- 1Functional Neuroimaging Laboratory, School of Psychology, University of Newcastle, Callaghan, NSW, Australia
- 2Hunter Medical Research Institute, Newcastle, NSW, Australia
- 3Priority Research Centre for Stroke and Brain Injury, The University of Newcastle, Callaghan, NSW, Australia
- 4Melbourne Brain Centre, Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia
- 5Sydney Partnership for Health, Education, Research and Enterprise, Sydney, NSW, Australia
Background and Purpose: Altered executive functions and resting-state functional connectivity (rsFC) are common following a minor stroke or transient ischemic attack (TIA). However, the long-term persistence of these abnormalities is not well-studied. We investigated whether there were cognitive and rsFC differences between (a) controls and minor cerebrovascular event (CVE) patients and (b) between CVE patients with and without an imaging confirmed infarct (i.e., minor stroke and TIA, respectively) at an average of 3.8 years following their event.
Methods: Structural and resting-state imaging and cognitive assessments including the Montreal Cognitive Assessment, the Trail Making Task and the National Institute of Health (NIH) Cognition Toolbox were conducted on 42 patients (minor stroke = 17, TIA = 25) and 20 healthy controls (total N = 62).
Results: Controls performed better than patients on two measures of executive functioning (both p < 0.046) and had reduced rsFC between the frontoparietal and default mode networks (FPN and DMN, respectively; p = 0.035). No cognitive differences were found between minor stroke and TIA patients, however, rsFC differences were found within the FPN and the DMN (both p < 0.013). Specifically, increased connectivity within the FPN was associated with faster performance in the minor stroke group but not the TIA group (p = 0.047).
Conclusions: These findings suggest that transient or relatively minor cerebrovascular events are associated with persistent disruption of functional connectivity of neural networks and cognitive performance. These findings suggest a need for novel interventions beyond secondary prevention to reduce the risk of persistent cognitive deficits.
Introduction
Minor stroke and transient ischemic attack (TIA) are cerebrovascular events (CVEs) characterized by a brief mild ischaemic episode with transient clinical and neurological symptoms. Disturbances in executive functions are well recognized following CVEs and include impairments to attention, memory, planning and cognitive flexibility (1, 2). Executive dysfunction can reduce a patient's ability to undertake the activities of daily living which can reduce one's quality of life and the likelihood of returning to premorbid levels of functioning (3). Studies show substantial variability in the reported prevalence of moderate (29–68%) or severe (8–22%) executive dysfunction following a minor CVE. These discrepancies may arise from a range of methodological differences, including inconsistency in neurological event definition. The classical, clinical definition differentiates between minor stroke and TIA based on symptom duration, however, the more recent definition, based on diffusion-weighted magnetic resonance imaging (MRI) (4) differentiates minor stroke from TIA using confirmation of acute tissue infarction (5). Many studies, however, continue to use the classical definition (6–8) due to convenience or lack of access to advanced brain imaging which can be problematic, as studies comparing patients using the two definitions show that up to 30% of conventionally-defined TIA patients display cerebral infarction on MRI (9, 10). Moreover, many studies do not control for variables that independently affect cognition [e.g., age, presence of vascular risk factors, premorbid functioning (10)] or they pool minor stroke and TIA patients together, making it unclear whether there are structural or functional differences between minor CVE patients with persistent ischaemic damage (i.e., minor stroke) and without damage (i.e., TIA). Finally, most studies assess patients in the acute or subacute stages after a minor CVE when variability is likely to be maximal (10), and only a few have investigated the long-term impacts of such events (11, 12).
By using resting-state functional connectivity (rsFC) analysis of functional MRI (fMRI), previous work (13–15) have found that compared to healthy controls, TIA patients typically display patterns of rsFC indicative of reduced executive functions, including decreased rsFC in prefrontal neural regions and increased connectivity between task-positive and task-negative networks [e.g., the frontoparietal network; FPN and the default mode network; DMN, respectively (16, 17)]. Moreover, Guo et al. (18) found these functional connectivity differences in TIA patients were associated with reduced cognitive ability on a broad screening test. Moreover, Bivard et al. (19) reported that patients with a TIA (defined as a baseline hemispheric perfusion deficit without evidence of an ischemic lesion on 24 h MRI) displayed significantly greater gray matter atrophy and poorer performance on the Montreal Cognitive Assessment 90 days following their event, compared to healthy controls. Thus, there is strong evidence for widespread disruption of rsFC and cognition in acute and subacute periods following a TIA. However, there is limited evidence regarding the longer-term cognitive and functional connectivity outcomes. Further, there is little evidence of the functional connectivity outcomes of a minor stroke.
This study aims to assess patients with a clinically and radiologically diagnosed minor stroke or TIA on average 4 years post-event to identify whether they have persistent neurological impairments on resting-state MRI and general cognitive assessments. We hypothesize that, compared to healthy controls, CVE patients will show (a) reduced executive functions and (b) reduced internetwork (between the FPN and DMN) and increased intranetwork (within the FPN and the DMN) rsFC. Further, as a CVE with persistent tissue damage may have a greater impact on brain function, we hypothesize that patients with MRI-defined infarction (i.e., minor stroke patients) will display lower cognitive ability, reduced internetwork rsFC and increased intranetwork rsFC compared to patients with no evidence of infarction (i.e., TIA patients).
Finally, we will test the above hypotheses after correcting for the effects of vascular risk factors and age which both increase the risk of developing a CVE and are independently associated with executive functions (20–22). These parameters will assist in determining whether cognitive or functional connectivity differences, if present, are associated with the minor CVE or a chronic cerebrovascular disease process which may be associated with aging.
Methods
Participants
Minor CVE patients were recruited from two sources. Fifteen patients were recruited from a general neurology clinic in the Hunter region based on a clinical diagnosis of a possible minor stroke or TIA within the last 3 years. Eighty-two patients were recruited from the International Systems of Care and Patient Outcomes in Minor Stroke and TIA (INSIST) study, a longitudinal, community-based inception cohort study that recruited patients with a possible stroke or TIA diagnosis from general practices, emergency departments, and acute cerebrovascular units in the Hunter and Manning Valley regions of New South Wales, Australia (23). In the INSIST study, event adjudication was completed by an expert panel using a standardized clinical definition along with clinical notes and medical history (see Table 1). The INSIST-COG study was approved by Hunter New England Health and the University of Newcastle Human Research Ethics Committees (12/04/18/4.02; H-2012-0154). All patients provided written informed consent.
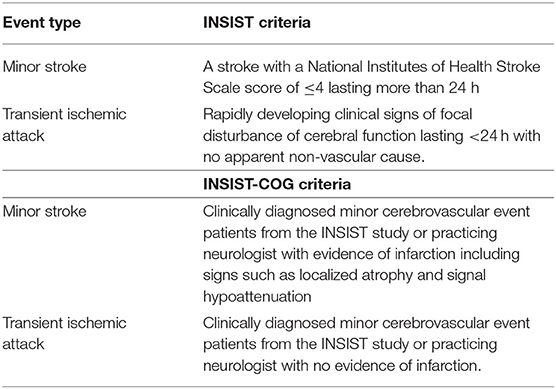
Table 1. Criteria for adjudication of minor stroke and TIA event types in the INSIST and INSIST-COG cohorts.
Healthy controls were recruited from the Hunter Medical Research Institute (HMRI) volunteer registry, community groups and social media. Controls were selected to have no history of a neurological or vascular event, radiation therapy within the last 5 years, any neurodegenerative disorders and to have fewer than three vascular risk factors. All participants gave written informed consent and the study was approved by the Hunter New England Human Research Ethics Committee (12/04/18/4.02).
All INSIST-COG participants completed an MRI scan (see below). Minor CVE patients were reclassified based on the presence of an infarct on the diffusion weighted MRI evidence by a radiologist blinded to prior event history as either having a minor stroke or a TIA. Specifically, patients were classified as having had a minor stroke if there was evidence of infarction including signs such as localized atrophy and signal hypoattenuation. If there was no evidence of infarction, they were classified as having had a TIA (see Table 1). Thus, patients in the current study were initially clinically adjudicated as having had a minor CVE (INSIST or clinical notes), and later the DWI was used to confirm the absence or presence of an infarct and classify as TIA or minor stroke, respectively (see Table 2).
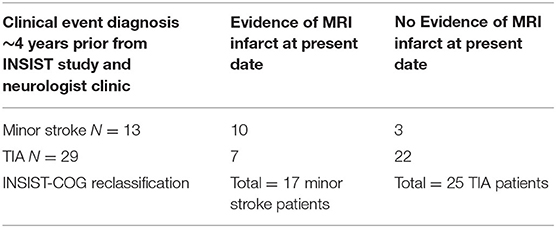
Table 2. Reclassification of clinically diagnosed minor cerebrovascular event patients following diffusion weighted MRI ~4 years after their event.
Procedure
Participants attended two testing sessions [median 13 days apart: interquartile range (IQR) 5 and 32]. Demographic information, health and lifestyle history, and brief cognitive and affective questionnaires were obtained in the telephone interview (session 1). Cognitive testing and an MRI were completed during session 2.
Imaging
Scans were undertaken on a 3T Prisma (Siemens Healthineers, Erlangen, Germany) MRI scanner with a 64-channel head and neck coil. For each subject, a high-resolution T1-weighted anatomical image was acquired in the sagittal plane (TR = 2.3 s, TE = 2.96 ms, inversion time = 900 ms, flip angle = 9°, FOV = 256 mm, voxel size = 1.0 × 1.0 × 1.0 mm, total acquisition time = 5.12 min). Structural images were used for registration and to create lesion masks to optimize registration. rs-fMRI BOLD (blood oxygenation level-dependent) T2*-weighted series were obtained with a gradient echo-planar-imaging (EPI) acquisition (160 volumes, TR = 3.12 s, TE = 21 ms, flip angle = 90°, FOV = 288 mm, voxel size=3.0 × 3.0 × 3.0 mm, total acquisition time = 8.3 min). This voxel size was used to ensure an appropriate compromise between signal-to-noise ratio and spatial accuracy. During resting-state series acquisition, patients were instructed to lie still with their eyes open. Diffusion-weighted imaging, fluid-attenuated inversion recovery and fast low-angle shot sequences were also obtained but are not used in the current study. Total scanning time was 45 min.
For participants with an identified lesion, axial T1 images were used to draw a region of interest mask around the primary infarct using fslView (24). Masks were drawn by a trained neuroimaging researcher (MV), quality checked and modified as necessary by a senior neurologist (MP) to ensure they accurately represented the infarct. These masks were used during fMRIprep pre-processing for spatial normalization with Advanced Normalization Tools as a loss function. Lesion masks were also used in a second pipeline based on fMRIprep using methods suggested by Siegel and colleagues (25).
Functional Data Processing and Stroke-Specific Processes
fMRIprep (version 1.2.5) was used to preprocess the anatomical and functional data (26) using a Nipype-based tool (27). Following pre-processing, manual quality assurance assessment was undertaken. Lesion masks were resampled from MNI anatomical resolution (1 mm iso) to the MNI functional resolution (3 mm iso).
Each T1w (T1-weighted) volume was corrected for INU (intensity non-uniformity) using N4BiasFieldCorrection v2.1.0 (28) and skull-stripped using antsBrainExtraction v2.1.0 (using the OASIS template). Brain surfaces were reconstructed using recon-all from FreeSurfer v6.0.1 (29), and the brain mask estimated previously was refined with a custom variation of the method to reconcile ANTs-derived and FreeSurfer-derived segmentation of the cortical gray-matter of Mindboggle (30). Spatial normalization to the ICBM 152 Non-linear Asymmetrical template version 2009c (31) was performed through non-linear registration with the antsRegistration tool of ANTs v2.1.0 (32), using brain-extracted versions of both T1w volume and template. Brain tissue segmentation of cerebrospinal fluid (CSF), white matter (WM) and gray-matter (GM) were performed on the brain-extracted T1w using fast (33) (FSL v5.0.9).
Functional data were slice time corrected using 3dShift from AFNI v16.2.07 (34) and motion-corrected using mcflirt [FSL v5.0.9 (24)]. “Fieldmap-less” distortion correction was performed by co-registering the functional image to the same-subject T1w image with intensity inverted (35) constrained with an average fieldmap template (36), implemented with antsRegistration (ANTs). This was followed by co-registration to the corresponding T1w using boundary-based registration (37) with 9 degrees of freedom, using bbregister (FreeSurfer v6.0.1). Motion correcting transformations, field distortion correcting warp, BOLD-to-T1w transformation and T1w-to-template (MNI) warp were concatenated and applied in a single step using antsApplyTransforms (ANTs v2.1.0) using Lanczos interpolation.
Frame-wise displacement (38) was calculated for each functional run using the implementation of Nipype. ICA-based Automatic Removal Of Motion Artifacts (AROMA) was used to generate aggressive noise regressors as well as to create a variant of data that is non-aggressively denoised (39). Many internal operations of FMRIPREP use Nilearn (40), principally within the BOLD-processing workflow. For more details of the pipeline see https://fmriprep.readthedocs.io/en/latest/workflows.html.
Exclusions, Cleaning, and Smoothing
Participants with Framewise Displacement (41) >0.5 mm, visually incomplete separation of MELODIC motion components from known resting-state networks or artifact identified on the carpet plots (including high-frequency noise and motion-related spikes) were removed from further analysis. Unsmoothed, unfiltered and MNI normalized fMRI volumes were entered into a second pipeline for cleaning and smoothing. The pipeline was programmed using Python and made use of Numpy (42), Scipy, Pandas (43), Nipype (27) and Deepdish (https://github.com/uchicago-cs/deepdish). Motion and physiologically related artifact were regressed from the functional time series using Ordinary Least Squares Regression. The design matrix for all participants consisted of the time series from motion-related components identified by AROMA, CSF and a discrete high pass cosine filter (0.007 Hz/128 s).
Lesion Masks
Residual images were z-scored, smoothed with a 6 mm full width half-maximum Gaussian kernel and parcellated with the MDSL atlas (44) using Nilearn (40). Lesions masks were used to attenuate the effects of the lesion related artifact on the ICA-smoothed BOLD signal using the method outlined by Yourganov et al. (45). Briefly, the overlap between the lesion mask and z score thresholded (p < 0.05) MELODIC component spatial maps was assessed using the Jaccard index (Scipy). If the Jaccard index was equal to or >5%, the component was considered to be significantly overlapping with the lesion mask to contribute mostly aberrant signal and added to the design matrix. If the component was already identified by AROMA as motion-related, it was not re-added.
Partial correlation was used to examine the functional connectivity between parcellations. As hypotheses referred to specific networks, we only examined parcellations associated with the left and right frontoparietal and the default mode networks (see Figure 1 for information regarding the spatial distribution and number of extracted nodes for each network). The differences between functional connections between groups were investigated with and without the addition of confounders age and vascular risk factors (i.e., the corrected and raw models, respectively).
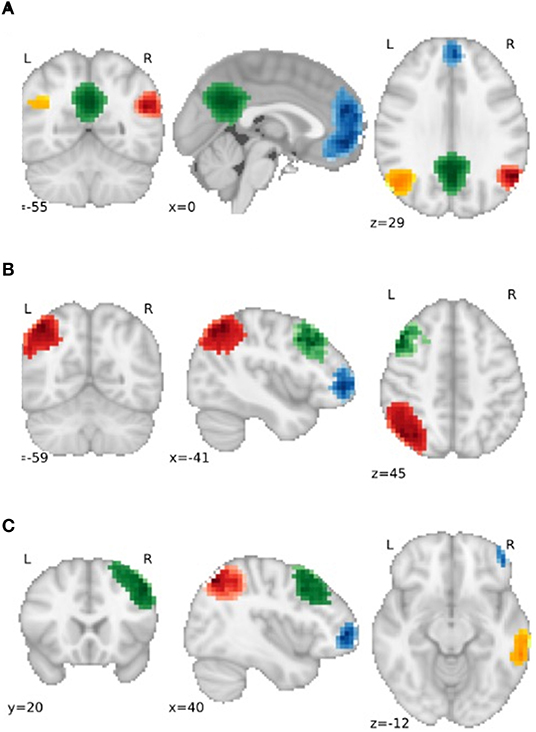
Figure 1. Gray matter regions used as nodes for the (A) default mode network (DMN), (B) Left Frontoparietal Network (FPN) and (C) Right FPN. For (A), Blue = Frontal DMN, Green = Medial DMN, Orange = Left DMN, Red = Right DMN. For (B) and (C), Blue = Dorsolateral Prefrontal node, Red = Parietal node, Green = Frontal Pole node, Orange = Posterior Temporal node.
Cognitive Measures
Montreal Cognitive Assessment (MoCA)
The MoCA assesses short-term memory, visuospatial skills, executive functions, attention, language, and orientation. Scores below 23 indicate mild cognitive impairment and below 18 suggest dementia (46, 47).
Trail Making Test (TMT)
The TMT measures visual attention and task switching and is a validated indicator of brain damage (48). In part A, participants are instructed to connect numbers in ascending order from 1 to 25 and in Part B, in an ascending pattern, alternating between numbers and letters (i.e., 1-A-2-B, etc.). The TMT is scored by subtracting the total time taken for Part A from total time for Part B with higher scores indicating poorer performance.
National Institute of Health (NIH) Toolbox
Six cognitive tests from the NIH Toolbox cognition battery measuring crystallized intelligence, processing speed, memory, and executive functions were administered using the application on an iPad following standardized procedures as per the administration manual (49, 50). The NIH Toolbox shows acceptable internal consistency, test-retest reliability and validity (51, 52).
List Sorting Working Memory Task
List sorting working memory task assesses working memory. Participants recall and sequence a list of foods and animals presented orally and visually from smallest to largest. The first condition includes only food or animals and the second condition involves both food and animals, where foods are listed first from smallest to largest followed by animals. Scores were calculated using item response theory (IRT; a score derived from each participant's overall ability).
Pattern Comparison Processing Speed Task
Pattern comparison processing speed task measures processing speed by using IRT and raw performance values [average reaction time (RT) and accuracy] to score participants ability to decide whether two images presented side by side are the same or not.
Auditory Verbal Learning Task
Auditory verbal learning task measures short-term episodic memory. Participants are asked to verbally recall as many words as possible from a list of 15 unrelated words. The list was presented orally three times and the total score was determined by each correctly remembered word. Scores were determined by the number of total words recalled.
Dimensional Change Card Sort Task
Dimensional change card sort task assesses executive functions by asking participants to match bivalent stimuli to a target picture with two dimensions (shape and color) according to a word cue (shape or color). This requires participants to either switch or repeat the task (e.g., shape, shape, color—where the color trial is a switch trial). A computed score was created based on a combination of accuracy and RT performance. Raw performance values (average accuracy and RT) were also obtained.
Oral Reading Recognition Task
Examines reading decoding skills. Participants pronounce words displayed on an iPad with the test administrator scoring correct or incorrect. Scores were calculated using IRT.
Picture Vocabulary Task
Measures receptive vocabulary. After hearing a word, participants select one of four pictures that most closely matches the meaning of the word. Scores were calculated using IRT.
Crystallized Ability Composite Score
It was computed by averaging the Oral Reading Recognition and Picture Vocabulary Tests.
All scores, excluding the Auditory Verbal Learning Test, were corrected against the entire NIH Toolbox national US registry with a normative mean of 100 and a standard deviation of 15. A score of 85 and 115 indicates performance one standard deviation below and above the mean, respectively. Raw scores from the auditory verbal learning task were uncorrected, however, NIH normative data indicated a mean of 19.04 (SD = 9.08) for participants 60–69 years and a mean of 15.14 (SD = 6.14) for people 70–85 years.
Statistical Analysis
SPSS (version 25.0) was used to examine the main effects of group (CVE vs. control and minor stroke vs. TIA) on cognitive performance. Differences were examined between the control and the cerebrovascular Event (CVE) groups and between CVE subgroups with minor stroke vs. TIA. Continuous variables were investigated using a one-way ANOVA and categorical variables using a chi-square test. An ANCOVA was used to investigate the effect of group on each cognitive measure while controlling for the hypothesized confounders: age and number of vascular risk factors. These confounders were chosen based on Munir et al. (11) and Nicolas et al. (6). As the current study aims to investigate the independent effect of CVE on cognitive ability, adjusting for age and the presence of vascular risk factors appeared appropriate.
Permutation analysis was used to investigate the significant group differences in rsFC between nodes (results shown in Table 4). Specifically, a null distribution was created using 10,000 permutations for each of the statistical connections (i.e., correlations between two parcellations) between nodes and compared to the observed t-value. There were a total of 11 nodes examined of which there were 55 pairwise combinations. MaxT correction was used for multiple comparisons (53), which is robust to outliers. Then, to investigate these group differences after controlling for the effects of age and vascular risk factors, these variables were regressed from each cognitive score using the Huber Regression (a method robust to the effects of outliers) in sci-kit learn (54) (epsilon =1.35, alpha = 1e-3, tol = 1e-5).
Significant associations in rsFC between nodes and cognitive performance and group interaction effects between rsFC and cognitive performance were determined using Manley's method {i.e., generating the null distribution using unresricted sampling. [See (55)]} and permutation tests (10,000 permutations were used to calculate a null distribution for each parameter). A Huber Regression was used to investigate the linear association between cognitive performance and functional connectivity, and the differences in these associations between groups. Then, to investigate these interactions without the effects of age and vascular risk factors, the effects of these variables were regressed from each cognitive and rsFC score. The cognitive and rsFC residuals without the effects of age and vascular risk factors are shown in Figure 4. As we focus on interaction effects between rsFC, group and cognitive performance, no multiple comparison correction was used for the regression analysis.
Results
In total, 123 participants were recruited, including 15 patients from a neurology clinic, 82 patients from the INSIST study and 26 healthy controls.
Participants were excluded from analyses if they did not complete the second testing session (19 participants), showed a large neural abnormality (i.e., cyst or tumor; 2 participants), did not complete the MRI (9 participants) or had severe motion artifacts in the MRI data (10 participants). Patients from the larger INSIST-COG [see (6)] study that did not have a clinically adjudicated vascular event (i.e., patients who were adjudicated as having had a mimic CVE including seizure, migraine or visual disturbance N = 21) were not included in the current analysis as the current study was interested only in the differences between minor CVE patients and controls. One control was excluded due to a neural abnormalities that may have affected the functional connectivity analysis. The final sample included 62 participants of whom 17 had a minor stroke as identified on MRI, 25 had a TIA and 20 were controls.
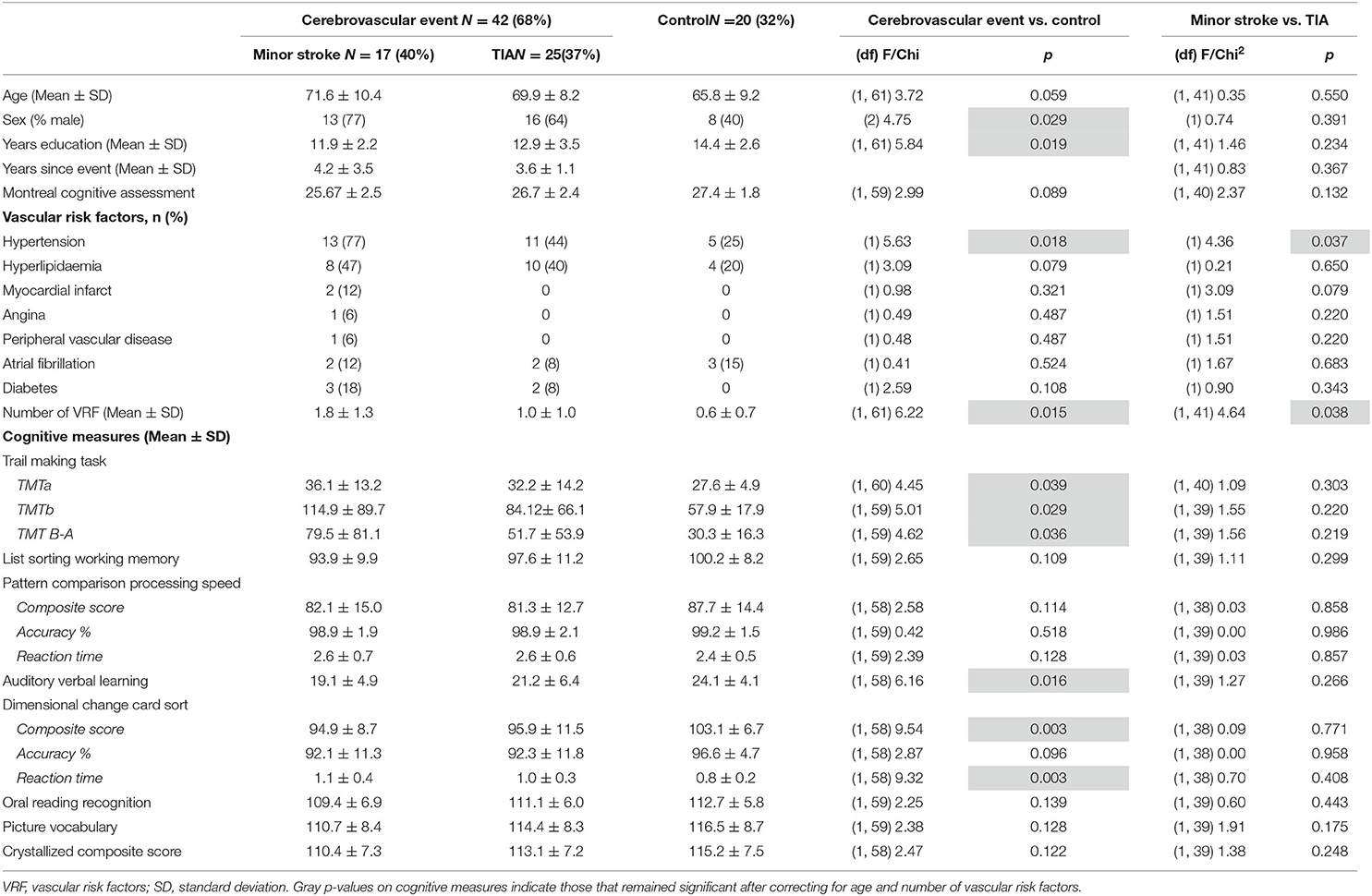
As shown in Table 3, compared to the Control group, the CVE group had significantly fewer years of formal education and greater prevalence of males, hypertension and vascular risk factors (all p < 0.029). Minor stroke patients also had a significantly greater prevalence of hypertension and vascular risk factors than TIA patients (both p < 0.038). Of the minor stroke patients, structural imaging showed that three patients (18%) had a right-hemisphere ischaemic lesion, six (36%) had a left-hemisphere lesion, four (24%) had a lesion in both hemispheres, one (6%) had a lesion in the pons, one (6%) had a lesion in the basal ganglia and two (12%) had multiple middle cerebral artery infarcts. As the sample was small, we could not examine the effects of lesion location.
Cognitive Measures
The CVE group performed significantly lower than the Control group on the Auditory Verbal Learning task, the composite and reaction time (RT) measures of the Dimensional Change Card Sort task, and all components of the Trail Making Task (all p < 0.039; Table 3). After controlling for age and number of vascular risk factors, group differences remained only for the composite and RT components of the Dimensional Change Card Sort task (p = 0.033 and p = 0.046, respectively; data not tabulated). Minor stroke and TIA patients did not differ on any cognitive tasks (all p > 0.132, Table 3).
Resting-State Functional Connectivity Differences
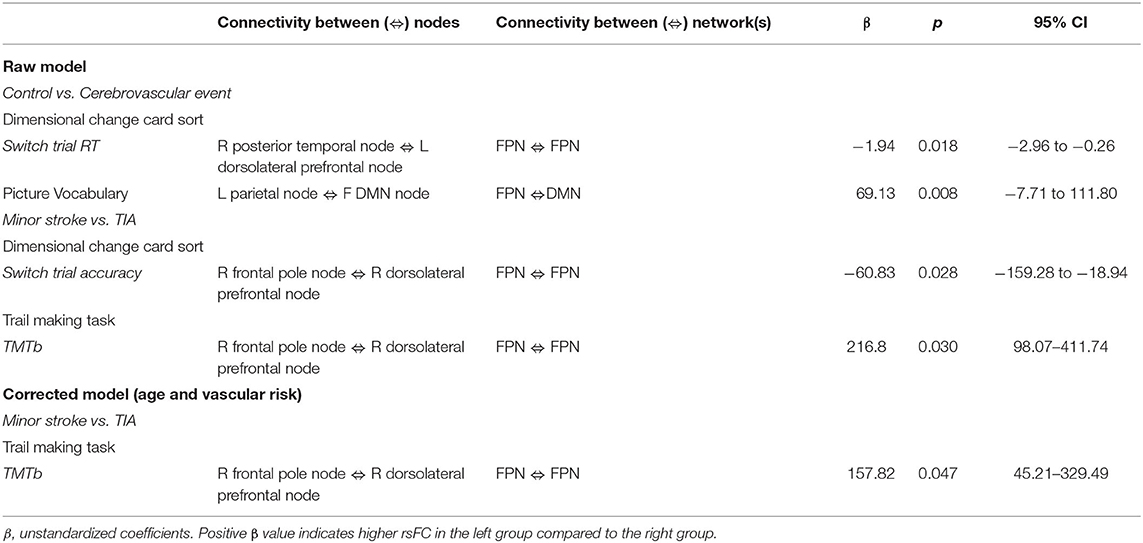
Table 4 shows significant group differences in the strength of rsFC between nodes extracted from the DMN and the left and right FPN network (see Figure 2 for a visual schematic of significant differences). Node location was then mapped to the corresponding network, e.g., connectivity between the left parietal FrP node (located in the FPN) and the left DMN node (located in the DMN) corresponds to connectivity between the FPN and the DMN.
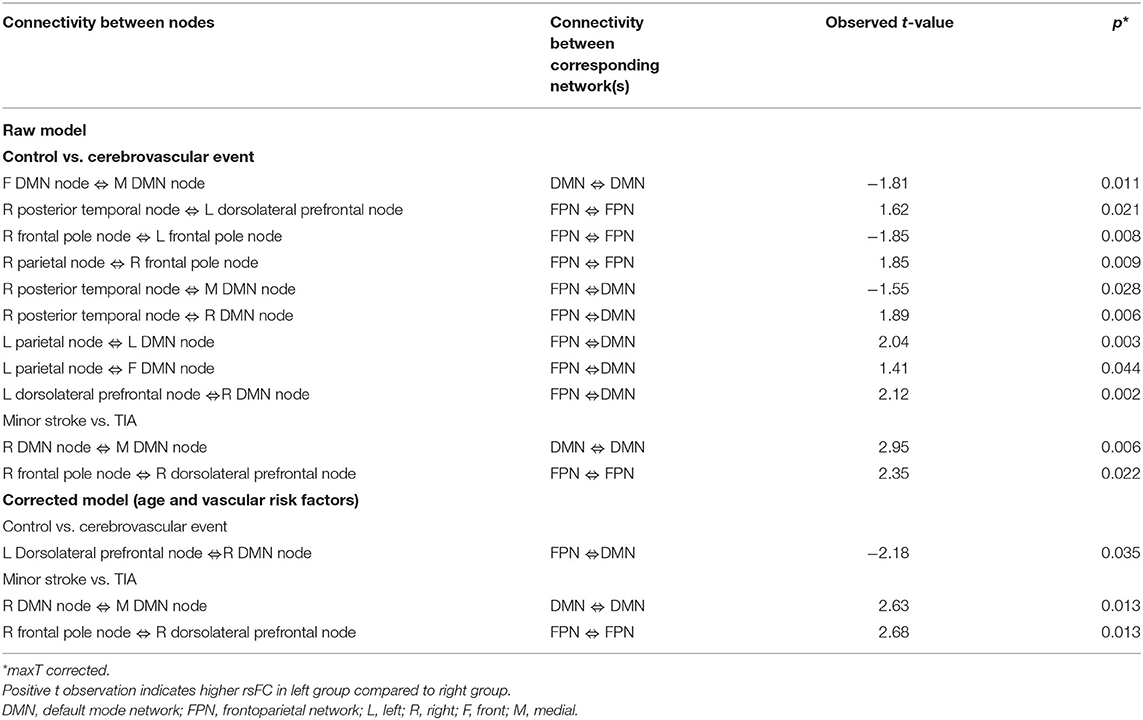
Table 4. Significant groups differences in the strength of rsFC between nodes extracted from the DMN and the FPN network.
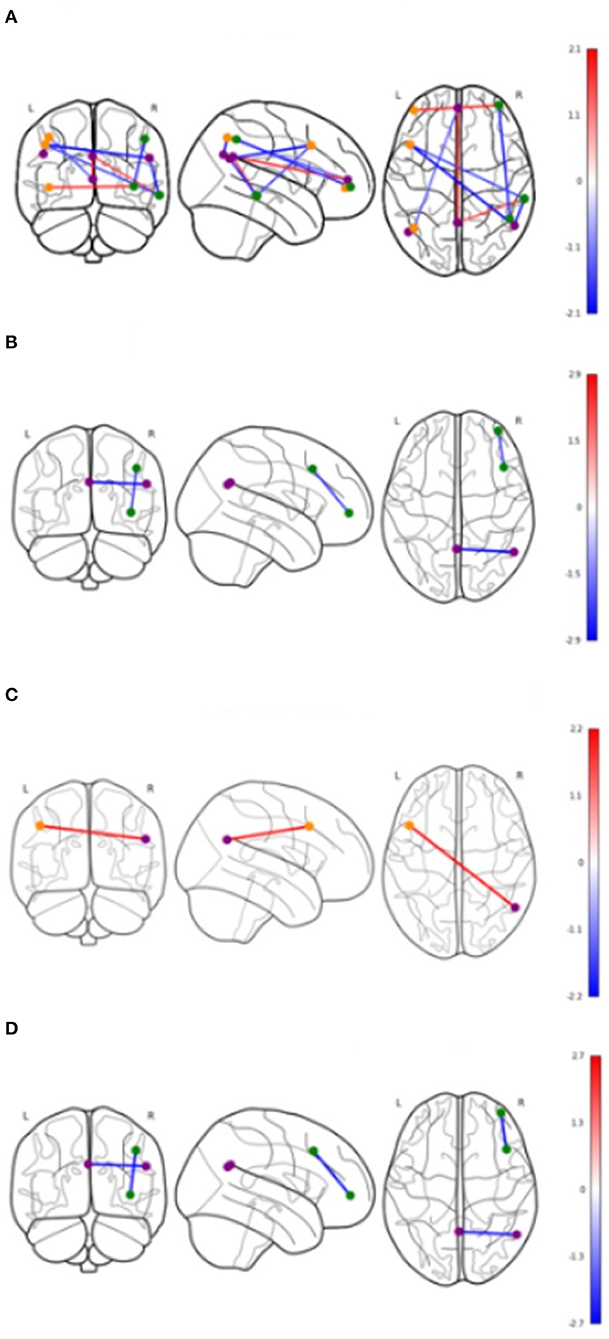
Figure 2. Significant differences in functional connectivity between nodes by groups. Values shown in color bars are t-values. Line color represents t-values (warm = positive, cool = negative). Nodes are colored based on the network they belong to. Purple = DMN, Orange = Left FPN, Green = Right FPN. (A) Cerebrovascular event vs. Control (uncorrected). (B) Minor stroke vs. TIA (uncorrected). (C) Cerebrovascular event vs. Control (corrected). (D) Minor stroke vs. TIA (corrected).
Control vs. Cerebrovascular Event Group
In Table 4, the raw model (top) refers to results before correcting for the effects of age and vascular risk factors, whereas the corrected model (bottom) refers to results that remained significant after correction. In the raw model, control and CVE groups differed in rsFC strength between nodes within DMN and FPN networks (all p < 0.021), as well as connectivity between nodes belonging to DMN and FPN networks (all p < 0.044). Compared to the CVE group, controls showed greater and reduced connectivity between nodes within the FPN, reduced connectivity between nodes within the DMN and both greater and reduced connectivity between nodes in the two different networks.
After controlling for age and number of vascular risk factors (corrected model), the only significant group difference was that rsFC between the left dorsolateral prefrontal node (FPN) and the right DMN node was weaker in controls than the CVE group (p = 0.035).
Minor Stroke vs. TIA
The minor stroke group showed significantly stronger rsFC between nodes within both DMN and FPN than the TIA group (p = 0.006 and p = 0.022, respectively), which remained significant after controlling for confounders (both p = 0.013).
Interactions Between Cognition, Resting-State Functional Connectivity, and Group
Table 5 shows significant associations between cognitive performance and rsFC, and the differences in these associations between groups. Figures 3, 4 display the interaction of rsFC between two nodes, cognitive performance and group.
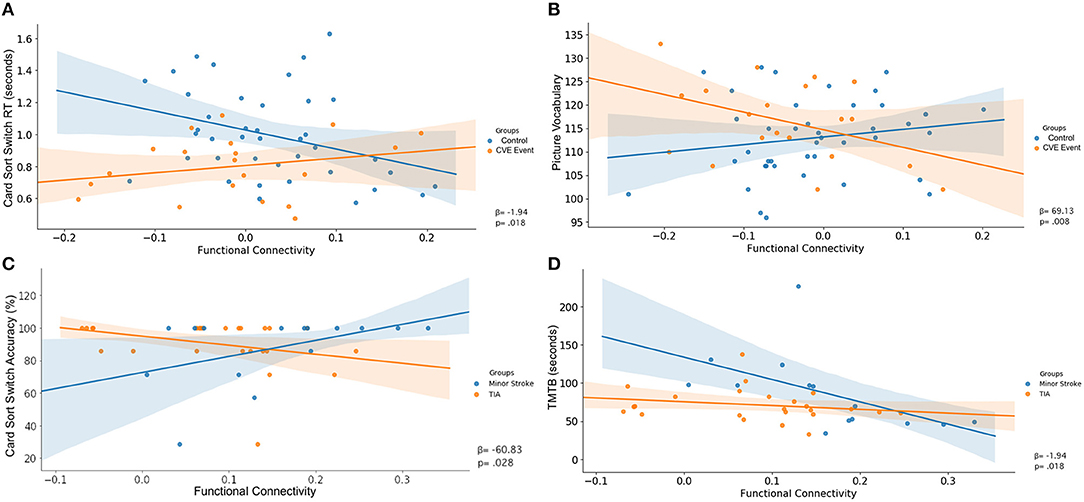
Figure 3. Uncorrected interaction of functional connectivity between two nodes (X-axis), cognitive performance (Y-axis) and group (legend). Shaded areas indicated the 95%-confidence intervals around the correlation coefficients. (A) FPN ⇔ FPN, (B) FPN ⇔ DMN, (C) FPN ⇔ FPN, (D) FPN ⇔ FPN.
Control vs. Cerebrovascular Event
There was an interaction between group, RT on the switch trials of the Dimensional Change Card Sort test and connectivity across nodes within the FPN (β = −1.94, p = 0.018; Table 5). As shown in Figure 3A, greater rsFC in FPN was associated with faster switch trial RT for the control group, but this CVE group showed a weak effect in the opposite direction.
Further, there was an interaction between group, performance on the Picture Vocabulary test and rsFC between nodes comprising the FPN and DMN (β = 69.13, p = 0.008). As shown in Figure 3B, greater connectivity between FPN and DMN was associated with higher vocabulary scores in the control group, whereas CVE patients showed a strong trend in the opposite direction.
Both of these interactions were removed after controlling for potential confounders (both p > 0.050).
Minor Stroke vs. TIA
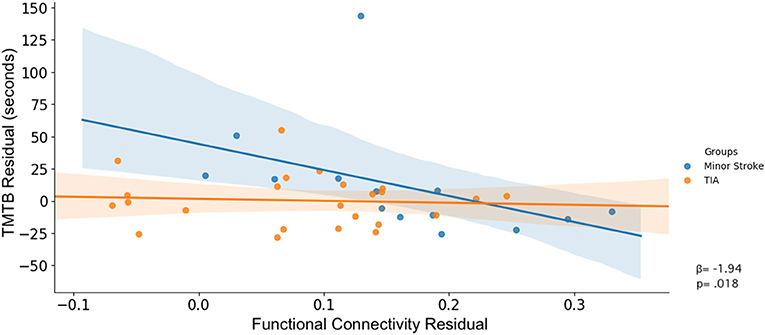
There was an interaction between group, accuracy on the switch trials of the Dimensional Change Card Sort test and connectivity across nodes within the FPN (β = −60.83, p = 0.028; Table 5). As shown in Figure 3C, increased rsFC within the FPN was associated with greater accuracy in minor stroke patients and with little effect on TIA patients.
There was an interaction between group, TMTb performance and connectivity across nodes within the FPN (β = 216.80, p = 0.030). As shown in Figure 3D, increased rsFC within the FPN was associated with faster completion time for minor stroke patients with little effect on TIA patients.
After controlling for confounders, only the latter interaction remained (β = 157.82, p = 0.047; Figure 4).
Discussion
We examined whether there were persistent cognitive and resting-state functional connectivity differences between (a) healthy controls and cerebrovascular event patients and (b) between minor stroke and TIA patients approximately 4 years following their event. In line with our hypothesis, CVE patients had poorer performance on measures of executive functioning and greater inter-network rsFC between nodes connecting the FPN and DMN when compared to healthy controls. Interestingly, when compared to TIA patients, minor stroke patients with MRI-defined brain infarction displayed greater intra-network rsFC in the DMN and the FPN. Finally, the strength of this intra-network rsFC within the FPN was associated with differential performance in minor stroke and TIA patients on the B component of the TMT, a measure sensitive to executive functioning.
Executive functioning ability is associated with rsFC between task-positive (FPN) and task-negative (DMN) networks (56). Reduced connectivity between these networks is believed to demonstrate the ability to suppress task-negative networks and increase activation of task-positive networks during a cognitive task (57, 58). After controlling for confounders, controls displayed better performance on measures of executive functions and reduced rsFC between task-positive and task-negative networks, when compared to CVE patients. So controls appear to more successfully suppress DMN activity and activate FPN when completing executive function tasks. While the absence of this relationship between performance and rsFC in participants with a prior CVE is consistent with persistent neural changes, these findings need to be interpreted with caution, as we found no significant interaction between rsFC, cognitive performance and group. This is likely due to the relatively small and heterogeneous CVE group which included patients with and without a permanent infarct, as well as heterogenous lesion locations in the minor stroke group. Moreover, although the CVE group had more males than the control group, we did not control for this as it is consistent with the consistent with sex distribution in typical stroke populations and the small sample size limits the effectiveness of correction.
While there were no cognitive differences between CVE patients with and without an infarct (i.e., minor stroke and TIA, respectively), minor stroke patients displayed greater intranetwork rsFC within both the DMN (right to medial DMN regions) and the FPN (right frontal pole to right DLPFC). Each of these regions is associated with executive functions including monitoring outcomes and working memory (59–61). Previous studies have found the DMN is linked to internally oriented mentation (62) and the DLPFC and frontal pole have been demonstrated to be involved in information manipulation, controlling working memory and organizing and cataloging information (63, 64) which are all components of executive functions. While it is unknown why these neural regions were over-activated in minor stroke compared to TIA patients, it is well-known that both acute stroke (65, 66) and TIA (14, 15) can lead to abnormal rsFC. However, to the best of our knowledge, there are currently no studies investigating the differences between rsFC in minor stroke and TIA patients and thus, future research is required to further investigate these findings. Moreover, increased connectivity within the FPN was associated with better performance on the B component of the TMT task in minor stroke patients but appeared to have little effect in TIA patients. As minor stroke patients in the current study are characterized by the presence of a persistent infarction, we speculate that differences between groups may be attributed to the use of compensatory processes (e.g., recruiting additional frontal neural resources or functionally reorganizing neural regions) to overcome infarct-related damage which in turn resulted in them performing as well as TIA patients. This conclusion, however, requires further investigation using a larger cohort of patients with homogenous brain lesions and demographics to successfully generalize and interpret such results. Together, these findings provide evidence that the presence of ischaemia following a minor neurological event results in significant, long-term connectivity differences, the strength of which can differentially affect executive functioning.
There are potential limitations with deriving measures of rsFC in ischaemic patients. Although we used the current gold standards for quantifying stroke-affected BOLD (67) to reduce the effect of individual differences across patients (e.g., differing lesion/occlusion locations and the severity of neural damage), it is still possible that there was some signal artifact. Additionally, the parcellation scheme assumes that individuals have common functional parcellations in standard space, so individual differences due to different lesion/occlusion locations may result in measurement error (68). Further, functional reorganization can occur following an ischemic event (67) and this can shift functional boundaries and delay the BOLD response, resulting in individual rsFC estimate errors. We used an Independent Component Analysis method (45) to overcome this delay in minor stroke patients. However, such a method is unavailable for TIA patients who may also experience a delay. Finally, while BOLD is an indirect measure of neural activity, the precise functional significance of rsFC derived from the BOLD signal and its relationship to excitatory or inhibitory brain processes remains under investigation.
In conclusion, we found poorer executive functions and disrupted inter-network rsFC between the DMN and FPN in minor CVE patients ~4 years after the event. These findings indicate that the presence of a minor CVE is independently associated with persistent neurobiological and functional consequences. Further, while there were no cognitive differences between minor cerebrovascular patients with and without evidence of a neural infarct, these subgroups showed different patterns of rsFC within nodes of the FPN which differentially affected performance on a measure of executive functioning. Together, these findings provide evidence that patients with and without a neural infarct show different patterns of rsFC which can affect cognitive performance. This emphasizes the importance of event prevention and the need for the development of restorative approaches aimed at reducing the impact of minor stroke and TIA on long-term cognition and quality of life.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Hunter New England Human Research Ethics Committee (12/04/18/4.02). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KN: investigation, writing—original draft, project administration, data curation, formal analysis, methodology, and conceptualization. PG: software, formal analysis, writing—review & editing, methodology, and visualization. MV: writing—review & editing and formal analysis. PM: supervision and writing—review & editing. AB: supervision, writing—review & editing, conceptualization, methodology, and funding acquisition. CL: supervision, writing—review & editing, and funding acquisition. MP: writing—review & editing. FK: supervision, writing—review & editing, and conceptualization. All authors contributed to the article and approved the submitted version.
Funding
KN was supported by a UNRSC 50:50 scholarship by the University of Newcastle, Australia and the CRE-Stroke Recovery and Rehab.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the practice nurses, neurologists, and GPs for their contributions to TIA/minor stroke identification and patient recruitment.
References
1. Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Koschera A. Progression of cognitive impairment in stroke patients. Neurology. (2004) 63:1618–23. doi: 10.1212/01.WNL.0000142964.83484.DE
2. Soros P, Harnadek M, Blake T, Hachinski V, Chan R. Executive dysfunction in patients with transient ischemic attack and minor stroke. J Neurol Sci. (2015) 354:17–20. doi: 10.1016/j.jns.2015.04.022
3. Moran GM, Fletcher B, Feltham MG, Calvert M, Sackley C, Marshall T. Fatigue, psychological and cognitive impairment following transient ischaemic attack and minor stroke: a systematic review. Eur J Neurol. (2014) 21:1258–67. doi: 10.1111/ene.12469
4. Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, Marco De Marchis G, et al. What is a minor stroke? Stroke. (2010) 41:661–6. doi: 10.1161/STROKEAHA.109.572883
5. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack. Stroke. (2009) 40:2276–93. doi: 10.1161/STROKEAHA.108.192218
6. Nicolas K, Levi C, Evans T-J, Michie PT, Magin P, Quain D, et al. Cognition in the first year after a minor stroke, transient ischemic attack, or mimic event and the role of vascular risk factors. Front Neurol. (2020) 11:216. doi: 10.3389/fneur.2020.00216
7. van Rooij FG, Schaapsmeerders P, Maaijwee NA, van Duijnhoven DA, de Leeuw FE, Kessels RP, et al. Persistent cognitive impairment after transient ischemic attack. Stroke. (2014) 45:2270–4. doi: 10.1161/STROKEAHA.114.005205
8. Winward C, Sackley C, Metha Z, Rothwell PM. A population-based study of the prevalence of fatigue after transient ischemic attack and minor stroke. Stroke. (2009) 40:757–61. doi: 10.1161/STROKEAHA.108.527101
9. Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PAG, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. (2014) 75:67–76. doi: 10.1002/ana.24026
10. van Rooij FG, Kessels RP, Richard E, De Leeuw FE, van Dijk EJ. Cognitive impairment in transient ischemic attack patients: a systematic review. Cerebrovasc Dis. (2016) 42:1–9. doi: 10.1159/000444282
11. Munir M, Ursenbach J, Reid M, Gupta Sah R, Wang M, Sitaram A, et al. Longitudinal brain atrophy rates in transient ischemic attack and minor ischemic stroke patients and cognitive profiles. Front Neurol. (2019) 10:18. doi: 10.3389/fneur.2019.00018
12. Sachdev PS, Lipnicki DM, Crawford JD, Wen W, Brodaty H. Progression of cognitive impairment in stroke/TIA patients over 3 years. J Neurol Neurosurg Psychiatry. (2014) 85:1324–30. doi: 10.1136/jnnp-2013-306776
13. Li R, Wang S, Zhu L, Guo J, Zeng L, Gong Q, et al. Aberrant functional connectivity of resting state networks in transient ischemic attack. PloS ONE. (2013) 8:e71009. doi: 10.1371/journal.pone.0071009
14. Lv Y, Li L, Song Y, Han Y, Zhou C, Zhou D, et al. The local brain abnormalities in patients with transient ischemic attack: a resting-state fMRI study. Front Neurosci. (2019) 13:24. doi: 10.3389/fnins.2019.00024
15. Zhu T, Li L, Song Y, Han Y, Zhou C, Zhou D, et al. Altered functional connectivity within default mode network in patients with transient ischemic attack: a resting-state functional magnetic resonance imaging study. Cerebrovas Dis. (2019) 48:61–9. doi: 10.1159/000502884
16. Keller JB, Hedden T, Thompson TW, Anteraper SA, Gabrieli JDE, Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. (2015) 64:271–80. doi: 10.1016/j.cortex.2014.12.001
17. Reineberg AE, Gustavson DE, Benca C, Banich MT, Friedman NP. The relationship between resting state network connectivity and individual differences in executive functions. Front Psychol. (2018) 9:1600. doi: 10.3389/fpsyg.2018.01600
18. Guo J, Chen N, Li R, Wu Q, Chen H, Gong Q, et al. Regional homogeneity abnormalities in patients with transient ischaemic attack: a resting-state fMRI study. Clin Neurophysiol. (2014) 125:520–525. doi: 10.1016/j.clinph.2013.08.010
19. Bivard A, Lillicrap T, Maréchal B, Garcia-Esperon C, Holliday E, Krishnamurthy V, et al. Transient ischemic attack results in delayed brain atrophy and cognitive decline. Stroke. (2018) 49:384–90. doi: 10.1161/STROKEAHA.117.019276
20. Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. (2011) 137:753–84. doi: 10.1037/a0023262
21. Strom JO, Tavosian A, Appelros P. Cardiovascular risk factors and TIA characteristics in 19,872 Swedish TIA patients. Acta Neurol. Scand. (2016) 134:427–33. doi: 10.1111/ane.12560
22. Tekin S, Cummings JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry an update. J Psychosom Res. (2002) 53:647–54. doi: 10.1016/S0022-3999(02)00428-2
23. Levi CR, Lasserson D, Quain D, Valderas J, Dewey HM, Alan Barber P, et al. The International comparison of Systems of care and patient outcomes In minor Stroke and Tia (InSIST) study: a community-based cohort study. Int. J. Stroke. (2019) 14:186–90. doi: 10.1177/1747493018799983
24. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. (2002) 17:825–41. doi: 10.1006/nimg.2002.1132
25. Siegel JS, Shulman GL, Corbetta M. Measuring functional connectivity in stroke: approaches and considerations. J Cereb. Blood Flow Metab. (2017) 37:2665–78. doi: 10.1177/0271678X17709198
26. Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. (2019) 16:111. doi: 10.1038/s41592-018-0235-4
27. Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinf. (2011) 5:13. doi: 10.3389/fninf.2011.00013
28. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. (2010) 29:1310. doi: 10.1109/TMI.2010.2046908
29. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. (1999) 9:179–94. doi: 10.1006/nimg.1998.0395
30. Klein A, Ghosh SS, Bao FS, Giard J, Häme Y, Stavsky E, et al. Mindboggling morphometry of human brains. PLoS Comput. Biol. (2017) 13:e1005350. doi: 10.1371/journal.pcbi.1005350
31. Fonov VS, Evans AC, McKinstry RC, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. (2009) 47:S102. doi: 10.1016/S1053-8119(09)70884-5
32. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Anal. (2008) 12:26–41. doi: 10.1016/j.media.2007.06.004
33. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. (2001) 20:45–57. doi: 10.1109/42.906424
34. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. (1996) 29:162–73. doi: 10.1006/cbmr.1996.0014
35. Wang S, Peterson DJ, Gatenby JC, Li W, Grabowski TJ, Madhyastha TM. Evaluation of field map and nonlinear registration methods for correction of susceptibility artifacts in diffusion MRI. Front Neuroinf. (2017) 11:17. doi: 10.3389/fninf.2017.00017
36. Treiber JM, White NS, Steed TC, Bartsch H, Holland D, Farid N, et al. Characterization and correction of geometric distortions in 814 diffusion weighted images. PLoS ONE. (2016) 11:e0152472. doi: 10.1371/journal.pone.0152472
37. Greve DN, Fischl B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage. (2009) 48:63–72. doi: 10.1016/j.neuroimage.2009.06.060
38. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. (2014) 84:320–41. doi: 10.1016/j.neuroimage.2013.08.048
39. Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. (2015) 112:267–77. doi: 10.1016/j.neuroimage.2015.02.064
40. Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinf. (2014) 8:14. doi: 10.3389/fninf.2014.00014
41. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. (2012) 59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018
42. Van Der Walt S, Colbert SC, Varoquaux G. The NumPy array: a structure for efficient numerical computation. Comput Sci Eng. (2011) 13:22. doi: 10.1109/MCSE.2011.37
43. McKinney W. Data structures for statistical computing in python. In: Paper Presented at the Proceedings of the 9th Python in Science Conference. Austin, TX (2010). doi: 10.25080/Majora-92bf1922-00a
44. Varoquaux G, Gramfort A, Pedregosa F, Michel V, Thirion B. Multi-subject dictionary learning to segment an atlas of brain spontaneous activity. In: Székely G, Hahn HK, editors. Information Processing in Medical Imaging. Berlin; Heidelberg: Springer (2011). p. 562–73. doi: 10.1007/978-3-642-22092-0_46
45. Yourganov G, Fridriksson J, Stark B, Rorden C. Removal of artifacts from resting-state fMRI data in stroke. NeuroImage. (2018) 17:297–305. doi: 10.1016/j.nicl.2017.10.027
46. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
47. Freitas S, Simões MR, Alves L, Santana I. Montreal cognitive assessment. Alzheimer Dis Assoc Disord. (2013) 27:37–43. doi: 10.1097/WAD.0b013e3182420bfe
48. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. (1958) 8:271–6. doi: 10.2466/pms.1958.8.3.271
49. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. (2013) 80:S2–S6. doi: 10.1212/WNL.0b013e3182872e5f
50. Slotkin J, Kallen M, Griffith J, Magasi S, Salsman J, Nowinski C, et al. NIH Toolbox Technical Manual (2012).
51. Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, et al. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J Int Neuropsychol Soc. (2014) 20:588–98. doi: 10.1017/S1355617714000241
52. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology.(2013) 80(11 Suppl. 3):S54. doi: 10.1212/WNL.0b013e3182872ded
53. Ge Y, Dudoit S, Speed TP. Resampling-based multiple testing for microarray data analysis. Test. (2003) 12:1–77. doi: 10.1007/BF02595811
54. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. (2011) 12:2825–30.
55. Manly BF. Randomization, Bootstrap and Monte Carlo Methods in Biology. New York, NY: Chapman and Hall/CRC (2018). doi: 10.1201/9781315273075
56. Xin F, Lei X. Competition between frontoparietal control and default networks supports social working memory and empathy. Soc Cogn Affect Neurosci. (2015) 10:1144–52. doi: 10.1093/scan/nsu160
57. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. From the cover: the human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
58. Wen X, Liu Y, Yao L, Ding M. Top-down regulation of default mode activity in spatial visual attention. J Neurosci. (2013) 33:6444–53. doi: 10.1523/JNEUROSCI.4939-12.2013
59. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. (2001) 98:4259. doi: 10.1073/pnas.071043098
60. Koechlin E, Danek A, Burnod Y, Grafman J. Medial prefrontal and subcortical mechanisms underlying the acquisition of motor and cognitive action sequences in humans. Neuron. (2002) 35:371–81. doi: 10.1016/S0896-6273(02)00742-0
61. Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, et al. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. (2008) 46:1114–23. doi: 10.1016/j.neuropsychologia.2007.10.022
62. Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network. Ann. N. Y. Acad. Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
63. Dobbins IG, Foley H, Schacter DL, Wagner AD. (2002). Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. (2002) 35:989–96. doi: 10.1016/S0896-6273(02)00858-9
64. Ku Y, Bodner M, Zhou Y-D. (2015). Prefrontal cortex and sensory cortices during working memory: quantity and quality. Neurosci Bull. (2015) 31:175–82. doi: 10.1007/s12264-014-1503-7
65. Chen L, Li C, Zhai J, Wang A, Song Q, Liu Y, et al. Altered resting-state signals in patients with acute stroke in or under the thalamus. Neurosci Bul. (2016) 32:585–90. doi: 10.1007/s12264-016-0064-3
66. Park C-H, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. (2011) 42:1357–62. doi: 10.1161/STROKEAHA.110.596155
67. Siegel JS, Seitzman BA, Ramsey LE, Ortega M, Gordon EM, Dosenbach NU, et al. Re-emergence of modular brain networks in stroke recovery. Cortex. (2018) 101:44–59. doi: 10.1016/j.cortex.2017.12.019
Keywords: transient ischaemic attack, minor stroke, cognitive impairment, functional connectivity, executive function
Citation: Nicolas K, Goodin P, Visser MM, Michie PT, Bivard A, Levi C, Parsons MW and Karayanidis F (2021) Altered Functional Connectivity and Cognition Persists 4 Years After a Transient Ischemic Attack or Minor Stroke. Front. Neurol. 12:612177. doi: 10.3389/fneur.2021.612177
Received: 01 October 2020; Accepted: 30 April 2021;
Published: 07 June 2021.
Edited by:
Peter Sörös, University of Oldenburg, GermanyReviewed by:
Daniele Corbo, University of Brescia, ItalyWen Qin, Tianjin Medical University General Hospital, China
Copyright © 2021 Nicolas, Goodin, Visser, Michie, Bivard, Levi, Parsons and Karayanidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frini Karayanidis, ZnJpbmkua2FyYXlhbmlkaXNAbmV3Y2FzdGxlLmVkdS5hdQ==
 Korinne Nicolas
Korinne Nicolas Peter Goodin
Peter Goodin Milanka M. Visser3,4
Milanka M. Visser3,4 Patricia T. Michie
Patricia T. Michie Andrew Bivard
Andrew Bivard Christopher Levi
Christopher Levi Mark W. Parsons
Mark W. Parsons Frini Karayanidis
Frini Karayanidis