2016.02.046
54. Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. (2017) 23:721–33. doi: 10.1177/1352458517690821
55. Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RH. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler. (2007) 13:52–7. doi: 10.1177/1352458506070750
56. Sokolov AA, Grivaz P, Bove R. Cognitive deficits in multiple sclerosis: recent advances in treatment and neurorehabilitation. Curr Treat Options Neurol. (2018) 20:53. doi: 10.1007/s11940-018-0538-x
57. Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. J Neurol Neurosurg Psychiatry. (2003) 74:443–6. doi: 10.1136/jnnp.74.4.443
58. Rogers JM, Panegyres PK. Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J Clin Neurosci. (2007) 14:919–27. doi: 10.1016/j.jocn.2007.02.006
59. Hsu WY, Ku Y, Zanto TP, Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging. (2015) 36:2348–59. doi: 10.1016/j.neurobiolaging.2015.04.016
60. Katz B, Au J, Buschkuehl M, Abagis T, Zabel C, Jaeggi SM, et al. Individual differences and long-term consequences of tDCS-augmented cognitive training. J Cogn Neurosci. (2017) 29:1498–508. doi: 10.1162/jocn_a_01115
61. O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. (2008) 137:96–111. doi: 10.1016/j.pain.2007.08.024
62. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. (2009) 32:1–32. doi: 10.1146/annurev.neuro.051508.135531
63. Cuypers K, Leenus DJ, van den Berg FE, Nitsche MA, Thijs H, Wenderoth N, et al. Is motor learning mediated by tDCS intensity? PLoS ONE. (2013) 8:e67344. doi: 10.1371/journal.pone.0067344
64. Nistico R, Mango D, Mandolesi G, Piccinin S, Berretta N, Pignatelli M, et al. Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS ONE. (2013) 8:e54666. doi: 10.1371/journal.pone.0054666
65. Mori F, Rossi S, Sancesario G, Codeca C, Mataluni G, Monteleone F, et al. Cognitive and cortical plasticity deficits correlate with altered amyloid-beta CSF levels in multiple sclerosis. Neuropsychopharmacology. (2011) 36:559–68. doi: 10.1038/npp.2010.187
66. Amtmann D, Bamer AM, Noonan V, Lang N, Kim J, Cook KF. Comparison of the psychometric properties of two fatigue scales in multiple sclerosis. Rehabil Psychol. (2012) 57:159–66. doi: 10.1037/a0027890
67. Colosimo C, Millefiorini E, Grasso MG, Vinci F, Fiorelli M, Koudriavtseva T, et al. Fatigue in MS is associated with specific clinical features. Acta Neurol Scand. (1995) 92:353–5. doi: 10.1111/j.1600-0404.1995.tb00145.x
68. Johnson SK, Lange G, DeLuca J, Korn LR, Natelson B. The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. (1997) 4:145–53. doi: 10.1207/s15324826an0403_1
69. Paul RH, Beatty WW, Schneider R, Blanco CR, Hames KA. Cognitive and physical fatigue in multiple sclerosis: relations between self-report and objective performance. Appl Neuropsychol. (1998) 5:143–8. doi: 10.1207/s15324826an0503_5
70. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. (2016) 9:641–61. doi: 10.1016/j.brs.2016.06.004
71. Evans C, Bachmann C, Lee JSA, Gregoriou E, Ward N, Bestmann S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. (2020) 13:125–36. doi: 10.1016/j.brs.2019.10.004
72. Jones K, Zanto T, Ostrand A, Hsu WY, Gazzaley A. Individual differences in neuroanatomy predict neurostimulation related multitasking gains in older adults. In: Cognitive Neuroscience Society Conference 2020 Virtual Meeting. (2020).
73. Kasten FH, Duecker K, Maack MC, Meiser A, Herrmann CS. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat Commun. (2019) 10:5427. doi: 10.1038/s41467-019-13417-6
74. Ho KA, Taylor JL, Chew T, Galvez V, Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. (2016) 9:1–7. doi: 10.1016/j.brs.2015.08.003
75. Winker C, Rehbein MA, Sabatinelli D, Junghofer M. Repeated noninvasive stimulation of the ventromedial prefrontal cortex reveals cumulative amplification of pleasant compared to unpleasant scene processing: a single subject pilot study. PLoS ONE. (2020) 15:e0222057. doi: 10.1371/journal.pone.0222057
Keywords: cognition, mood, pain, fatigue, multiple sclerosis, transcranial direct current stimulation
Citation: Hsu W-Y, Cheng C-H, Zanto TP, Gazzaley A and Bove RM (2021) Effects of Transcranial Direct Current Stimulation on Cognition, Mood, Pain, and Fatigue in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front. Neurol. 12:626113. doi: 10.3389/fneur.2021.626113
Received: 04 November 2020; Accepted: 10 February 2021;
Published: 08 March 2021.
Edited by:
Roberta Lanzillo, Federico II University Hospital, ItalyReviewed by:
John Foley, Independent Researcher, Salt Lake City, UT, United StatesRosa Iodice, University of Naples Federico II, Italy
Copyright © 2021 Hsu, Cheng, Zanto, Gazzaley and Bove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-Yu Hsu, d2FuLXl1LmhzdUB1Y3NmLmVkdQ==
 Wan-Yu Hsu
Wan-Yu Hsu Chia-Hsiung Cheng
Chia-Hsiung Cheng Theodore P. Zanto
Theodore P. Zanto Adam Gazzaley
Adam Gazzaley Riley M. Bove
Riley M. Bove