
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 10 June 2021
Sec. Endovascular and Interventional Neurology
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.622457
This article is part of the Research Topic Advances in the Endovascular Treatment for Cerebrovascular Diseases and its Complications View all 31 articles
Background: Treatment of unruptured vertebral artery aneurysm involving posterior inferior cerebellar artery (PICA) is challenging. The experience of pipeline embolization device (PED) therapy for these lesions is still limited.
Objective: To evaluate the safety and efficacy of the PED for unruptured vertebral artery aneurysm involving PICA.
Methods: Thirty-two patients with unruptured vertebral artery aneurysm involving PICA underwent treatment with PED were retrospectively identified. Procedure-related complications, PICA patency, clinical, and angiographic outcomes were analyzed.
Results: Thirty-two aneurysms were successfully treated without any procedure-related complications. Images were available in 30 patients (93.8%) during a period of 3–26 months follow-up (average 8.4 months), which confirmed complete occlusion in 17 patients (56.5%), near-complete occlusion in 9 patients (30%), and incomplete occlusion in one patient (3.3%). Parent artery occlusion (PAO) was occurred in 3 patients (10%). Twenty-eight of 30 PICA remained patent. The two occlusions of PICA were secondary to PAO. At a mean of 20.7 months (range 7–50 months) clinical follow-up, all the patients achieved a favorable outcome without any new neurological deficit.
Conclusion: PED seems to be a safe and effective alternative endovascular option for patients with unruptured vertebral artery aneurysm involving PICA.
Vertebral artery aneurysm account for 11% of posterior circulation aneurysms and 3–5% of subarachnoid hemorrhage (SAH) (1–3). The outcome of ruptured vertebral artery aneurysm is dismay with a high mortality rate of up to 50% (4). Therefore, it is necessary to take more aggressive treatment for the unruptured vertebral artery aneurysm considering the risk of progression and rupture, especially vertebral artery aneurysm involving posterior inferior cerebellar artery (PICA). Previous study reported that PICA involvement is risk factor of progression (5), and it has the highest morbidity among the ruptured vertebral artery aneurysm (6). However, Treatment of vertebral artery aneurysm involving PICA is challenging. The major consideration including preservation of the PICA and complete occlusion of the aneurysm (7). Selection of appropriate treatment modalities for these lesions remains controversial, the most acceptable conventional endovascular methods include internal coil trapping with revascularization of PICA and stent-assisted coiling. However, each method has its own drawback and limitation in terms of retaining the patency of PICA (8). PED has emerged as a popular treatment option for intracranial aneurysms and achieved promising results (9). This device can preserve parent vessels as well as major side branches while excluding aneurysm from circulation by disrupting flow within the aneurysm and remodeling vessel (10). These characteristics may be suitable for the treatment for vertebral artery aneurysm involving PICA. However, few articles have been published describing patient outcome following PED in treatment of these lesions. In this study, we report our experience regarding the efficacy and safety of the treatment of unruptured vertebral artery aneurysm involving PICA with PED.
In accordance with our institutional review board, a retrospective study by extracting patient data from a prospectively maintained database was performed. Three hundred and twenty-four patients with vertebral artery aneurysms treated with endovascular methods in our institution between January 2016 and October 2019 were reviewed. Cases were excluded if the aneurysm did not involve PICA, the patients presented with subarachnoid hemorrhage, or the aneurysms were not treated with PED. Thirty-two patients with unruptured vertebral artery aneurysm involving PICA and using PED as treatment modality were included. Every patient was discussed by at least 3 experienced senior interventional radiologists for indications, which include high risk of rupture and uncontrolled progressive clinical symptoms (headache, dizziness, ataxia, vomiting, dysphagia; Table 1). All the patients provided written informed content and the study was approved by ethics committee of our institution.
All endovascular procedures were performed under general anesthesia and systemic heparinization was administered after placement of the sheath, a 6-F guiding catheter (Codman, Raynham, Massachusetts, USA) was placed in the distal V2 segment. Using a coaxial system, Marksman (EV3, Irvine, California, USA) was navigated over a 0.014-inch microwire in the target artery beyond the aneurysm. Once the PED reached scheduled position, it was released carefully by a combination of withdrawing the Marksman catheter and advancing the delivery wire. For the aneurysms with necessity of adjunctive coil embolization, additional coiling was conducted through a pre-jailed Echelon-10 catheter (EV3, Plymouth, Minnesota, USA) to loosely pack the aneurysmal sac. Coil embolization was performed to facilitate the thrombosis of the aneurysm, instead of completely occlude the aneurysm. A final angiogram was obtained after deployment to assess stent placement and vessel patency. Cone-beam CT was performed to ascertain the wall apposition of the device.
Patients were pre-medicated with a dual antiplatelet regimen (75 mg of clopidogrel and 100 mg of aspirin daily) for at least 5 days before treatment. During the procedure, we administered an intravenous bolus dose of heparin (70 IU/kg) and continued heparinization to maintain an activated clotting time throughout the procedure of two to three times greater than the baseline value. Dual antiplatelet therapy was continued for 6 months after the procedure, and aspirin was continued indefinitely to prevent thrombi forming in the stents.
Clinical outcome was measured by the Modified Rankin Scale (MRS) at the latest available follow-up. Angiographic outcomes were determined by digital subtraction angiography (DSA)or computed tomographic angiography (CTA) which was scheduled at 3–6 months and 1–2 years postoperatively. A follow-up MRS scores of 0–2 were defined as a favorable outcome; MRS scores of 3–6 were considered a poor outcome. Aneurysms occlusion at follow- up was categorized as complete (100%), near-complete (≥90%) or incomplete (<90%). Follow-up angiographic images were also reviewed for patency of PICA and parent vessels.
Since the number of patients is relatively small and there is no sub-group analysis in the study, the data were presented in descriptive methods. For continuous variables, data that obeyed normal distribution are presented as mean and standard deviation. Data that did not obey normal distribution are presented as median and inter-quartile range. For categorical variables, data are presented as the absolute value followed by percentage.
A total 32 patients with vertebral artery aneurysm involving PICA were included in the present study. The mean age was 52 years old (range 17–67 years old). This study had greater proportion of males (71.9%) compared with that of females (28.1%). Patients risk factors include hypertension (43.8%), diabetes (12.5%) and smoking (21.9%). Of the 32 patients, twenty-six presents various clinical symptoms including headache (43.8%), dizziness (18.8%), ataxia (6.3%), dysphagia (6.3%), and vomiting (6.3%). The other six were incidentally identified without any symptoms.
The mean length and diameters of aneurysms were 13.94mm (Ranges from 7 to 27 mm) and 8.88 mm (Ranges from 4 to 18 mm), respectively. In terms of morphology, the majority of cases were fusiform (71.9%) compared to saccular (28.1 %). In terms of VA dominance, the majority of cases were codominant (62.5%) compared to single dominant (37.5%). All patients and aneurysm characteristics were summarized in Table 1.
Patients with aneurysms of an irregular morphology and clinical symptoms (headache, dizziness, ataxia, vomiting, and dysphagia) were defined as having indications for treatment. All aneurysms were treated successfully with endovascular reconstruction including PED alone (n = 28) and pipeline-assisted coiling (n = 4). Thirty-three PEDs were used in 32 procedures. Thirty-one cases were treated with one PED, and double PEDs were used in one patient. Balloon angioplasty was used in one patient with parent artery stenosis. The time of contrast within aneurysms increased moderately in all the patients after PED placement. The aneurysm, parent artery and stent details are listed in Table 2.
There was no intra-procedural complication. Three patients developed delayed complication (parent artery occlusion PAO) during follow up which did not lead to any neurological symptoms or neurological deficits.
Of the 32 patients, follow-up DSA or CTA was available in 30 patients. The average angiographic time was 8.3 months (3–26 months) after procedure. Seventeen (56.7%) of follow-up cases were completely occluded angiographically including four patients with adjunctive coiling (Figure 1). Nine (30%) cases were near completely occluded, and one case (3.3%) was incompletely occluded (Figure 2). Parent artery occlusion occurred in 3 (10%) cases. Involved PICA remained patency in 28 of 30 patients including one of PAO with retrograde filling from contralateral VA (Figure 3). The other two patients with PAO showed PICA occlusion. Clinical follow-ups were available in all 32 patients. the mean time of follow-up was 20.7 months (7–50 months). All patients achieved favorable outcome at the last follow-up. There were no morbidity and mortality. All follow-up outcomes were summarized in Table 3.
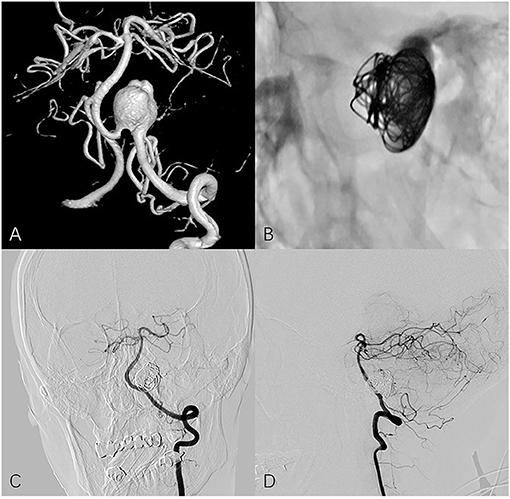
Figure 1. Fifty four year-old female with left vertebral artery aneurysm. (A) A 3-dimensional reconstruction image of a vertebral angiogram shows an irregular aneurysm involving PICA. (B) Non-subtracted oblique view shows PED in place and loosely filling coils. Follow-up frontal (C) and lateral (D) angiography shows the aneurysm is completely occluded and PICA remains patency.
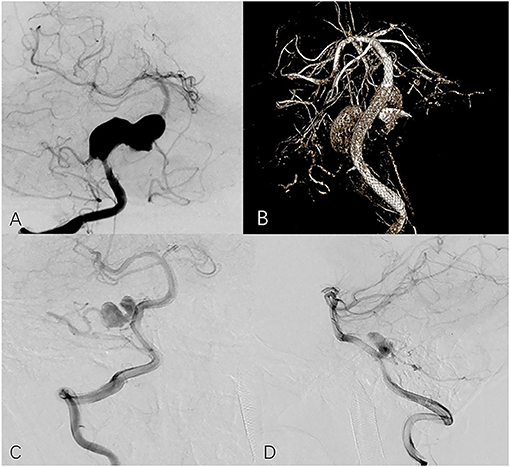
Figure 2. Seventeen year-old male with right vertebral aneurysm extending to basilar artery. (A) Preoperative frontal angiogram shows a giant vertebrobasilar aneurysm involving PICA. (B) Postoperative DynaCT indicates good opening of the PED and apposition. The 13-month follow-up frontal (C) and lateral (D) angiogram shows the aneurysm is incompletely occluded and the PICA is preserved.
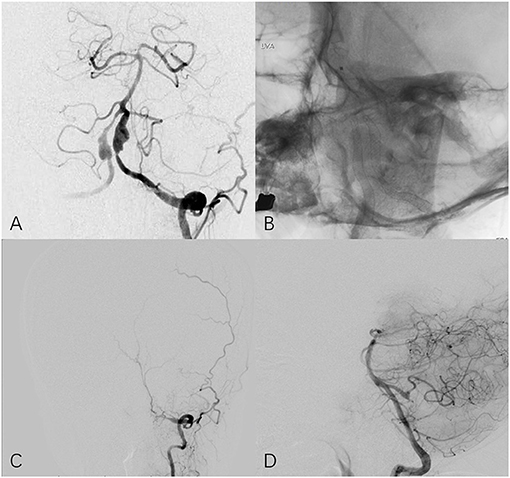
Figure 3. Fifty four year-old female with bilateral vertebral aneurysm. (A) Frontal angiogram of the left vertebral artery shows bilateral dissecting aneurysm and the left involved PCIA. (B) Non-subtracted frontal view shows bilateral PED in place. (C) The 25-month follow-up left (C) and right (D) angiogram indicates left vertebral artery is occluded at V3 segment and PICA remains patency through contralateral circulation.
PICA is an important branch originate from intracranial VA, which supplies part of cerebellum and medulla. The sacrifice of PICA will result in severe ischemic stroke and poor clinical outcome. Studies have shown that ischemic complications happened in 21.7% cases in which the PICA is sacrificed (8). When vertebral artery aneurysm involves the ostium of PICA, the treatment become complicated and challenging and the options are also limited (11). One consideration is to maintain the PICA patency. Various techniques were utilized for this purpose including loosely filling aneurysm, stent placement from VA to PICA, and surgical bypass using an occipital artery (OA)-to-PICA, PICA side-to-side anastomosis or PICA transposition. However, each approach has its own limitation and is unable to be an ideal and routine method. Loosely filling when using stent-assisted coiling result in high rate of incomplete occlusion which is thought to be an important factor to predict the postprocedural recurrence. A previous study for vertebral artery aneurysm treated with stent assisted coiling found 18% vertebral artery aneurysm involving PCIA experienced recurrence, whereas only 5% occurred in those without PICA involvement (12). The uncertainty of releasing a stent in a vessel <2 mm diameter and the difficulty of introducing a catheter into PICA due to the disadvantaged angel made the using of stent placement from VA to PICA only for a few selective patients (13, 14). Surgical bypass in posterior circulation is technically challenging and may require a lengthy operative time. Furthermore, bypass associated with various of surgical complications such as lower cranial nerve palsy and anastomosis failure (15). A review conducted by Chen et al. (16) noted two graft occlusion and three infarction occurred in 19 patients with vertebral artery aneurysm involving PICA treated by bypass, suggesting this method has been typically reserved for the cases not amendable to endovascular treatment. In the present study, all the PEDs were successfully deployed without technical events. one PED was used in thirty-one patients (96.9%) and the exceptional case (3.1%) using two devices harbored a giant vertebral artery aneurysm extending to basilar artery. For the four large saccular aneurysms, one or two coils were used to facilitate the thrombosis of the aneurysm instead of intensely coiling. Of the 30 patients with follow-up imaging, 28 remained the patency of PICA and two occurred PICA occlusions secondary to PAO. Mazur et al. (17) reported that all the PICA with origin covered by PED remained patency in eight patients and suggested when appropriately sized to the vessel wall and positioned in the VA, the device may cover the origin of PICA without impairing flow through the branching artery. Levitt et al. (18) reported that all the PICA maintained patency in six cases with vertebral artery aneurysms involving PICA treated by flow diverting stent. Similar results were reported by Adeeb et al. (19) and Wu et al. (20). These data suggested that the PICA can be effectively and safely preserved when using PED in treatment of these lesions. Additionally, in terms of conserving PICA, PED appears to be more convenient than conventional endovascular methods.
Nearly 86.7% of patients with angiographic follow-up achieved complete or nearly complete occlusion at mean 7.8 months follow-up without recurrence and retreatment. The only one incomplete occlusion (3.3%) was occurred in a patient with a giant vertebral artery aneurysm extending to basilar artery which was substantially attenuated. In addition, complete occlusion also occurred in three PAOs without any recanalization and recurrence. Likewise, Zhang et al. (21) report 93.3% nearly complete and complete occlusion rate of unruptured vertebral no-saccular aneurysm after PED placement in 32 cases series (21). Kuhn et al. (10) reported 100% nearly complete and complete occlusion rate in six patients with unruptured vertebral aneurysms. The similar results were reported by Yeung et al. (22). These data suggested that unruptured vertebral artery aneurysm may be effectively managed with PED. On the other hand, Stent assisted coiling is associated with high risk of incomplete occlusion and recurrence. Zhao et al. (12) reported 52.7% partial obliteration rate and 10.3% recurrence in 97 patients with vertebral artery aneurysm treated by stent assisted coiling, suggesting recurrence is closely associated with PICA involvement and immediate occlusion degree. Kim et al. (23) also reported 13% recurrence rate after conventional endovascular treatment of vertebrobasilar dissecting aneurysms, and concluded PICA involvement was the independent risk factor for recurrence. Despite the promising results in the present study, long-term angiographic follow-up and large study are needed to assess the efficacy of the PED in unruptured vertebral artery aneurysm involved PICA.
PAO is one of serious complications related to PED. Becsek et al. (24) reported 5 cases of PAO in their study for PED and concluded that non-compliance or resistance to antiplatelet therapy and severe in-stent stenosis might lead to PAO and evaluating antiplatelet effectiveness might be useful to reduce this complication. Oishi et al. (25) reported a patient who developed PAO during the 28 months follow-up after treatment with PED and found thrombus development at the non-covered part with endothelium due to discontinuation of antiplatelet and incomplete occlusion of the aneurysm. In the present study, PAO were developed in three cases during the follow-up. One patient developed PAO at 5 months due to the stenosis of the parent artery, another patient occurred PAO at 11 months attribute to discontinuation of antiplatelet at 6 months after procedure. We are not sure the mechanism of the last patient. There is no in-situ stenosis in the parent artery, the antiplatelet therapy was continued, and the deployment of PED was successful. A reasonable explanation might be the resistance of the antiplatelet medication. It might be useful to decline this complication with in-situ stenosis angioplasty, longer antiplatelet treatment and routinely monitoring antiplatelet effectiveness. Three PAOs resulted in obliteration of two involving PICA, fortunately, these patients did not develop any new neurological deficit, and there were no postoperative strokes in the treated PICA territory. This may due to slow progress and sufficient time for the establishment of collateral circulation.
The limitations of the current study include the retrospective property and variable follow-up intervals, which may cause selection bias of patients and add difficulties for replication. This study will provide some initial experience for the treatment of such lesions, while the result needs to be consolidated by large multi-center prospective studies.
Our preliminary experience of using PED in the treatment of unruptured vertebral artery aneurysm involving PICA demonstrated that this method is effective and safe with favorable angiographic and clinical outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Beijing Tiantan Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
WF, HJ, and YL designed, conceptualized the study, and analyzed and interpreted the data. WF, HJ, HG, GL, XM, and JW collected the data. WF drafted the manuscript. HJ, HG, GL, XM, JW, and YL revised the manuscript for intellectual content. All authors agreed and approved the publication.
This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFB1304400) and the Youth Program of the National Natural Science Foundation of China (Grant No. 81901197).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. (1991) 75:874–82. doi: 10.3171/jns.1991.75.6.0874
2. Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg. (1988) 69:345–9. doi: 10.3171/jns.1988.69.3.0345
3. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida TJN. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. (1995) 36:905–11. doi: 10.1097/00006123-199505000-00003
4. Santos-Franco JA, Zenteno M, Lee A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev. (2008) 31:131–40. doi: 10.1007/s10143-008-0124-x
5. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Uemura A, Niimi Y. Basilar extension and posterior inferior cerebellar artery involvement as risk factors for progression of the unruptured spontaneous intradural vertebral artery dissection. J Neurol Neurosurg Psychiatry. (2014) 85:1049–54. doi: 10.1136/jnnp-2013-306931
6. Yasui T, Komiyama M, Nishikawa M, Nakajima H. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery: report of two cases and review of the literature. Neurosurgery. (2000) 46:196–200; discussion 200–1. doi: 10.1093/neurosurgery/46.1.196
7. Kim YS, Kim TS, Yang IC, Joo SP. Staged, combined management of ruptured vertebral artery dissecting aneurysms involving the posterior inferior cerebellar artery: report of 4 cases and review of the literature. World Neurosurg. (2019) 128:444–7. doi: 10.1016/j.wneu.2019.05.146
8. Shi L, Xu K, Sun X, Yu J. Therapeutic progress in treating vertebral dissecting aneurysms involving the posterior inferior cerebellar artery. Int J Med Sci. (2016) 13:540–55. doi: 10.7150/ijms.15233
9. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ, et al. Long-Term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. (2017) 80:40–8. doi: 10.1093/neuros/nyw014
10. Kuhn AL, Kan P, Massari F, Lozano JD, Hou SY, Howk M, et al. Endovascular reconstruction of unruptured intradural vertebral artery dissecting aneurysms with the pipeline embolization device. J Neurointerv Surg. (2016) 8:1048–51. doi: 10.1136/neurintsurg-2015-012028
11. Satow T, Ishii D, Iihara K, Sakai N; JR-NET study group. Endovascular treatment for ruptured vertebral artery dissecting aneurysms: results from japanese registry of neuroendovascular therapy (JR-NET) 1 and 2. Neurol Med Chir. (2014) 54:98–106. doi: 10.2176/nmc.oa.2013-0184
12. Zhao KJ, Zhao R, Huang QH, Xu Y, Hong B, Fang YB, et al. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with stent(s)-assisted coiling. Eur Radiol. (2014) 24:2088–96. doi: 10.1007/s00330-014-3225-7
13. Chung J, Kim BS, Lee D, Kim TH, Shin YS. Vertebral artery occlusion with vertebral artery-to-posterior inferior cerebellar artery stenting for preservation of the PICA in treating ruptured vertebral artery dissection. Acta Neurochir. (2010) 152:1489–92. doi: 10.1007/s00701-010-0725-3
14. Kim MJ, Chung J, Kim SL, Roh HG, Kwon BJ, Kim B, et al. Stenting from the vertebral artery to the posterior inferior cerebellar artery. AJNR Am J Neuroradiol. (2012) 33:348–52. doi: 10.3174/ajnr.A2741
15. Sanai N, Tarapore P, Lee AC, Lawton MT. The current role of microsurgery for posterior circulation aneurysms: a selective approach in the endovascular era. Neurosurgery. (2008) 62:1236–49; discussion 1249–53. doi: 10.1227/01.neu.0000333295.59738.de
16. Chen JA, Garrett MC, Mlikotic A, Ausman JI. Treatment of intracranial vertebral artery dissecting aneurysms involving the posterior inferior cerebellar artery origin. Surg Neurol Int. (2019) 10:116. doi: 10.25259/SNI-281-2019
17. Mazur MD, Kilburg C, Wang V, Taussky P. Pipeline embolization device for the treatment of vertebral artery aneurysms: the fate of covered branch vessels. J Neurointerv Surg. (2016) 8:1041–7. doi: 10.1136/neurintsurg-2015-012040
18. Levitt MR, Park MS, Albuquerque FC, Moon K, Kalani MY, McDougall CG. Posterior inferior cerebellar artery patency after flow-diverting stent treatment. AJNR Am J Neuroradiol. (2016) 37:487–9. doi: 10.3174/ajnr.A4550
19. Adeeb N, Griessenauer CJ, Dmytriw AA, Shallwani H, Gupta R, Foreman PM, et al. Risk of branch occlusion and ischemic complications with the pipeline embolization device in the treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol. (2018) 39:1303–9. doi: 10.3174/ajnr.A5696
20. Wu X, Tian Z, Liu J, Zhang Y, Li W, Zhang Y, et al. Patency of posterior circulation branches covered by flow diverter device: a hemodynamic study. Front Neurol. (2019) 10:658. doi: 10.3389/fneur.2019.00658
21. Zhang Y, Liang F, Zhang Y, Yan P, Liang S, Ma C, et al. Exploring the feasibility of pipeline embolization device compared with stent-assisted coiling to treat non-saccular, unruptured, intradural vertebral artery aneurysms. Front Neurol. (2019) 10:275. doi: 10.3389/fneur.2019.00275
22. Yeung TW, Lai V, Lau HY, Poon WL, Tan CB, Wong YC. Long-term outcome of endovascular reconstruction with the pipeline embolization device in the management of unruptured dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. (2012) 116:882–7. doi: 10.3171/2011.12.JNS111514
23. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. (2011) 42:2425–30. doi: 10.1161/STROKEAHA.111.617381
24. Becske T, Kallmes DF, Saatci I, Mcdougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. (2013) 267:858–68. doi: 10.1148/radiol.13120099
Keywords: pipeline embolization device, vertebral artery, posterior circulation aneurysms, dissecting aneurysms, pica
Citation: Fu W, Ge H, Luo G, Meng X, Wang J, Jin H and Li Y (2021) Treatment of Unruptured Vertebral Artery Aneurysm Involving Posterior Inferior Cerebellar Artery With Pipeline Embolization Device. Front. Neurol. 12:622457. doi: 10.3389/fneur.2021.622457
Received: 28 October 2020; Accepted: 20 April 2021;
Published: 10 June 2021.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Carmen Parra-Farinas, University of Toronto, CanadaCopyright © 2021 Fu, Ge, Luo, Meng, Wang, Jin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengwei Jin, amluaGVuZ3dlaTE5ODdAMTYzLmNvbQ==; Youxiang Li, ZHJsaXlvdXhpYW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.