
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 February 2021
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.604052
This article is part of the Research TopicNews and Views in the Management of Myasthenia GravisView all 24 articles
 Ji-Lan Han1,2
Ji-Lan Han1,2 Yao-Xian Yue3
Yao-Xian Yue3 Xiang Gao4
Xiang Gao4 Yan-Chen Xie5
Yan-Chen Xie5 Hong-Jun Hao6
Hong-Jun Hao6 Hong-Yan Li3
Hong-Yan Li3 Xiao-Long Yu7
Xiao-Long Yu7 Jie Li3
Jie Li3 Rui-Sheng Duan1*†
Rui-Sheng Duan1*† Hai-Feng Li8*†
Hai-Feng Li8*†Myasthenia gravis (MG) is an autoimmune disease in which antibodies bind to acetylcholine receptors (AChR) or other functional molecules in the postsynaptic membrane at the neuromuscular junction. Vitamin D (VD) has a number of pluripotent effects, which include immune-regulation and bone metabolism. The immunomodulatory actions of 1,25(OH)2D3 are mediated by its binding to a vitamin D receptor (VDR). In the study, we undertook a case-control study to explore the association between VDR gene polymorphism and the susceptibility and severity of MG patients. Four hundred and eighty MG patients and 487 healthy controls were included and gene polymorphisms of VDR were determined with improved multiplex ligation detection reaction technique and SNPscanTM technique. MG patients were classified into subgroups by essential clinical features and by a comprehensive classification. The frequencies of alleles and genotypes were compared between the MG group and the control group, between each MG subgroup and the control group, and between each pair of MG subgroups. There were no significant differences in frequencies of alleles and genotypes between MG patients and healthy controls, between MG subgroups and healthy controls, or between each pair of MG subgroups in the analysis of subgroups classified by essential clinical features (onset age, gender, thymoma, AChRAb positivity, onset involvement) and the maximal severity (modified Oosterhuis score). In the analysis of subgroups with a comprehensive classification, the frequencies of alleles and genotypes in rs731236 showed significant differences between adult non-thymoma AChRAb negative MG subgroup and the control group, as well as the adult non-thymoma AChRAb positive MG group. In the Chinese Han population, rs731236 was found to be possibly associated with adult non-thymoma AChRAb negative MG patients, although this needs further confirmation.
Myasthenia gravis (MG) is an autoimmune disease in which antibodies bind to acetylcholine receptors (AChR) or to other functional molecules, such as muscle-specific kinase (MuSK) and lipoprotein-receptor-related protein 4 (LRP4), in the postsynaptic membrane at the neuromuscular junction (NMJ) (1). Various immune cells involving innate and adaptive immunity participate in the pathogenesis of MG, including dendritic cells and B and T lymphocytes (2). Immune-modulating molecules, such as cytokines, are major mediators. An aberrant regulation of the immune system is presumed to be involved in the susceptibility and the severity of MG. Immune response is also modulated by other molecules, such as vitamin D (3).
Vitamin D (VD) has a number of pluripotent effects, which include immune-regulation and bone metabolism. 1,25(OH)2D3 promotes the differentiation of monocytes and inhibits the maturation of dendritic cells (4). 1,25(OH)2D3 inhibits the proliferation and differentiation of T helper 1 cells and modulates cytokine production by reducing the expression of pro-inflammatory interleukin-2 and interferon-γ, stimulates T helper 2 cells with upregulation of the production of anti-inflammatory cytokines, suppresses the development of Th17 cells, inhibits the production of interleukin-17, and induces proliferation of regulatory T cells (5, 6). Moreover, 1,25(OH)2D3 inhibits proliferation and differentiation of plasma cells (6).
The immunomodulatory actions of 1,25(OH)2D3 are mediated by its binding to VDR. The Vitamin D receptor (VDR) is a transcription factor belonging to the glucocorticoid nuclear receptor family, which binds to 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (7). VDRs are expressed in most immune cells, including monocytes/macrophages, dendritic cells, B lymphocytes, and T lymphocytes (3). The formation of the VD-VDR complex results in gene expression at the transcriptional level (3). The VDR gene is located in 12q14, which includes six untranslated exons (exon1a-1f) and eight coding exons (exons 2–9). The VDR gene could affect the expression of VDR (8). VDR genes' polymorphism has been associated with many autoimmune disorders such as autoimmune thyroid disease (9), idiopathic inflammatory myopathy (10), multiple sclerosis (11), type 1 diabetes mellitus (12), and systemic lupus erythematosus (13).
Our previous study found that VDR gene Tru9I (rs757343) polymorphism was associated with risk of MG in females older than 15 years (14). In this study, we undertook a case-control study to further explore the association between VDR gene polymorphisms and the susceptibility and severity of MG patients in a systematic way.
Four hundred and eighty patients who were diagnosed and treated in the Affiliated Hospital of Qingdao University and Beijing Friendship Hospital, Capital Medical University and 487 randomly recruited healthy controls in the same area were included in this study. All MG patients met the following diagnostic criteria: typical symptoms of fluctuating muscle weakness, positive result of neostigmine test, and the presence of AChRAb or MuSK antibody and/or amplitude decrement response >10% in low frequency repetitive nerve stimulation test (15, 16). MG patients were followed up with at least twice a year, with additional follow-ups if symptoms worsened and within 2–3 months thereafter (15, 16). Maximal severity was acquired by the history of MG patients and follow-ups and qualified by Oosterhuis score (17). Chronic infections (with relevant tests to exclude suspected infection when possible) and commonly associated autoimmune diseases (by self-reported questionnaire and tests including anti-thyroid antibodies and ENA) were excluded in both MG patients and healthy controls. All MG patients and healthy controls were Han Chinese in origin and non-consanguineous. Informed consent was obtained from all adult participants and the guardians of juvenile MG patients. The study was approved by the ethical committees of the two hospitals.
Patients were classified by essential clinical characteristics such as gender, onset age (<15/15–50/>50 years) (1), thymoma (typical CT and/or pathology), AChRAb, onset involvement (ocular/generalized), and the maximal severity (modified Oosterhuis score 0–2/3–5). AChRAb was detected with ELISA kits (RSR Limited, Cardiff, UK) and MuSK antibodies were detected and measured with RIA method (RSR Limited, Cardiff, UK) in AChR antibody negative MG patients. A natural history study showed that 82% of MG patients reached maximum worsening within 2 years after onset (18); therefore, the maximum Oosterhuis score was analyzed only in patients with a clinical course of 2 years or more. Because of potential interactions among the essential clinical characteristics (16, 19), a comprehensive classification (Figure 1) (20) was also used in the analysis. The new classification used the combination of essential clinical characteristics of MG to classify biologically and clinically meaningful subgroups. It ensured that any MG patients with sufficient data were assigned into one subgroup and only to one subgroup.
SNPs were selected systematically, including the functional loci [rs4516035 (5′ near gene), rs2228570 (exon 2), rs9729 (3′UTR)], hot SNPs [rs1544410 (9, 10, 12), rs731236 (9, 10, 12), rs7975232 (9, 10, 12), rs757343 (21), rs2238136 (22)], and tag SNPs (rs3847987, rs10875692, rs2107301, rs2239186, rs2853564, rs11574027, rs7136534, rs739837, and rs2239181). Nine tag SNPs were selected using the UCLA Association Study Design Server online software package (http://design.cs.ucla.edu), based on HapMap database (23). The minor allele frequency (MAF) of each SNP was more than 5% in the Han Chinese population (1,000 Genomes Project Phase 3). Genomic DNA was extracted using peripheral blood genomic DNA purification kit (Biochain, Newark, CA, USA). Rs1544410 and rs2107301 were genotyped by using improved multiplex ligation detection reaction (iMLDR) technique (Shanghai Genesky Biotechnologies Inc. China). The remaining fifteen SNPs were genotyped with SNPscanTM technique Kit (Cat#: G0104, Genesky Biotechnologies Inc. Shanghai, China). The primers and probes will be provided on request. Forty samples were randomly selected for double-blind quality control, and the results were consistent with the original genotyping results.
The online SNPstats software (https://snpstats.net/start.htm) was used to test the Hardy-Weinberg equilibrium in the control group. Genotype frequencies were analyzed under codominant and additive inheritance models in SNPstats software. The χ2 test or Fisher exact test was used to compare the allele frequencies between MG group and the control group, between each MG subgroup and the control group, and between each pair of MG subgroups (SPSS17.0). Bonferroni correction was applied for the multiple-testing. When there were significant differences in allele frequencies between MG subgroups and the control group or among MG subgroups, Logistic regression (SPSS 17.0) was used to adjust for potential confounding factors. The Haploview 4.2 software was used to calculate the linkage disequilibrium of SNPs and construct haplotype blocks. Post-hoc statistical power was calculated by Quanto program (version 1.2.4). P ≤ 0.05 was considered as statistically significant.
The successful genotyping rates of the seventeen SNPs were 96.07%~99.79%. Among the 17 selected SNPs, frequencies of rs2853564 in the healthy controls (P < 0.05) were not consistent with the Hardy-Weinberg equilibrium and were excluded from further analysis (Table 1).
In MG patients, 189 were males and 291 were females. Onset age was 1–86 years old (median 40, interquartile range 32). The disease duration of MG ranged from 8 to 220 months (median 43, interquartile range 61). There were 107 patients with thymoma and 367 patients without thymoma. Three hundred and thirty eight patients were AChRAb positive and 124 patients were AChRAb negative. Three hundred and forty two patients were ocular presenting and 135 patients were generalized presenting at onset. The others had no relevant information. The maximum Oosterhuis score was available in 370 patients (77%). Two hundred and sixteen patients were classified into the mild subgroup (Oosterhuis score 0–2) and 154 patients into the severe subgroup (Oosterhuis score 3–5) (Supplementary Table 1).
Four hundred and eighty seven healthy controls, including 249 males and 238 females, were 14–78 years old (median 45, interquartile range 24).
There were no significant differences in allele frequencies between the MG group and the control group, and in genotype frequencies between the MG group and the control group under the codominant or additive inheritance model among the 16 SNPs. There were no significant differences in allele or genotype frequencies between each MG subgroup (gender, onset age, thymoma, AChRAb, onset involvement, and maximal severity) and the control group, or between each pair of MG subgroups (Supplementary Table 1).
According to the comprehensive classification, 71 patients were juvenile MG (onset age <15) and 409 patients were adult MG. In adult MG patients, 104 patients had thymoma and 300 patients were without thymoma. In adult non-thymoma patients, 84 patients were AChRAb negative and 204 patients were AChRAb positive. In adult non-thymoma AChRAb negative patients, 15 patients were MuSK antibody positive and 69 patients were MuSK antibody negative. In adult non-thymoma AChRAb positive patients, 126 patients were early onset MG (EOMG, onset age 15–50) and 78 patients were late onset MG (LOMG, onset age >50); 135 patients were ocular presenting and 67 patients were generalized presenting. The others had no relevant information (Supplementary Table 2).
The G allele frequency in rs731236 was significantly higher in the adult non-thymoma AChRAb negative MG subgroup than that in the control group (Pbon = 0.032, OR = 2.42) and in the adult non-thymoma AChRAb positive MG group (Pbon = 0.032, OR = 2.90) (Table 2). Post-hoc statistical power was 0.9791 and 0.9929 based on Log-additive inheritance mode for the two comparisons. There were significant differences in genotype frequencies between adult non-thymoma AChRAb negative MG and the control group (P = 0.017 and P = 0.0044) as well as between adult non-thymoma AChRAb negative MG and adult non-thymoma AChRAb positive MG (P = 0.0092 and P = 0.003) in rs731236 under the codominant and additive inheritance model (Table 2).
We further compared between the MuSK antibody positive and MuSK antibody negative (double negative) group within the AChRAb negative MG patients. The G allele frequency in rs731236 was significantly higher in the double negative group than those in the control group (Pbon = 0.016, OR = 2.65). There were no significant differences in allele frequency in rs731236 between the MuSK positive and the double negative group, as well as between the MuSK positive and the control group (Supplementary Table 2).
Logistic regression analysis was performed in adult non-thymoma MG patients with AChRAb (positive and negative) as a dependent variable, and with gender (male and female), onset age (15–50 and >50 years), muscle involvement at onset (ocular and generalized), and genotypes of rs731236 (codominant model) as independent variables. The genotype and muscle involvement at onset were found to be independent risk factors (Table 3).
Linkage disequilibrium analysis amongst 16 SNPs was shown in Figure 2A. Two haplotype blocks were constructed. Block 1 was constructed by rs9729, rs3847987, rs739837, rs731236, rs7975232, rs10875692, and rs757343, and block 2 was constructed by rs11574027 and rs7136534. There were no significant differences in haplotype frequencies between MG and the control group (Table 4.1). We also performed linkage disequilibrium analysis between the adult non-thymoma AChRAb negative MG subgroup and the control group (Figure 2B). Two haplotype blocks were constructed. Block 1 was constructed by rs9729, rs3847987, rs739837, rs731236, rs7975232, rs10875692, rs757343, and rs1544410, and block 2 was constructed by rs11574027 and rs7136534. There were also no significant differences in haplotype frequencies between the adult non-thymoma AChRAb negative MG subgroup and the control group (Table 4.2).
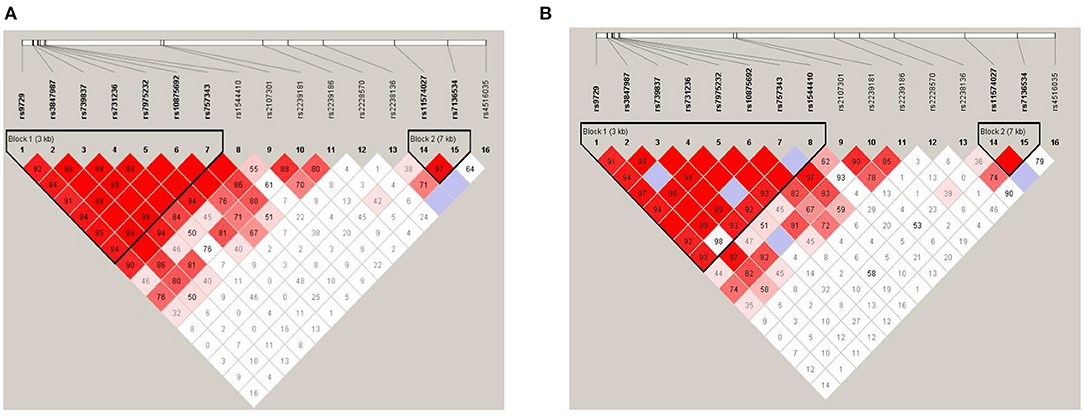
Figure 2. Linkage disequilibrium (LD) plot and haplotype block construction. MG patients and healthy controls (A); adult non-thymoma AChRAb negative MG patients and healthy controls (B). The locations of each SNP were indicated by the straight lines. The D' value corresponding to each SNP pair was expressed as a percentage and shown within the respective square (D' = 1, not shown). Higher D' values were indicated in darker red.
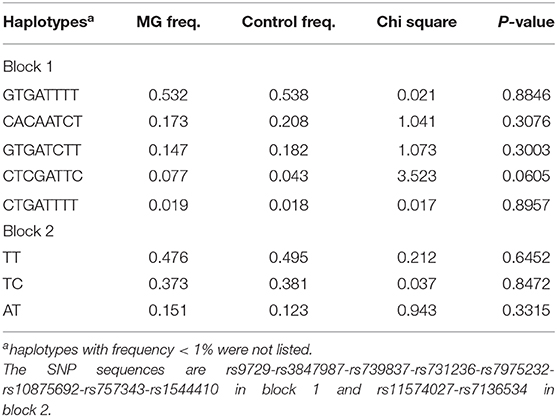
Table 4.2. Haplotypes of the VDR gene between adult non-thymoma AChRAb negative MG and the control group.
In our study, we found there were no significant differences between MG patients and healthy controls, between MG subgroups and healthy controls, or between each pair of MG subgroups in the analysis of subgroups classified by essential clinical features and the maximal severity. In subgroup analysis by the comprehensive classification, the frequencies of alleles and genotypes in rs731236 showed significant differences between the adult non-thymoma AChRAb negative MG subgroup and the control group, as well as the adult non-thymoma AChRAb positive MG group (Table 2). The statistical power of this association was high. We further compared between the MuSK antibody positive and the double negative group within the AChRAb antibody negative MG patients. There was significant difference between the double negative group and the control group. However, there were no significant differences between the MuSK antibody positive group and double negative group, as well as the control group. Although the comprehensive classification eliminates some of the confounding factors, other clinical variables (early or late onset, gender, and initial muscular involvement) might lead to confounding effects. Logistic analysis revealed that rs731236 was an independent risk factor in the adult non-thymoma AChRAb negative MG subgroup.
Rs731236 (also known as TaqI) is within exon 9. Its allele polymorphism yields a synonymous coding sequence. Nevertheless, it is located near the 3′UTR of VDR, which is known to be involved in the regulation of gene expression through the regulation of mRNA stability and protein translation efficiency (8). The rs731236 was found to be located in a CpG imposing a direct cis effect on site-specific and regional methylation (24). Children carrying the C allele for TaqI were more likely to develop asthma, and interleukin-10 levels were significantly low in asthmatics with the TC genotype for TaqI due to a decrease in expression of VDR (25). Therefore, it is presumed that rs731236 can affect the expression of VDR, and thus affect the expression of cytokines, thereby exerting immunomodulatory effects. rs731236 was also found to be associated with allergic diseases (8), autoimmune thyroid disease (9), and multiple sclerosis (26).
MG is a heterogeneous autoimmune disease with distinct immunogenetic characteristics in different MG subtypes (27). In AChRAb negative MG patients, antibodies against other NMJ proteins, such as MuSK and LRP4, are found (28). Moreover, some of the AChR antibody negative patients might be shown to be AChR antibody positive when more sensitive testing methods are used (29). In our study, there were no significant differences between the MuSK antibody positive and double negative group, as well as the control group. Hence, the double (AChR and MuSK antibodies) negative MG patients should be analyzed with further antibody testing.
Previous studies found that rs7975232, rs731236, and rs1544410 variants were in strong linkage disequilibrium (30), and we also performed haplotype analysis. We found that rs9729, rs3847987, rs739837, rs731236, rs7975232, rs10875692, and rs757343 were in high linkage disequilibrium, but there were no significant differences in haplotype frequencies between MG and the control group, which suggested that these haplotypes were not significantly related to the susceptibility of MG. There were also no significant differences in haplotype frequencies between the adult non-thymoma AChRAb negative MG subgroup and the control group.
Our previous research (14) found that VDR gene Tru9I (rs757343) polymorphism may be associated with risk of MG in females older than 15 years. However, the association was only found in a subgroup and the sample size was small. Moreover, AChR antibodies were not included in that study and no Logistic analysis was performed, which might lead to bias. Therefore, we recruited a new cohort with a larger sample size, selected SNPs of VDR gene in a systematic strategy, and performed Logistic analysis. In this study, we used both the subgroups classified by single clinical features and a new comprehensive scheme. We cannot confirm the previous association in this study.
There are several limitations to our study. The first limitation is that we did not measure the serum vitamin D levels. Our study mainly explores the association between vitamin D receptor gene polymorphism and the susceptibility and maximal severity of MG. Although vitamin D levels may be related with severity of MG by its immune-modulating effects, the serum vitamin D levels on blood collecting did not reflect the vitamin D levels during the entire disease course and at the maximal severity, due to factors such as steroid use, dietary habits, and sun exposure. This study cannot exclude the effects of vitamin D level by its design. Another limitation is that severity is analyzed by Oosterhuis score. It is relatively crude but has been used in other association studies (17). However, our study analyzes the maximum severity during the whole disease course of the patients. Oosterhuis scores can be accessed from the medical history, while more accurate measurements of severity, such as QMG scores recommended by MGFA, require on-site measurement, and hence are unfit for collecting information of maximal severity. A final limitation is that the sample size of the MuSK antibody positive MG subgroup is small and other related antibodies were not examined.
In conclusion, in the Chinese Han population, rs731236 was found to be possibly associated with adult non-thymoma AChRAb negative MG patients. Our study is a preliminary study, which needs further confirmation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found here: https://www.ncbi.nlm.nih.gov/SNP/snp_viewTable.cgi?handle=VDR-MG-CHINESE.
The studies involving human participants were reviewed and approved by the ethical committees of Affiliated Hospital of Qingdao University, and Beijing Friendship Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
J-LH, Y-XY, and H-FL designed the study, interpreted the data, and wrote the manuscript. Y-XY and H-FL designed the experiment. J-LH and Y-XY performed statistical analysis. Y-CX and H-JH contributed to discussion. H-FL, Y-CX, and XG diagnosed and treated patients. XG, H-YL, X-LY, and JL maintained the database. R-SD and H-FL revised the manuscript. All authors approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 81070963 and 81771362).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Genesky Biotechnologies Inc. (Shanghai) for their help in SNP genotyping.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.604052/full#supplementary-material
2. Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. (2014) 52:90–100. doi: 10.1016/j.jaut.2013.12.011
3. Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. (2011) 134:123–39. doi: 10.1111/j.1365-2567.2011.03482.x
4. Alhassan Mohammed H, Saboor-Yaraghi AA, Mirshafiey A, Vahedi H, Shiri-Shahsavar MR, Mousavi Nasl Khameneh A. Immunomodulatory and Immunosuppressive roles of 1α,25(OH)2D3 In Autoimmune Diseases. Scand J Immunol. (2017) 85:95–103. doi: 10.1111/sji.12512
5. Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int. (2020) 106:58–75. doi: 10.1007/s00223-019-00577-2
6. Skrobot A, Demkow U, Wachowska M. Immunomodulatory role of vitamin D: a review. Adv Exp Med Biol. (2018) 1108:13–23. doi: 10.1007/5584_2018_246
7. Maestro MA, Molnár F, Mouriño A, Carlberg C. Vitamin D receptor 2016: novel ligands and structural insights. Expert Opin Ther Pat. (2016) 26:1291–306. doi: 10.1080/13543776.2016.1216547
8. Zhang L, Zhang S, He C, Wang X. VDR gene polymorphisms and allergic diseases: evidence from a meta-analysis. Immunol Invest. (2020) 49:166–77. doi: 10.1080/08820139.2019.1674325
9. Feng M, Li H, Chen SF, Li WF, Zhang FB. Polymorphisms in the vitamin D receptor gene and risk of Autoimmune. Thyroid diseases: a meta-analysis. Endocrine. (2013) 43:318–26. doi: 10.1007/s12020-012-9812-y
10. Bodoki L, Chen JQ, Zeher M, Nagy-Vincze M, Griger Z, Zilahi E, et al. Vitamin D receptor gene polymorphisms and haplotypes in hungarian patients with idiopathic inflammatory myopathy. Biomed Res Int. (2015) 2015:809895. doi: 10.1155/2015/809895
11. Narooie-Nejad M, Moossavi M, Torkamanzehi A, Moghtaderi A. Positive association of vitamin D receptor gene variations with multiple sclerosis in South East Iranian population. Biomed Res Int. (2015) 2015:427519. doi: 10.1155/2015/427519
12. Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E. Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clin Immunol. (2009) 133:276–81. doi: 10.1016/j.clim.2009.08.004
13. Xiong J, He Z, Zeng X, Zhang Y, Hu Z. Association of vitamin D receptor gene polymorphisms with systemic lupus erythematosus: a meta-analysis. Clin Exp Rheumatol. (2014) 32:174–81.
14. Han JL, Li HF, Xie YC, Sun L, Wang ZX, Xu X, et al. Association between vitamin D receptor gene Tru9I Polymorphism and myasthenia gravis. Zhonghua Yi Xue Za Zhi. (2012) 92:2028–33.
15. Jiang P, Yue YX, Hong Y, Xie Y, Gao X, Gu CK, et al. IL-4Rα polymorphism is associated with myasthenia gravis in Chinese Han population. Front Neurol. (2018) 9:529. doi: 10.3389/fneur.2018.00529
16. Yue YX, Hong Y, Xie Y, Hao HJ, Sui Y, Gu CK, et al. Association study between IL-17A and IL-17F gene polymorphism and myasthenia gravis in Chinese patients. Neurol Sci. (2016) 37:123–30. doi: 10.1007/s10072-015-2375-y
17. Skeie GO, Pandey JP, Aarli JA, Gilhus NE. TNFA and TNFB polymorphisms in myasthenia gravis. Arch Neurol. (1999) 56:457–61. doi: 10.1001/archneur.56.4.457
18. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. (2008) 37:141–9. doi: 10.1002/mus.20950
19. Zhang X, Ding XJ, Wang Q, Yue YX, Xie Y, Hao HJ, et al. Rs3761389 polymorphism in autoimmune regulator (AIRE) gene is associated with susceptibility of myasthenia gravis in Chinese patients. J Clin Neurosci. (2017) 40:180–4. doi: 10.1016/j.jocn.2017.02.049
20. Li HF, Xie Y, Yue YX. Myasthenia gravis: subgroup classifications. Lancet Neurol. (2016) 15:355–6. doi: 10.1016/S1474-4422(16)00032-6
21. Ramos-Lopez E, Jansen T, Ivaskevicius V, Kahles H, Klepzig C, Oldenburg J, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. (2006) 1079:327–34. doi: 10.1196/annals.1375.050
22. Fang Y, Yu B, Yang Y, Liu G, Tu SY. Association between vitamin D receptor genetic polymorphisms and haplotypes and risk of lumbar degenerative disc disease in a Chinese population. Int J Clin Exp Pathol. (2017) 10:8695–702.
23. Han B, Kang HM, Seo MS, Zaitlen N, Eskin E. Efficient association study design via power-optimized tag SNP selection. Ann Hum Genet. (2008) 72:834–47. doi: 10.1111/j.1469-1809.2008.00469.x
24. Andraos C, Koorsen G, Knight JC, Bornman L. Vitamin D receptor gene methylation is associated with ethnicity, tuberculosis, and TaqI polymorphism. Hum Immunol. (2011) 72:262–8. doi: 10.1016/j.humimm.2010.12.010
25. Hutchinson K, Kerley CP, Faul J, Greally P, Coghlan D, Louw M, et al. Vitamin D receptor variants and uncontrolled asthma. Eur Ann Allergy Clin Immunol. (2018) 50:108–16. doi: 10.23822/EurAnnACI.1764-1489.46
26. Imani D, Razi B, Motallebnezhad M, Rezaei R. Association between vitamin D receptor (VDR) polymorphisms and the risk of multiple sclerosis (MS): an updated meta-analysis. BMC Neurol. (2019) 19:339. doi: 10.1186/s12883-019-1577-y
27. Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the guidelines of the German neurological society. J Neurol. (2016) 263:1473–94. doi: 10.1007/s00415-016-8045-z
28. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
29. Hong Y, Zisimopoulou P, Trakas N, Karagiorgou K, Stergiou C, Skeie GO, et al. Multiple antibody detection in‘seronegative'myasthenia gravis patients. Eur J Neurol. (2017) 24:844–50. doi: 10.1111/ene.13300
Keywords: myasthenia gravis, vitamin D receptor, polymorphism, susceptibility, severity
Citation: Han J-L, Yue Y-X, Gao X, Xie Y-C, Hao H-J, Li H-Y, Yu X-L, Li J, Duan R-S and Li H-F (2021) Vitamin D Receptor Polymorphism and Myasthenia Gravis in Chinese Han Population. Front. Neurol. 12:604052. doi: 10.3389/fneur.2021.604052
Received: 08 September 2020; Accepted: 05 January 2021;
Published: 09 February 2021.
Edited by:
Amelia Evoli, Catholic University of the Sacred Heart, ItalyReviewed by:
Carlo Provenzano, Catholic University of the Sacred Heart, ItalyCopyright © 2021 Han, Yue, Gao, Xie, Hao, Li, Yu, Li, Duan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Sheng Duan, cnVpc2hlbmdfZHVhbkB5YWhvby5jb20=; Hai-Feng Li, ZHJsaGZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.