- 1Department of Neurosurgery, College of Medicine, The First Affiliated Hospital, Zhejiang University, Hangzhou, China
- 2Department of Neurosurgery, Quzhou People's Hospital, Quzhou, China
Background: Patients with poor-grade aneurysmal subarachnoid hemorrhage (aSAH), defined as World Federation of Neurosurgical Societies (WFNS) grades IV–V have high rates of disability and mortality. The objective of this study was to accurately prognosticate the outcomes of patients with poor-grade aSAH by developing a new scoring model.
Methods: A total of 147 poor-grade aSAH patients in our center were enrolled. Risk variables identified by multivariate logistic regression analysis were used to devise a scoring model (total score, 0–9 points). The scores were estimated on the basis of β coefficients. A cohort of 68 patients from another institute was used to validate the model.
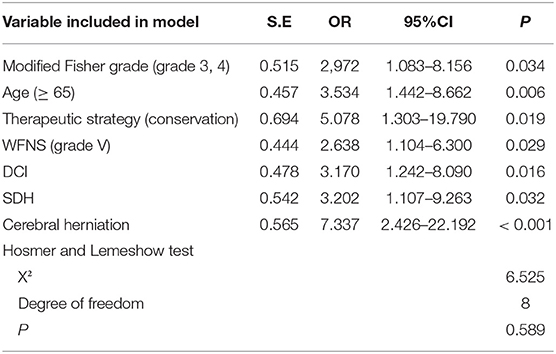
Results: Multivariate logistic regression analysis revealed that modified Fisher grade >2 [odds ratio [OR], 2.972; P = 0.034], age ≥65 years (OR, 3.534; P = 0.006), conservative treatment (OR, 5.078; P = 0.019), WFNS grade V (OR, 2.638; P = 0.029), delayed cerebral ischemia (OR, 3.170; P = 0.016), shunt-dependent hydrocephalus (OR, 3.202; P = 0.032), and cerebral herniation (OR, 7.337; P < 0.001) were significant predictors for poor prognosis [modified Rankin Scale [mRS] ≥3]. A scoring system was constructed by the integration of these factors and divided the poor-grade aSAH patients into three categories: low risk (0–1 points), intermediate risk (2–3 points), and high risk (4–9 points), with predicted risks of poor prognosis of 11, 52, and 87%, respectively (P < 0.001). The area under the curve in the derivation cohort was 0.844 (95% CI, 0.778–0.909). The AUC in the validation cohort was 0.831 (95% CI, 0.732–0.929).
Conclusions: The new scoring model can improve prognostication and help decision-making for subsequent complementary treatment in patients with aSAH.
Introduction
Intracranial aneurysms are abnormal protrusions of the intracranial arterial wall arising from various causes (1, 2). The prevalence rate of intracranial aneurysms in the global population (mean age, 50 years) is up to 3.2% (3). A previous report described that approximately, 1–2% of these aneurysms will rupture (4). According to statistics, the global incidence of aneurysmal subarachnoid hemorrhage (aSAH) is 9–11 per 100,000 people/year. Furthermore, poor-grade aSAH [World Federation of Neurological Surgeons [WFNS] grades IV–V] accounts for 18–30% of all aSAH cases (5, 6). A meta-analysis by Han et al. (7) reported a 26% mortality rate for poor-grade aSAH. At present, most related literature indicates that the disability rate for poor-grade aSAH exceeds 60% (8).
Regarding prognosis prediction, the International Subarachnoid Aneurysm Trial (ISAT) could achieve an accurate prediction of 60-day mortality after aSAH (9). Meanwhile, the Subarachnoid Hemorrhage International Trialists (SAHIT) model successfully predicted long-term outcomes and was used to counsel patients with aSAH and their family members (10). An external validation of the SAHIT model using the Barrow Ruptured Aneurysm Trial (BRAT) cohort revealed that its area under the curve (AUC) for unfavorable outcomes was 0.734 (11). It is worth pointing out that these studies included patients exposed to different subgroups of various treatment procedures, and that most of them were good-grade aSAH patients eligible for surgical treatment. Although good-grade and poor-grade aSAH patients differ in disease progression and survival prognosis (5, 12), previous studies typically combined these patients for analysis without detailed stratification (9–11). Therefore, the previous predictive models have some limitations for the accurate prediction of outcomes in poor-grade aSAH patients. The objective of the present study was to devise a new scoring system that can evaluate the prognosis of patients with poor-grade aSAH intuitively.
Materials and Methods
Study Design
The derivation cohort comprised poor-grade aSAH patients who were treated in the Department of Neurosurgery at our center from January 2013 to January 2019. The validation cohort was composed of aSAH patients treated in the Department of Neurosurgery at another institute from January 2016 to January 2019. The inclusion criteria were: (1) aSAH diagnosed by computed tomography (CT) or lumbar puncture in the medical center; (2) aneurysm confirmed as the cause of SAH on digital subtraction angiography (DSA), three-dimensional CT angiography, or magnetic resonance angiography; (3) WFNS grade IV and V; (4) signed informed consent from family members of patients to cooperate with clinical treatment procedures; and (5) patients without surgical treatment in referral centers. The exclusion criteria were: (1) traumatic, mycotic, or arteriovenous malformation-related aneurysms or SAH of unknown etiology; (2) WFNS grade less than or equal to III; (3) absence of important medical information for patients; and (4) patients treated with medical instruments or drugs that were not approved. The STROBE statement guideline has been implemented in this manuscript.
Clinical Therapeutic Protocol
All patients admitted under emergency conditions received early resuscitation, early CT angiography, multidisciplinary consensus consultation, conservative treatment, or surgical treatment. A multidisciplinary team of neurosurgeons and anesthesiologists made therapeutic decisions on the basis of the clinical conditions and family members' consent. The treatment mode in our study was divided into two categories: (1) the conservative group: patients who received pure medicinal conservative treatment or patients who received other basic surgical methods without treatment of the underlying aneurysm, such as external drainage surgery, hematoma evacuation, and decompressive craniectomy; and (2) the clipping or coiling group: patients who underwent primary aneurysm embolization or clipping alone, or combined with a basic surgical operation involving coiling or clipping. Patients underwent surgical treatment in accordance with an early treatment strategy (within 72 h). All aSAH patients were treated with routine SAH treatments, including mannitol, anticonvulsants, triple-H (hypervolemia/hypertension/hemodilution) treatment, and nimodipine treatment. Antiplatelets were administered to prevent thrombosis after stent-assisted embolization.
Clinical Data and Variable Definitions
The clinical variables were collected retrospectively from the hospital database. Patient's baseline information and imaging information were collected by two doctors separately, and any conflicting items were evaluated again by a senior doctor. Age was divided into two subcategories in accordance with the cutoff age of 65 years. The modified Fisher grade was divided into two subcategories in accordance with the cutoff value of grade 2. A wide-necked aneurysm was defined as an aneurysm with a neck width ≥4 mm or a neck ratio exceeding 1:2. Cerebral herniation was diagnosed based on CT results and corresponding signs, including deterioration of consciousness disturbance, some focal signs, oculomotor palsy, respiratory distress, and decorticate or decerebrate rigidity (13). Among the complications, shunt-dependent hydrocephalus (SDH) was defined as clinical deterioration occurring on the 14th day after aSAH and no other causes were found except for hydrocephalus, at the same time, it was observed in CT that the ventricular size progressively increased and the Evans index exceeded 0.30 (14). Epilepsy was defined as rhythmic jerking, with or without preceding tonic spasms, that was focal or generalized in nature, with or without loss of consciousness. Even one late seizure was considered to be post-stroke epilepsy (15, 16). Aneurysm rebleeding was defined as a sudden clinical deterioration accompanied by increased subarachnoid, intracerebral, or ventricular blood flow on subsequent CT scans (17). Cerebral vasospasm (CVS) was defined as arterial stenosis found on the CT angiography examination when the patient's clinical symptoms deteriorated, or vasospasm was detected during DSA (18). Delayed cerebral ischemia (DCI) was defined as: (1) occurrence of focal neurological impairment or decrease of ≥ 2 points on the Glasgow Coma Scale that could not be attributed to another cause, such as cerebral rebleeding or encephaledema; or (2) a new low-density area not seen on the previous CT scan and not attributable to other causes such as surgical treatment, or a low-density shadow after absorption of a hematoma (19).
Outcome Measures
A dynamic follow-up evaluation was performed at 6 months after discharge by neurosurgeons in accordance with the modified Rankin score (mRS) via telephone call or outpatient appointment. The assessment of neurological prognosis mainly focused on whether or not the patients presented with self-care ability. Functional prognosis was classified as good (mRS scores 0–2) or poor (mRS scores 3–6).
Statistical Analysis
Data were analyzed using the SPSS Version 23.0 software (IBM, Armonk, NY). Continuous variables were reported as mean ± standard deviation and compared between favorable and poor outcomes using an unpaired t-test. Categorical variables were reported as proportion and percentile and analyzed by the chi-square or Fisher exact test, as appropriate. Univariate and multivariate logistic regression analyses were performed using poor outcomes as the outcome variable in the derivation cohort. Variables with P ≤ 0.1 in the univariate analyses were entered into the multivariate logistic regression analysis with stepwise backward selection. Risk variables independently associated with prognosis were entered into the new scoring model. The points for individual factors were assigned on the basis of their corresponding β coefficients in the multivariate analysis. The discrimination of the prognostic model was assessed by the AUC in a receiver operating characteristic curve analysis. The Hosmer–Lemeshow goodness-of-fit test and a calibration plot were used to evaluate the calibration of the prediction model.
Results
Basic Information of Patients
The detailed processes for the selection and exclusion of patients in the derivation group and validation group are shown in Figure 1. In total, 147 patients were included in the derivation study and 68 patients were included in the validation cohort.
In the derivation cohort, 55 (37%) patients were male and 92 (63%) were female. Among these patients, the age range was 37–87 years, the mean age was 61.3 ± 11.5 years, and ~39% were aged ≥65 years. The baseline characteristics of the 147 patients with poor-grade aSAH are presented in Table 1. In total, 124 (84.3%) patients received surgical therapies including coiling (29.2%) and clipping (55.1%), and 23 (15.7%) patients received conservative treatment. In addition, there was no significant statistical difference between the treatment approach and the WFNS grade (P = 0.110). The distribution of mRS scores among the 147 poor-grade aSAH patients with different treatments is shown in Figure 2A. As shown in Figures 2B–E, patients who received coiling or clipping had a better prognosis than patients who received conservative treatment, but there was no significant difference in prognosis between patients who received coiling or clipping. There were 114 (77.6%) poor-grade aSAH patients with a modified Fisher grade >2 and 85 (57.8%) patients with WFNS grade V. The distribution of mRS scores among the 147 poor-grade aSAH patients with different modified Fisher grades is shown in Figure 2F. The influences of different modified Fisher grades on the prognosis of patients are shown in Figures 2G–J. During the 6-month follow-up after discharge, 85 patients (58%) had poor outcomes.
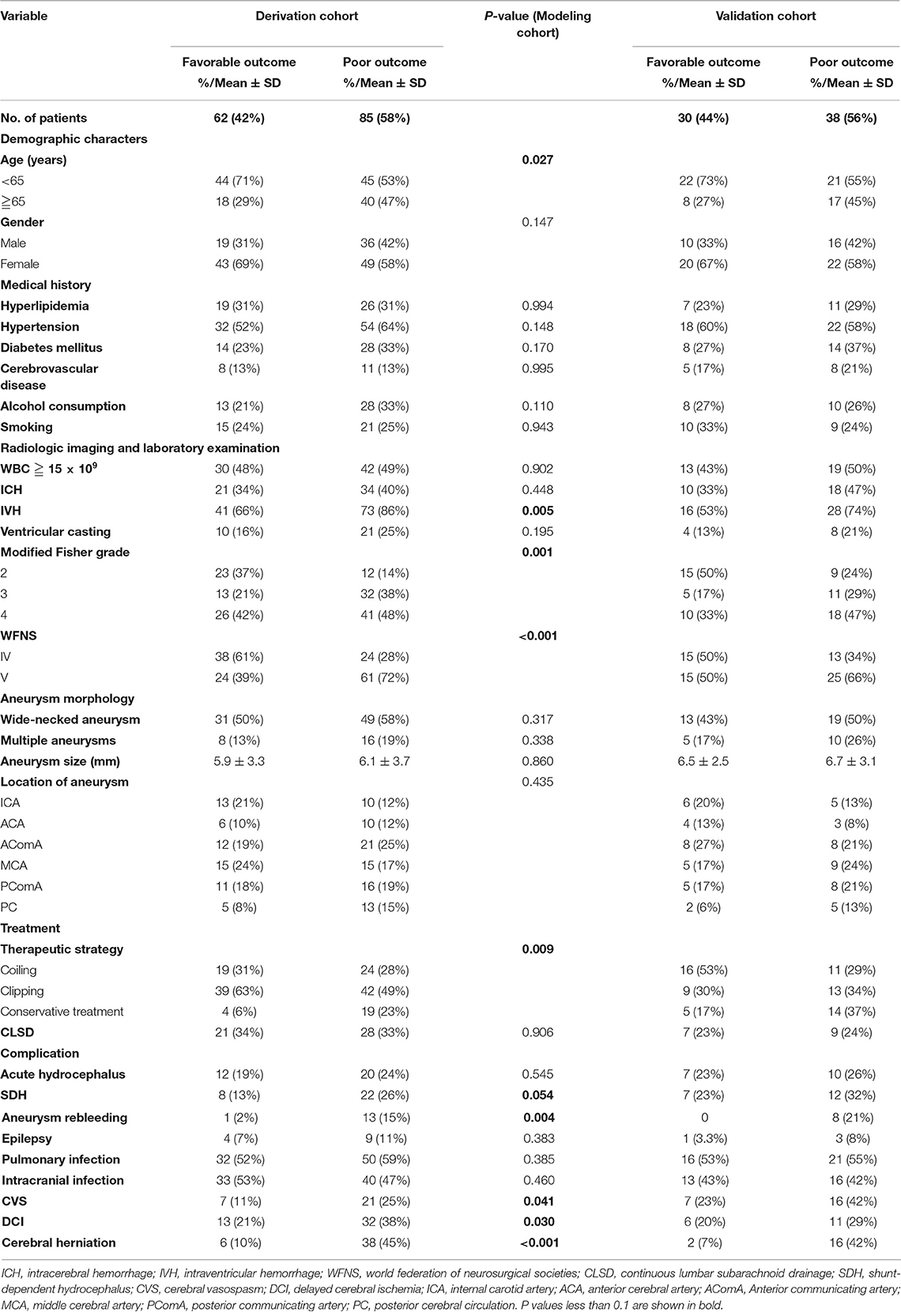
Table 1. Demographic and baseline characteristics of the study population and univariate analysis results of modeling cohorts.
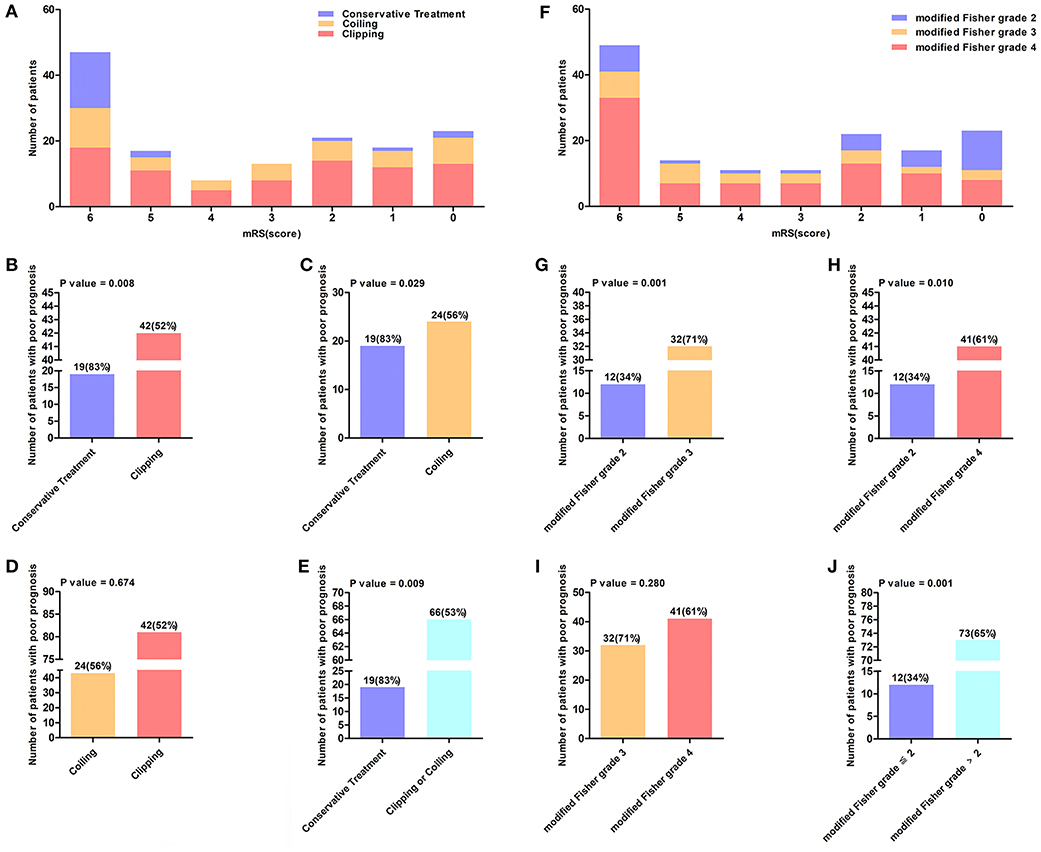
Figure 2. (A) The distribution of mRS score of 147 poor-grade aSAH patients who accepted different treatment methods. The value above the histogram shows the number of patients with poor prognosis and their percentage, for instance, the interpretation of 19 (83%) in (B) is that 19 (83%) patients had a poor outcome among 23 patients who received conservation treatment. (B–E) Reflects the influence of different treatment methods on the prognosis of patients. (F) Shows the distribution of mRS score of 147 poor-grade aSAH patients in different modified Fisher grade. (G–J) Reflects the influence of different modified Fisher grade groups on the prognosis of patients.
Of the 68 patients in the validation cohort, 25 (36.7%) patients were aged ≥65 years. A total of 44 (64.7%) poor-grade aSAH patients had a modified Fisher grade >2 and 40 (58.8%) patients presented with WFNS grade V. Forty-nine (72%) patients underwent surgical therapies. At the 6-month follow-up after discharge, 38 (56%) patients had poor outcomes. Specific data for the validation cohort are presented in Table 1.
Univariate Analyses of Poor Outcomes
The associations between clinical variables and poor outcomes identified by univariate analyses are shown in Table 1. Poor prognosis was associated with age ≥65 years (P = 0.027), intraventricular hemorrhage (IVH) (P = 0.005), WFNS grade V (P < 0.001), conservative treatment (P = 0.009), modified Fisher grade >2 (P = 0.001), emergence of cerebral herniation (P < 0.001), aneurysm rebleeding (P = 0.004), CVS (P = 0.041), and DCI (P = 0.030). Medical histories of patients and data for aneurysms were not significantly correlated with clinical outcomes.
Multivariate Regression Analysis of Poor Outcome
Ten variables with P < 0.1 in the univariate analyses were entered into the multivariate logistic regression analysis (Table 2). The results showed that age ≥65 years (OR, 3.534; P = 0.006), modified Fisher grade >2 (OR, 2,972; P = 0.034), cerebral herniation (OR, 7.337; P < 0.001), WFNS grade V (OR, 2.638; P = 0.029), SDH (OR, 3.202; P = 0.032), conservative treatment (OR, 5.078; P = 0.019), and DCI (OR, 3.170; P = 0.016) were independent risk factors for poor outcomes. The Hosmer–Lemeshow test reflected a satisfactory degree of consistency between the predicted risk of the model and the actual risk (P = 0.589; Table 2).
Development of the Scoring System
By integration of the seven independent risk factors, namely modified Fisher grade >2, age ≥65 years, conservative treatment, WFNS grade V, DCI, SDH, and cerebral herniation, a scoring system designated Poor-Grade Aneurysmal Subarachnoid Hemorrhage Prognostic Scoring System (PASHPSS) was constructed (Table 3). On the basis of the β coefficients in the multivariate analysis, scores of 2 were assigned to cerebral herniation and conservative treatment, and scores of 1 were assigned to the other five risk factors; otherwise, a score of 0 points was assigned. In accordance with the sum of the scores (range, 0–9), the new model divided poor-grade aSAH patients into three prognostically different categories (Table 4): low risk category, 11% prediction risk of poor prognosis in patients with total scores of 0–1 point; intermediate risk category, 51% prediction risk of poor prognosis in patients with total scores of 2–3 points; high risk category, 87% prediction risk of poor prognosis in patients with total scores of ≥4 points.
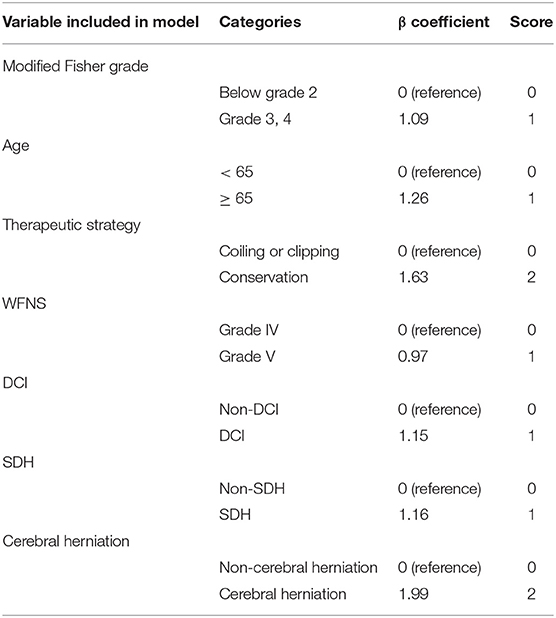
Table 3. Poor-Grade Aneurysmal Subarachnoid Hemorrhage Prognostic Scoring System (PASHPSS) derived from the β coefficients.
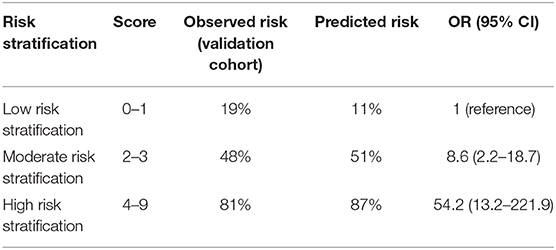
Table 4. Risk of poor prognosis for low, intermediate, and high-risk individuals according to the PASHPSS risk score.
Discrimination and Calibration of the Scoring System
In the derivation cohort, the AUC of the PASHPSS was 0.844 (95% CI: 0.778–0.909; Figure 3), and the Hosmer–Lemeshow test showed good calibration (P = 0.589). In the validation cohort, the PASHPSS also showed good discrimination with an AUC of 0.831 (95% CI, 0.732–0.929; Figure 3) and good calibration by the Hosmer–Lemeshow test (P = 0.984). Also in the validation cohort, the observed risks in the three risk categories were close to the predicted risks (Table 3): low risk category, actual observed risk of poor prognosis was 19%; intermediate risk category, actual observed risk of poor prognosis was 48%; high risk category, actual observed risk of poor prognosis was 81%.
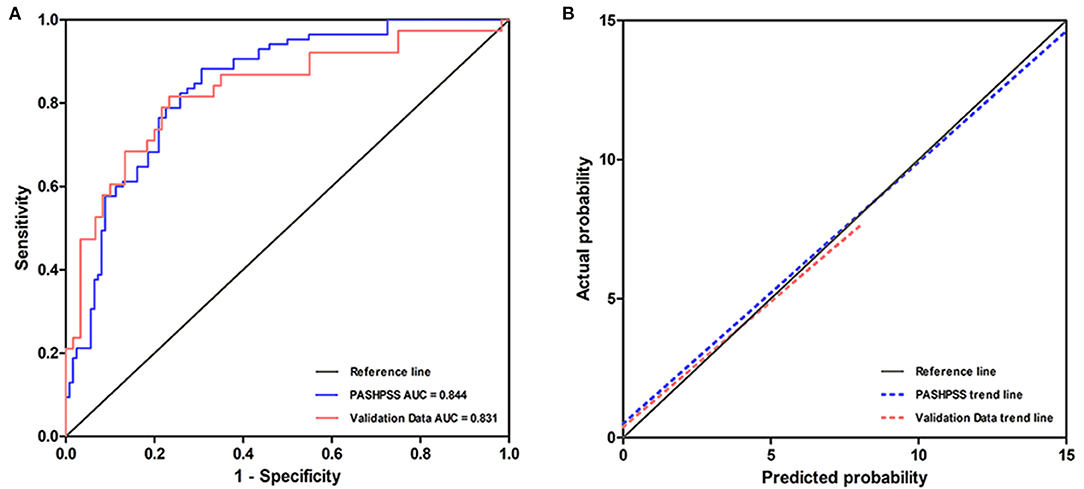
Figure 3. (A) The AUC of the PASHPSS is 0.844 (95% CI, 0.778–0.909) in our center's derivation data, while it is 0.831 (95% CI, 0.732–0.929) in validation data. (B) A slope of 1 (45 degrees) with an intercept of 0 represents perfect calibration, the deviation from the reference line is smaller, the calibration is better. PASHPSS has a good calibration in derivation cohort and validation cohort.
Discussion
As a serious cerebrovascular disease, poor-grade aSAH has high rates of mortality and disability. In this study, the rates of poor prognosis of patients in the modeling cohort and validation cohort both exceeded 55%. Although active and effective treatments can be provided, some aSAH patients still present with neurological dysfunctions and life disorders that have great impacts on society and family members (1, 4, 6, 8). It is necessary to explore the relevant risk factors and evaluate the prognosis of these patients. Several modifiable and non-modifiable risk factors for poor prognosis in poor-grade aSAH patients are currently known, with the most common risk factors being elderly age, cerebral herniation, WFNS grade V, and higher modified Fisher grade (9, 10, 12, 20–24). These risk factors were also identified in the present study.
The choice of treatment method is significantly related to the prognosis of patients with poor-grade aSAH. In a systematic review of 815 patients with aSAH, researchers reported that the rates of good prognosis in patients with clipping, embolization, and conservative treatment were 45.3, 36.3, and 9.0%, respectively (25). In our study, the rate of poor prognosis in patients with clipping or coiling was significantly lower than that in patients with conservative treatment, but there was no statistically significant difference in prognosis between patients with clipping or embolization. Combining our center's experience with previous literature on poor-grade aSAH patients, more aggressive treatment of the underlying aneurysm by surgery is associated with a better therapeutic prognosis than conservative treatment.
Post-operative complications play important roles in the prognosis of poor-grade aSAH patients. As a critical complication, aneurysmal rebleeding usually causes a sharp increase in intracranial pressure, damages the nerve function, and increases the risk of death in the short term (26–28). CVS is generally considered a risk factor for poor prognosis. However, immediate vasospasm is usually difficult to detect, and nimodipine is routinely used in clinical practice to prevent its occurrence, leading to an overall reduction in the incidence of CVS (29). A more commonly observed and easily detected complication during clinical treatment is DCI caused by CVS, which is a strong independent risk factor for poor prognosis in patients with poor-grade aSAH (19, 30, 31). DCI continues to be an important cause of cognitive impairment and disability after aSAH despite aggressive management (32–34). A single-center study on 888 aSAH patients found that SDH was a strong independent risk factor for unfavorable functional outcomes (35). Our final results confirmed predictive roles for the above-mentioned factors.
Some other risk factors have also been raised in recent articles, but have not been widely recognized. IVH was considered as a risk factor for poor outcomes in many reports (36). IVH was identified in our univariate analyses, but subsequently eliminated in the multivariate regression analysis. The possible reason may be that IVH caused impairment of cerebrospinal fluid absorption by blocking arachnoid villi and brain capillaries, thereby affecting the prognosis by developing into chronic hydrocephalus (37, 38). Whether or not aneurysm location and size are predictive factors for poor prognosis of aSAH patients remains inconclusive (24). These inconsistent results may be explained by treatment selection biases in different studies. In the present study, there was no correlation between aneurysm location and size and long-term prognosis. In a multicenter study on poor-grade aSAH patients, Zhao et al. (21) demonstrated that wide-necked aneurysms and post-operative pneumonia were also poor prognostic factors. However, these two risk factors were not identified in our study. Leukocytosis (WBC >15 × 109 /L) was regarded as a predictive factor for poor prognosis in a 9-year cohort study (22), but was not reported in other articles.
Although the current literature on poor-grade aSAH patients has focused on reporting risk factors for prognosis, prognostic predictive models for poor-grade aSAH patients are rare. A recent systematic review assessed 11 clinical prediction models for aSAH patients and found that the most common factors associated with outcomes were age (8 of 11 studies), neurologic grade on admission (10 of 11 studies), and amount of blood detected by CT examination on admission (6 of 11 studies) (24). Although the WFNS and modified Fisher grade scales were commonly used, both scales are not completely reliable in patients because of the subjective nature of the parameters on which the models were built (39). For example, the WFNS and Hunt–Hess scales are generally unreliable in intubated patients. Furthermore, in two articles that established predictive scores in poor-grade aSAH patient populations, the factors were applied, but no additional risk factors were added to circumvent the errors caused by the inter-rater and intra-rater variabilities (22, 23). Undeniably, more valuable risk variables added into a risk score can improve its predictivity. Treatment methods, SDH, and DCI are three factors that affect the long-term neurological prognosis and cognitive impairment of patients, and their roles in predicting the prognosis of patients are worthy of recognition (19, 35, 37, 40). Our PASHPSS showed significantly improved discrimination compared with other risk scores by including these risk factors. For example, the AUC of the SAHIT model was 0.734 (11), while the AUC of the WAP score for poor-grade aSAH patients was 0.74 (23). Meanwhile, the AUC of the PASHPSS was 0.844, which can be regarded as excellent, especially when predicting the prognosis of poor-grade aSAH patients.
At present, several studies have proposed prognosis models for poor-grade aSAH patients, but most of these models have limitations in reporting calibration, discrimination, and external validation. Clinicians generally do not use existing models for the prediction of prognosis in poor-grade aSAH patients, even though their internal effectiveness is not inferior to the PASHPSS (22–24), partly because they lack external validity. However, the PASHPSS showed good discrimination in the validation data. Specifically, its AUC was 0.831, meaning that the system still performed well when it was applied to a new patient cohort.
The present study shows that the PASHPSS developed with identified risk factors can predict the future risk of poor prognosis in aSAH patients very well. Furthermore, it can help guide clinical decisions and patient consultations, and may also reduce the cost of treatment by ensuring effective resource allocation. Such benefits may be particularly important in the management of patients with poor-grade aSAH.
Limitations
Some limitations of our risk score need to be discussed. First, the statistical data were retrospectively collected. Second, the results of the study only represent the subgroup of poor-grade aSAH patients. Therefore, the scoring model is applicable to the prediction of poor prognosis among poor-grade aSAH patients only. With regard to functional neurologic outcomes, we selected 6 months after discharge as the follow-up point on the basis of the critical period for neurological recovery. However, if data on long-term follow-up can be acquired, the prediction of prognosis will be more accurate. Furthermore, the modeling data were acquired from a single center, which may lead to some inevitable bias in the analysis and conclusions.
Conclusions
The obtained results have allowed us to draw the following conclusions. The main risk factors affecting the prognosis of patients with poor-grade aSAH are modified Fisher grade, elderly age, therapeutic schedule, WFNS grade, DCI, SDH, and cerebral herniation. The PASHPSS is an efficient tool for predicting the prognosis of poor-grade aSAH, can be easily measured, and is helpful for decision-making on subsequent complementary treatment and in reducing the cost of treatment by ensuring effective resource allocation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethics approval has been obtained from the ethics committee of First Affiliated Hospital of Zhejiang University. Non-essential identifiable details have been omitted from all manuscripts. The patients next of kin provided written informed consent to participate in this study.
Author Contributions
JS and JY contributed to writing the manuscript, acquisition of the data, and analysis and interpretation of the data. SH contributed to the acquisition of follow-up data and preliminary revision of the manuscript content. RM corrected the English language used in the manuscript. KH contributed to the acquisition of the data and preliminary revision of the manuscript content. XP contributed to preliminary revision of the manuscript content. GY provided the external validation data. ZX, LZ, ZL, and DC contributed to the literature review. JP and RZ contributed to the critical revision of the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Key Research & Development (R&D) Plan of Zhejiang Province (No. 2019C03034).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks for the data provided by An Wu, director of the Department of Neurosurgery of The Quzhou Municipal People's Hospital. This manuscript has been released as a pre-print at Research Square (41).
Abbreviations
aSAH, Aneurysmal subarachnoid hemorrhage; WFNS, World Federation of Neurosurgical Societies; OR, Odds ratio; CI, Confidence interval; ICU, Intensive care unit; DCI, Delayed cerebral ischemia; CVS, Cerebral vasospasm; GOS, Glasgow Outcome Scale; IVH, Intraventricular hemorrhage; ICH, Intracerebral hemorrhage; CT, Computed tomography; mRS, Modified Rankin score; ISAT, International Subarachnoid Aneurysm Trail; SAHIT, Subarachnoid Hemorrhage International Trialists; BRAT, Barrow Ruptured Aneurysm Trial; CLSD, Continuous lumber subarachnoid drainage; AUC, Area under the curve; WBC, White blood cell count; SDH, Shunt-dependent hydrocephalus; PASHPSS, Poor-Grade Aneurysmal Subarachnoid Hemorrhage Prognostic Scoring System.
References
1. De Rooij NK, Linn FH, Van Der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. (2007) 78:1365–72. doi: 10.1136/jnnp.2007.117655
2. Wiebers D, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
3. Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2368–400. doi: 10.1161/STR.0000000000000070
4. Ellis JA, Nossek E, Kronenburg A, Langer DJ, Ortiz RA. Intracranial aneurysm: diagnostic monitoring, current interventional practices, and advances. Curr Treat Options Cardiovasc Med. (2018) 20:94. doi: 10.1007/s11936-018-0695-y
5. Steiner T. Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, et al. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. (2013) 35:93–112. doi: 10.1159/000346087
6. Howard. BM, Barrow. DL. Outcomes for patients with poor-grade subarachnoid hemorrhage: to treat or not to treat? World Neurosurgery. (2016) 85:125–9. doi: 10.1016/j.wneu.2015.10.034
7. Han Y, Ye F, Long X, Li A, Xu H, Zou L, et al. Ultra-Early treatment for poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. World Neurosurg. (2018) 115:e160–71. doi: 10.1016/j.wneu.2018.03.219
8. Wartenberg KE. Critical care of poor-grade subarachnoid hemorrhage. Curr Opin Crit Care. (2011) 17:85–93. doi: 10.1097/MCC.0b013e328342f83d
9. Risselada R, Lingsma HF, Bauer-Mehren A, Friedrich CM, Molyneux AJ, Kerr RS, et al. Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the International Subarachnoid Aneurysm Trial (ISAT). Eur J Epidemiol. (2010) 25:261–6. doi: 10.1007/s10654-010-9432-x
10. Jaja BNR, Saposnik G, Lingsma HF, Macdonald E, Thorpe KE, Mamdani M, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. (2018) 360:j5745. doi: 10.1136/bmj.j5745
11. Mascitelli JR, Cole T, Yoon S, Nakaji P, Albuquerque FC, Mcdougall CG, et al. External validation of the subarachnoid hemorrhage international trialists (SAHIT) predictive model using the barrow ruptured aneurysm trial (BRAT) cohort. Neurosurgery. (2020) 86:101–6. doi: 10.1093/neuros/nyy600
12. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. (2002) 360:1267–74. doi: 10.1016/S0140-6736(02)11314-6
13. Riveros Gilardi B, Munoz Lopez JI, Hernandez Villegas AC, Garay Mora JA, Rico Rodriguez OC, Chavez Appendini R, et al. Types of cerebral herniation and their imaging features. Radiographics. (2019) 39:1598–610. doi: 10.1148/rg.2019190018
14. Suzuki H, Kinoshita N, Imanaka-Yoshida K, Yoshida T, Taki W. Cerebrospinal fluid tenascin-C increases preceding the development of chronic shunt-dependent hydrocephalus after subarachnoid hemorrhage. Stroke. (2008) 39:1610–2. doi: 10.1161/STROKEAHA.107.505735
15. Lin CL, Dumont AS, Lieu AS, Yen CP, Hwang SL, Kwan AL, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. (2003) 99:978–85. doi: 10.3171/jns.2003.99.6.0978
16. Engel J Jr. Report of the ILAE classification core group. Epilepsia. (2006) 47:1558–68. doi: 10.1111/j.1528-1167.2006.00215.x
17. Schuss P, Hadjiathanasiou A, Borger V, Wispel C, Vatter H, Guresir E. Poor-Grade aneurysmal subarachnoid hemorrhage: factors influencing functional outcome–a single-center series. World Neurosurg. (2016) 85:125–9. doi: 10.1016/j.wneu.2015.08.046
18. Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. (2009) 40:1963–8. doi: 10.1161/STROKEAHA.108.544700
19. Vergouwen MD, Vermeulen M, Van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. (2010) 41:2391–5. doi: 10.1161/STROKEAHA.110.589275
20. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. (2015) 385:691–7. doi: 10.1016/S0140-6736(14)60975-2
21. Zhao B, Yang H, Zheng K, Li Z, Xiong Y, Tan X, et al. Preoperative and postoperative predictors of long-term outcome after endovascular treatment of poor-grade aneurysmal subarachnoid hemorrhage. J Neurosurg. (2017) 126:1764–71. doi: 10.3171/2016.4.JNS152587
22. Szklener S, Melges A, Korchut A, Zaluska W, Trojanowski T, Rejdak R, et al. Predictive model for patients with poor-grade subarachnoid haemorrhage in 30-day observation: a 9-year cohort study. BMJ Open. (2015) 5:e007795. doi: 10.1136/bmjopen-2015-007795
23. Zheng K, Zhong M, Zhao B, Chen SY, Tan XX, Li ZQ, et al. Poor-Grade aneurysmal subarachnoid hemorrhage: risk factors affecting clinical outcomes in intracranial aneurysm patients in a multi-center study. Front Neurol. (2019) 10:123. doi: 10.3389/fneur.2019.00123
24. Jaja BN, Cusimano MD, Etminan N, Hanggi D, Hasan D, Ilodigwe D, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. (2013) 18:143–53. doi: 10.1007/s12028-012-9792-z
25. Ohkuma H, Shimamura N, Naraoka M, Katagai T. Aneurysmal subarachnoid hemorrhage in the elderly over age 75: a systematic review. Neurol Med Chir. (2017) 57:575–83. doi: 10.2176/nmc.ra.2017-0057
26. Van Donkelaar CE, Bakker NA, Veeger NJ, Uyttenboogaart M, Metzemaekers JD, Luijckx GJ, et al. Predictive factors for rebleeding after aneurysmal subarachnoid hemorrhage: rebleeding aneurysmal subarachnoid hemorrhage study. Stroke. (2015) 46:2100–6. doi: 10.1161/STROKEAHA.115.010037
27. Larsen CC, Astrup J. Rebleeding after aneurysmal subarachnoid hemorrhage: a literature review. World Neurosurg. (2013) 79:307–12. doi: 10.1016/j.wneu.2012.06.023
28. Lu VM, Graffeo CS, Perry A, Carlstrom LP, Rangel-Castilla L, Lanzino G, et al. Rebleeding drives poor outcome in aneurysmal subarachnoid hemorrhage independent of delayed cerebral ischemia: a propensity-score matched cohort study. J Neurosurg. (2019) 133:360–8. doi: 10.3171/2019.4.JNS19779
29. Findlay JM, Nisar J, Darsaut T. Cerebral vasospasm: a review. Can J Neurol Sci. (2016) 43:15–32. doi: 10.1017/cjn.2015.288
30. Al-Mufti F, Roh D, Lahiri S, Meyers E, Witsch J, Frey HP, et al. Ultra-early angiographic vasospasm associated with delayed cerebral ischemia and infarction following aneurysmal subarachnoid hemorrhage. J Neurosurg. (2017) 126:1545–51. doi: 10.3171/2016.2.JNS151939
31. Leclerc JL, Blackburn S, Neal D, Mendez NV, Wharton JA, Waters MF, et al. Haptoglobin phenotype predicts the development of focal and global cerebral vasospasm and may influence outcomes after aneurysmal subarachnoid hemorrhage. Proc Natl Acad Sci USA. (2015) 112:1155–60. doi: 10.1073/pnas.1412833112
32. Wang X, Han C, Xing D, Wang C, Ding X. Early management of poor-grade aneurysmal subarachnoid hemorrhage: a prognostic analysis of 104 patients. Clin Neurol Neurosurg. (2019) 179:4–8. doi: 10.1016/j.clineuro.2019.02.003
33. Etminan N, Vergouwen Md Rl. M. Angiographic vasospasm versus cerebral infarction as outcome measures after aneurysmal subarachnoid hemorrhage. Acta Neurochirurgica. (2013) 115:33–40. doi: 10.1007/978-3-7091-1192-5_8
34. Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. (2002) 33:200–8. doi: 10.1161/hs0102.101080
35. Paisan GM, Ding D, Starke RM, Crowley RW, Liu KC. Shunt-Dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: predictors and long-term functional outcomes. Neurosurgery. (2018) 83:393–402. doi: 10.1093/neuros/nyx393
36. Nguyen HS, Li L, Patel M, Kurpad S, Mueller W. Radiodensity of intraventricular hemorrhage associated with aneurysmal subarachnoid hemorrhage may be a negative predictor of outcome. J Neurosurg. (2018) 128:1032–6. doi: 10.3171/2016.11.JNS152839
37. De Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, et al. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery. (2007) 61:924–33; discussion 933–24. doi: 10.1227/01.neu.0000303188.72425.24
38. Lai L, Morgan MK. Predictors of in-hospital shunt-dependent hydrocephalus following rupture of cerebral aneurysms. J Clin Neurosci. (2013) 20:1134–8. doi: 10.1016/j.jocn.2012.09.033
39. Degen LA, Dorhout Mees SM, Algra A, Rinkel GJ. Interobserver variability of grading scales for aneurysmal subarachnoid hemorrhage. Stroke. (2011) 42:1546–9. doi: 10.1161/STROKEAHA.110.601211
40. Van Donkelaar CE, Dijkland SA, Van Den Bergh WM, Bakker J, Dippel DW, Nijsten MW, et al. Early circulating lactate and glucose levels after aneurysmal subarachnoid hemorrhage correlate with poor outcome and delayed cerebral ischemia: a two-center cohort study. Crit Care Med. (2016) 44:966–72. doi: 10.1097/CCM.0000000000001569
41. Jie S, Jianbo Y, Sicong H, Rajneesh M, Kaiyuan H, Xinfa P, et al. A Scoring Model to Predict the Prognosis of Patients With Poor-Grade Aneurysmal Subarachnoid Hemorrhage Research Square 2020. (2020) Available online at: https://researchsquare.com/article/rs-69333/v1 (accessed Sep 2, 2020).
Keywords: scoring system, prognosis, poor-grade, aneurysmal subarachnoid hemorrhage, model
Citation: Shen J, Yu J, Huang S, Mungur R, Huang K, Pan X, Yu G, Xie Z, Zhou L, Liu Z, Cheng D, Pan J and Zhan R (2021) Scoring Model to Predict Functional Outcome in Poor-Grade Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 12:601996. doi: 10.3389/fneur.2021.601996
Received: 02 September 2020; Accepted: 20 January 2021;
Published: 18 February 2021.
Edited by:
Andrew Bivard, The University of Melbourne, AustraliaReviewed by:
Marialuisa Zedde, Local Health Authority of Reggio Emilia, ItalyR. Loch Macdonald, University of Toronto, Canada
Copyright © 2021 Shen, Yu, Huang, Mungur, Huang, Pan, Yu, Xie, Zhou, Liu, Cheng, Pan and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renya Zhan, MTE5NjA1N0B6anUuZWR1LmNu; Jianwei Pan, MTIwMjA1M0B6anUuZWR1LmNu
†These authors have contributed equally to this work
 Jie Shen1†
Jie Shen1† Jianbo Yu
Jianbo Yu Sicong Huang
Sicong Huang Xinfa Pan
Xinfa Pan Jianwei Pan
Jianwei Pan Renya Zhan
Renya Zhan