- Department of Neurology and Institute of Neurology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Animals acquire motor skills to better survive and adapt to a changing environment. The ability to learn novel motor actions without disturbing learned ones is essential to maintaining a broad motor repertoire. During motor learning, the brain makes a series of adjustments to build novel sensory–motor relationships that are stored within specific circuits for long-term retention. The neural mechanism of learning novel motor actions and transforming them into long-term memory still remains unclear. Here we review the latest findings with regard to the contributions of various brain subregions, cell types, and neurotransmitters to motor learning. Aiming to seek therapeutic strategies to restore the motor memory in relative neurodegenerative disorders, we also briefly describe the common experimental tests and manipulations for motor memory in rodents.
Introduction
Motor learning implies a process of change or improvement in motor action to perform the requested task by practicing and refining (1). There are three components of motor learning: motor skill learning, motor adaptation, and motor action selection (2). Motor skill learning, including motor sequence learning, consists of a series of relatively slow changes in motor functions leading to improved performance. Motor adaptation refers to faster changes in motor behavior that preserve stable performance of learned behavior despite small fluctuations in the environment. Besides the abovementioned motor learning categories, motor action selection is an intermediate one, which is described as the task of choosing which of several possible behaviors to execute (2, 3). In daily life, different categories of motor learning often overlap (4). In this review, we mainly concentrate on motor skill learning. The learning of new skills involves three stages: the initial acquisition phase with fast amelioration in performance, the following consolidation phase with more gradual ameliorations as skills are automatized, and the final retention phase in which the long-lasting memory is formed (5–9). The basal ganglia, cerebellum, and motor cortex are the brain areas involved in motor learning through their circuits (5, 10–12). Motor skill learning is extremely critical for optimizing behavior (13, 14). From a computation-neurobiological perspective, a good motor task should be able to be repeated, emulating a reference model as precisely as possible, aiming to attain the best performance (15). On the other hand, extensive studies have proved that motor skill learning is damaged in patients with Parkinson's disease (PD) (16–20), presymptomatic and symptomatic Huntington's disease (HD) (21–23), and primary dystonia (24). However, the mechanisms on how the brain links various actions together into fluid chains of behavior which are able to be recalled later are still not clear (25). This review addresses the latest findings in motor skill learning, aiming to better comprehend the functional contribution of various brain subregions, cell types, and neurotransmitter systems to this type of memory, evaluate the impact of genetic and pharmacological manipulations, and identify potential treatments for related neurological disorders.
Common Behavior Tests for Motor Skill Learning in Rodents
In the investigations of motor skill learning, smaller animals like rodents are preferred by investigators due to rapid reproduction and relatively low costs. The single use of one test may not be comprehensive for detecting all aspects of learning dysfunction. In light of this, a few of behavioral tests have been applied to evaluate and quantify the presence of motor skill learning impairments in rodents.
Rotarod Test
The rotarod test is a frequently used paradigm to measure a rodent's ability to keep itself on a rotating rod at accelerating speeds (26). This requires mice to keep balance on the rod and measures their latency to drop down which generally correlates motor skill learning (26, 27). The speed is gradually accelerated from 0 to 40 rpm over 5 min, and the rodents are tested for several trials a day (28). Motor skill learning can be assessed by repeated daily testing and suggested by the decrease in latency to drop down during sequential testing sessions (29). Most performance improvements occur on the first day of training (30). However, with longitudinally repeated tests, the animals can learn that the consequences of falling are harmless (28). Thus, individual animals may refuse testing and directly fall once they are put on the rod. In this case, we tend to appropriately increase the sample size, so as to remove the individual outliers with extremely abnormal results in statistical analysis.
Food-Reaching Task
Animals are trained to reach for a food pellet through a narrow slot with a preferred limb (31–33). As a reinforcement learned behavior, it requires acquisition of a skilled reaching movement through daily training over several weeks (33). A successful reach is scored when the animal grasps the food pellet and brings it into the cage and to its mouth without dropping the pellet (34). The basic measures include (a) total number of reach attempts, (b) number of sensory errors, and (c) percentage of successful reaches (33). The shortcoming is that the evaluation is a readout of the learning sequence order rather than focusing on improvements of motor behavior itself (2). After all, the reaching movements themselves are simple without speed-accuracy constraint (35).
Wheel-Skill Learning
Although developed to measure the animal's voluntary activity in home cage (28), the running wheel is also used to investigate procedural learning (36, 37). At the beginning of the training, rats usually could not run on the wheel without causing it to swing (37). Over continued training sessions, they gradually learn how to adjust their movements on the wheel so as to stabilize it and avoid swinging. Within the first training sessions, rats learn the wheel skill fast and the wheel swings provide a measurement of performance error during skill learning (37). The factors that affect wheel-skill learning include the number of trails in each session and the total amount of training sessions (38). However, it does not depend on motivational manipulations, such as forced locomotion in rotarod, food deprivation in instrumental learning, and electric shocks in avoidance learning (39). It is noteworthy that the running-wheel behavior in female animals could be affected by estrous cycle (40). Thus, mixed-gender cohorts should be avoided in these tests (28).
In addition to the tests described above, there are also other assays being used to assess motor learning, such as the beam-walking test. With several days of training followed by one day of testing, the goal is for the tested rodent to keep balance and walk through a narrow viaduct beam to a safe platform (41). Performance of the subjects, including the time to walk across the beam and the times of paw slips during the test, has been validated as a measure of fine coordination. The beam walking test can be useful especially when assessing balancing capacity and subtle deficits in motor skills which are uneasy to be detected by other tests (41).
Specific Brain Subregions and Cell Types Involved in Motor Skill Learning
By means of neuroimaging, lesions, electrical stimulation, and electrophysiological recordings, the major brain regions involved in motor skill learning have been disclosed, including primary motor cortex (M1), basal ganglia (BG), and cerebellum. Each region consists of various intermingled cell types connected in specific circuitry and motor skill occurs via changes in neuronal excitability, synaptic strength, and circuit connectivity (42).
Primary Motor Cortex (M1)
Compared to other nuclei involved in motor learning, M1 acts as a controller which sends commands directly or indirectly to motor neurons (2, 43, 44). The motor cortex provides independent limb control to execute specific actions with high speed and precision and allows flexible synergies of performance related with novel tasks or objects (45, 46). Motor training can induce functional and structural synaptic plasticity in motor map organization (47–50), which is not simply caused by increased use (51). Not like pure exercise or recall of learned skills, a new motor skill learning is able to efficiently trigger the spine formation of pyramidal neurons in the layer V motor cortex (51). Moreover, the tested subjects' performance is closely related with the degree of new spine formation (51–53). In addition, learning-triggered newly formed spines provide a structural basis for enhancing synaptic strength, which are given priority to be stabilized and retained with new skill memory (52, 53). However, longer training could lead to increased spine elimination, indicating that skill refinement might be based on removal of inappropriate connections (52, 53). The skill learning-related spinogenesis could be further induced in the same place where baseline control originates by training the pretrained animals (52, 53). In the sensorimotor cortex, functional synaptic plasticity including long-term depression (LTD) and long-term potentiation (LTP) is the crucial mechanism for acquiring motor skills (54). In humans, improved performance of sequential finger movements are identified to be related with elevation of blood-oxygen level-dependent (BOLD) signal in M1 (55, 56), which is enhanced by transcranial direct current stimulation of M1 (56, 57) and inhibited by repetitive transcranial magnetic stimulation (TMS) of M1 (10). Moreover, TMS could induce piano-playing behaviors in pianists but not in controls, suggesting that M1 can encode novel skills via continuous practicing (58). In rats, the skill acquisition can be completely abolished by destroying dopaminergic (DAergic) projections to the motor cortex, demonstrating that skill learning needs to occur in M1 directly (59).
Basal Ganglia (BG)
The BG, preponderantly involved in movement control and skill learning (60–66), are highly conserved in both anatomy and neurotransmitter localization that consist of cortico-striato-pallido-thalamocortical loops (67). In rodents, BG circuits are critical in task improvement through promoting execution quality. The tested mice's improvement of their performance in rotarod is correlated with synaptic strength enhancement in the striatum (6, 68). In the food reaching task, protein synthesis inhibition in the striatum could impair early stages of learning in rats (69).
The striatum is the main input nucleus of the BG. It works as the central meeting point which compiles and integrates the information from the thalamus, the cortex, and the midbrain DAergic innervation before processing of motor output (70–74). The ventral striatum is involved in reward-related learning due to its anatomical connection with limbic structures (75–78). The dorsal striatum gets involved in movement and action selection, and it mainly receives innervation from the substantia nigra (SN) and cortex (79–84). For the direct pathway, the net effect of activating D1-expressing medium spiny neurons (MSNs) is facilitation of movement by disinhibiting neurons in the motor cortex (85). For the indirect pathway, the net effect of activating D2-expressing MSNs is suppression of movement by inhibiting neurons in the motor cortex (85). When dopamine (DA) is released from DAergic neurons of the substantia nigra pars compacta (SNc) to the dorsal striatum, the direct and indirect pathways are enhanced and attenuated respectively, and vice versa (25). To be noted, the cortical inputs to the BG are unevenly distributed across the two pathways, with the indirect pathway receiving more from the motor cortex and the direct pathway receiving more from somatosensory and limbic systems (74). Within the dorsal striatum, the medial and lateral parts also play various roles in instrumental learning (25, 86). In the rotarod task, improvement of early stage (action selection) depends on striatal projection to the prefrontal cortex (6, 8), while improvement across training days (execution of sequence elements) depends on striatal projection to the sensorimotor cortex (6). In the BG, the motor functions are closely related with non-motor functions. For instance, most of striatal neurons are involved in both reward- and movement-related activities through combining both reward information and motor actions to obtain the reward (87–89). A neuronal system showing such property usually indicates its role in habit learning and goal-directed behavior (89–91).
Cerebellum
From the phylogenetic perspective, the cerebellum is a highly conserved brain architecture across all the vertebrates (92, 93), indicating a sustained evolutionary requirement for a specific computation ability (2). The cerebellum is necessary for adaptation of eye and limb movements, which is engaged in finetuning movement and learning novel motor tasks in real-time (94), through its feed-forward structures from parallel fibers to Purkinje cells, which inhibit the inferior olive and the deep nuclei of the cerebellum (15). The cerebellum is believed to be a site of supervised learning, aiming to adjust the movement pattern by using feedback from the system and further improve future performance (95). Generally, our procedural memories formed in the cerebellum exhibit at least two types of information coding: rate coding and temporal coding (96–99). In the cerebellum, different coding schemes are used within different modules to produce and express various memories. For example, zebrin-negative zones predominantly form the memories by inhibition mechanisms and express the memories partially by temporal coding. While zebrin-positive zones mainly form the memories by enhancement mechanisms and express the memories by rate coding (100). The rotarod performance can be damaged by inhibiting the LTD at parallel fiber-Purkinje cells in the cerebellum (101). The cortico-BG-thalamo-cortical loop is essential for skilled motor coordination, and the LTD in cerebellum plays a role in movement optimization for environmental conditions (102).
Differential cortical and subcortical regions activated by long (days to weeks) (5) and short (minutes to hours) (103, 104) times of motor learning have been shown by numerous functional studies. Among all these brain areas, M1 is a critical structure for skill execution but it is still in the location for stereotypy which is learned initially through BG dependent processes (2). During the motor skill learning process, BG is likely to infuse variability for exploration and then when the best performance matures, variability is decreased and stereotypy and automatization arise (105). The cerebellum controls fine motor skills as well as motor adaptation and coordination (95).
Neurotransmitters and Neuromodulators
A huge emphasis has been put into new methodologies for precise cellular localization of neurotransmitters and neuromodulators, enzymes involved in their synthesis or degradation, receptors, and transporters, which markedly improved our understanding of the molecular pathways that govern motor skill learning. In this section, we review current progress on the mechanisms by which differential modulators get involved in a pathway-specific manner during motor skill learning.
Dopaminergic System
DA functions by binding to its receptors, which are a group of G protein-coupled receptors (GPCR) and function through the second-messenger system (106, 107). In the primary motor cortex, inhibition of either D1 or D2 receptors could impair skill acquisition (108). In the motor cortex, D1 and D2 receptors play different roles in the regulation of synaptic plasticity: predominantly modulate spine elimination and spine formation, respectively (51). As mentioned above, there are two distinct pathways for DAergic modulation of primary motor cortex including (1) mesocortical projections: directly project from VTA and SNc to directly modulate primary motor cortex (108–110) and (2) nigrostriatal projections: activate a set of BG nuclei and indirectly modulate the primary motor cortex (111, 112).
In physiological conditions, DA exerts an irreplaceable effect in regulating bidirectional plasticity of MSNs (113). For the striatal MSNs, the spine density is significantly decreased in the absence of DA by means of 6-hydroxydopamine lesion of the medial forebrain bundle (MFB) (114). In the motor cortex of various mouse models of PD, abnormal remodeling of neuronal circuits has been disclosed by 2-photon in vivo imaging microscopy (115). DA is required for the formation of LTP, which likely is a fingerprint mechanism of a motor memory trace within M1 (116). In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected mice, DA depletion leads to marked instability of synaptic connections and significant spine remodeling in the motor cortex (115). In such mice, motor learning-induced newly formed spines failed to stay stable and were eliminated then, which is associated with impaired retainment of motor memory in PD (115). Although motor symptoms of PD can be alleviated by levodopa (L-dopa) treatment, it was unable to ameliorate functional plasticity in the motor cortex (117), as well as motor skill learning (118).
Cholinergic System
The cholinergic system is correlated with a wide range of neural processes, such as motor, attention, learning, and memory functions (119). In the striatum, acetylcholine (ACh) mainly arises from cholinergic interneurons (CINs), which is involved in controlling the late component of the motor skill learning (120, 121). The cholinergic inputs are activated by ACh binding to nicotinic receptors (nAChRs) on the DAergic axons (122). Thus, the striatum DA release can be directly triggered by CINs tonic firing, independent of the activity of DAergic neuron activity (123, 124). However, in the absence of ACh, DA release is found to be proportional to the firing rate of DAergic neurons (122). Meanwhile, the computational modeling study of PD showed that the lower DA concentration in turn leads to shortening of CIN activity pause in the striatum, and the phasic DA excursion drives learning (125).
In the primary motor cortex, the diffuse cholinergic afferents regulate the synaptic efficiency of horizontal connections (126). Blocking cholinoreceptors is able to alter the learned reaching task in rats (127). Another study of rat showed that increase in ACh levels during early sleep prevented motor memory consolidation in experiments with physostigmine (128). The role of cholinergic connections in motor cortex plasticity also highlights how inhibition of interfering coordinations forms when new movements are learned (129).
Endocannabinoid (eCB) System
In the brain, cannabinoid receptor 1 (CB1) abundantly distribute across the cerebellum, cortical layers I and IV, BG, CA1 pyramidal cell layer, and dentate gyrus (130). Cannabinoid receptor 2 (CB2) was later identified to be highly expressed in the immune system (131, 132). Then, CB1 and CB2 could be activated by the lipids anandamide and 2-arachidonoyl-glycerol (2-AG) with high affinity and efficacy in the brain and intestinal system, which were named eCBs (133–135). The eCB system also includes enzymes involved in eCB biosynthesis and inactivation. The biosynthesis of 2-AG and other monoacylglycerols is catalyzed by diacylglycerol lipase α and β (DAGLα, DAGLβ) (136). The hydrolysis of 2-AG and other monoacylglycerols is catalyzed by monoacylglycerol lipase (MAGL) (137). CB1 is predominantly located in presynaptical membranes of inhibitory and excitatory neurons, which can suppress vesicular release of gamma-aminobutyric acid (GABA) or glutamate and voltage-gated Ca2+ channels in a feedback way (138, 139). In addition, DAGLα is located in postsynaptic membranes and MAGL is located in axon terminals (138). It is suggested that eCBs, especially 2-AG, are inhibitory retrograde neuromodulators (140).
The endocannabinoids, acting as retrograde messengers, are critical for fine-tuning neuronal excitability and synaptic plasticity and involved in neurobiological mechanisms underlying mood, perception, cognition, locomotion, reward-seeking, and motivation-processing (141–144). The cannabinoids have been found with neuroprotective functions in animal models of stroke, epilepsy, HD, PD, multiple sclerosis, and Alzheimer disease (145–150). Biphasic dysregulation of CB1 was disclosed in different PD animal models: hypoactivity at presymptomatic/early stages and hyperactivity at later stages (151–153). The key influence of eCBs in motor behavior, especially in motor learning, has been highlighted (94, 154). The mice without CB1 receptors show less voluntary running behavior in a housed running wheel than wild-type littermates (155). It is suggested that CB1 receptors control the running behavior rather than the locomotor behavior (156, 157). CB1 knockout mice were demonstrated with impairment of cerebellum-dependent discrete motor learning (158). In turn, the motor skill training was found to rescue nicotine-induced damage of synaptic plasticity mediated by eCB in the dorsolateral striatum (159).
GABA and Glutamate
In the BG circuitry, the strength of glutamatergic neurons is dynamically adjusted through long-term plasticity, which regulates motor function and information flow within the BG network (160). In the BG, long-term plasticity of glutamatergic synapses is an essential contributor to adapted motor execution, among which LTD is the most common form of synaptic adaptation (160). Ninety five percent of striatal neurons are MSNs, including direct-pathway MSNs (dMSNs) and indirect-pathway MSNs (iMSNs): (I) dMSNs expressing dynorphin and substance P bear M4 muscarinic and D1 receptors on the membrane and send projections to the BG output structures and (II) iMSNs expressing enkephalin bear adenosine 2A (A2A) and D2 receptors and send projections to GPe (161–163). In the striatum, LTD exerts effects on the postsynaptic activation of metabotropic glutamate receptors (mGluR), leading to eCB production and CB1-mediated reduction of presynaptic glutamate release. Meanwhile, SNr-LTD depends on N-methyl-D-aspartic acid receptor (NMDAR)-triggered endocytosis of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and is independent of mGluR and eCB (160).
A magnetic resonance spectroscopy (MRS) study in human showed that motor sequence learning is associated with a reduced GABA concentration in M1 motor cortex (164, 165). In rat, motor learning-dependent plasticity was found to be regulated by AMPA/GABA receptors of the horizontal connection layer II/III in M1 (102). Motor training could rapid eliminate inhibitory boutons of distal dendrites in layer II/III (166). Specifically, an immediate decrease of axonal boutons occurred on somatostatin (SST) expressing GABAergic neurons after motor training began (166).
In summarization, motor skill learning is the integrative result of different neuromodulator systems, among which every part contributes to a different aspect of learning. Different neural mechanisms check and balance each other to acquire and store motor skills efficiently. The circuitries involved in motor skill learning are summarized in Figure 1, representing the main connections and neuromodulator systems among BG, M1, and cerebellum of rodent brain in a sagittal diagram.
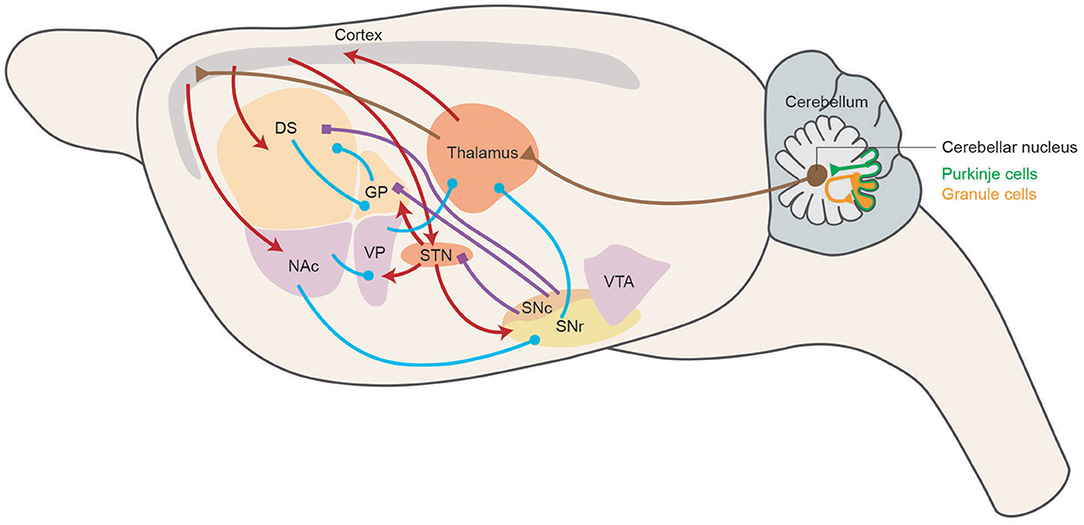
Figure 1. Motor skill learning circuitry in rodents. Schematic representing the main connections of the basal ganglia network in a sagittal section of the rodent brain. Dopaminergic, GABAergic, and glutamatergic projections are depicted in blue, purple, and red, respectively. Cerebellar related circuitry is in brown. DS, dorsal striatum; NAc, nucleus accumbens; GP, globus pallidus; VP, ventral pallidum; STN, subthalamic nucleus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; VTA, ventral tegmental area.
Genetic Manipulations
Optogenetics and chemogenetics are now widely used circuit-based techniques to acutely and reversibly suppress or activate cell-type-specific neuronal firing activity through the use of a genetically mediated actuator expressed on the cell membrane. Here we introduce the mechanisms and applications of major genetic manipulations in exploring specific brain areas and cell types related with motor skill learning.
Optogenetics
Optogenetics, by using genetically encodable light-activated proteins, allows for cell-type (167–169) and projection-specific (170, 171) manipulation of neural circuit elements with precise temporal control. In neural systems, the most commonly used are the channelrhodopsins (ChR2, ChR1, VChR1, and SFOs) to excite neurons, as well as archaerhodopsin-3 (Arch) and enhanced halorhodopsin (eNpHR2.0 and eNpHR3.0) to inhibit neuronal activity (168, 172–174). Within the striatum, optogenetics helped characterize the inhibition of MSNs by CINs as well as confirm the opposing relationship between direct and indirect pathway MSNs (175). In the dorsal striatum, through expressing ChR2 in iMSNs and dMSNs, activation of dMSNs increased locomotion and reduced freezing, while activation of iMSNs induced freezing gait, bradykinesia, and hindered locomotor initiations (176). Using a similar method, dMSNs and iMSNs of the dorsal striatum also showed opposing influences on reinforcement (177). In the mouse cerebellum, the memory of oculomotor learning could be artificially implanted by optogenetic stimulation of the Purkinje cells or the climbing fibers (178). Optogenetic suppressions of different brain regions at different stages of skill training enable us to better understand when and how each region gets involved into learning: (1) primary visual cortex (V1) suppression could reduce accuracy across all training stages; (2) anterior cingulate cortex (ACC) suppression decreased accuracy during learning; and (3) hippocampus suppression affected learning more mildly (179). The combination of optogenetics, in vivo imaging, and pharmacological manipulations revealed that sensory experience transduced through the granule neuron pathway could orchestrate motor learning through remodeling chromatin architecture and neural circuit activity in the anterior dorsal cerebellar vermis of mouse brain (180).
Chemogenetics
By means of mall molecules that activate engineered receptors targeting to specific cell types, genetically encoded neuron manipulation tools have been developed to remotely control diverse neuronal/non-neuronal functions (181). Early chemogenetic technologies were based on GPCRs (182). According to the downstream effector system initiated, GPCRs could suppress or excite neuronal firing (183). However, these early-generation tools have not been broadly adopted in vivo studies due to the relatively weak potency of synthetic ligands (184) and adenosine (185–187) or given modest signaling (188). To overcome these problems, a new platform called DREADD (designer receptor exclusively activated by designer drug) was developed (189), which uniquely get activated by inert molecule and influence neural processes (190, 191). The most commonly used DREADD receptors include the human muscarinic excitatory and inhibitory receptors (hM3Dq and hM4Di), which can be activated by clozapine-N-oxide (CNO) or low concentration of clozapine (CLZ) (191, 192). In the lever-pushing learning paradigm, by combining chemogenetics and two-photon imaging, mice were trained to perform the task in response to a sound cue, followed by monitoring striatal neuron activity. It helped distinguish that D1 neuron silencing impaired initiation of the learned motor, while D2 neuron silencing increased false performance of lever pushing (193). In the MPTP-injection mouse model, chemogenetic re-activation of SST inhibitory interneurons could alleviate the structural and functional deficits of dendritic spines, as well as enhance rotarod learning (194).
To be noted, it is critical to set stringent experimental controls, because even a slight alteration in designing behavior tests or in choosing DREADD receptor or opsin can make cross-study comparisons difficult (195). Therefore, it is strongly emphasized to study the replication and pay attention to the reported technical challenges or negative findings.
Pharmacological Manipulations
6-Hydroxydopamine (6-OHDA)
6-OHDA, also known as oxidopamine or 2,4,5-trihydroxyphenethylamine, is a catecholamine neurotoxin used to destroy DAergic and noradrenergic neurons in the brain (196). Although there are different techniques for DA depletion, the most commonly employed way is to inject 6-OHDA into the striatum or into MFB (33). Intrastriatal injections consist of four infusions of 6-OHDA spanning the entire length of the striatum. This induces direct toxic damage to the DAergic axon terminals and gradual DA depletion occurs over 4 weeks (197). However, MFB injection involves one infusion into the DAergic projections from the SN to striatum and DA depletion and Parkinson's symptoms occur more rapidly and usually within 48 h. The degree of DA depletion can be verified by using immunostaining to assess the levels of tyrosine hydroxylase (TH) (197). Previous studies have demonstrated impaired rotarod behavior in rats with 6-OHDA lesion of striatum during the pre-motor stage of PD (197, 198).
1-Methyl-4-phenyl-1,2,3,6-Tetrahydropyridine (MPTP)
MPTP is a highly lipophilic compound and easy to cross the blood–brain barrier. In the brain, under the catalysis of enzyme monoamine oxidase-B (MAO-B), it is converted to the active metabolite, 1-methyl-4-phenylpyridinium (MPP+) (199). A series of cytotoxic mechanisms leading to apoptotic cell death are induced by MPP+, such as oxidative stress, mitochondrial dysfunction, and energetic failure (199). MPP+ induces vesicular DA into the cytoplasm, leading to the production of cytotoxic substances (200). MPTP-injected primate model, manifesting profound parkinsonian syndrome, has been widely used for development of novel therapeutics of PD (201). However, MPTP has been less used in rats due to the absence of MAO-B leading to limited toxicity of MPTP in rat brain. Conversely, MPTP does produce obvious DA depletion in mice through downregulating the activity of TH in the biosynthetic pathway for catecholamines in DAergic neurons (201).
Rotenone
Rotenone is an insecticide and piscicide that has been related to a high risk of PD (202, 203). Rotenone impairs mitochondrial transport and abolishes the potentiation of the synapse by inhibiting mitochondrial electron transport chain complex I and inducing mitochondrial reactive oxygen species (ROS) generation (204, 205). Rotenone also inhibits microtubule formation from tubulin (206–208). Chronic administration of rotenone could induce a dose- and time-dependent nigro-striatal degeneration by oral administration for mice or intravenous or s.c. infusion for rats (209–212). The administration of rotenone can impair motor behavior, learning, and memory functions in animal model (213–215). In the rotarod test, rotenone-infused rats showed a significantly decreased balancing ability with an increased falling frequency in comparison with control group (216).
How to Restore Motor Learning and Clinical Application
DAergic Enhancement
The DAergic system, critical for motor learning, experiences a parallel decline even with normal aging (217–219). This age-dependent decline, contributing to the faded learning ability, involves DA metabolism, receptors, and transporters (219–221). Thus, pharmacologic strategies that enhance DAergic neurotransmission have been tried in patients with motor learning deficiency during stroke recovery and have been proven a promising adjuvant therapy in motor rehabilitation (222–224). In animal studies, DA and DA-receptor agonists have been proved with a positive role in synaptic plasticity, recovery, and learning after brain lesions (220, 225, 226). Experimental studies in healthy humans showed that premedication with L-dopa (precursor of DA) (227) and cabergoline (D2R agonist) (228) improved the elementary motor memory formation (228). The deficiency in motor skill learning in the PITx3(−/−) mice, a commonly used DA deficiency model (229–231), could be rescued with levodopa treatment (232). However, the PITx3(−/−) mice showed a gradual deterioration after cessation of L-dopa treatment (232). Although the clinical strategies of alleviating DA-related symptoms in PD by DAergic replacement have been proved highly successful in treating the motor symptoms (233), the effects on motor learning ability remained controversial probably due to different motor tasks being used. One clinical trial on the effect of L-dopa on patients with mild-moderate PD showed improved learning of upper extremity task (234). Another two clinical trials suggested that the L-dopa medication did not significantly alter learning performance of the stepping task in PD patients (235, 236). However, some studies hold that, since exogenous DA is delivered systemically, it may suppress the striatal activation during the acquisition stage of motor learning (237–239). Moreover, long-term administration of L-dopa could lead to L-dopa-induced dyskinesia in advanced stage of PD (240–242).
Deep Brain Stimulation (DBS)
Deep brain stimulation (DBS) is the gold standard for surgical treatment in PD patients by modulating specific neural pathways (243). Recently, a clinical trial on PD patients engaged in a visuomotor tracking task disclosed that the impaired sequence motor learning in PD could be partially restored through subthalamic nucleus (STN)-DBS (4). Actually, the disynaptic connections between the cerebellum and BG have been proved in nonhuman primates by viral tracing (244). STN output projects to the ipsilateral cerebellum through pontine synapse and dentate projections form the thalamo-striatal circuitry. Another clinical study in PD showed an obvious positive association of functional connectivity between cerebellar and DBS contacts during STN-DBS. In addition, the PD patients treated with STN-DBS showed the significant learning-related spatial covariance pattern including increased activity in the para-hippocampal gyrus, dorsal premotor cortex, and lateral cerebellum, with covarying reduction in the orbitofrontal cortex and supplementary motor area (SMA) (236). It is suggested that the pathological STN activities could interfere cerebellar functions due to higher firing rate and lacking desynchronization, whereas the electrical stimulation of DBS could liberate or decorrelate the cerebellum from abnormal BG input (245).
Neurofeedback (NFB) Training
NFB works as a biofeedback technology. In NFB, by displaying the sensory signals (reflecting real-time neural activity) to subjects, they can learn to modulate activity in targeted neural areas involved in specific behaviors or brain functions (246, 247). By using functional magnetic resonance imaging (fMRI), researchers are able to monitor the task-induced changes in neural activation and provide neural signal feedback to the participant in a real-time way (rt-fMRI-NFB) (246, 248). In stroke, PD, and Huntington's disease, rt-fMRI-NFB was proved to alter neural activity in motor-associated areas and to modify specific motor behaviors after the self-regulation training was completed (249–251). A clinical study of PD patients of early stage with NFB training showed an improvement in motor speed during tasks as well as activation in STN and GP, which are connected to SMA (252). This model is consistent with a motor learning study in a healthy population, where the functional coupling between BG and SMA increased with practice (253). The increased activation of SMA could raise the input to STN and the activity of GPi, leading to a changed neural activation pattern within the BG network and thus causing an improvement of symptoms (252).
Physical Exercise
Given that physical exercise leads to synaptic reorganization and neuroplasticity changes in the corresponding motor cortex (52, 53). Such exercises usually need specially designed movement patterns, which should consider multiple key factors, such as the visual and other external cues, compliance, and attrition of the patients (254). There are many types of exercise which can be grouped into “motor-skill” exercises, such as various kinds of dancing or Tai Chi, which require participants to keep learning and training of novel movements (254, 255). It seems reasonable that sustained “motor skill” exercises may be more beneficial for PD patients (51). In addition, patients diagnosed with Alzheimer's disease taking part into a waltz dance training were significantly improved with procedural learning (256). To be noted, extra auditory cues could be provided by the music during these physical exercises that access the motor cortex via the cerebellum and SMA via the thalamus, causing improvement of gait speed, initiation, coordination, and cadence (254). There is no doubt about the clear benefits to physical exercises, including the increase of endurance, strength, and balance stability. However, the current overall level of evidence for studies in human beings with neurodegenerative disease is still very low, and thus much remains to be known regarding the mechanisms of exercise-mediated relief of motor symptoms (257).
Conclusion
Here we briefly reviewed the current discoveries of motor learning across rodent and clinical studies on the basis of neural circuitries and neurotransmitter systems in M1, BG, and cerebellum involved in motor learning. In the past few decades, the exploration of the mechanism underneath motor learning has never stopped and kept guiding us better comprehend how the motor memories are formed, stored, recalled, faded, or disturbed as well as restored. With the development of neuro-computational and neuroimaging technologies, along with the combination of genetic and pharmacological manipulations, we could see more essence through the surface than ever. Yet, we believe that more work combining the theoretical progress in rodent models, the use of well-controlled experiments of in vivo neuroimaging, and the newly discovered biomarkers for neuron subtypes by genomics and proteomics methods helps to understand the precise nature and the determinants of specific roles played by precisely-defined cell subtypes when spatiotemporally participating into this form of memory.
Author Contributions
WT contributed by writing the manuscript and creating the figure. SC contributed to the conceptualization and critical review of the manuscript. Both authors contributed equally to drafting the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 81430022, 81971187, and 81771374) and Doctoral Innovation Fund of Shanghai Jiao Tong University School of Medicine (No. BXJ201913).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to Dr. Huaibin Cai for the helpful suggestions of the manuscript. We sincerely apologize to researchers whose work could not be acknowledged due to space constraints.
References
1. Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson's disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. (2009) 15 (Suppl 3):S53–8. doi: 10.1016/S1353-8020(09)70781-3
2. Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. (2011) 72:469–76. doi: 10.1016/j.neuron.2011.10.017
3. Prescott TJ, Bryson JJ, Seth AK. Introduction. Modelling natural action selection. Philos Trans R Soc Lond B Biol Sci. (2007) 362:1521–9. doi: 10.1098/rstb.2007.2050
4. de Almeida Marcelino AL, Horn A, Krause P, Kühn AA, Neumann WJ. Subthalamic neuromodulation improves short-term motor learning in Parkinson's disease. Brain. (2019) 142:2198–206. doi: 10.1093/brain/awz152
5. Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. (1998) 95:861–8. doi: 10.1073/pnas.95.3.861
6. Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. (2009) 12:333–41. doi: 10.1038/nn.2261
7. Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci. (2004) 24:5560–9. doi: 10.1523/JNEUROSCI.0562-04.2004
8. Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. (1997) 115:1–5. doi: 10.1007/PL00005669
9. Hikosaka O, Rand MK, Nakamura K, Miyachi S, Kitaguchi K, Sakai K, et al. Long-term retention of motor skill in macaque monkeys and humans. Exp Brain Res. (2002) 147:494–504. doi: 10.1007/s00221-002-1258-7
10. Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, et al. Early consolidation in human primary motor cortex. Nature. (2002) 415:640–4. doi: 10.1038/nature712
11. Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. (2002) 296:2043–6. doi: 10.1126/science.1068524
12. Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. (2002) 78:553–64. doi: 10.1006/nlme.2002.4091
13. Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci. (2013) 17:434–41. doi: 10.1016/j.tics.2013.07.001
14. Kupferschmidt DA, Juczewski K, Cui G, Johnson KA, Lovinger DM. Parallel, but dissociable, processing in discrete corticostriatal inputs encodes skill learning. Neuron. (2017) 96:476–89.e5. doi: 10.1016/j.neuron.2017.09.040
15. Kiper P, Szczudlik A, Venneri A, Stozek J, Luque-Moreno C, Opara J, et al. Computational models and motor learning paradigms: could they provide insights for neuroplasticity after stroke? An overview. J Neurol Sci. (2016) 369:141–8. doi: 10.1016/j.jns.2016.08.019
16. Doyon J, Gaudreau D, Laforce R Jr, Castonguay M, Bédard PJ, Bédard F, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn. (1997) 34:218–45. doi: 10.1006/brcg.1997.0899
17. Doyon J, Laforce R Jr, Bouchard G, Gaudreau D, Roy J, Poirier M, et al. Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia. (1998) 36:625–41. doi: 10.1016/S0028-3932(97)00168-1
18. Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson's disease or cerebellar lesions. J Cogn Neurosci. (2003) 15:1232–43. doi: 10.1162/089892903322598175
19. Stefanova ED, Kostic VS, Ziropadja L, Markovic M, Ocic GG. Visuomotor skill learning on serial reaction time task in patients with early Parkinson's disease. Mov Disord. (2000) 15:1095–3. doi: 10.1002/1531-8257(200011)15:6<1095::AID-MDS1006>3.0.CO;2-R
20. Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis. (2015) 82:226–34. doi: 10.1016/j.nbd.2015.06.014
21. Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. (2006) 59:53–9. doi: 10.1002/ana.20684
22. Willingham DB, Koroshetz WJ. Evidence for dissociable motor skills in Huntington's disease patients. Psychobiology. (1993) 21:173–82.
23. Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington's disease. Behav Neurosci. (1988) 102:141–7. doi: 10.1037/0735-7044.102.1.141
24. Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the dYT1 dystonia mutation. Ann Neurol. (2003) 54:102–9. doi: 10.1002/ana.10610
25. Garr E. Contributions of the basal ganglia to action sequence learning and performance. Neurosci Biobehav Rev. (2019) 107:279–95. doi: 10.1016/j.neubiorev.2019.09.017
26. Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. (2004) 81:211–6. doi: 10.1016/j.nlm.2004.01.001
27. Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking nMDAR1 in the striatum. Proc Natl Acad Sci USA. (2006) 103:15254–9. doi: 10.1073/pnas.0601758103
28. Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user's guide. Nat Rev Neurosci. (2009) 10:519–29. doi: 10.1038/nrn2652
29. Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of acra7-deficient mice. Learn Mem. (1998) 5:302–16.
30. Ölveczky BP. Motoring ahead with rodents. Curr Opin Neurobiol. (2011) 21:571–8. doi: 10.1016/j.conb.2011.05.002
31. Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, et al. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. (2008) 170:229–44. doi: 10.1016/j.jneumeth.2008.01.015
32. Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. (2008) 192:124–36. doi: 10.1016/j.bbr.2007.12.026
33. Plowman EK, Kleim JA. Behavioral and neurophysiological correlates of striatal dopamine depletion: a rodent model of Parkinson's disease. J Commun Disord. (2011) 44:549–56. doi: 10.1016/j.jcomdis.2011.04.008
34. Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. (1991) 42:77–91. doi: 10.1016/S0166-4328(05)80042-7
35. Hikosaka O, Rand MK, Miyachi S, Miyashita K. Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol. (1995) 74:1652–61. doi: 10.1152/jn.1995.74.4.1652
36. Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioural context. Eur J Neurosci. (2003) 17:1053–66. doi: 10.1046/j.1460-9568.2003.02525.x
37. Willuhn I, Steiner H. Motor-skill learning-associated gene regulation in the striatum: effects of cocaine. Neuropsychopharmacology. (2006) 31:2669–82. doi: 10.1038/sj.npp.1300995
38. Willuhn I, Steiner H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience. (2008) 153:249–58. doi: 10.1016/j.neuroscience.2008.01.041
39. Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. (1998) 56:11–27. doi: 10.1006/anbe.1998.0836
40. Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol Behav. (1991) 49:1079–84. doi: 10.1016/0031-9384(91)90334-K
41. Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. (2011) 49:2376. doi: 10.3791/2376
42. Papale AE, Hooks BM. Circuit changes in motor cortex during motor skill learning. Neuroscience. (2018) 368:283–97. doi: 10.1016/j.neuroscience.2017.09.010
43. Metz GA, Dietz V, Schwab ME, van de Meent H. The effects of unilateral pyramidal tract section on hindlimb motor performance in the rat. Behav Brain Res. (1998) 96:37–46. doi: 10.1016/S0166-4328(97)00195-2
44. Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. (1996) 75:1126–37. doi: 10.1152/jn.1996.75.3.1126
45. Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature. (2011) 478:387–90. doi: 10.1038/nature10436
46. Gritsenko V, Kalaska JF, Cisek P. Descending corticospinal control of intersegmental dynamics. J Neurosci. (2011) 31:11968–79. doi: 10.1523/JNEUROSCI.0132-11.2011
47. Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. (1996) 16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996
48. Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. (1998) 80:3321–5. doi: 10.1152/jn.1998.80.6.3321
49. Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. (2002) 934:1–6. doi: 10.1016/S0006-8993(02)02239-4
50. Pearce AJ, Thickbroom GW, Byrnes ML, Mastaglia FL. Functional reorganisation of the corticomotor projection to the hand in skilled racquet players. Exp Brain Res. (2000) 130:238–43. doi: 10.1007/s002219900236
51. Xu T, Wang S, Lalchandani RR, Ding JB. Motor learning in animal models of Parkinson's disease: aberrant synaptic plasticity in the motor cortex. Mov Disord. (2017) 32:487–97. doi: 10.1002/mds.26938
52. Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. (2009) 462:915–9. doi: 10.1038/nature08389
53. Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. (2009) 462:920–4. doi: 10.1038/nature08577
54. Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. (2004) 125:329–36. doi: 10.1016/j.neuroscience.2004.01.048
55. Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. (1995) 377:155–8. doi: 10.1038/377155a0
56. Stagg CJ, Bachtiar V, Johansen-Berg H. The role of gABA in human motor learning. Curr Biol. (2011) 21:480–4. doi: 10.1016/j.cub.2011.01.069
57. Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. (2009) 106:1590–5. doi: 10.1073/pnas.0805413106
58. Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. (2010) 20:1869–74. doi: 10.1016/j.cub.2010.09.045
59. Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. (2011) 31:2481–7. doi: 10.1523/JNEUROSCI.5411-10.2011
60. McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. (1994) 61:260–70. doi: 10.1016/S0163-1047(05)80009-3
61. Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. (1995) 5:733–41. doi: 10.1016/0959-4388(95)80100-6
62. Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. (1998) 70:119–36. doi: 10.1006/nlme.1998.3843
63. Salmon DP, Butters N. Neurobiology of skill and habit learning. Curr Opin Neurobiol. (1995) 5:184–90. doi: 10.1016/0959-4388(95)80025-5
64. Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. (1996) 273:1399–402. doi: 10.1126/science.273.5280.1399
65. White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. (1997) 7:164–9. doi: 10.1016/S0959-4388(97)80004-9
66. White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. (2002) 77:125–84. doi: 10.1006/nlme.2001.4008
67. Baladron J, Hamker FH. Habit learning in hierarchical cortex-basal ganglia loops. Eur J Neurosci. (2020) 52:4613–38. doi: 10.1111/ejn.14730
68. Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. (2004) 14:1124–34. doi: 10.1016/j.cub.2004.06.053
69. Wächter T, Röhrich S, Frank A, Molina-Luna K, Pekanovic A, Hertler B, et al. Motor skill learning depends on protein synthesis in the dorsal striatum after training. Exp Brain Res. (2010) 200:319–23. doi: 10.1007/s00221-009-2027-7
70. Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. (1994) 344:1–19. doi: 10.1002/cne.903440102
71. Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. (1998) 18:4722–31. doi: 10.1523/JNEUROSCI.18-12-04722.1998
72. Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. (2000) 196 (Pt 4):527–42. doi: 10.1046/j.1469-7580.2000.19640527.x
73. Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, et al. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS ONE. (2015) 10:e0123381. doi: 10.1371/journal.pone.0123381
74. Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. (2013) 79:347–60. doi: 10.1016/j.neuron.2013.05.014
75. Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. (1982) 7:2321–35. doi: 10.1016/0306-4522(82)90198-1
76. Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat–an anatomical study by anterograde and retrograde tracing methods. Neuroscience. (1982) 7:615–30. doi: 10.1016/0306-4522(82)90067-7
77. McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. (1989) 29:503–37. doi: 10.1016/0306-4522(89)90128-0
78. Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, et al. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. (2002) 116:553–67. doi: 10.1037/0735-7044.116.4.553
79. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. (1989) 12:366–75. doi: 10.1016/0166-2236(89)90074-X
80. Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. (1994) 265:1826–31. doi: 10.1126/science.8091209
81. Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp Brain Res. (2002) 142:67–80. doi: 10.1007/s00221-001-0918-3
82. Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. (2002) 418:413–7. doi: 10.1038/nature00892
83. Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. (2003) 26:317–30. doi: 10.1016/j.jchemneu.2003.10.003
84. Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. (2007) 27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007
85. Oldenburg IA, Sabatini BL. Antagonistic but not symmetric regulation of primary motor cortex by basal ganglia direct and indirect pathways. Neuron. (2015) 86:1174–81. doi: 10.1016/j.neuron.2015.05.008
86. Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. (2012) 72:389–95. doi: 10.1016/j.biopsych.2012.02.024
87. Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol. (1998) 80:947–63. doi: 10.1152/jn.1998.80.2.947
88. Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. (2005) 310:1337–40. doi: 10.1126/science.1115270
89. Schultz W. Reward functions of the basal ganglia. J Neural Transm (Vienna). (2016) 123:679–93. doi: 10.1007/s00702-016-1510-0
90. Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. (2004) 19:181–9. doi: 10.1111/j.1460-9568.2004.03095.x
91. Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. (2005) 22:513–23. doi: 10.1111/j.1460-9568.2005.04218.x
92. Bell CC. Evolution of cerebellum-like structures. Brain Behav Evol. (2002) 59:312–26. doi: 10.1159/000063567
93. Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. (2008) 31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225
94. El Manira A, Kyriakatos A. The role of endocannabinoid signaling in motor control. Physiology (Bethesda). (2010) 25:230–8. doi: 10.1152/physiol.00007.2010
95. Raymond JL, Medina JF. Computational principles of supervised learning in the cerebellum. Annu Rev Neurosci. (2018) 41:233–53. doi: 10.1146/annurev-neuro-080317-061948
96. De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. (2011) 12:327–44. doi: 10.1038/nrn3011
97. Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. Elife. (2013) 2:e01574. doi: 10.7554/eLife.01574
98. Heck DH, De Zeeuw CI, Jaeger D, Khodakhah K, Person AL. The neuronal code(s) of the cerebellum. J Neurosci. (2013) 33:17603–9. doi: 10.1523/JNEUROSCI.2759-13.2013
99. Person AL, Raman IM. Synchrony and neural coding in cerebellar circuits. Front Neural Circuits. (2012) 6:97. doi: 10.3389/fncir.2012.00097
100. De Zeeuw CI, Ten Brinke MM. Motor learning and the cerebellum. Cold Spring Harb Perspect Biol. (2015) 7:a021683. doi: 10.1101/cshperspect.a021683
101. Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, et al. D-serine regulates cerebellar LTD and motor coordination through the δ2 glutamate receptor. Nat Neurosci. (2011) 14:603–11. doi: 10.1038/nn.2791
102. Kida H, Mitsushima D. Mechanisms of motor learning mediated by synaptic plasticity in rat primary motor cortex. Neurosci Res. (2018) 128:14–8. doi: 10.1016/j.neures.2017.09.008
103. Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. (1997) 277:821–5. doi: 10.1126/science.277.5327.821
104. Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, et al. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol. (2004) 91:924–33. doi: 10.1152/jn.00675.2003
105. Costa RM. A selectionist account of de novo action learning. Curr Opin Neurobiol. (2011) 21:579–86. doi: 10.1016/j.conb.2011.05.004
106. Dawson TM, Barone P, Sidhu A, Wamsley JK, Chase TN. Quantitative autoradiographic localization of d-1 dopamine receptors in the rat brain: use of the iodinated ligand [125I]SCH 23982. Neurosci Lett. (1986) 68:261–6. doi: 10.1016/0304-3940(86)90499-4
107. Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine d2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H] raclopride. Proc Natl Acad Sci USA. (1989) 86:6412–6. doi: 10.1073/pnas.86.16.6412
108. Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE. (2009) 4:e7082. doi: 10.1371/journal.pone.0007082
109. Bertuzzi M, Tang D, Calligaris R, Vlachouli C, Finaurini S, Sanges R, et al. A human minisatellite hosts an alternative transcription start site for nPRL3 driving its expression in a repeat number-dependent manner. Hum Mutat. (2020) 41:807–824. doi: 10.1002/humu.23974
110. Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. (1987) 7:279–90. doi: 10.1523/JNEUROSCI.07-01-00279.1987
111. Fuxe K, Manger P, Genedani S, Agnati L. The nigrostriatal dA pathway and Parkinson's disease. J Neural Transm Suppl. (2006) 70:71–83. doi: 10.1007/978-3-211-45295-0_13
112. Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. (2011) 34:441–66. doi: 10.1146/annurev-neuro-061010-113641
113. Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. (2008) 321:848–51. doi: 10.1126/science.1160575
114. Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. (1989) 503:334–8. doi: 10.1016/0006-8993(89)91686-7
115. Guo L, Xiong H, Kim JI, Wu YW, Lalchandani RR, Cui Y, et al. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson's disease. Nat Neurosci. (2015) 18:1299–309. doi: 10.1038/nn.4082
116. Hosp JA, Luft AR. Dopaminergic meso-cortical projections to m1: role in motor learning and motor cortex plasticity. Front Neurol. (2013) 4:145. doi: 10.3389/fneur.2013.00145
117. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. (2007) 35:222–33. doi: 10.1016/j.neuroimage.2006.11.047
118. Kishore A, Joseph T, Velayudhan B, Popa T, Meunier S. Early, severe and bilateral loss of lTP and lTD-like plasticity in motor cortex (M1) in de novo Parkinson's disease. Clin Neurophysiol. (2012) 123:822–8. doi: 10.1016/j.clinph.2011.06.034
119. Miwa JM, Walz A. Enhancement in motor learning through genetic manipulation of the lynx1 gene. PLoS ONE. (2012) 7:e43302. doi: 10.1371/journal.pone.0043302
120. Shapovalova KB. Current concepts of the neuromorphology and neurochemistry of the striatal cholinergic system and its role in regulating movement. Zh Vyssh Nerv Deiat Im I P Pavlova. (1996) 46:656–73.
121. Shapovalova KB. The cholinergic system of the striatum: its participation in the motor and sensory components of motor behavior. Zh Vyssh Nerv Deiat Im I P Pavlova. (1997) 47:393–411.
122. Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. (2004) 7:583–4. doi: 10.1038/nn1244
123. Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. (2012) 2:33–41. doi: 10.1016/j.celrep.2012.05.011
124. Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. (2012) 75:58–64. doi: 10.1016/j.neuron.2012.04.038
125. Kim T, Capps RA, Hamade KC, Barnett WH, Todorov DI, Latash EM, et al. The functional role of striatal cholinergic interneurons in reinforcement learning from computational perspective. Front Neural Circuits. (2019) 13:10. doi: 10.3389/fncir.2019.00010
126. Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. (1995) 97:382–6. doi: 10.1016/0924-980X(95)00194-P
127. Zhuravin IA, Dubrovskaia NM. The participation of the cholinergic system of the rat sensorimotor cortex in regulating different types of movements. Zh Vyssh Nerv Deiat Im I P Pavlova. (2000) 50:103–12.
128. Inayat S, Nazariahangarkolaee M, Singh S, McNaughton BL, Whishaw IQ, Mohajerani MH. Low acetylcholine during early sleep is important for motor memory consolidation. Sleep. (2020) 43:zsz297. doi: 10.1093/sleep/zsz297
129. Hess G, Donoghue JP. Facilitation of long-term potentiation in layer iI/III horizontal connections of rat motor cortex following layer i stimulation: route of effect and cholinergic contributions. Exp Brain Res. (1999) 127:279–90. doi: 10.1007/s002210050797
130. Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. (1998) 51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A
131. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. (1993) 365:61–5. doi: 10.1038/365061a0
132. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. (1990) 346:561–4. doi: 10.1038/346561a0
133. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. (1992) 258:1946–9. doi: 10.1126/science.1470919
134. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. (1995) 50:83–90. doi: 10.1016/0006-2952(95)00109-D
135. Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. (1995) 215:89–97. doi: 10.1006/bbrc.1995.2437
136. Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. (2003) 163:463–8. doi: 10.1083/jcb.200305129
137. Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. (2002) 99:10819–24. doi: 10.1073/pnas.152334899
138. Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. (2008) 14:923–30. doi: 10.1038/nm.f.1869
139. Mátyás F, Urbán GM, Watanabe M, Mackie K, Zimmer A, Freund TF, et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both gABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. (2008) 54:95–107. doi: 10.1016/j.neuropharm.2007.05.028
140. Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. (2001) 410:588–92. doi: 10.1038/35069076
141. Melis M, Muntoni AL, Pistis M. Endocannabinoids and the processing of value-related signals. Front Pharmacol. (2012) 3:7. doi: 10.3389/fphar.2012.00007
142. Melis M, Pistis M. Hub and switches: endocannabinoid signalling in midbrain dopamine neurons. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3276–85. doi: 10.1098/rstb.2011.0383
143. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handb Exp Pharmacol. (2015) 231:59–93. doi: 10.1007/978-3-319-20825-1_3
144. Wang H, Lupica CR. Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes. Prog Neuropsychopharmacol Biol Psychiatry. (2014) 52:24–7. doi: 10.1016/j.pnpbp.2014.01.019
145. Alger BE. Endocannabinoids and their implications for epilepsy. Epilepsy Curr. (2004) 4:169–73. doi: 10.1111/j.1535-7597.2004.04501.x
146. de Lago E, Fernández-Ruiz J, Ortega-Gutiérrez S, Cabranes A, Pryce G, Baker D, et al. UCM707, an inhibitor of the anandamide uptake, behaves as a symptom control agent in models of Huntington's disease and multiple sclerosis, but fails to delay/arrest the progression of different motor-related disorders. Eur Neuropsychopharmacol. (2006) 16:7–18. doi: 10.1016/j.euroneuro.2005.06.001
147. Jackson SJ, Pryce G, Diemel LT, Cuzner ML, Baker D. Cannabinoid-receptor 1 null mice are susceptible to neurofilament damage and caspase 3 activation. Neuroscience. (2005) 134:261–8. doi: 10.1016/j.neuroscience.2005.02.045
148. Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. (2005) 19:96–107. doi: 10.1016/j.nbd.2004.11.009
149. Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci. (2004) 19:1691–8. doi: 10.1111/j.1460-9568.2004.03285.x
150. Grotenhermen F. Cannabinoids. Curr Drug Targets CNS Neurol Disord. (2005) 4:507–30. doi: 10.2174/156800705774322111
151. García-Arencibia M, García C, Kurz A, Rodríguez-Navarro JA, Gispert-Sáchez S, Mena MA, et al. Cannabinoid cB1 receptors are early downregulated followed by a further upregulation in the basal ganglia of mice with deletion of specific park genes. J Neural Transm Suppl. (2009) 73:269–75. doi: 10.1007/978-3-211-92660-4_22
152. Walsh S, Mnich K, Mackie K, Gorman AM, Finn DP, Dowd E. Loss of cannabinoid cB1 receptor expression in the 6-hydroxydopamine-induced nigrostriatal terminal lesion model of Parkinson's disease in the rat. Brain Res Bull. (2010) 81:543–8. doi: 10.1016/j.brainresbull.2010.01.009
153. Rojo-Bustamante E, Abellanas MA, Clavero P, Thiolat ML, Li Q, Luquin MR, et al. The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol Dis. (2018) 118:64–75. doi: 10.1016/j.nbd.2018.06.019
154. Chaouloff F, Dubreucq S, Bellocchio L, Marsicano G. Endocannabinoids and motor behavior: cB1 receptors also control running activity. Physiology (Bethesda). (2011) 26:76–7; author reply 78. doi: 10.1152/physiol.00050.2010
155. Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. (2010) 224:106–13. doi: 10.1016/j.expneurol.2010.01.017
156. Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. (2009) 8:685–98. doi: 10.1111/j.1601-183X.2009.00512.x
157. Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in cB1 receptor knockout mice. Science. (1999) 283:401–4. doi: 10.1126/science.283.5400.401
158. Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. (2006) 26:8829–37. doi: 10.1523/JNEUROSCI.1236-06.2006
159. Licheri V, Eckernäs D, Bergquist F, Ericson M, Adermark L. Nicotine-induced neuroplasticity in striatum is subregion-specific and reversed by motor training on the rotarod. Addict Biol. (2020) 25:e12757. doi: 10.1111/adb.12757
160. Dupuis JP, Bioulac BH, Baufreton J. Long-term depression at distinct glutamatergic synapses in the basal ganglia. Rev Neurosci. (2014) 25:741–54. doi: 10.1515/revneuro-2014-0024
161. Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, et al. D1 and d2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. (1990) 250:1429–32. doi: 10.1126/science.2147780
162. Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. (2008) 28:10814–24. doi: 10.1523/JNEUROSCI.2660-08.2008
163. Bertran-Gonzalez J, Hervé D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. (2010) 4:136. doi: 10.3389/fnana.2010.00136
164. Kolasinski J, Hinson EL, Divanbeighi Zand AP, Rizov A, Emir UE, Stagg CJ. The dynamics of cortical GABA in human motor learning. J Physiol. (2019) 597:271–282. doi: 10.1113/JP276626
165. Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. (2006) 95:1639–44. doi: 10.1152/jn.00346.2005
166. Chen SX, Kim AN, Peters AJ, Komiyama T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci. (2015) 18:1109–15. doi: 10.1038/nn.4049
167. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. (2005) 8:1263–8. doi: 10.1038/nn1525
168. Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. (2007) 446:633–9. doi: 10.1038/nature05744
169. Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. (2008) 28:7025–30. doi: 10.1523/JNEUROSCI.1954-08.2008
170. Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. (2011) 471:358–62. doi: 10.1038/nature09820
171. Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. (2011) 72:721–33. doi: 10.1016/j.neuron.2011.10.028
172. Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. (2010) 141:154–65. doi: 10.1016/j.cell.2010.02.037
173. Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O'Shea DJ, Prakash R, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. (2011) 9:159–72. doi: 10.1038/nmeth.1808
174. Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. (2010) 463:98–102. doi: 10.1038/nature08652
175. Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. (2013) 1511:73–92. doi: 10.1016/j.brainres.2012.11.001
176. Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of Parkinson ian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. (2010) 466:622–6. doi: 10.1038/nature09159
177. Paxinos G. Brain, behaviour and evolution. Adv Exp Med Biol. (2015) 821:1. doi: 10.1007/978-3-319-08939-3_1
178. Nguyen-Vu TD, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, et al. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. (2013) 16:1734–6. doi: 10.1038/nn.3576
179. Weible AP, Posner MI, Niell CM. Differential involvement of three brain regions during mouse skill learning. eNeuro. (2019) 6:19. doi: 10.1523/ENEURO.0143-19.2019
180. Yamada T, Yang Y, Valnegri P, Juric I, Abnousi A, Markwalter KH, et al. Sensory experience remodels genome architecture in neural circuit to drive motor learning. Nature. (2019) 569:708–13. doi: 10.1038/s41586-019-1190-7
181. Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. (2014) 37:387–407. doi: 10.1146/annurev-neuro-071013-014048
182. Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at g protein-coupled receptors. Annu Rev Pharmacol Toxicol. (2011) 51:117–44. doi: 10.1146/annurev-pharmtox-010510-100553
183. Farrell MS, Roth BL. Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res. (2013) 1511:6–20. doi: 10.1016/j.brainres.2012.09.043
184. Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, et al. A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a “salt-bridge disruption” mechanism. J Pharmacol Exp Ther. (2000) 293:735–46.
185. Gao ZG, Duong HT, Sonina T, Kim SK, Van Rompaey P, Van Calenbergh S, et al. Orthogonal activation of the reengineered A3 adenosine receptor (neoceptor) using tailored nucleoside agonists. J Med Chem. (2006) 49:2689–702. doi: 10.1021/jm050968b
186. Jacobson KA, Gao ZG, Chen A, Barak D, Kim SA, Lee K, et al. Neoceptor concept based on molecular complementarity in GPCRs: a mutant adenosine a(3) receptor with selectively enhanced affinity for amine-modified nucleosides. J Med Chem. (2001) 44:4125–36. doi: 10.1021/jm010232o
187. Jacobson KA, Ohno M, Duong HT, Kim SK, Tchilibon S, Cesnek M, et al. A neoceptor approach to unraveling microscopic interactions between the human A2A adenosine receptor and its agonists. Chem Biol. (2005) 12:237–47. doi: 10.1016/j.chembiol.2004.12.010
188. Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. (2009) 458:1025–9. doi: 10.1038/nature07926
189. Armbruster BN, Roth BL. Mining the receptorome. J Biol Chem. (2005) 280:5129–32. doi: 10.1074/jbc.R400030200
190. Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved g protein-coupled receptors. Neuron. (2009) 63:27–39. doi: 10.1016/j.neuron.2009.06.014
191. Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of g protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. (2007) 104:5163–8. doi: 10.1073/pnas.0700293104
192. Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Michaelides M Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. (2017) 357:503–7. doi: 10.1126/science.aan2475
193. Sheng MJ, Lu D, Shen ZM, Poo MM. Emergence of stable striatal D1R and D2R neuronal ensembles with distinct firing sequence during motor learning. Proc Natl Acad Sci USA. (2019) 116:11038–47. doi: 10.1073/pnas.1901712116
194. Chen K, Yang G, So KF, Zhang L. Activation of cortical somatostatin interneurons rescues synapse loss and motor deficits after acute MPTP infusion. iScience. (2019) 17:230–41. doi: 10.1016/j.isci.2019.06.040
195. Carr MR, de Vries TJ, Pattij T. Optogenetic and chemogenetic approaches to manipulate attention, impulsivity and behavioural flexibility in rodents. Behav Pharmacol. (2018) 29:560–8. doi: 10.1097/FBP.0000000000000425
196. Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. (1997) 50:55–66. doi: 10.1007/978-3-7091-6842-4_7
197. Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. (1998) 152:259–77. doi: 10.1006/exnr.1998.6848
198. Gambhir H, Mathur R, Behari M. Progressive impairment in motor skill learning at 12 and 20 weeks post 6-OHDA- SNc lesion in rats. Parkinsonism Relat Disord. (2011) 17:476–8. doi: 10.1016/j.parkreldis.2010.12.017
199. Przedborski S, Vila M. MPTP: a review of its mechanisms of neurotoxicity. Clin Neurosci Res. (2001) 1:407–18. doi: 10.1016/S1566-2772(01)00019-6
200. Lotharius J, O'Malley KL. The Parkinson ism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem. (2000) 275:38581–8. doi: 10.1074/jbc.M005385200
201. Dunnett SB, Lelos M. Behavioral analysis of motor and non-motor symptoms in rodent models of Parkinson's disease. Prog Brain Res. (2010) 184:35–51. doi: 10.1016/S0079-6123(10)84003-8
202. Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, et al. Pesticide/environmental exposures and Parkinson's disease in east texas. J Agromedicine. (2008) 13:37–48. doi: 10.1080/10599240801986215
203. Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. (2011) 119:866–72. doi: 10.1289/ehp.1002839
204. Schuler F, Casida JE. Functional coupling of PSST and ND1 subunits in nADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim Biophys Acta. (2001) 1506:79–87. doi: 10.1016/S0005-2728(01)00183-9
205. Schönfeld P, Reiser G. Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J Biol Chem. (2006) 281:7136–42. doi: 10.1074/jbc.M513198200
206. Choi WS, Palmiter RD, Xia Z. Loss of mitochondrial complex i activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. J Cell Biol. (2011) 192:873–82. doi: 10.1083/jcb.201009132
207. Brinkley BR, Barham SS, Barranco SC, Fuller GM. Rotenone inhibition of spindle microtubule assembly in mammalian cells. Exp Cell Res. (1974) 85:41–6. doi: 10.1016/0014-4827(74)90210-9
208. Marshall LE, Himes RH. Rotenone inhibition of tubulin self-assembly. Biochim Biophys Acta. (1978) 543:590–4. doi: 10.1016/0304-4165(78)90315-X
209. Inden M, Kitamura Y, Takeuchi H, Yanagida T, Takata K, Kobayashi Y, et al. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem. (2007) 101:1491–504. doi: 10.1111/j.1471-4159.2006.04440.x
210. Inden M, Kitamura Y, Abe M, Tamaki A, Takata K, Taniguchi T. Parkinsonian rotenone mouse model: reevaluation of long-term administration of rotenone in c57BL/6 mice. Biol Pharm Bull. (2011) 34:92–6. doi: 10.1248/bpb.34.92
211. Takeuchi H, Yanagida T, Inden M, Takata K, Kitamura Y, Yamakawa K, et al. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson's disease models. J Neurosci Res. (2009) 87:576–85. doi: 10.1002/jnr.21869
212. Bové J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. (2012) 211:51–76. doi: 10.1016/j.neuroscience.2011.10.057
213. Salama M, Helmy B, El-Gamal M, Reda A, Ellaithy A, Tantawy D, et al. Role of l-thyroxin in counteracting rotenone induced neurotoxicity in rats. Environ Toxicol Pharmacol. (2013) 35:270–7. doi: 10.1016/j.etap.2012.12.008
214. Sharma N, Nehru B. Beneficial effect of vitamin e in rotenone induced model of PD: behavioural, neurochemical and biochemical study. Exp Neurobiol. (2013) 22:214–23. doi: 10.5607/en.2013.22.3.214
215. Salama M, Ellaithy A, Helmy B, El-Gamal M, Tantawy D, Mohamed M, et al. Colchicine protects dopaminergic neurons in a rat model of Parkinson's disease. CNS Neurol Disord Drug Targets. (2012) 11:836–43. doi: 10.2174/1871527311201070836
216. Gopi M, Arambakkam Janardhanam V. Asiaticoside: attenuation of rotenone induced oxidative burden in a rat model of hemi Parkinson ism by maintaining the phosphoinositide-mediated synaptic integrity. Pharmacol Biochem Behav. (2017) 155:1–15. doi: 10.1016/j.pbb.2017.02.005
217. Carlsson A. Age-dependent changes in central dopaminergic and other monoaminergic systems. Adv Exp Med Biol. (1978) 113:1–13. doi: 10.1007/978-1-4684-8893-7_1
218. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. (1991) 114(Pt. 5):2283–301. doi: 10.1093/brain/114.5.2283
219. Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. (1998) 155:344–9.
220. Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. (2003) 69:375–90. doi: 10.1016/S0301-0082(03)00085-6
221. Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. (2004) 5:483–94. doi: 10.1038/nrn1406
222. Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology. (2005) 65:472–4. doi: 10.1212/01.wnl.0000172340.56307.5e
223. Scheidtmann K, Fries W, Müller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. (2001) 358:787–90. doi: 10.1016/S0140-6736(01)05966-9
224. Rösser N, Heuschmann P, Wersching H, Breitenstein C, Knecht S, Flöel A. Levodopa improves procedural motor learning in chronic stroke patients. Arch Phys Med Rehabil. (2008) 89:1633–41. doi: 10.1016/j.apmr.2008.02.030
225. Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. (2001) 412:79–83. doi: 10.1038/35083586
226. Grondin R, Zhang Z, Gerhardt GA, Gash DM. Dopaminergic therapy improves upper limb motor performance in aged rhesus monkeys. Ann Neurol. (2000) 48:250–3. doi: 10.1002/1531-8249(200008)48:2<250::AID-ANA16>3.0.CO;2-1
227. Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, et al. Dopaminergic influences on formation of a motor memory. Ann Neurol. (2005) 58:121–30. doi: 10.1002/ana.20536
228. Meintzschel F, Ziemann U. Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex. (2006) 16:1106–15. doi: 10.1093/cercor/bhj052
229. Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of pitx3-deficient aphakia mice. Brain Res Mol Brain Res. (2003) 114:123–31. doi: 10.1016/S0169-328X(03)00162-1
230. Smits SM, Mathon DS, Burbach JP, Ramakers GM, Smidt MP. Molecular and cellular alterations in the pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. (2005) 30:352–63. doi: 10.1016/j.mcn.2005.07.018
231. Beeler JA, Cao ZF, Kheirbek MA, Zhuang X. Loss of cocaine locomotor response in pitx3-deficient mice lacking a nigrostriatal pathway. Neuropsychopharmacology. (2009) 34:1149–61. doi: 10.1038/npp.2008.117
232. Beeler JA, Cao ZF, Kheirbek MA, Ding Y, Koranda J, Murakami M, et al. Dopamine-dependent motor learning: insight into levodopa's long-duration response. Ann Neurol. (2010) 67:639–47. doi: 10.1002/ana.21947
233. Schapira AH. Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology. (2009) 72(7 Suppl):S44–50. doi: 10.1212/WNL.0b013e3181990438
234. Paul SS, Dibble LE, Olivier GN, Walter C, Duff K, Schaefer SY. Dopamine replacement improves motor learning of an upper extremity task in people with Parkinson disease. Behav Brain Res. (2020) 377:112213. doi: 10.1016/j.bbr.2019.112213
235. Paul SS, Schaefer SY, Olivier GN, Walter CS, Lohse KR, Dibble LE. Dopamine replacement medication does not influence implicit learning of a stepping task in people with Parkinson's disease. Neurorehabil Neural Repair. (2018) 32:1031–42. doi: 10.1177/1545968318809922
236. Mure H, Tang CC, Argyelan M, Ghilardi MF, Kaplitt MG, Dhawan V, et al. Improved sequence learning with subthalamic nucleus deep brain stimulation: evidence for treatment-specific network modulation. J Neurosci. (2012) 32:2804–13. doi: 10.1523/JNEUROSCI.4331-11.2012
237. Dan X, King BR, Doyon J, Chan P. Motor sequence learning and consolidation in unilateral de novo patients with Parkinson's disease. PLoS One. (2015) 10:e0134291. doi: 10.1371/journal.pone.0134291
238. Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord. (2013) 28:1920–9. doi: 10.1002/mds.25687
239. Kwak Y, Müller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. J Neurophysiol. (2010) 103:942–9. doi: 10.1152/jn.00197.2009
240. Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. (2004) 351:2498–508. doi: 10.1056/NEJMoa033447
241. A randomized controlled trial comparing pramipexole with levodopa in early Parkinson's disease: design and methods of the CALM-PD Study. Parkinson Study Group. Clin Neuropharmacol. (2000) 23:34–44. doi: 10.1097/00002826-200001000-00007
242. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicenter study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. (2005) 20:190–9. doi: 10.1002/mds.20324
243. Hariz M, Blomstedt P, Zrinzo L. Future of brain stimulation: new targets, new indications, new technology. Mov Disord. (2013) 28:1784–92. doi: 10.1002/mds.25665
244. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. (2010) 107:8452–6. doi: 10.1073/pnas.1000496107
245. Wilson CJ. Active decorrelation in the basal ganglia. Neuroscience. (2013) 250:467–82. doi: 10.1016/j.neuroscience.2013.07.032
246. Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. (2017) 18:86–100. doi: 10.1038/nrn.2016.164
247. Wang T, Mantini D, Gillebert CR. The potential of real-time fMRI neurofeedback for stroke rehabilitation: a systematic review. Cortex. (2018) 107:148–65. doi: 10.1016/j.cortex.2017.09.006
248. Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. (2011) 334:1413–5. doi: 10.1126/science.1212003
249. Linden DE, Turner DL. Real-time functional magnetic resonance imaging neurofeedback in motor neurorehabilitation. Curr Opin Neurol. (2016) 29:412–8. doi: 10.1097/WCO.0000000000000340
250. Papoutsi M, Weiskopf N, Langbehn D, Reilmann R, Rees G, Tabrizi SJ. Stimulating neural plasticity with real-time fMRI neurofeedback in Huntington's disease: a proof of concept study. Hum Brain Mapp. (2018) 39:1339–53. doi: 10.1002/hbm.23921
251. Chiew M, LaConte SM, Graham SJ. Investigation of fMRI neurofeedback of differential primary motor cortex activity using kinesthetic motor imagery. Neuroimage. (2012) 61:21–31. doi: 10.1016/j.neuroimage.2012.02.053
252. Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, et al. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson's disease. J Neurosci. (2011) 31:16309–17. doi: 10.1523/JNEUROSCI.3498-11.2011
253. Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, et al. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. (2010) 1318:64–76. doi: 10.1016/j.brainres.2009.12.073
254. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. (2009) 41:475–81. doi: 10.2340/16501977-0362
255. Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. (2012) 366:511–9. doi: 10.1056/NEJMoa1107911
256. Rösler A, Seifritz E, Kräuchi K, Spoerl D, Brokuslaus I, Proserpi SM, et al. Skill learning in patients with moderate Alzheimer's disease: a prospective pilot-study of waltz-lessons. Int J Geriatr Psychiatry. (2002) 17:1155–6. doi: 10.1002/gps.705
Keywords: motor skill learning, neurotransmitter, neural circuitry, neurodegeneration, Parkinson's disease, Huntington's disease
Citation: Tian W and Chen S (2021) Neurotransmitters, Cell Types, and Circuit Mechanisms of Motor Skill Learning and Clinical Applications. Front. Neurol. 12:616820. doi: 10.3389/fneur.2021.616820
Received: 14 October 2020; Accepted: 18 January 2021;
Published: 25 February 2021.
Edited by:
Emily Keshner, Temple University, United StatesReviewed by:
Jean-Sébastien Blouin, University of British Columbia, CanadaMarta Llansola, Principe Felipe Research Center (CIPF), Spain
Copyright © 2021 Tian and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengdi Chen, chensd@rjh.com.cn
 Wotu Tian
Wotu Tian Shengdi Chen*
Shengdi Chen*