
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 23 February 2021
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.617526
This article is part of the Research Topic Tackling Chronic Traumatic Encephalopathy: A Transdisciplinary Series of Original Research Articles to Advance Knowledge on Disease Diagnosis, Risk Factors, and Mechanisms View all 8 articles
 Jeff Schaffert1
Jeff Schaffert1 Nyaz Didehbani1,2
Nyaz Didehbani1,2 Christian LoBue1,3
Christian LoBue1,3 John Hart2,4,5
John Hart2,4,5 Heidi Rossetti1
Heidi Rossetti1 Laura Lacritz1,5
Laura Lacritz1,5 C. Munro Cullum1,3,5*
C. Munro Cullum1,3,5*Traumatic encephalopathy syndrome (TES) is proposed to represent the long-term impact of repetitive head-injury exposure and the clinical manifestation of chronic traumatic encephalopathy (CTE). This study aimed to evaluate the frequency of TES in a cohort of retired professional contact sport athletes, compare the frequency of TES to clinical consensus diagnoses, and identify predictors that increase the likelihood of TES diagnosis. Participants were 85 retired professional contact sport athletes from a prospective cohort at the University of Texas Southwestern Medical Center and the University of Texas at Dallas. Participants ranged in age from 23 to 79 (M = 55.95, SD = 13.82) and obtained 7 to 19 years of education (M = 16.08, SD = 1.03). Retirees were either non-Hispanic white (n = 62) or African-American (n = 23). Retired athletes underwent a standard clinical evaluation, which included a clinical interview, neurological exam, neuroimaging, neuropsychological testing, and consensus diagnosis of normal, mild cognitive impairment, or dementia. TES criteria were applied to all 85 athletes, and frequencies of diagnoses were compared. Fourteen predictors of TES diagnosis were evaluated using binary logistic regressions, and included demographic, neuropsychological, depression symptoms, and head-injury exposure variables. A high frequency (56%) of TES was observed among this cohort of retired athletes, but 54% of those meeting criteria for TES were diagnosed as cognitively normal via consensus diagnosis. Games played in the National Football League (OR = 0.993, p = 0.087), number of concussions (OR = 1.020, p = 0.532), number of concussions with loss of consciousness (OR = 1.141 p = 0.188), and years playing professionally (OR = 0.976, p = 0.627) were not associated with TES diagnosis. Degree of depressive symptomatology, as measured by the total score on the Beck Depression Inventory-II, was the only predictor of TES diagnosis (OR = 1.297, p < 0.001). Our results add to previous findings underscoring the risk for false positive diagnosis, highlight the limitations of the TES criteria in clinical and research settings, and question the relationship between TES and head-injury exposure. Future research is needed to examine depression in retired professional athletes.
In recent years, the long-term impact of sports-related concussion has gathered a great deal of scientific and public-health interest, in part due to the identification of chronic traumatic encephalopathy (CTE) among former National Football League (NFL) players and other professional athletes. CTE is thought to constitute a progressive neurodegenerative disease found in individuals with a history of repetitive brain trauma (1). However, there remains a great deal of controversy surrounding CTE, including a debate as to whether CTE is neurodegenerative in nature (2, 3), the risk factors for developing clinical symptoms associated with CTE (4), and the potential media bias that has accompanied recent publications (5). Furthermore, although several in-vivo diagnostic classifications have been proposed (6–9), CTE can only be diagnosed posthumously via autopsy and the clinical presentation remains poorly understood.
In 2014, Montenigro et al. proposed research diagnostic criteria for Traumatic Encephalopathy Syndrome (TES), which aimed to represent the clinical manifestation of CTE and chronic post-concussive deficits (6). These criteria were derived from a comprehensive literature review of available case series among athletes with possible CTE using Jordan's et al. criteria (8). A diagnosis of TES was “meant to describe the clinical presentation of CTE as well as other possible long-term consequences of repetitive head impacts.” The proposed diagnosis of TES consists of 5 general criteria, three core clinical features, and nine supportive features (see Table 1 for an extraction of TES criteria from Montenigro et al.). Individuals must meet all 5 general criteria, one core clinical feature, and two or more supportive features to render a diagnosis of TES. In addition, several subtypes of TES were proposed depending on the combination of the presenting symptoms, along with likelihood of CTE based on biomarker data. The three core clinical features include cognitive (attention, executive functioning, and memory) behavioral (explosive tendencies or violence), and mood symptoms (depression, apathy, worthlessness). To be considered a core clinical feature, the symptom must have been reported in at least 70% of a limited number of autopsy-confirmed CTE cases (N = 36) that were described in a previous publication (10). The nine supportive features include impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, documented decline, and delayed onset. Supportive features were selected from a literature review which identified in total 56 possible clinical features of CTE, but a more specific quantification and justification for the inclusion of these nine supportive features in the TES diagnostic criteria was not reported (6). The authors noted the selection of TES criteria was meant to favor sensitivity of the clinical features of CTE rather than specificity, and would be refined in further study.

Table 1. Traumatic encephalopathy syndrome clinical research criteria from Montenigro et al. (6).
The 2014 TES criteria were adapted for use in the ongoing UNITE study (Understanding Neurologic Injury and Traumatic Encephalopathy) with the aim of identifying the correlation of CTE pathology and clinical symptoms of brain donors at presumably high risk of CTE (11). A recent abstract presented at the 2020 American Academy of Neurology Conference aimed to examine the validity of TES criteria compared to the presence of CTE pathology in the UNITE study. Eleven TES symptoms were collected via retrospective interviews posthumously through family interview and medical record review of 302 brain donors aged 14 to 89 years old (12). TES criteria had high sensitivity, but very low specificity of 0.23 for CTE pathology, with a high percentage of those meeting criteria for TES possessing a different neurodegenerative or vascular disease. There have been no peer-reviewed clinicopathological studies validating the sensitivity and specificity of TES criteria.
The low specificity of TES criteria to CTE is not surprising for two primary reasons. First, many of the proposed symptoms of TES are common in those with and without head-injury exposure, and in patients with other diseases. For example, apathy and suicidality occur in high frequency among those with depression, and cognitive declines seen on neuropsychological testing are frequent in those with and without neurodegenerative disease. A series of studies from Iverson et al. observed high frequencies of anger control problems (13) and depression (14) among men in the general population. Apathy, disinhibition, agitation, and other behavioral control problems are also very common among those with Alzheimer's disease (15). From a cognitive perspective, one or two low scores that could be classified as “deficits” are frequently seen among even those with normal cognition when administered a comprehensive battery of neuropsychological tests (16). Second, the nature of how TES criteria were developed (i.e., case reports from retrospective next of kin interviews) likely contributes to the poor specificity of the diagnosis. Clinical diagnostic criteria for other neurodegenerative conditions, such as Alzheimer's disease, have been developed and refined over decades of prospective studies investigating clinicopathological relationships, and as such provide a much better framework for making a clinical diagnosis despite containing overlapping symptoms with other neurodegenerative diseases. TES and/or additional CTE clinical criteria currently have no validated criteria through prospective clinicopathological studies (17). Next of kin interviews are prone to multiple biases that may influence reported symptoms. For example, retrospective informant ratings of emotional symptoms (e.g., internal mood states of depression and worthlessness) are inherently speculative. Furthermore, determining the validity of cognitive changes solely based on retrospective review or informant review (i.e., without substantiated neuropsychological impairment) is extremely difficult, especially when factors such as depression and other emotional symptoms are associated with over reporting of cognitive symptoms (18). As such, the low specificity of TES criteria to CTE is likely multifactorial.
To our knowledge, the frequency of TES symptoms has not been evaluated in a prospective cohort design of those thought to be at high risk of TES and/or CTE, and there have not been any comparisons of TES diagnoses and other clinical diagnoses. Such an investigation would speak to the ease in which retired contact sport athletes may meet criteria for TES, while being diagnosed as normal via clinical consensus conference. Also, despite the fact that a diagnosis of TES is assumed to be associated with cortical and/or subcortical brain damage from a history of repetitive head trauma (6), there have not been any studies specifically evaluating the relationship between head-injury exposure or other characteristics and the likelihood of obtaining a diagnosis of TES.
The present study had three primary aims. First, we aimed to document the frequency of TES diagnoses and TES symptoms (see Table 1) in retired contact sport athletes using standard clinical methods (e.g., clinical interview, clinical questionnaires, neuropsychological testing, neurological exam, and neuroimaging). Second, we aimed to compare the rates of TES diagnoses to clinical consensus diagnoses of normal cognition, mild cognitive impairment, and dementia. We hypothesized, based on non-specificity of symptoms and the presence of high base rate symptoms (e.g., depression, anxiety, etc.), that we would observe a high frequency of TES diagnoses in our sample, but also find a high percentage of those diagnosed with TES determined to be “normal” via consensus diagnosis. Lastly, we aimed to test the hypothesis that higher levels of head-injury exposure is associated with TES diagnosis in our sample of retired contact sport athletes. For this last aim, we hypothesized that TES diagnosis would not be associated with head-injury exposure due to the non-specificity of TES symptoms to long-term head-injury exposure.
Participants were 85 retired professional athletes who participated in a prospective cohort study at the University of Texas Southwestern Medical Center (UTSW) and the University of Texas at Dallas (UTD) which aimed to investigate the neurocognitive impact of repetitive head-injury exposure. Athletes were recruited via local NFL player gatherings, word of mouth, and local flyers. Recruitment was open to all retired professional athletes with history of concussion and/or head-injury exposure, but were more targeted for retired NFL players given their research interest and availability. There were no specific symptomatic inclusion criteria for the study, but participants were excluded if they were found to have a history of major territory stroke, epilepsy, or pervasive developmental disorder. The study was approved by both the UTSW and UTD IRBs and all participants completed informed consent. Of the 85 athletes who participated in the study, 77 (90.6%) were retired NFL players. Of the remaining 8 athletes, 2 were retired National Hockey League (NHL) players, 3 were retired professional bull riders, 2 were retired boxers/kickboxers, and 1 was a retired professional skier. Participants ranged in age from 23 to 79 (M = 55.95, Median = 58, SD = 13.82). Age distribution of the sample was as follows: 15.3% were 40 years old or younger, 30.6% were between 41 and 55 years old, 37.6% were between 56 and 70 years old, and 16.5% were older than 70 years of age. Retirees obtained 7 to 19 years of education (M = 16.08, SD = 1.03). Retirees were mostly non-Hispanic white (N = 62 [72.9%]), with a sizeable minority of African-Americans (N = 23 [27.1%]). Head-injury exposure was defined as the number of self-reported concussions and number of concussions with loss of consciousness (LOC) as well as number of games and years played at the professional level. A concussion was defined by the clinical interviewer via 1997 American Academy of Neurology standards (19). Games played and years playing professionally for all retirees were obtained via online databases when available (obtained via pro-football-reference.com, pro-hockey-reference.com, or boxrec.com; see Table 2 for demographic information and athletic career info). Unfortunately, years of exposure was not initially collected in this study and is unavailable. However, online data are available from several sources due to the high-profile nature of these athletes. Additional online searches (Wikipedia, profootballarchives.com, etc.) for each athlete were used to confirm a least 6 years of contact sport participation (at least two at the college level or higher) as required by TES criteria.
Participants completed a semi-structured clinical interview, neuropsychological testing, a neurological exam, neuroimaging (magnetic resonance imaging), and self-report symptom questionnaires, including measures of cognitive and psychiatric complaints. Participants underwent consensus clinical diagnosis of normal, mild cognitive impairment, or dementia by two neuropsychologists (N.D. and M.C.) and a behavioral neurologist (J.H.) utilizing the available clinical information. Standard clinical criteria were used to determine MCI diagnoses and dementia (20). Additionally, the clinical histories were reviewed and a suspected etiology was determined based on clinical features of each disorder (e.g., Alzheimer's, Lewy body disease, Frontotemporal lobar degeneration, or unknown). TES was not considered as a diagnosis during clinical consensus conferences, as our goal was to compare frequencies of clinical diagnoses to TES.
Regarding general criteria for TES (see Table 1), all participants either sustained multiple concussions or “subconcussive trauma” through at least 6 years of contact sports participation, satisfying criterion 1. We considered all participants who reported symptoms to meet criterion 2, as comorbid psychiatric and neurodegenerative disease as reported in the Montenigro criteria are acceptable comorbid diagnoses along with TES. All symptomatic participants reported clinical features longer than 12 months, which satisfied criterion 3 (6). The last two general TES criteria, criterion 4 and criterion 5, state that one core feature and two supportive features features must be present, and are discussed below.
TES core features were obtained via self- or informant- report, semi-structured clinical interview, neurological exam, and neuropsychological testing (see Table 3 for sources of information).
Cognitive features included cognitive complaints that were collected via self-report of cognitive decline during the semi-structured clinical interview or during completion of a symptom questionnaire that collected information regarding memory, language, attention, problem solving, and daily functioning. These complaints were then examined via a comprehensive neuropsychological battery of tests assessing multiple cognitive domains, including episodic memory as measured by the California Verbal Learning Test-2nd Edition (21) (CVLT-II) delayed recall trial and Rey Complex Figure Test (22) (RCFT) delayed recall trial; executive functioning as measured by the Texas Card Sorting Test (23) (TCST) logical sorts and Trail Making Test B (24) (TMTB); and attention as measured by the Wechsler Adult Intelligence Scale-4th Edition Digit Span subtest (25) (WAIS-IV DS) and CVLT-II (21) Trial 1 score. Scores at least 1.5 standard deviations below the mean were classified as impaired, per TES specifications. Tests were administered and scored by a neuropsychologist (N.D.), a neuropsychology fellow/doctoral intern, or a qualified psychometrist.
Behavioral core features were collected via semi-structured clinical interview and self-report on the aforementioned symptom questionnaire. On this questionnaire, participants were specifically asked if they were experiencing “Emotional outbursts or uncontrollable feelings.”
Mood core features were collected via self-report during the semi-structured clinical interview, endorsement of “depressed mood” on the symptom questionnaire, or a Beck Depression Inventory-2nd edition (BDI-II) score of 14 or greater.
Data were collected on seven of nine supportive features of TES. Only two supportive features were not collected or reported in this study. These included data on “impulsivity” and “documented decline” and thus were considered absent in all athletes for the purposes of this study. Documented decline was not available due to the need for repeat testing and/or evaluation at least 1 year apart, which was unavailable at baseline for our participants. Impulsivity was not collected systematically via our semi-structured clinical interview and/or questionnaire and thus was also not evaluated in this study. The majority of supportive features were collected via self-report during the semi-structured clinical interview or on the symptom questionnaire. Because severity or frequency of TES symptoms are not specified in the Montinegro et al. criteria, we did not delineate endorsement based on severity or intensity. Anxiety was defined as self-reported anxiety during the semi-structured clinical interview or endorsement of frequent feelings of “worry/anxiety” on the symptom questionnaire. Apathy was defined as difficulty with initiation on the symptom questionnaire or endorsement of item 12 on the BDI-II, which measures a loss of interest in other people or activities. Paranoia was collected via self-report during the semi-structured clinical interview or endorsement on the symptom questionnaire. A question regarding paranoia was added after the start of the study and only available in approximately half of the sample. Data on suicidality was collected via self-report during the semi-structured clinical interview or endorsement of item 9 on the BDI-II measuring suicidal thoughts or wishes. Headache data was obtained via self-report during the semi-structured clinical interview. Motor signs or parkinsonism were obtained during a neurological exam by a board-certified neurologist (J.H.). Data on the course, i.e., delayed onset, was collected during the semi-structured clinical interview.
Chi-square analyses and frequency analyses were used to compare the percentage of retired athletes who met consensus conference diagnosis for cognitively normal, MCI, or dementia and criteria for TES.
Binary logistic regressions were used to evaluate potential predictors of a TES diagnosis, which included: demographics (age, education, and race), neuropsychological scores (CVLT-II Trial 1, WAIS-IV Digit Span, TCST logical sorts, TMT B, CVLT-II delayed recall, and RCFT delayed recall), depressive symptom scores (BDI-II total score), and repetitive head-injury exposure (number of concussions, number of concussions with LOC, games played, and years played professionally). It should be noted that games played differ substantially between sports due to season length. As such, games played was restricted to only the retired NFL players. Each variable was first examined in isolation using separate binary logistic regressions, and then included in a comprehensive model to examine the relative impact of each variable while holding other variables constant. Missing data were excluded case wise for each analysis, with p < 0.05 set as the level of significance.
Four retired athletes were missing self-reported concussion data (5%), and two retired athletes were missing data on number of concussions with LOC (3%). For concussion history and career statistics, see Table 2. Number of concussions with and without LOC, in addition to playing statistics varied widely among the retired professional athletes. Five NFL retirees had a career statistic of 0 games played, but were rostered for either 1 (n = 2), 2 (n = 2), or 3 years (n = 1). It should be noted that the number of concussions reported by two players were statistical outliers (i.e., 50 and 40 self-reported concussions >3.29 SD away from the mean). However, results were very similar and significance levels did not change when these outliers were removed from the analyses, and as such, results are reported with these players retained in the analyses.
Sixty-two retirees (72.9%) reported at least one cognitive complaint. Fourteen players had at least one cognitive complaint but no objective cognitive impairment. Forty-eight retirees (56.5%) had at least one score 1.5 SD below the mean on neuropsychological testing. By domain, 25 retirees (29.4%) had at least one low score on executive functioning measures, 28 (33.7%) had at least one low score on attention measures, and 28 (32.9%) had at least one low score on memory measures.
In total, 54 retirees (63.5%) met the criteria for at least one core clinical feature. Thirty-eight retirees (46.9%) met criteria definition for core clinical feature number 1 (i.e., “cognitive”), 20 retirees (23.5%) met criteria for core clinical feature number 2 (i.e., “behavioral”), and 39 retirees (45.9%) met criteria for core clinical feature number 3 (i.e., “mood”). Regarding the “cognitive” core clinical feature, which required both a cognitive complaint and a cognitive impairment, 48 players obtained a cognitively impaired score of 1.5 SD below the mean but only 38 players met the core “cognitive” clinical feature because of the absence of cognitive complaints in 10 players. Twenty-five retirees met criteria for just one core clinical feature (29.4%), while 29 (34%) met criteria for more than one. Notably, of the 39 retirees who met criteria for the “mood” core clinical feature, only 69% obtained a BDI-II score of 14 or higher (M = 18.21, SD = 10.05), with the other 31% reporting depressed mood while obtaining a BDI-II score under 13.
Regarding supplemental features, 36 (42.4%) retirees reported anxiety, 50 (58.8%) reported apathy, 7 of 37 (19%) with available data reported paranoia, 18 reported suicidal ideation (21.2%), 26 reported headaches (30.6%), and 6 were found to have parkinsonism on motor exam (7.1%). In total, 76 retirees had a mood, behavioral, and/or cognitive complaint and all symptomatic players met the delayed onset criterion (n = 76, 100%).
In total, 48 (56.5%) retirees met criteria for TES. If the delayed onset criteria was not considered (all of the symptomatic sample met this criteria), 38 (44.7%) retirees would still meet criteria for TES. Out of the 48 retirees who met criteria for TES, 20 (23.5%) met criteria for TES-behavioral/mood variant, 4 (4.7%) met criteria for TES-cognitive variant, 18 (21.2%) met criteria for TES-mixed variant, and 6 (7.1%) met criteria for TES-dementia. Out of 20 retirees who met criteria for TES-behavioral/mood variant, only 1 player exclusively met the “behavioral” core clinical feature and only 6 met the “behavioral” and “mood” core clinical feature, while the remaining 13 met the definition of the “mood” core clinical feature. Five retirees (5.9%) met criteria for TES with motor features specifier (see Table 4 for frequency of core and supportive features of TES in the sample). Among those 37 retirees who did not satisfy TES criteria, 9 reported no TES symptom criterion (10.5%). Thirty-one retirees (including the 9 non-symptomatic retirees) did not meet any core clinical criteria and thus did not achieve a TES diagnosis. An additional 6 retirees reported at least one core clinical feature but did not have at least two supportive clinical features, and thus did not meet TES criteria.
Through clinical consensus diagnosis, 63 retired athletes were diagnosed as normal (74%), 15 with MCI (18%), and 7 with dementia (8%). Of the 22 athletes with a clinical diagnosis of MCI or dementia, suspected etiologies included AD (n = 14), Lewy body disease (n = 3), Vascular (n = 1), and unknown etiology (n = 4). Retirees with and without TES diagnoses significantly differed in the frequency of consensus diagnoses of normal, MCI, or dementia (χ2 = 6.143, p = 0.046). In the 63 retired athletes diagnosed as cognitively normal through clinical consensus diagnosis, 34 (56%) met criteria for TES. In the 15 retired athletes diagnosed with MCI via clinical consensus conference, 8 (46%) met criteria for TES. The significant difference observed via chi-square analysis was driven largely by the dementia diagnoses. All 7 retired athletes diagnosed with dementia via clinical consensus conference met criteria for TES. To further explore this relationship, dementia and MCI categories were collapsed into a “cognitively impaired” diagnosis vs. normal. No significant differences were found in the ratios of cognitive impairment via consensus diagnosis compared to those with and without TES (χ2 = 0.620, p = 0.431). Using this collapsed category, 22 retired athletes were diagnosed as having MCI or dementia through clinical consensus conference, 14 (64%) of which met criteria for TES (see Table 5 for chi-square results and frequency analyses).
To evaluate if subtypes of TES differed by age, a chi-square of TES subtypes by age (median split of ≤58 and >58 years old) was conducted (see Table 6). No statistically significant differences in frequencies were observed between subtypes of TES and a median split of age (χ2 = 1.698, p = 0.791).
Binary logistic regressions were used to evaluate each of the 14 predictors (Age, race, education, number of concussions, number of concussions with LOC, years playing professionally, games played, CVLT-II Trial 1, WAIS-IV Digit Span, TCST logical sorts, TMT B, CVLT-II delayed recall, RCFT delayed recall, BDI-II total score) independently prior to including it into a comprehensive model. Worse performance on CVLT-II trial 1 was associated with TES diagnosis (B = −0.576, Wald χ2 = 5.754, OR = 0.562 [0.351–0.900], p = 0.016) in addition to higher endorsement of depression symptoms on the BDI-II (B = 0.260, Wald χ2 = 17.432, OR = 1.297 [1.148–1.465], p < 0.001). The remaining univariate binary logistic regressions were not statistically significant (all p's > 0.05—see Table 7 for binary logistic regressions).
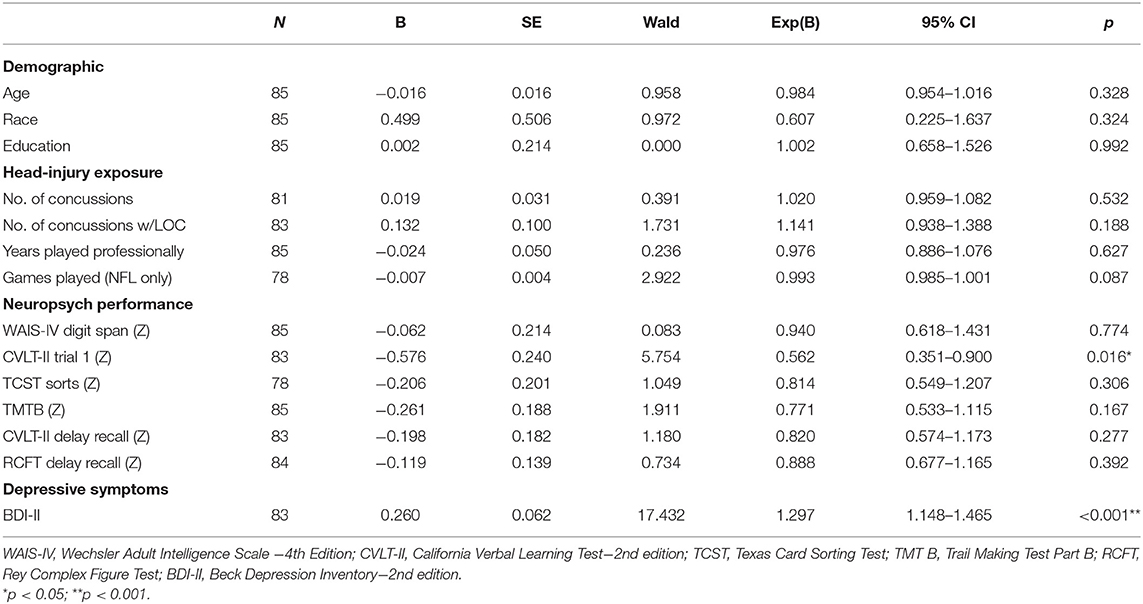
Table 7. Univariate binary logistic regressions assessing demographic, head-injury exposure, cognitive, and mood predictors of TES diagnosis.
When evaluating the 13 predictors (games played was dropped due to extreme differences in season length between sports) in a multivariate binary logistic regression model, the overall model was significant (χ2 = 46.295, p < 0.001) with good fit (Hosmer and Lemeshow Test p = 0.932; Nagelkerke R2 = 0.628). The classification accuracy improved from 59.5% in the null model to 84.9%. However, only BDI-II score significantly predicted TES diagnoses (B = 0.379, Wald χ2 = 12.107, OR = 1.461 [1.180–1.809], p < 0.001). No demographic, head-injury exposure variables, or neuropsychological scores significantly predicted TES diagnosis (all p's < 0.05). To further examine if any additional variables improved specification of the model, the model was evaluated in a forward step-wise fashion with p < 0.05 as the entry criteria, and only BDI-II scores was statistically significant (B = 0.250, Wald χ2 = 15.542, OR = 1.284 [1.134–1.454], p < 0.001) out of the 13 predictors (see Table 8 for additional statistics on the multivariate binary logistic regression).
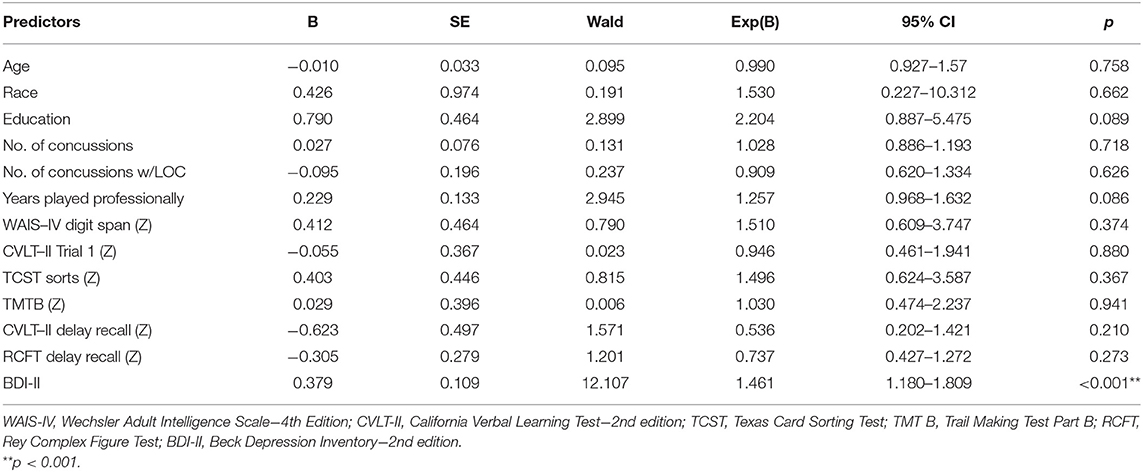
Table 8. Multivariate binary logistic regression assessing demographic, head-injury exposure, cognitive, and mood predictors of TES diagnosis (N = 74).
An additional post-hoc analysis was conducted to analyze BDI-II scores and likelihood of obtaining a TES diagnosis. BDI-II scores were removed from the TES diagnostic criterion for the purposes of this analysis in order to assess if this association would remain significant if BDI-II scores were not considered in the diagnosis of TES. By excluding BDI-II in the criterion, TES frequency reduced from 48 out of 85 (56.8%) to 43 out of 85 (50.5%). BDI-II score remained a significant predictor of TES diagnosis, B = 0.089, Wald χ2 = 9.718, OR = 1.094 (1.034–1.157), p = 0.002) and increased classification accuracy from 51.8 to 67.5%.
This study aimed to evaluate the frequency of TES diagnoses and TES symptoms collected prospectively in a cohort of retired professional athletes with head-injury exposure, compared the frequency of TES diagnoses to clinical consensus diagnoses, and evaluated predictors of TES diagnosis. To our knowledge, this was the first study to evaluate the frequency of TES diagnoses and TES criterion obtained through direct clinical evaluations of living retired professional athletes with head-injury exposure. We found a high percentage of the retired athletes in our sample met criteria for TES (56%), and over half of the athletes (54%) who met criteria for TES were diagnosed as normal based on comprehensive clinical evaluation and interdisciplinary consensus diagnosis. Furthermore, the only significant predictor of TES diagnosis was level of depressive symptomatology. No significant associations were found between likelihood of TES diagnosis and demographic characteristics, head-injury exposure, or neuropsychological functioning. Our findings underscore the limitations of the TES criteria, and call into question the utility of using these criteria in clinical and research settings, due to the potential for false positive determinations.
Although there have been several investigations linking head-injury exposure and CTE pathology (26, 27), to our knowledge there have been no investigations of head-injury exposure and likelihood of obtaining a diagnosis of TES. Because TES is considered to reflect long-term difficulties related to repetitive head-injury, it is logical to assume that increased head-injury exposure would increase likelihood of a TES diagnosis. However, this study did not observe a relationship between TES diagnoses and years playing professional sports, games played in the NFL, number of concussions, or number of concussions with LOC. We also did not find an association between neuropsychological functioning and likelihood of TES diagnosis in our multivariate model. Research investigating the link between head-injury exposure and cognitive functioning has been equivocal at best (28). A well-cited study by Stamm et al. in 2015 suggested worse executive functioning deficits in those who began playing football earlier than 12 years of age (29), though this study has limitations in the form of inequity between groups in regards to learning disabilities, substance use, and steroid use, as well as the lack of a control group (30). A 2016 study by Solomon et al. and a 2019 study by Fields et al. failed to find a statistically significant linear association between head-injury exposure and neuropsychological functioning among retired NFL players (30, 31). Our findings did, however, suggest that greater depressive symptomatology increased the likelihood of TES diagnosis in our sample, which appear to be heavily emphasized in TES diagnoses (e.g., “mood variant,” apathy, worthlessness, anxiety, etc.) and are not surprising in this context.
Large scale survey studies have suggested that more concussions are associated with a higher prevalence of self-reported depression in retired NFL players (32, 33). Despite this, investigations utilizing objective psychometric measures to assess the relationship between depression and head-injury exposure have been more mixed. A 2013 study found that number of concussions was specifically associated with cognitive symptoms of depression (e.g., pessimism, feelings of guilt, self-dislike) in retired NFL players (34), and a follow-up investigation found depression symptoms to be significantly associated with white matter changes (35). However, other investigations have failed to find an association between depressive symptoms and number of concussions or years played in the NFL (31), or between depressive symptoms and years of pre-high school football (30). In a recent article by Brett and colleagues, 43 retired NFL players aged 60 or younger were administered the BDI-II (M = 9.26) and the PHQ-15 to measure somatic symptoms. The authors found that somatic symptom reporting significantly moderated the relationship between concussions and depressive symptoms, such that somatic symptoms had an ~2-fold influence on depressive symptom reporting compared to concussion history (36). Their findings suggest that additional factors beyond head-injury exposure may be responsible for the relationship between head-injury exposure and depressive symptoms, and underscore the importance of future investigations aimed at examining depression and moderators of depression following head-injury exposure in contact sport athletes.
The multifactorial etiology of depressive symptoms and the presence of depression in other neurodegenerative diseases (e.g., AD, Parkinson's disease, etc.) adds additional concerns regarding the specificity of depression for its inclusion as a core diagnostic criterion for TES. Development of clinical diagnostic criteria for a neurodegenerative disorder that may share features with other diseases requires clear consensus on the most pertinent clinical features of the disorder. Ideally, this is informed through thorough study of many patients with the suspected disorder and longitudinal follow up of individuals in whom pathological confirmation can be made, so that clinical-pathological correlates can be reviewed to help refine the diagnostic process. Brett and colleagues (18) compare the processes in which clinical and neuropathological diagnoses of CTE have been derived in comparison with disorders such as AD and Lewy body disease (LBD), highlighting the rigorous approach needed to develop criteria that have good sensitivity and specificity and are clinically useful. For example, clinical and neuropathological criteria for AD and LBD have undergone numerous iterations (AD = 8; LBD = 4), involving diverse consensus work groups spanning many different institutions and followed by validation studies. While the clinical criteria for AD have remained largely similar over time, yet better refined, the pathological criteria have clearly evolved through this process. LBD is an example of a neurodegenerative disorder in which initial clinical criteria were not very specific, resulting in many inaccurate diagnoses. Through refinement of core and supportive clinical features over time (e.g., moving rapid eye movement behavioral disorder from a supportive to core feature), the likelihood of making an accurate diagnosis of LBD has improved considerably (accuracy of diagnosis 82 to 91%). CTE is in its infancy with respect to development of precise diagnostic criteria, which has to date relied heavily on a small number of cases reviewed by a limited number of professionals for the pathological diagnosis and largely non-empirical methods of deriving clinical syndromic criteria. Exclusion of other potential causes for the various presenting symptoms is part of the diagnostic criteria for AD and LBD, underscoring that other disorders can share similar features and need to be ruled out. While the criteria for TES also includes the exclusion of other disorders that can account for all of the clinical features, allowing comorbid disorders such as AD and other dementias, as well as various psychiatric disorders almost guarantees a high overlap in symptoms when these disorders are present.
In the current TES diagnostic framework, general criterion 2 states “No other neurological disorder (including chronic residual symptoms from a single TBI or persistent post-concussion syndrome) that likely accounts for all clinical features, although concomitant diagnoses of substance abuse, post-traumatic stress disorder (PTSD), mood/anxiety disorders, or other neurodegenerative diseases (for example, AD and frontotemporal dementia) or a combination of these can be present.” When this criterion was applied to the current sample, it became difficult to discern if the presence of other neurological disease may or may not be exclusionary criteria on a case by case basis, especially in the presence of potential neurodegenerative disease and lack of specificity of the core and supportive features criteria. As written, if a clinician suspects that even a single supportive feature may not be due to a comorbid neurodegenerative disease (such as AD), this would satisfy this criterion. Almost all of the supportive features for TES (e.g., impulsivity, apathy, anxiety, paranoia, and motor signs) may or may not be present in neurodegenerative conditions such as AD (15), making it difficult to determine if AD “accounts for all clinical features.” The Montenigro et al. criteria outline two case examples that meet criteria for AD as well as TES, both of which were diagnosed with TES-dementia subtype, which suggest that comorbid neurodegenerative conditions are often present. In addition, this criterion as written is not clear if commonly referred to psychiatric conditions such as post-traumatic stress disorder, major depressive disorder, or generalized anxiety disorder are considered “neurological diseases” given their mention in the same sentence, and can be particularly difficult to distinguish as TES criteria are heavily influenced by psychiatric symptoms. If these criteria are applied as written, any retired professional contact sport athlete who had onset of depressed mood 2 years following the end of their career would easily meet criteria for TES. The ease with which retired athletes can meet criteira for TES, which was designed to represent clinical features of the neurodegenerative disease CTE, is not suprising when poor specificity, allowance of comorbid conditions that may account for the majority of clinical features, and the high base rates of many of the core and supportive features are considered.
Several of the proposed clinical features for TES are non-specific and relatively common conditions/traits occurring in the general population. For example, among 3,933 males in a national survey on mental disorders, 15% reported having an alcohol use disorder, 16% a depression-related mood disorder, 13% an anxiety disorder, 25% aggression/hostility, and 10% anger during their lifetime (13). In the normative database of the National Institutes of Health Toolbox, anger (25%) and some aggression/hostility (11%) within the past month were also common for the male cohort (37). Thus, many of the psychological problems that have been proposed for TES are not unique, and there have been no studies to date comparing even active professional athletes to the general population on base rates of the purported conditions/traits. It is possible that professional athletes may be prone to having some of the conditions/traits at higher rates than the general population, which has been found for alcohol/substance use disorders (38). For contact sport athletes specifically, it is reasonable to think that aggression/hostility could also be more prevalent, especially since there is typically a lifetime of playing fairly aggressive sports. With that said, retired professional athletes could be more prone to developing these features during aging, but the underlying etiology may not necessarily be a neurodegenerative disease. For 101 and 206 males meeting diagnostic criteria for Major Depressive Disorder and Intermittent Explosive Disorder in a larger psychological disorders national survey, ~35% met core and supplemental features criteria for TES (13, 14). This was due to irritability (48%), aggression/hostility (31%), anxiety (56%), alcohol use disorder (25%) and suicidal ideation (10%) in the past 12 months, with apathy (18%) and headaches (22%) frequently co-occurring in individuals with such psychological problems. Therefore, many of the clinical features for TES are co-morbid with psychological problems, which would make it challenging to have good sensitivity/specificity in classifying TES vs. unrelated psychological conditions. Indeed, retired professional athletes may be susceptible to experiencing problems adjusting to a shortened career, chronic physical problems, and other life stressors during aging. Misattributing symptoms to a neurodegenerative disorder rather than a treatable condition can have serious consequences. Moving forward, it will be important for future research with TES criteria to identify clinical features that are distinct from common psychological conditions/traits in the general population.
The extraordinary media coverage conflating sports, concussion, and CTE has colored both public and clinician perception of this issue, and influenced decision-making (e.g., avoiding sports, seeking financial restitution). In this context, it is possible that more common disorders or diseases may be overlooked or dismissed in the differential diagnosis, and symptoms could be instead attributed to a poorly understood, rare neuropathological entity, in stark contrast to basic diagnostic approaches across disciplines and despite repeated calls for caution (39). Common comorbidities that overlap with TES, including depression, anxiety, and substance abuse, have an array of effective, empirically based treatments and a diverse range of causes, and it is possible that a diagnosis of TES may influence either course of treatment and/or motivation to participate in treatment. Similarly, some individuals may misinterpret symptoms such as headaches, anger, and apathy as signs of an untreatable progressive disease related to past head impact exposure and fail to present clinically for proper evaluation and intervention. Furthermore, retired players have high rates of depression, chronic pain, and opioid use, and those with this constellation of problems also report greater financial strain and life stress, and it is not difficult to imagine that adding a premature diagnosis based on broad and non-specific criteria to this mix may have adverse or iatrogenic effects [for a review of suicide and CTE, see Iverson 2016—(40)]. This has been tragically illustrated by cases in which athletes have died by suicide after being told they had CTE or after their personal online research led them to this conclusion (41). Thus, determining the specific cause of these commonly occurring symptoms in any one individual can be difficult, and practitioners should be wary of providing false-positive diagnoses of progressive, neurodegenerative diseases.
Although the present study has numerous strengths, including prospective collection of potential TES symptoms, a wide age-range of retirees, and the richness of the available clinical data, there a number of limitations. First, as is the case with most studies, concussion data were collected via self-report and was not verifiable and may be prone to recall bias. Second, the current study did not collect data on impulsivity, although this likely would have increased the already high frequency of TES diagnoses in the sample. Third, because the sample was recruited without strict criteria, it is possible that word of mouth among the retirees participating may have increased the recruitment of other retirees with neurological and psychiatric disorders due to the clinical nature of the study. As such, although we cannot speak to the prevalence of TES diagnoses in all retired professional contact sport athletes, our findings still underscore the ease at which normally functioning retired athletes can be diagnosed with a syndrome purported to represent a neurodegenerative disease. Fourth, in the logistic regression analyses, BDI-II and neuropsychological scores were entered as predictors of TES diagnosis while also being used in making the TES diagnosis, which may have inflated findings of these specific predictors in these models. Nonetheless, our only consistent finding in this regard was the athlete's BDI-II score, and this variable remained significant after removing BDI-II as a diagnostic criterion. As stated earlier, our finding that depressive symptoms predict a TES diagnosis are not surprising in the context of the high base rate of depressive symptoms and the high frequency of depressive symptom criterion in the published TES criteria. Fifth, the Montenigro criteria attempt to increase the specificity of TES criteria to CTE using biomarker data, which was lacking in the current study. Lastly, although not the primary aim of the study, it should be noted that we are unable to provide an accurate estimate of the specificity and sensitivity of the TES criteria, as this would require in vivo biomarkers (which have not been validated) or autopsy-verification of CTE among both clinical and healthy control samples in addition to individuals with repetitive head-injury exposure. The only published data using autopsy-verification has not been peer reviewed (12). Still, we feel our findings underscore the potential for false-positive diagnoses of TES and CTE in those who may not be suffering from a neurodegenerative disease, and adds to the existing literature in this regard (13, 14).
Despite these limitations, the current study is the first to evaluate the frequency of TES diagnoses and TES criteria obtained through direct clinical evaluations of retired professional contact sport athletes, and the first to examine potential predictors of TES diagnosis. Our findings highlight the lack of specificity of the assumed features of CTE and the ease at which a retired player can achieve a diagnosis of TES, despite being clinically diagnosed as normal. We did not find TES diagnoses to be associated with greater head-injury exposure, and only depressive symptoms increased likelihood of a diagnosis, which is not surprising given the strong emphasis placed on potential psychiatric symptoms in the TES criteria. Our findings underscore the need for future study investigating the long-term cognitive correlates of head-injury exposure, the development of biomarkers to aid in vivo clinical diagnoses of CTE, and longitudinal clinicopathological studies of CTE that would add to our understanding regarding the potential relationship between clinical symptoms and CTE pathology.
The datasets presented in this article are not readily available because due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available. Requests to access the datasets should be directed to Munro Cullum, at bXVucm8uY3VsbHVtQHV0c291dGh3ZXN0ZXJuLmVkdQ==.
The studies involving human participants were reviewed and approved by University of Texas Southwestern Medical Center Institutional Review Board and The University of Texas at Dallas Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
JS contributed to the study design, conceptualization, data collection, data analysis, and writing of the manuscript. ND, CL, JH, and CMC contributed to the study design, conceptualization, data collection, and writing of the manuscript. HR and LL contributed to the study design, interpretation of the results, and writing of the manuscript. All authors contributed to the article and approved the submitted version.
This study was made possible, in part, by the Texas Alzheimer's Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer's Disease and Related Disorders. This work was supported in part by an Alzheimer's Association Research Grant (2019-AARG643558) and the US Army Medical Research and Development Program (W81XWH-20-1-0493). Support was also provided by the Texas Institute for Brain Injury and Repair (TIBIR), a state-funded initiative as part of the Peter J. O'Donnell Jr. Brain Institute at The University of Texas Southwestern Medical Center. This study was additionally supported by The University of Texas at Dallas, and in part by grant 5K23AG030006 from the National Institute on Aging.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the retired NFL players whose partnership made this study possible.
1. McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg Clin N Am. (2016) 27:529–35. doi: 10.1016/j.nec.2016.05.009
2. Randolph C. Chronic traumatic encephalopathy is not a real disease. Arch Clin Neuropsychol. (2018) 33:644–8. doi: 10.1093/arclin/acy063
3. Iverson GL, Gardner AJ, Shultz SR, Solomon GS, McCrory P, Zafonte R, et al. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain. (2019) 142:3672–93. doi: 10.1093/brain/awz286
4. Asken BM, Sullan MJ, Snyder AR, Houck ZM, Bryant VE, Hizel LP, et al. Factors influencing clinical correlates of chronic traumatic encephalopathy (CTE): a review. Neuropsychol Rev. (2016) 26:340–63. doi: 10.1007/s11065-016-9327-z
5. Wolfson DI, Kuhn AW, Kerr ZY, Brett BL, Yengo-Kahn AM, Solomon GS, et al. Chronic traumatic encephalopathy research viewed in the public domain: what makes headlines? Brain Inj. (2020) 34:528–34. doi: 10.1080/02699052.2020.1725843
6. Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. (2014) 6:68. doi: 10.1186/s13195-014-0068-z
7. Montenigro PH, Bernick C, Cantu RC. Clinical features of repetitive traumatic brain injury and chronic traumatic encephalopathy. Brain Pathol. (2015) 25:304–17. doi: 10.1111/bpa.12250
8. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. (2013) 9:222. doi: 10.1038/nrneurol.2013.33
9. Reams N, Eckner JT, Almeida AA, Aagesen AL, Giordani B, Paulson H, et al. A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol. (2016) 73:743–9. doi: 10.1001/jamaneurol.2015.5015
10. Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. (2013) 81:1122–9. doi: 10.1212/WNL.0b013e3182a55f7f
11. Mez J, Solomon TM, Daneshvar DH, Murphy L, Kiernan PT, Montenigro PH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther. (2015) 7:62. doi: 10.1186/s13195-015-0148-8
12. Mez J, Alosco M, Daneshvar D, Saltiel N, Baucom Z, Tripodis Y, et al. Validity of traumatic encephalopathy syndrome clinical research criteria for chronic traumatic encephalopathy pathology: the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) study (2433). Neurology. (2020) 94(Suppl. 15):2433.
13. Iverson GL, Gardner AJ. Risk for misdiagnosing chronic traumatic encephalopathy in men with anger control problems. Front Neurol. (2020) 11:739. doi: 10.3389/fneur.2020.00739
14. Iverson GL, Gardner AJ. Risk of misdiagnosing chronic traumatic encephalopathy in men with depression. J Neuropsychiatry Clin Neurosci. (2020) 32:139–46. doi: 10.1176/appi.neuropsych.19010021
15. Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dementia. (2011) 7:532–9. doi: 10.1016/j.jalz.2011.05.2410
16. Palmer BW, Boone KB, Lesser IM, Wohl MA. Base rates of “Impaired” neuropsychological test performance among healthy older adults. Arch Clin Neuropsychol. (1998) 13:503–11. doi: 10.1016/S0887-6177(97)00037-1
17. Brett BL, Wilmoth K, Cummings P, Solomon GS, McCrea MA, Zuckerman SL. The neuropathological and clinical diagnostic criteria of chronic traumatic encephalopathy: a critical examination in relation to other neurodegenerative diseases. J Alzheimers Dis. (2019) 68:591–608. doi: 10.3233/JAD-181058
18. Denney DA, Prigatano GP. Subjective ratings of cognitive and emotional functioning in patients with mild cognitive impairment and patients with subjective memory complaints but normal cognitive functioning. J Clin Exp Neuropsychol. (2019) 41:565–75. doi: 10.1080/13803395.2019.1588229
19. Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology. (1997) 48:581–5. doi: 10.1212/WNL.48.3.581
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association (2000).
21. Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. 2nd ed. San Antonio, TX: Adult Version Manual The Psychological Corporation (2000).
22. Meyers J. The Meyers Scoring System for the Rey Complex Figure and the Recognition Trial: Professional Manual. Odessa, FL: Psychological Assessment Resources (1995).
23. Lacritz LH, Cullum CM, O'Bryant S, Hall J, Massman P, Waring S, et al. Normative data for the Texas Card Sorting Test: a brief new executive function measure. J Int Neuropsychol Soc. (2009) 15:231–2.
24. Heaton R, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African, American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources (2004).
25. Wechsler D. Wechsler Adult Intelligence Scale (WAIS–IV). 4th ed. San Antonio, TX: NCS Pearson (2008).
26. Alosco ML, Kasimis AB, Stamm JM, Chua AS, Baugh CM, Daneshvar DH, et al. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl Psychiatry. (2017) 7:e1236. doi: 10.1038/tp.2017.197
27. Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT, et al. Duration of American Football Play and chronic traumatic encephalopathy. Ann Neurol. (2020) 87:116–31. doi: 10.1002/ana.25611
28. Schaffert J, LoBue C, Fields L, Wilmoth K, Didehbani N, Hart J Jr, et al. Neuropsychological functioning in ageing retired NFL players: a critical review. Int Rev Psychiatry. (2019) 32:71–88. doi: 10.1080/09540261.2019.1658572
29. Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. (2015) 84:1114–20. doi: 10.1212/WNL.0000000000001358
30. Solomon GS, Kuhn AW, Zuckerman SL, Casson IR, Viano DC, Lovell MR, et al. Participation in pre-high school football and neurological, neuroradiological, and neuropsychological findings in later life: a study of 45 retired national football league players. Am J Sports Med. (2016) 44:1106–15. doi: 10.1177/0363546515626164
31. Fields L, Didehbani N, Hart J Jr, Cullum CM. No linear association between number of concussions or years played and cognitive outcomes in retired NFL players. Arch Clin Neuropsychol. (2019) 35:233–9. doi: 10.1093/arclin/acz008
32. Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. (2007) 39:903–9. doi: 10.1249/mss.0b013e3180383da5
33. Kerr ZY, Marshall SW, Harding HP Jr, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med. (2012) 40:2206–12. doi: 10.1177/0363546512456193
34. Didehbani N, Munro Cullum C, Mansinghani S, Conover H, Hart J Jr. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. (2013) 28:418–24. doi: 10.1093/arclin/act028
35. Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA, et al. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. (2013) 81:25–32. doi: 10.1212/WNL.0b013e318299ccf8
36. Brett BL, Mummareddy N, Kuhn AW, Yengo-Kahn AM, Zuckerman SL. The relationship between prior concussions and depression is modified by somatic symptomatology in retired NFL athletes. J Neuropsychiatry Clin Neurosci. (2019) 31:17–24. doi: 10.1176/appi.neuropsych.18040080
37. Iverson GL, Terry DP, Luz M, Zafonte R, McCrory P, Solomon GS, et al. Anger and depression in middle-aged men: implications for a clinical diagnosis of chronic traumatic encephalopathy. J Neuropsychiatry Clin Neurosci. (2019) 31:328–36. doi: 10.1176/appi.neuropsych.18110280
38. Gil F, de Andrade AG, Castaldelli-Maia JM. Discussing prevalence, impacts, and treatment of substance use disorders in athletes. Int Rev Psychiatry. (2016) 28:572–8. doi: 10.1080/09540261.2016.1212821
39. Stewart W, Allinson K, Al-Sarraj S, Bachmeier C, Barlow K, Belli A, et al. Primum non nocere: a call for balance when reporting on CTE. Lancet Neurol. (2019) 18:231–3. doi: 10.1016/S1474-4422(19)30020-1
40. Iverson GL. Suicide and chronic traumatic encephalopathy. J Neuropsychiatry Clin Neurosci. (2016) 28:9–16. doi: 10.1176/appi.neuropsych.15070172
41. Hunsinger Benbow D. Wabash Football Player Searched CTE on Laptop Then Took His Own Life (2020). Available online at: https://www.indystar.com/story/sports/college/2020/02/13/wabash-football-player-searched-cte-laptop-then-took-his-own-life/4750427002/ (accessed October 13, 2020).
Keywords: traumatic encephalopathy syndrome, chronic traumatic encephalopathy, concussion and sports, dementia, athletes
Citation: Schaffert J, Didehbani N, LoBue C, Hart J, Rossetti H, Lacritz L and Cullum CM (2021) Frequency and Predictors of Traumatic Encephalopathy Syndrome in a Prospective Cohort of Retired Professional Athletes. Front. Neurol. 12:617526. doi: 10.3389/fneur.2021.617526
Received: 14 October 2020; Accepted: 28 January 2021;
Published: 23 February 2021.
Edited by:
Thor Stein, Boston University, United StatesReviewed by:
Maria Carmela Tartaglia, University of Toronto, CanadaCopyright © 2021 Schaffert, Didehbani, LoBue, Hart, Rossetti, Lacritz and Cullum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Munro Cullum, bXVucm8uY3VsbHVtQHV0c291dGh3ZXN0ZXJuLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.