
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 14 May 2021
Sec. Dementia and Neurodegenerative Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.610302
This article is part of the Research Topic Dementia in Low and Middle Income Countries View all 37 articles
 Aline Maria M. Ciciliati1
Aline Maria M. Ciciliati1 Izabela Ono Adriazola1
Izabela Ono Adriazola1 Daniela Souza Farias-Itao2
Daniela Souza Farias-Itao2 Carlos Augusto Pasqualucci2
Carlos Augusto Pasqualucci2 Renata Elaine Paraizo Leite2
Renata Elaine Paraizo Leite2 Ricardo Nitrini3
Ricardo Nitrini3 Lea T. Grinberg2,4
Lea T. Grinberg2,4 Wilson Jacob-Filho1
Wilson Jacob-Filho1 Claudia Kimie Suemoto1*
Claudia Kimie Suemoto1*Background: Body mass index (BMI) in midlife is associated with dementia. However, the association between BMI and late-life obesity is controversial. Few studies have investigated the association between BMI and cognitive performance near the time of death using data from autopsy examination. We aimed to investigate the association between BMI and dementia in deceased individuals who underwent a full-body autopsy examination.
Methods: Weight and height were measured before the autopsy exam. Cognitive function before death was investigated using the Clinical Dementia Rating (CDR) scale. The cross-sectional association between BMI and dementia was investigated using linear regression models adjusted for sociodemographic and clinical variables.
Results: We included 1,090 individuals (mean age 69.5 ± 13.5 years old, 46% women). Most participants (56%) had a normal BMI (18.5–24.9 kg/m2), and the prevalence of dementia was 16%. Twenty-four percent of the sample had cancer, including 76 cases diagnosed only by the autopsy examination. Moderate and severe dementia were associated with lower BMI compared with participants with normal cognition in fully adjusted models (moderate: β = −1.92, 95% CI = −3.77 to −0.06, p = 0.042; severe: β = −2.91, 95% CI = −3.97 to −1.86, p < 0.001).
Conclusion: BMI was associated with moderate and severe dementia in late life, but we did not find associations of BMI with less advanced dementia stages.
Dementia affects 46 million people worldwide, and 58% of these people live in low-/middle-income countries (1). Obesity is a known risk factor for dementia, but dementia itself can also affect body weight through changes in appetite and other behavioral problems (2). Body mass index (BMI) is an easy measure to assess nutritional status. Besides, BMI has a strong correlation with total-body and visceral adiposity (3).
In several studies, overweight and obesity evaluated with BMI in midlife (between the fifth and the sixth decades of life) were linked to worse cognitive performance (4–16). However, in older individuals, BMI was not associated with higher dementia risk in some studies, and even higher BMI values were related to lower dementia risk (9, 11, 15, 17). These unexpected findings could be due to reverse causation, as dementia has an insidious onset and a long and asymptomatic preclinical phase, and the weight loss may be secondary to the cognitive and neuropsychiatric changes that occur early during the disease course (11, 12, 18). Survival bias could be another possible explanation since individuals with higher BMI may not survive until old age to develop dementia symptoms, as they suffer a higher burden of cardiovascular disease (8). Another reason for BMI to be found to be protective against dementia could be the presence of consumptive conditions (e.g., cancer), which lead to weight loss, but were not fully adjusted in previous studies (6, 7, 17, 19). Indeed, the presence of undiagnosed cancer could bias the association between BMI and adverse health outcomes (20). Therefore, we investigated the relationship between BMI near the time of death and cognitive performance in 1,090 deceased individuals submitted to a full-body autopsy, which allowed the detection of undiagnosed cancer.
This study used the Biobank for Aging Studies (BAS) collection from the University of São Paulo Medical School (Brazil). Data were collected at the São Paulo Autopsy Service that receives individuals who died from non-traumatic deaths and required a full-body autopsy to determine the cause of death (21). Trained nurses/gerontologists interviewed family members using a semistructured questionnaire that included sociodemographic variables, clinical history, and functional and cognitive assessments. To ensure data reliability, informants had to have at least weekly contact with the deceased (22). Exclusion criteria for the BAS are individuals with macroscopic cerebral lesions that required brain examination by the pathologist to determine the cause of death or the presence of severe acidosis (pH < 6.5) (21).
For this study, we also excluded individuals under 50 years old, those with incomplete data on weight, height, the Clinical Dementia Rating (CDR) scale, or covariates. Body weight was obtained using an electronic scale, and height was measured with a stadiometer with the deceased individuals in the supine position without clothes and shoes (23). All participants underwent a full-autopsy exam. Assessment of the death certificates was carried out to include possible cases of undiagnosed cancer during life. The local ethical committee approved the research (protocol number 04655612.9.0000.0065), and family members of the deceased signed an informed consent document.
The CDR is an extensively validated instrument, translated into several languages (24). It is based on clinical symptoms of dementia, not depending on any other psychometric test for its application. It can be applied by non-medical professionals and offers different modes of interpretation, including the possibility of comparative use of a patient's total score over the years (25). It classifies cognitive performance into six categories (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) through a semistructured interview applied to the individual and an informant. By design, we only used the informant part of the CDR in this study. CDR scores range from 0 (no dementia) to 0.5 (questionable dementia), 1 (mild dementia), 2 (moderate dementia), and 3 (severe dementia) (24). The scoring system is based on individual scores of all categories. Memory was considered the primary category, and others are secondary. It is also possible to compute the total CDR sum of the boxes (CDR-SOB) score by summing the points in each of the six cognitive ability categories (26, 27). The CDR-SOB scores range from 0 to 18.
Possible confounders for the association of BMI and CDR were age, sex (male or female), race (White, Black, or Asian), years of education, physical inactivity (defined by <three times of physical activity per week), alcohol use (never consumed any alcohol, current or previous history of alcohol use), current smoking (never smoked, current smoker, or previous use of tobacco), and history of previous medical diagnosis of hypertension, diabetes, coronary heart disease, heart failure, and cancer, which was later compared to death certificates to identify possible non-diagnosed cases during the lifetime.
We compared BMI categories with categorical variables using the chi-square test and continuous variables using one-way ANOVA or the Kruskal–Wallis tests. We hypothesized that dementia presence and severity would determine weight at the time of death. Therefore, we used a linear regression in which the predictor variable was the CDR categories, and the outcome was the measured BMI (Table 1). Age, sex, race, education, hypertension, diabetes mellitus, heart failure, dyslipidemia, cancer, sedentary lifestyle, smoking, and alcohol use were considered confounding factors in the association between BMI and CDR. Besides, we performed a sensitivity analysis for this association using the CDR sum of boxes as the outcome. Moreover, since cancer and current smoking could be a strong predictor of BMI (28), we conducted an additional sensitivity analysis excluding participants with these conditions and using adjusted linear models for the same set of covariates.
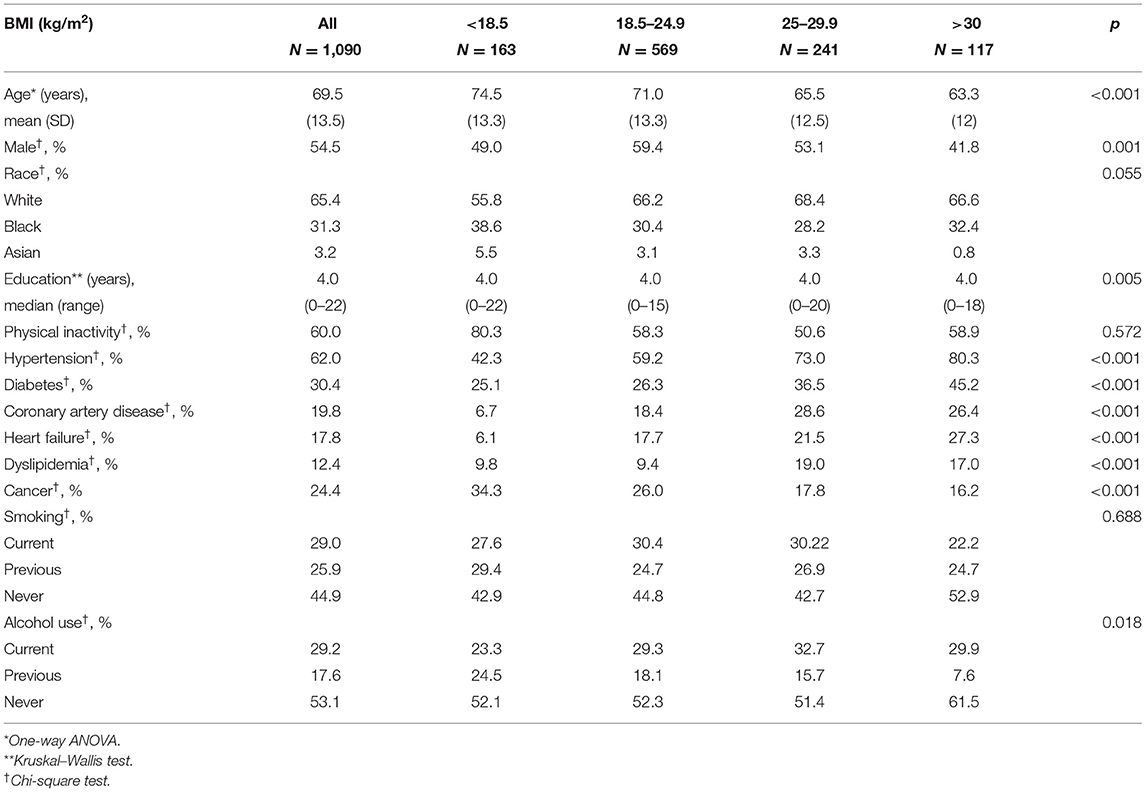
Table 1. Sociodemographic and clinical characteristics by body mass index (BMI) categories (n = 1,090).
To explore the diversity of race/ethnicity in our sample, we investigated whether the association between BMI and cognition was different between Black and White participants. For this analysis, we excluded Asians (n = 35) and created an interaction term between race and CDR. In addition, we conducted stratified analyses by race for the association between BMI and CDR. Statistical analysis was performed with STATA 15 (StataCorp. 2017, College Station, TX). The level of significance was set at 0.05 in two-tailed tests.
A total of 1,090 participants were included in this study. The final sample consisted mostly of men (55%), with a mean age of 69.5 ± 13.5 years old and median educational attainment of 4 years (range: 0–22 years). The most frequent clinical conditions were hypertension (62%), diabetes (31%), and physical inactivity (60%) (Table 1). The majority of the sample had a normal BMI (52%) with a mean BMI of 23.5 ± 5.5 kg/m2 (Figure 1). The prevalence of cognitive impairment (CDR ≥ 1) was 16%. Approximately 24% of individuals were diagnosed with cancer, and 76 (7%) of the cancers were detected during the autopsy exam only.
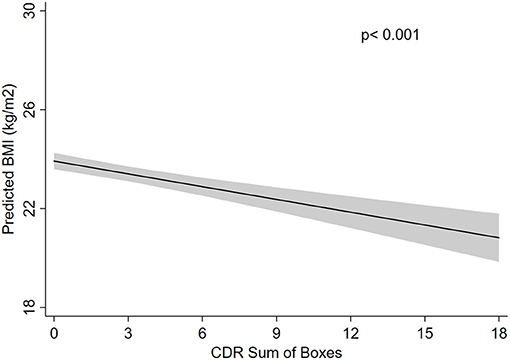
Figure 1. Absolute frequency of participants regarding the Clinical Dementia Rating (CDR) scores and body mass index (BMI) categories.
We observed that underweight was associated with older age, while obesity (BMI > 30 kg/m2) was more prevalent in younger individuals. Men had a lower BMI, accounting for almost 60% of malnourished individuals. Higher education level was associated with higher BMI, and Black individuals had, on average, lower BMI than White individuals (Table 1). Regarding clinical characteristics, hypertension, diabetes, coronary artery disease, heart failure, and dyslipidemia were associated with overweight and obesity (Table 1). Alcohol use was associated with lower BMI levels (Table 1).
In the unadjusted model, we did not observe BMI differences among participants with normal cognition, questionable dementia, and mild dementia, while moderate and severe dementia were associated with lower BMI (moderate dementia: β = −2.77, 95% CI = −4.73 to −0.81, p = 0.005; severe dementia: β = −4.47, 95% CI = −5.49 to −3.45, p < 0.001) (Table 2). Even after adjusting the analysis for all confounding variables, these associations remained significant. Compared with participants with normal cognition, participants with moderate dementia had on average 1.92 kg/m2 of BMI less than participants without dementia (β = −1.92, 95% CI = −3.77 to −0.06, p = 0.042). Participants with severe dementia had on average 2.91 kg/m2 less than those with normal cognition (β = −2.91, 95% CI = −3.97 to −1.86, p < 0.001) (Table 2). Association between BMI and all covariates is presented in Supplementary Table 1. Age, education, heart failure, cancer, and current alcohol use were related to BMI.
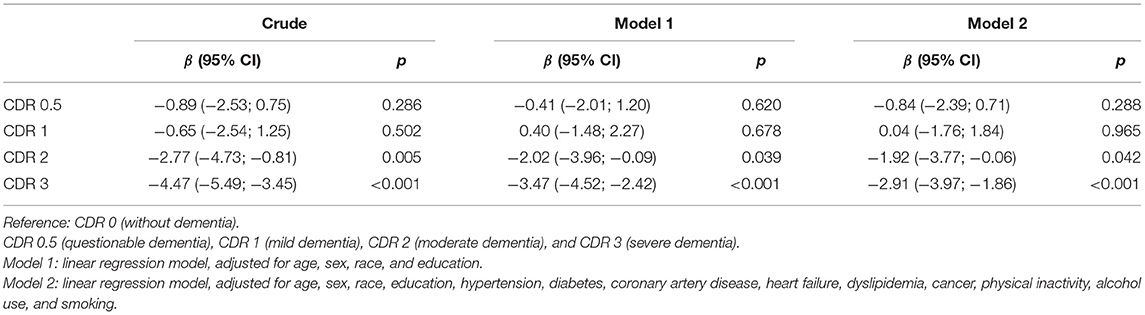
Table 2. Association between body mass index (BMI) and clinical dementia rating (CDR) categories (n = 1,090).
We performed two different sensitivity analyses that confirm our finding. We first excluded participants with a cancer diagnosis or current smoking. The exclusion of these participants did not change our results (moderate dementia: β = −2.24, 95% CI = −4.48 to 0.06, p = 0.044; severe dementia: β = −3.16, 95% CI = −4.52 to −1.85, p < 0.001) (Table 3). In another approach, we used the CDR sum of boxes as the exposure variable, and we found on average a 0.178-kg/m2 decrease in BMI for each 1-unit increase in the CDR sum of boxes in the fully adjusted model (β = −0.178, 95% CI = −0.238 to −0.119, p < 0.001) (Figure 2).
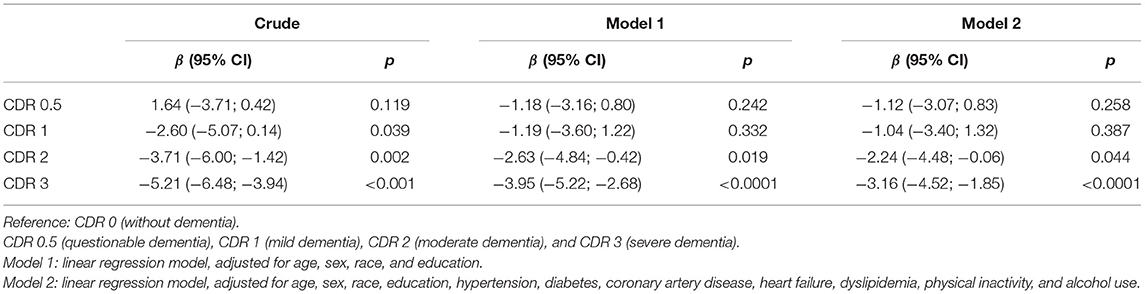
Table 3. Association between body mass index (BMI) and clinical dementia rating (CDR) categories, excluding participants with cancer and current smokers (n = 575).
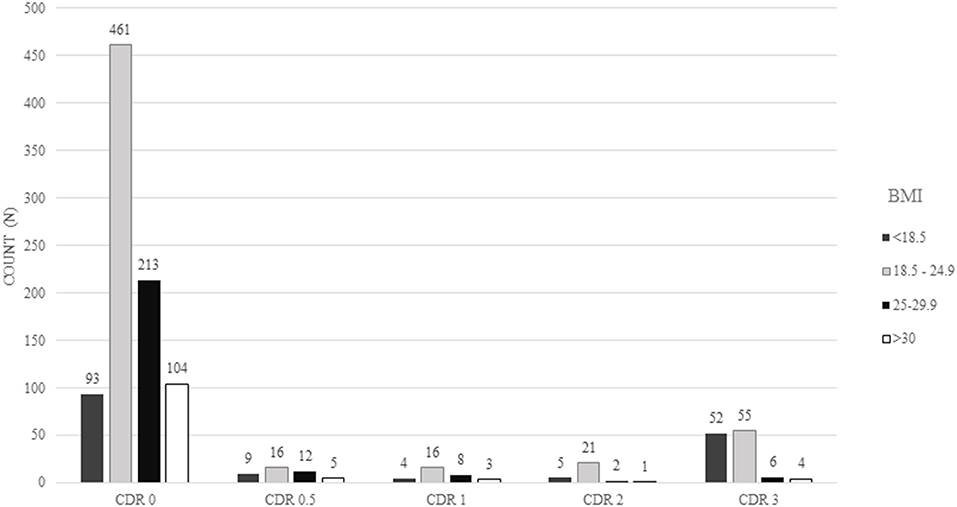
Figure 2. Predicted body mass index (BMI) according to Clinical Dementia Rating sum of boxes (CDR-SOB). Predicted BMI was calculated using a linear regression model adjusted for age, sex, race, education, hypertension, diabetes mellitus, heart failure, dyslipidemia, cancer, sedentary lifestyle, smoking, and alcohol use.
We also investigated the interaction between race and CDR on BMI values. We did not find a significant interaction between race and CDR (p = 0.328). Moreover, we found similar associations between CDR and BMI in White and Black participants in stratified analyses by race, with lower BMI values among participants with severe dementia compared with participants with normal cognition (Supplementary Table 2).
We found an association of BMI with moderate and severe dementia, suggesting that individuals with more advanced dementia are more likely to have lower body weight by the time of death. We did not find associations between BMI and early dementia stages. When we excluded individuals with cancer and current smokers, moderate and severe dementia were still associated with lower BMI. Moreover, we did not find evidence of interaction between race and cognitive status on BMI.
The relationship between BMI and dementia varies along the life course. In middle-aged adults, obesity was linked to brain atrophy (29), worse cognitive performance, and higher mortality (30, 31). This association may be explained by diverse pathophysiological pathways, including systemic and central nervous system inflammation (32), triggered by the production of hormones like leptin and other inflammatory cytokines (e.g., tumor necrosis factor-α and interleukins) by the adipose tissue. After long-term exposure, inflammation is believed to damage cerebral arteries, raising the risk for vascular dementia and Alzheimer's disease (30, 33–39). In animal models, intestinal microbiota dysbiosis linked to obesity also stimulates the neuroinflammatory response, but human data regarding this hypothesis are still lacking (35).
On the other hand, obesity was not associated with higher dementia risk in late life, while lower BMI was related to higher dementia risk (8, 9, 11, 15). Along with these findings, we found that moderate and severe dementia were related to lower BMI. One possible explanation for the lower BMI to be related to dementia could be reverse causation. Even decades before the dementia diagnosis, deposition of beta-amyloid and tau proteins in the olfactory bulb leads to impaired smelling capacity. In addition, the deposition of beta-amyloid protein on the hypothalamic nuclei is among the earliest neuropathological changes in Alzheimer's disease (36). The impaired smell sensibility can impact food tasting, decreased calorie intake, and lead to weight loss (37, 38). Deposition of beta-amyloid proteins in areas of the brain responsible for energy and hunger regulation can also decrease hormones, like leptin, cholecystokinin, and serotonin. Phosphorylated tau proteins are also linked to the avoidance of high-fat diets, related to higher BMI (39). However, we did not find that early dementia stages were related to lower BMI, only moderate and advanced dementia were associated with lower BMI values. These findings could be caused by neuropsychiatric symptoms that are common among individuals with more advanced dementia stages. Depressive symptoms often include appetite loss and apathy and the absence of motivation or initiative to obtain food and eat (18). Besides, the disturbance of neurons that produce neurotransmitters (e.g., norepinephrine) may result in anxiety and appetite dysfunction (38). Besides, patients with moderate and advanced dementia are at least partially dependent on basic activities of daily life, such as obtaining and cooking food (19). Another crucial factor to consider is dysphagia. A systematic review showed that 57% of patients with advanced dementia had dysphagia, which is an important cause of weight loss, malnutrition, dehydration, and additional functional loss (40).
A fundamental aspect of our study is that we could include data from the autopsy examination, while most studies on the association between BMI and dementia used clinical data (8, 9, 12). We were able to detect 76 additional cancer cases, which increased the frequency of neoplasms from 20 to 24% in our sample. The investigation of consumptive diseases like cancer is of paramount importance when studying the effect of BMI on health outcomes. Cancer is associated with severe inflammation and cachexia syndrome that lead to weight loss (41–44). Another strength of our study is that the data were collected at the São Paulo Autopsy Service, a general autopsy service. Therefore, most participants do not have cognitive impairment or have mild symptoms of dementia, which more accurately represent community-dwelling adults regarding dementia prevalence and severity. Another advantage is that this study was conducted in a sample with diverse races compared with other studies based on North American, European, and Asian populations (8, 9, 11, 14). Dementia risk seems to be higher in African Americans compared with non-Hispanic Whites (45). Since Brazil is a multiracial country (46), we were able to explore whether the association between BMI and cognition differed by race. Severe dementia was related to lower BMI in both White and Black participants, but we did not find an effect modification by race in this association.
Nonetheless, our study needs to be considered in light of its limitations. The cross-sectional design does not allow establishing cause–effect relationships. Moreover, despite extensive literature showing a high correlation between BMI and body adiposity (41), we did not directly measure body adipose composition. Instead, we used BMI, which is easy to perform in clinical settings and is correlated with body adiposity (3). Finally, we do not have available information on the neuropathological examination of these cases at this moment.
Our main finding was that individuals with moderate and severe stages of dementia had lower BMI, in agreement with previous studies (4–12, 16). However, no significant association was found with earlier dementia stages. Our study contributes to understanding the association between BMI and dementia at the end of life, including the information of undiagnosed cancer during life that could have contributed to weight loss unrelated to dementia. Further studies are needed to establish the pathophysiology and mechanisms directly leading to weight loss from preclinical to advanced dementia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of São Paulo's ethical committee. The patients/participants provided their written informed consent to participate in this study.
AC: Conception and design, data collection, data analysis, drafting, revision and final approval of the version to be published. IA: Conception and design, drafting, revision and final approval of the version to be published. DS, CP, RL, RN, and LG: Revision and final approval of the version to be published. WJ-F: Conception and design, data collection, data analysis, drafting, revision and final approval of the version to be published. CS: Conception and design, data analysis, revision and final approval of the version to be published. All authors contributed to the article and approved the submitted version.
This study received the following grants: FAPESP 06/55318-1, FAPESP 2013/12290-3, and 2017/24066-1 (to DS) and NIH K24AG053435 (to LG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.610302/full#supplementary-material
1. Wimo A, Prince M. Alzheimer's Disease International World Alzheimer Report 2010 The Global Economic Impact of Dementia. London: Alzheimer's Disease International (2010), p. 56.
2. Müller S, Preische O, Sohrabi HR, Gräber S, Jucker M, Dietzsch J, et al. Decreased body mass index in the preclinical stage of autosomal dominant Alzheimer's disease. Sci Rep. (2017) 7:1225. doi: 10.1038/s41598-017-01327-w
3. World Health Organization. WHO Expert Committee on physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee (1995).
4. Gustafson DR, Bäckman K, Joas E, Waern M, Östling S, Guo X, et al. 37 Years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis. (2012) 28:163–71. doi: 10.3233/JAD-2011-110917
5. Cova I, Clerici F, Maggiore L, Pomati S, Cucumo V, Ghiretti R, et al. Body mass index predicts progression of mild cognitive impairment to dementia. Dement Geriatr Cogn Disord. (2016) 41:172–80. doi: 10.1159/000444216
6. Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. (2015) 3:431–6. doi: 10.1016/S2213-8587(15)00033-9
7. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. (2008) 71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef
8. Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. (2018) 14:178–86. doi: 10.1016/j.jalz.2017.06.2637
9. Kivimäki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, Nyberg ST, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement. (2018) 14:601–9. doi: 10.1016/j.jalz.2017.09.016
10. Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Dementia severity and weight loss: a comparison across eight cohorts. The 10/66 study. Alzheimers Dement. (2013) 9:649–56. doi: 10.1016/j.jalz.2012.11.014
11. Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes. (2015) 39:1383–9. doi: 10.1038/ijo.2015.83
12. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies: BMI and risk of dementia. Obes Rev. (2011) 12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x
13. Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. (2011) 76:1568–74. doi: 10.1212/WNL.0b013e3182190d09
14. Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes. (2009) 33:893–8. doi: 10.1038/ijo.2009.104
15. Dahl AK, Löppönen M, Isoaho R, Berg S, Kivelä S-L. Overweight and obesity in old age are not associated with greater dementia risk: (see editorial comments by Dr. David S. Knodman, pp 2349–2350). J Am Geriatr Soc. (2008) 56:2261–6. doi: 10.1111/j.1532-5415.2008.01958.x
16. Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement Diagn Assess Dis Monit. (2017) 8:165–78. doi: 10.1016/j.dadm.2017.05.007
17. Luchsinger J, Cheng D, Tang M, Schupf N, Mayeux R. Central obesity in the elderly is related to late onset Alzheimer's disease. Alzheimer Dis Assoc Disord. (2012) 26:101–5. doi: 10.1097/WAD.0b013e318222f0d4
18. Brodaty H, Heffernan M, Draper B, Reppermund S, Kochan NA, Slavin MJ, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. (2012) 31:411–20. doi: 10.3233/JAD-2012-120169
19. Alhurani RE, Vassilaki M, Aakre JA, Mielke MM, Kremers WK, Machulda MM, et al. Decline in weight and incident mild cognitive impairment: mayo clinic study of aging. JAMA Neurol. (2016) 73:439. doi: 10.1001/jamaneurol.2015.4756
20. Banack HR, Kaufman JS, Wactawski-Wende J, Troen BR, Stovitz SD. Investigating and remediating selection bias in geriatrics research: the selection bias toolkit. J Am Geriatr Soc. (2019) 67:1970–6. doi: 10.1111/jgs.16022
21. Brazilian Aging Brain Study Group, Grinberg LT, de Lucena Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, et al. Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. (2007) 8:151–62. doi: 10.1007/s10561-006-9022-z
22. Jorm AF. Methods of screening for dementia: a meta-analysis of studies comparing an informant questionnaire with a brief cognitive test. Alzheimer Dis Assoc Disord. (1997) 11:158–62. doi: 10.1097/00002093-199709000-00008
23. Nishizawa A, Suemoto CK, Farias-Itao DS, Campos FM, Silva KCS, Bittencourt MS, et al. Morphometric measurements of systemic atherosclerosis and visceral fat: evidence from an autopsy study. PLoS ONE. (2017) 12:e0186630. doi: 10.1371/journal.pone.0186630
24. Fagundes Chaves ML, Camozzato AL, Godinho C, Kochhann R, Schuh A, de Almeida VL, et al. Validity of the clinical dementia rating scale for the detection and staging of dementia in Brazilian patients. Alzheimer Dis Assoc Disord. (2007) 21:210–7. doi: 10.1097/WAD.0b013e31811ff2b4
25. Isella V. Discriminative and predictive power of an informant report in mild cognitive impairment. J Neurol Neurosurg Psychiatry. (2006) 77:166–71. doi: 10.1136/jnnp.2005.069765
26. Morris JC. The clinical dementia rating (cdr): current version and scoring rules. Neurology. (1993) 43:2412. doi: 10.1212/WNL.43.11.2412-a
27. O'Bryant SE. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. (2008) 65:1091. doi: 10.1001/archneur.65.8.1091
28. Bowman K, Thambisetty M, Kuchel GA, Ferrucci L, Melzer D. Obesity and longer term risks of dementia in 65–74 year olds. Age Ageing. (2019) 48:367–73. doi: 10.1093/ageing/afz002
29. Driscoll I, Gaussoin SA, Wassertheil-Smoller S, Limacher M, Casanova R, Yaffe K, et al. Obesity and structural brain integrity in older women: the women's health initiative magnetic resonance imaging study. J Gerontol A Biol Sci Med Sci. (2016) 71:1216–22. doi: 10.1093/gerona/glw023
30. Bischof GN, Park DC. Obesity and aging: consequences for cognition, brain structure, and brain function. Psychosom Med. (2015) 77:697–709. doi: 10.1097/PSY.0000000000000212
31. Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. (2016) 45:14–21. doi: 10.1093/ageing/afv151
32. Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. (2005) 62:1545–8. doi: 10.1001/archneur.62.10.1545
33. Dorrance A, Matin N, Pires P. The effects of obesity on the cerebral vasculature. Curr Vasc Pharmacol. (2014) 12:462–72. doi: 10.2174/1570161112666140423222411
34. Lampe L, Zhang R, Beyer F, Huhn S, Kharabian-Masouleh S, Preusser S, et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol. (2018) 82:194–203. doi: 10.1002/ana.25396
35. Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. (2018) 13:1497–511. doi: 10.2147/CIA.S139163
36. Ishii M, Iadecola C. Metabolic and non-cognitive manifestations of Alzheimer's disease: the hypothalamus as both culprit and target of pathology. Cell Metab. (2015) 22:761–76. doi: 10.1016/j.cmet.2015.08.016
37. Devanand DP. Olfactory identification deficits, cognitive decline, and dementia in older adults. Am J Geriatr Psychiatry. (2016) 24:1151–7. doi: 10.1016/j.jagp.2016.08.010
38. Ehrenberg AJ, Suemoto CK, França Resende E de P, Petersen C, Leite REP, Rodriguez RD, et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer's disease. J Alzheimers Dis. (2018) 66:115–26. doi: 10.3233/JAD-180688
39. Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. (1997) 66:760–73. doi: 10.1093/ajcn/66.4.760
40. Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: a systematic review. Arch Gerontol Geriatr. (2013) 56:1–9. doi: 10.1016/j.archger.2012.04.011
41. Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer-associated anorexia and weight loss. Centre Rev. Dissemination. (2005) 83:1345–50. doi: 10.1093/ajcn/83.6.1345
42. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. (2006) 83:1345–50.
43. Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition. (2001) 17:S1–21. doi: 10.1016/S0899-9007(01)80001-0
44. Hernandez JL, Matorras P, Riancho JA, Gonzalez-Macias J. Involuntary weight loss without specific symptoms: a clinical prediction score for malignant neoplasm. QJM. (2003) 96:649–55. doi: 10.1093/qjmed/hcg107
Keywords: dementia, cognitive decline, body mass index, weight loss, epidemiology, aging
Citation: Ciciliati AMM, Adriazola IO, Souza Farias-Itao D, Pasqualucci CA, Leite REP, Nitrini R, Grinberg LT, Jacob-Filho W and Suemoto CK (2021) Severe Dementia Predicts Weight Loss by the Time of Death. Front. Neurol. 12:610302. doi: 10.3389/fneur.2021.610302
Received: 25 September 2020; Accepted: 30 March 2021;
Published: 14 May 2021.
Edited by:
Christopher Butler, University of Oxford, United KingdomReviewed by:
Jiu Chen, Nanjing Medical University, ChinaCopyright © 2021 Ciciliati, Adriazola, Souza Farias-Itao, Pasqualucci, Leite, Nitrini, Grinberg, Jacob-Filho and Suemoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Kimie Suemoto, Y2tzdWVtb3RvQHVzcC5icg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.