- Department of Neurosurgery, Joan C. Edwards School of Medicine, Marshall University, Huntington, WV, United States
Sedation is a ubiquitous practice in ICUs and NCCUs. It has the benefit of reducing cerebral energy demands, but also precludes an accurate neurologic assessment. Because of this, sedation is intermittently stopped for the purposes of a neurologic assessment, which is termed a neurologic wake-up test (NWT). NWTs are considered to be the gold-standard in continued assessment of brain-injured patients under sedation. NWTs also produce an acute stress response that is accompanied by elevations in blood pressure, respiratory rate, heart rate, and ICP. Utilization of cerebral microdialysis and brain tissue oxygen monitoring in small cohorts of brain-injured patients suggests that this is not mirrored by alterations in cerebral metabolism, and seldom affects oxygenation. The hard contraindications for the NWT are preexisting intracranial hypertension, barbiturate treatment, status epilepticus, and hyperthermia. However, hemodynamic instability, sedative use for primary ICP control, and sedative use for severe agitation or respiratory distress are considered significant safety concerns. Despite ubiquitous recommendation, it is not clear if additional clinically relevant information is gleaned through its use, especially with the contemporaneous utilization of multimodality monitoring. Various monitoring modalities provide unique and pertinent information about neurologic function, however, their role in improving patient outcomes and guiding treatment plans has not been fully elucidated. There is a paucity of information pertaining to the optimal frequency of NWTs, and if it differs based on type of injury. Only one concrete recommendation was found in the literature, exemplifying the uncertainty surrounding its utility. The most common sedative used and recommended is propofol because of its rapid onset, short duration, and reduction of cerebral energy requirements. Dexmedetomidine may be employed to facilitate serial NWTs, and should always be used in the non-intubated patient or if propofol infusion syndrome (PRIS) develops. Midazolam is not recommended due to tissue accumulation and residual sedation confounding a reliable NWT. Thus, NWTs are well-tolerated in selected patients and remain recommended as the gold-standard for continued neuromonitoring. Predicated upon one expert panel, they should be performed at least one time per day. Propofol or dexmedetomidine are the main sedative choices, both enabling a rapid awakening and consistent NWT.
Introduction
There is widespread use of sedation for patients in the intensive care unit (ICU) and neurocritical care unit (NCCU). This is a necessary practice to facilitate endotracheal intubation and mechanical ventilation, however, it is also Janus-faced. There is good clinical utility, such as controlling patient distress, attenuating anxiety, and abating pain recognition (1), with neuro-specific benefits including reduced metabolic demands to decrease energy consumption (2), decreased stress-related injury, as well as seizure, temperature, and intracranial pressure (ICP) control (3, 4). However, over sedation harbors complications, including increased morbidity (5, 6), prolonged ventilation with associated pneumonia (3), greater muscular atrophy, venous stasis, thrombosis, and a protracted ICU length of stay. Further, it may increase hospital costs secondary to ordering unnecessary neuroimaging (1). Too little sedation can magnify agitation and autonomic instability, leading to elevated ICP, hypertension, tachycardia, and cerebral oxygen consumption (1). Thus, risks and benefits must be carefully weighed when it comes to achieving optimal sedation.
Sedation also precludes an accurate neurologic examination, and continued sedation may mask significant changes in the patient's neurologic condition (7). This is concerning, as upwards of 40% of patients with a traumatic brain injury (TBI) demonstrate a significant deterioration of neurologic function within 10 days (8). There is a known secondary deterioration that evolves during the early period of brain injury that is heterogenous and hard to predict (9). This is due to secondary injury cascades that activate inflammatory, excitotoxic, metabolic, and vascular phenomena. This augments oxidative stress, elevates ICP and metabolic demands, causes cerebral edema, activates coagulation cascades, and impairs regional blood flow (7, 10). Continued sedation can also prevent the acquisition of an accurate Glasgow coma scale (GCS) score. This is imperative, as GCS scores are a robust prognostic marker and indicator of potential surgical intervention (11, 12), and are highly predictive of 6-month outcomes in TBI patients (13). This underlies the necessity for a brief cessation of sedation for an accurate neurologic assessment, termed the neurological wake-up test (NWT). The NWT is considered to be the gold-standard for neuro-monitoring (1, 3), and is the basis for neuroanatomical localization of pathology, identifying undiagnosed neurologic ailments, detecting early neurologic signs of insult, determining prognosis, and guiding appropriate therapy (3, 7, 14).
Serial NWTs are an integral part of continued ICU and NCCU assessment of neurologic functioning but are concerning because they require a temporary cessation of sedation. This results in a significant sympathetic nervous system (SNS) discharge, potentially resulting in neurologic injury via elevating ICP, increasing cerebral oxygen demand, and reducing cerebral perfusion. However, this must be weighed against the additional clinical information acquired. Moreover, with the increasing utilization of multimodality monitoring, there may be enough information gathered to make the NWT both harmful and redundant. Therefore, this paper will aim to elucidate the utility of the NWT, if it still has a role with multimodality monitoring, if there is an optimal frequency that imparts the most favorable risk to benefit profile, and if the choice of sedative has an influence on these quandaries. For this review, PubMed was searched for all existing literature using the terms brain injury, head injury, or TBI, with the terms sedation cessation, daily interruption of sedation, wake-up test, stopping sedation, spontaneous awakening trial, neurologic examination, multimodality monitoring, frequency of awakening, and/or frequency of neurologic exam.
Multimodality Neuromonitoring
A healthy brain has robust autoregulatory mechanisms to maintain constant cerebral blood flow (CBF) across a range of mean arterial pressures (MAPs) from 65 to 150 mmHg. In the presence of neurologic insult, there is often regional or global impairment of autoregulation (1). Thus, continued monitoring of ICP and CPP are often necessary. Utilizing transcranial doppler (TCD) may assist in assessing the degree of autoregulation failure (15). Other monitoring modalities include brain tissue oxygen tension monitoring (PbtiO2), jugular venous oxygen saturation (SjvO2), and brain neurochemistry by intracerebral microdialysis (MD). Newer, less invasive monitoring technologies including optic ultrasound, and the automated pupillometer to directly augment the NWT are also seeing increased use. Lastly, a role for brain injury biomarkers in diagnosis and prognosis is beginning to emerge.
ICP
Normal ICP values are between 5 and 15 mmHg (16). Increased ICP is defined as pressures >20 mmHg for more than 5 min. This monitoring can be achieved through insertion of a catheter into the lateral ventricle to act as an external ventricular drain (EVD), or via insertion of an intraparenchymal monitor (IPM) (7). There is frequent discordance in EVD vs. IPM measures, but little consistency exists about over vs. underestimating to allow for correction (17). The EVD measure is considered most accurate, but it also harbors increased rates of infection and hemorrhage. Furthermore, when maintained in a continuous open state, EVD measures are often erroneous (18). EVD has been suggested for use due to its ability to drain CSF, which can aid in controlling refractory ICP elevations (19).
ICP monitoring is recommended as part of the official TBI guidelines (20); consequently, there is widespread use of ICP monitoring of brain injured patients across NCCUs (7, 9, 21). The Brain Trauma Foundation (BTF) recommends monitoring for comatose patients [GCS of (3–8)] that have an abnormal CT scan (22). Based upon the International Multidisciplinary Consensus Conference on multimodality monitoring, ICP monitoring is strongly recommend, along with clinical examinations and other monitoring modalities to accurately prognosticate and guide treatment (11). ICP is frequently elevated following neurologic insult, and is a recognized cause of morbidity and especially mortality after TBI (7, 22–25). There is evidence to suggest that aggressive management of elevated ICP can improve outcomes in TBI patients (9, 22). Conversely, concerns have been raised regarding no improved clinical outcomes with ICP monitoring, and possibly increased mortality rates from its use, at least in TBI patients (7, 26). This was corroborated by the BEST-TRIP trial (27), which demonstrated that treatment guided by ICP-monitoring was not superior to treatment guided by the NWT and serial CT scans. In patients with hemispheric ischemic stroke and associated cerebral edema, ICP monitoring is not recommended (28).
Optic Ultrasound
Optic ultrasound is a quick, cost-effective, non-invasive method to measure ICP by evaluating optic nerve sheath diameter (ONSD). The optic nerve sheath is contiguous with the dura and contains CSF that communicates with cerebral subarachnoid components (29). Measuring 3-mm behind the globe at the anterior portion of the optic nerve, an ONSD of 5-mm translates to an ICP of ~20 mmHg (29). A meta-analysis of six studies calculated a sensitivity of 90%, and specificity of 85%, for detecting elevated ICP (30). Another prospective study calculated a sensitivity of 93.75%, and specificity of 86.67% for identifying increased ICP (31). This means that somewhere between 6 and 10% of patients with elevated ICP will be missed using this monitoring modality. This may be an acceptable trade-off given its quick bedside ease of use, and when coupled with clinical exam findings, may offset those limitations. Overall, measuring ONSD has established itself as a reliable initial screening tool for detecting ICP changes (32). It can also be useful to track individual changes quickly per patient, as ONSD changes occur within 5 min of ICP shifts (33). This may play an especially crucial role in situations where access to invasive monitoring techniques are unavailable.
CPP
rCPP can be calculated with ICP monitoring, as CPP = MAP – ICP. Uncertainty exists surrounding differing placements of zero reference points for ICP and MAP, which represents a technical hurdle in attaining accurate and consistent CPP values. This is a major issue, as many of the studies behind recommended CPP thresholds did not report their methods for obtaining CPP (34, 35). Moreover, the BTF guidelines state that CPP is calibrated to the level of the right atrium by convention (20). Most brain injured patients are maintained at 30° of head elevation, and the resultant 30 cm distance between the heart and head can overestimate CPP by up to 11 mmHg, and at elevations up to 50°, the CPP can be overestimated by 18 mmHg (36). Thus, in patients with head of the bed elevation, it is crucial that the arterial transducer is positioned at the level of the middle cranial fossa, which is approximated to the tragus, to ensure accurate CPP measurements (37).
The BTF recommends a CPP range between 60 and 70 mmHg (20). Crucially, CPP elevation past 70 mmHg has been linked to poor outcomes in TBI patients, along with lung damage, whereas CPP levels below 70 mmHg have been associated with worsening brain hypoxia (7). Andrews and colleagues found that low CPP along with hypotension was the best predictor of death in TBI patients (38). However, there is great variability in outcomes. In one study half of the patients benefited from higher CPP, and the other half benefited from lower CPP (39). To attenuate this variability, some recommend individualizing the CPP target (24, 40, 41) through the use of cerebral autoregulation monitoring (42).
SjvO2
SjvO2 monitoring is used to acquire information pertaining to cerebral oxygen supply, perfusion, and consumption. It is performed via fiberoptic catheters placed into the internal jugular bulb distal to the jugular foramen, or intermittently checking jugular venous blood samples (7, 9). Falsely elevated SjvO2 can be measured due to the presence of only extracerebral blood in the jugular bulb (43), which depends on contamination from extracerebral sources via aspirating too quickly, or misplacing the catheter by a couple of centimeters (44). This modality is of limited utility in severe global ischemia or very large infarcts, as SjvO2 can rebound upward due incomplete oxygen extraction by the ischemic tissue (43, 45).
Normal values range from 55 to 75% oxygen saturation (O2 sat). Lower values are indicative of ischemia, and values <50 and > 75% are both associated with poor patient outcomes (7, 9, 45). However, there are concerns about the clinical utility of this measurement, as one positron emission tomography (PET) study demonstrated that the SjvO2 value did not drop to <50% until ~13% of the brain became ischemic (46). Normal range SjvO2 values are also often obtained in the presence of ongoing focal ischemia, hyperemia, and/or shunting (9), along with frequent false positive desaturations (9, 47). Vidgeon et al. state that there is no solid evidence to support its use for ongoing clinical monitoring (9).
PbtiO2
Brain tissue oxygenation monitoring provides information pertaining to focal oxygenation, with typical values ranging from 15 to 30 mmHg (2–4 kPa), and a critical hypoxic threshold commonly established at 10 mmHg (<1.33 kPa) (7, 9). This monitoring is carried out through a thin electrode placed in either peri-ischemic at-risk tissue for focal measurements, or in frontal white matter to estimate global cerebral oxygenation in diffuse brain injury (7, 9). Ischemic changes with regional differences have been detected following TBI (48), and these transient periods of ischemia are correlated with worsened patient outcomes (49). Brain injured patients with brain hypoxia (PbtiO2 <10 mmHg) have significantly poorer outcomes and increased mortality (50). The BOOST-II trial demonstrated improved outcomes with less mortality when guiding therapy by PbtiO2 plus ICP monitoring vs. ICP alone (51). A systematic review comparing PbtiO2-based therapy alongside ICP/CPP monitoring to ICP/CPP-based therapy alone reported a favorable outcome for the PbtiO2-based group (52). Not all trials report a positive outcome, and they are largely based on low quality of evidence, therefore PbtiO2-guided therapy and clinical outcomes remain subject to debate (53).
Intracerebral Microdialysis (MD)
MD is utilized to measure brain neurochemistry. It is performed via the insertion of a microdialysis catheter that contains a semipermeable membrane, which is perfused with artificial CSF, allowing passive diffusion and measurement of various neurotransmitters, and metabolic substrates and products, like glucose, lactate, pyruvate, glycerol, glutamate, et cetera (7). The MD catheter may be placed adjacent to a focal lesion to detect early metabolic alterations, or in the non-dominant frontal region in the case of diffuse injury (9, 54). Its most promising application is detecting ischemia and neuronal damage prior to being clinically detectable, allowing for early intervention to salvage brain tissue (40, 55). LPR is a sensitive marker of brain ischemia and redox state (9, 54). Elevated LPR measurements correlate with symptom severity and fatal outcomes after brain injury (54, 55). Elevated LPR > 25 is associated with poor outcomes in TBI (9), and elevated LRP coupled with low glucose correlates with worsened outcomes in TBI and subarachnoid hemorrhage (SAH) patients (40, 56). One study showed that length of time spent with elevated LPR > 40 correlated with frontal lobe atrophy at 6 months (57). Thus, MD offers unique insight into cellular bioenergetics and their perturbations following brain injury. There is increasing use of MD, and certain protocols have been established, with alarm levels of LPR set at >30, and/or glucose levels <0.8 mmol/l (58). Despite the promising utility, its overall value as a tool for guiding clinical decision making has yet to be fully elucidated (7, 40, 58).
Regional CBF
Thermal diffusion flowmetry (TDF) can directly measure regional CBF (rCBF). This measurement is achieved through insertion of a probe into the brain with proximal and distal thermistors, which calculates the power required to maintain a temperature difference between them, and this is proportional to cerebral tissue perfusion (59). It is typically inserted nearby “at-risk” tissue, such as the white matter of a vascular territory at risk for vasospasm (60). Its use is limited due to sparse clinical data on how it can guide management, its ability to measure only a very small volume, and its high sensitivity to positioning (60, 61). Consistently, the consensus summary on multimodality monitoring state that “a TDF probe may be used to identify patients with focal ischemic risk within the vascular territory of the probe,” citing a weak recommendation with very low quality evidence (11). A recent systematic review found that both very low and very high rCBF measures were associated with poor outcome and correlated with intracranial hypertension, and that rCBF and PbtiO2 were largely congruent (59). However, they stress the lack of data, stating much more research needs to be undertaken prior to widespread adoption.
Brain Injury Biomarkers
Measurement of blood or CSF brain injury biomarkers is a cost effective and less invasive tool that may assist in triaging, prognosticating, and following the course of disease. The most studied of these include S100B, glial fibrillary acidic protein (GFAP), ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCH-L1), neuron-specific enolase (NSE), and neurofilament light chain (NFL). Among these, only S100B is part of an official set of TBI guidelines where it has suboptimal real-world performance (62), largely owing to poor specificity and large numbers of false positives (63, 64). In those guidelines, it is utilized to triage emergency department (ED) patients for CT scans based on S100B levels (65). In the ALERT-TBI study, they found a combination of GFAP and UCH-L1 was highly sensitive in triaging patients for CT scans (66). In another report, GFAP levels were highly predictive of CT positivity, and adding other biomarkers did not improve discrimination (62). Accordingly, an active role for biomarker measures in continued NCCU assessment and guiding therapies is tenuous, because rather than portending impending secondary insults, late elevations signify the occurrence of secondary damage (65).
Nevertheless, the various biomarkers are still robust markers of neuronal damage. Increased S100B levels within 24 h of severe TBI strongly correlates with mortality, as does elevated NSE and GFAP (67), while also correlating with lesion expansion and brain hypoxia (65). Therefore, serial measures in the NCCU can aid in establishing extent of damage, or if new damage is ongoing. This can be refined by taking differing kinetics into account, such as GFAP vs. S100B, whereas the former persists for days after an initial injury, the latter rises and falls within hours (68). The prognostic and diagnostic impact of such measures is obvious, but how they can guide continued management remains unclear. Furthermore, given their reliability as markers of neuronal damage, research utilizing biomarkers in patients undergoing NWTs will be an important step in its continued safety assessment.
Combination Monitoring
Each of these multimodal monitoring components provide unique and clinically relevant information pertaining to neurologic functioning. Multiple modalities can complement the information from one another synergistically, hence the rationale for using multiple monitoring modalities concurrently (9). When used in conjunction, the emergence of similar pathologic patterns can point to the cause of underlying deterioration or increase the likelihood of picking up early changes. It is also imperative to utilize a combination of regional (MD, PbtiO2, TDF) and global (ICP, CPP, SjvO2, biomarkers) monitoring to ensure a complete picture of ongoing processes.
For instance, Muizelaar, while evaluating the utility of multimodality monitoring to predict hypoperfusion, recommends utilizing a combination of ICP, CPP, and PbtiO2 monitoring (69). Aligning with this, Smith et al. state that the simultaneous measurement of ICP and brain tissue oxygenation is a simple, logical approach, as a single probe can monitor both measurements (41). Accordingly, the Seattle International Brain Injury Consensus Conference (SIBICC) recently stated that PbtiO2 should be the second monitored variable after ICP (70).
Multimodality Neuromonitoring Conclusion
Recently, the Neurocritical Care Society and the European Society of Intensive Care Medicine held a panel to evaluate and discuss the evidence of multimodality monitoring (24). They conclude that a single monitoring modality is demonstrably insufficient. Despite this, they state that there is no consensus on their use, and more studies are required to ascertain if utilization translates to improved patient outcomes (24). However, they underscore the importance of the neurologic exam and the NWT, stating that it remains a cornerstone in the accurate assessment of patients. The vast amounts of information gathered via these monitoring modalities, how to evaluate and integrate them, and their ability to guide optimal therapeutic plans is still being elucidated (11). Currently, though multimodality guided therapy can improve neurologic physiological variables, it has yet to show an improvement in outcomes (60).
The NWT
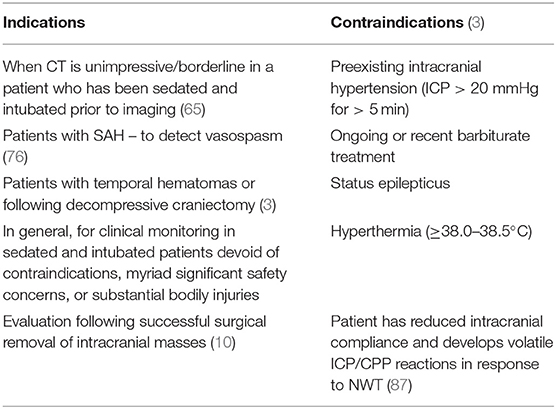
Temporarily and intermittently stopping sedation of ventilated patients to mitigate the harmful effects of over sedation is termed a daily interruption of continuous sedation (DIS) trial. Though widely utilized and studied, firm recommendations are tenuous because of large scale reviews with conflicting conclusions about their utility (3, 71). Despite lacking firm recommendations for DIS protocols, the NWT, which involves a daily cessation of sedation for neurological examination purposes, is regarded as the gold-standard for evaluating patients with brain injury in the ICU and NCCU (1, 3, 7, 14, 24, 72–74). Neither neuroimaging, nor multimodality monitoring can replace the neurologic examination (75), and it remains the most valuable tool for the assessment of brain injured patients, from stroke (74), to SAH (76, 77), and TBI (14, 24, 72). A recent intensive care symposium in Paris aiming to update neurocritical care recommendations states that the neurologic examination is indispensable for the accurate assessment of comatose patients, along with the simultaneous use of neuroimaging and multimodality monitoring (78). The SIBICC panel recommend a sedation holiday (an NWT) in TBI patients with ongoing ICP monitoring to facilitate an accurate neurologic exam (79). They could not reach consensus on absolute nor relative contraindications, but they do not endorse the NWT until ICP is within acceptable limits (<22 mmHg) for at least 24 h. This is in line with preexisting intracranial hypertension being regarded as an absolute contraindication to the NWT (14). Additional hard contraindications include barbiturate treatment, status epilepticus, or hyperthermia.
Importantly, the NWT is not akin to a true awakening response, but rather an arousal reaction (73). Essential components of the NWT include GCS rating of the motor component (GCS-M), by asking the patient to obey simple commands, such as moving an extremity, squeezing the practitioner's fingers, etc. If no responses are elicited, a painful stimulus is provided, such as a sternal rub, supraorbital pressure, mandibular pressure, or a trapezius squeeze, and the provoked motor response is recorded. The other essential components of the NWT include evaluation of pupil diameter with attention paid to anisocoria, both direct and indirect pupillary light reflexes, and any focal neurologic deficits along each extremity (3). The automated infrared pupillometer has emerged as a rapid, noninvasive neuromonitoring tool to provide an objective assessment of pupillary reactivity, and drastically increase reliability and sensitivity of the pupil examination (41, 80–82). Deterioration of the pupillary light reflex is a strong predictor of outcome after brain injury, and subtle pupillary changes are often a harbinger of elevated ICP, secondary brain injury, cerebral edema, hydrocephalus, and intracranial shift (80).
Neuroworsening, defined as a reduction in GCS-M ≥ 2 points, or development of pupillary anomalies, mandates further investigation (83). Irrespective of the utility of multimodality monitoring, the NWT remains integral in the overall evaluation of patients. Evolving pathology can be picked up earlier, and some deterioration may only be detected by physical examination (7). This helps in patient assessment, and in monitoring treatment effectiveness (24). Many patients may have ongoing damage without clear abnormalities picked up through other monitoring modalities (83). The NWT is especially useful following decompressive craniectomy, or cases of SAH-related vasospasm, as deterioration may occur before ICP elevations and neuroimaging changes are detected (76). In cases of temporal hematomas, brain herniation can occur without concomitant elevations in ICP (16, 84). In patients with cerebral edema secondary to hemispheric ischemic stroke, such as malignant middle cerebral artery (MCA) infarction, ICP monitoring is not recommended because ICP elevations do not occur for days, and midline shift and pupillary abnormalities often occur even with ICP values <20 mmHg (28, 74, 85). These cases necessitate NWTs for early detection. This may become more pertinent with the 2020 BTF update recommending decompressive craniectomy for control of late, medically refractory ICP elevation (86). Table 1 outlines the indications and contraindications.
NWT Safety
Despite presumed benefit provided by NWTs, some concerns have been raised pertaining to the acute stress response elicited with discontinuation of sedation. During each arousal, there is a significant acute stress response with SNS discharge that induces hypertension and tachycardia (73). This is reflected by significantly increased levels of stress hormones. Adrenocorticotrophic hormone (ACTH) can be increased by 72.5%, cortisol by 30.7%, epinephrine (E) by 87.5%, and norepinephrine (NE) by 40.4% (88). However, the increased levels of NE do not reach levels required for augmenting the risk of microthrombi formation (89). These changes are mirrored by significantly increased ICP and CPP values, with a mean increase of 3 and 8 mmHg, respectively. During the NWT, ICP reached an average value of 15.3 mmHg, and CPP reached an average value of 84.4 mmHg (88).
Skoglund et al. explored ICP and CPP changes in TBI and SAH patients (21 patients) undergoing NWTs (127 total NWTs). In pooling all patients, they demonstrated a mean increase in ICP of 8 mmHg, reaching an average value of > 20 mmHg. The mean CPP increase was 5.2 mmHg, reaching average values of > 81 mmHg (76). There were important subgroup differences, with TBI patients showing higher ICP values at baseline and during the NWT, and SAH patients presenting with higher CPP values at baseline and greater changes during the NWT, indicating the need for patient stratification based upon injury type and baseline characteristics. Furthermore, highlighting the heterogeneity of brain injuries, there was great variability in these metrics. Several patients developed a sustained increase in ICP levels to >30 mmHg for an average of 3.6 min. Similarly, some patients had a sustained decrease in CPP values to ≤ 50 mmHg for an average of 10 min, and these reductions were far more common in TBI patients. Overall, in 23/127 trials, CPP decreased to <50 mmHg due to ICP ≥ 25 mmHg, although these were generally short-lived deviations. The average ICP values peaking at >20 mmHg were interpreted to be safe and well-tolerated by the authors due to their mild and transient nature. Consistently, they refrained from performing NWTs in clearly unstable patients and those with plateau waves, which are sudden rapid elevations of ICP to 50–100 mmHg due to cerebral vasodilation (90). In contrast to the current study (76), the previously discussed research by Skoglund and colleagues showed less dramatic ICP and CPP elevations in response to the NWT (88). Stover hypothesizes that these discrepancies may reflect a learning curve to the NWT, or involve examinations undertaken on less severely injured patients with preserved autoregulation (73). In either case, it certainly indicates the heterogeneity of brain injury and the unpredictable clinical course that may ensue (9).
Excluding those with preexisting intracranial hypertension, the increases in ICP and CCP were transient and interpreted to be well-tolerated without advancing neurologic deterioration (3). Additionally, though these increases occur, they are not met with alterations in overall cerebral metabolism or oxygenation, suggesting its safety in most patients (1, 24). Components of multimodality monitoring were evaluated for changes occurring due to the NWT in 17 severe TBI patients (11 focal, 6 diffuse/mixed) (91). Patients were included if they had a severe TBI and were mechanically ventilated under propofol sedation, with ongoing MD, PbtiO2, and/or SjvO2 monitoring. They were excluded if they received recent or ongoing barbiturate infusion, required continuous propofol sedation for ICP control or tube tolerance, or if they were unstable. It was demonstrated that there were no significant changes in measures of glucose, lactate, pyruvate, glutamate, glycerol, or LPR as measured by MD. Similarly, changes in oxygenation as measured by both PbtiO2 and SjvO2 showed no significant alterations. Consistent with previous reports, ICP and CPP values significantly increased during the NWT, by 7.6 and 6 mmHg, respectively, reaching values of 16.7 and 94.4 mmHg. These reports demonstrate that although there is a stress response elicited by the NWT, and ICP and CPP elevations occur in parallel, neurochemical and cerebral perfusion alterations are minimal. This indicates that these perturbations are well-tolerated in this cohort of TBI patients, and are unlikely to cause secondary injury to the brain (7, 72).
In one prospective study of 20 patients (54 total NWTs), comprising 12 with TBI and nine with SAH, upwards of 34% of planned NWTs were not attempted as the patients were not seen as stable enough due to elevated ICP, hemodynamic instability, and need for continuous sedation (92). Of the 54 attempted NWTs, upwards of 33% were terminated early due primarily to ICP-crisis (>20 mmHg), agitation (22%), or systemic desaturation (11%). Moreover, in those patients with NWT cessation, they noted a statistically significant reduction in PbtiO2 measures, decreasing from an average of 28 mmHg to 19 mmHg, which is still well-above hypoxic thresholds (9, 29). Crucially, in none of their patients did PbtiO2 fall below 15 mmHg, which is within commonly cited normal ranges (9). While not rising to the level of statistical significance, a trend was noted between baseline lower brain glucose, higher total LPR, and neurologic deterioration from the NWT, which seems to corroborate the benefit of multiple simultaneous modalities of monitoring. Overall, however, there were no significant alterations in lactate, pyruvate, LPR, or glucose in patients undergoing NWTs. The authors speculate that MD is useful for baseline patient discrimination, without adding value during the NWT itself, at least in their cohort. Logically, this statement does not appear sound, as subtle metabolic alterations would necessarily precede injury progression, especially given their perilesional placement, potentially indicating the NWT is safer than presumed. Likewise, as shown by Skoglund et al. (76, 91), transiently increased ICP to >20 mmHg may not be as deleterious as assumed. If they are not coupled with concomitant metabolic indicators of ischemia or inadequate perfusion, it is unlikely that neurologic deterioration is occurring; in no reports on the NWT to date has there been evidence of secondary deterioration (3). Therefore, though patients that developed intracranial hypertension in response to the NWT had stoppage of the examination, the preponderance of data indicate most of these patients were likely to tolerate the exam without promoting neurologic injury. Nevertheless, differential findings in PbtiO2 measures between this report by Helbok et al. (92) and the aforementioned one by Skoglund et al. (91), further demonstrate the marked heterogeneity of brain injured patients and need for scrupulous patient stratification (73). An additional confounder between the two reports is that of considerably different sedative use. Helbok et al. (92) utilized a combination of propofol, midazolam, dexmedetomidine, and fentanyl, whereas Skoglund et al. (91) used continuous propofol infusion with intermittent morphine administration. These significant differences make conclusions questionable, however, it may give insight into optimal sedative practices to facilitate successful NWTs.
Additional predictors of NWT failure were shown by Esnault et al. in a large 7-year study at one center, with 242 patients, of whom 96 underwent an NWT (93). Some significant differences were found between patients that the authors felt should vs. should not undergo NWTs. Notably, patients that did not receive NWTs had higher ISS (26 vs. 16) and SAPS 2 scores (46 vs. 38), indicating more severe total body injuries, along with lower GCS scores (6 vs. 7), more shift (50 patients vs. 15), and the presence of more subdural hematomas (86 patients vs. 40). This underscores that in patients with severe comorbid injuries, and with significant shift, the NWT may be regarded as unsafe. Also, in patients with comorbid injuries, their brain injury, albeit severe, may be outweighed by more deleterious and imminent concerns. Nonetheless, this study demonstrated that 39.5% of their patients had discontinuation of NWTs. Of those patients with discontinued NWTs, it was due to neurologic deterioration in 71% of cases, with the remaining 26% due to respiratory distress. Most neurologic deteriorations were due to seeing no improvement in the neurologic exam (32% of stoppages), or intracranial hypertension [(ICP > 20 mmHg for > 5 min) 16% of stoppages]. As previously discussed, other research has demonstrated that these short-lived increases in ICP from the NWT are not met with alterations in cerebral metabolism or subsequent neurologic deterioration, making it likely that these elevations were not clinically relevant (91, 92). Not only that, the majority of premature stoppages were simply due to seeing no improvement in the exam, which does not constitute a safety concern in itself, bolstering the case for the NWT being well-tolerated in most patients in these studied cohorts. However, the significant number of patients displaying no improvement in their clinical exam is important, as a major function of the NWT is to accurately assess neurologic functioning. However, although the examination did not change in those patients from before to after sedation cessation, it may have still played a role in long-term assessment if subtle changes were later recognized, or if the lack of change remained consistent. They also identified two patient cohorts with a significantly increased probability of NWT failure: those with a subdural hematoma > 5 mm thick on first imaging, or initial GCS <5. Finally, they note that the patients that were unable to tolerate an NWT had significantly worse outcomes at 1 year, implicating the NWT as a long-term prognostic tool.
Does the NWT Provide Clinical Information?
While the NWT is widely used, there is little information about the clinical benefits procured by it in the literature. Theoretical rationale is a good starting point, but is incomplete on its own (72). Stocchetti et al. demonstrated that out of 449 TBI patients, 12.9% were misclassified as having a more severe brain injury due to sedation masking their neurologic functioning, precluding an accurate assessment (94). Compatibly, in the study by Esnault et al., 21% of patients that underwent a NWT were able to be extubated within 48 h (93). Only one randomized controlled trial of 97 patients (38 head-injured patients) on the NWT exists (95), which demonstrated a reduction in the duration of mechanical ventilation by an average of 3.9 days, and decreased ICU stay by 3 days in the head-injury group, albeit neither of these measures were statistically significant. The differences detected in the head-injury only group are stark in comparison to all pooled patients. The authors note that they estimated 45 patients were needed per group to detect significant changes, and the head-injury only subgroup had 21 in the intervention group and 17 in the control. Therefore, given the magnitude of those differences, it likely was not powered properly to achieve statistical significance in that patient cohort and requires further study in a larger patient population. Importantly, the authors did not comment on any relevant neurologic information gathered via this intervention, only noting that it was safe and well-tolerated. There has been only one study directly commenting on pertinent clinical information gained by performing NWTs (92), which showed that it rarely led to accrual of additional clinically relevant information (3, 96). In this study, when utilizing NWTs, they noted an increase in GCS and Full Outline of UnResponsiveness (FOUR) scores in half of their patients but did not comment on any change in treatment modality, nor associated prognosis. Although, that observation is still important, because it indicates a much more accurate neurologic examination and clinical representation was achieved in utilizing the NWT. In only one patient was a new focal neurologic deficit discovered. In that patient, increased brain lactate and decreased glucose was observed hours prior to the NWT, underscoring the utility of multimodality monitoring.
ICP Time-Dose Interaction
The transient elevations in ICP in response to the NWT is a point of clinical uncertainty. Most data indicate that sustained elevations in ICP, and ICP elevation refractory to medical management are deleterious and associated with worse outcomes (76, 83, 97). Consistently, the 2020 BTF update recommends decompressive craniectomy for ICP control only when prolonged and medically recalcitrant, noting late intervention improves mortality whereas early intervention does not (86, 98). The concept of “ICP dose” is becoming increasingly investigated. It recognizes that ICP thresholds are arbitrary and meaningless without accounting for the time spent at “deleterious” levels, and without consideration for the complex interactions of ICP, cerebral blood flow, cerebral metabolism, and the feedback mechanisms involved in cerebral autoregulation, neurovascular coupling, and CO2 reactivity (99). Multiple studies have begun to investigate this, demonstrating an association between poorer clinical outcomes with greater time spent above certain ICP thresholds (100–102). In one of these reports, it was shown that increased ICP time-dose was associated with higher mortality and poor outcomes, but no association was found between episodic ICP elevations (5 min > 20 mmHg) and outcomes (101). Helbok et al. point out that the injured brain is not aware of thresholds, and that even a “normal ICP” does not guarantee adequate cerebral perfusion, as ICP-dependent changes in CPP are dynamic, and the threshold-based approach is an oversimplification of a complex pathophysiological process (99). In patients with borderline ICP values, they can be stratified and managed appropriately with the addition of clinical examinations and other neuromonitoring to determine the presence or absence of brain hypoxia, cerebral hypoperfusion, or metabolic distress, allowing an individualized approach (99). This concept lends more credence toward the NWT and its associated transient ICP excursions as safe and well-tolerated in a majority of patients.
NWT Conclusion
The NWT remains contemporaneously regarded as the sine qua non for optimal assessment of the brain-injured patient (3, 7, 14, 24, 83, 92). The limited amount of published literature suggests safety and tolerability in most patients, including those with ICP elevations during the NWT itself. At present, patients with pre-existing sustained intracranial hypertension, those with status epilepticus, marked hyperthermia, or undergoing barbiturate treatment have absolute contraindications to the NWT. Other indicators that a patient may be unable to tolerate an NWT and require careful risk stratification include: hemodynamic instability, recent myocardial ischemia, patients with reduced intracranial compliance, ongoing midazolam sedation, or ongoing sedation for the purposes of controlling agitation, tube control, respiratory distress, seizure activity, or as a primary treatment for ICP control. Further predictors of an inability to tolerate an NWT may include: subdural hematoma > 5 mm thick on first imaging or initial GCS <5 (93), or emergence during the examination of shivering, cardiac arrythmias, or systemic desaturation (92). Individual patients with volatile ICP or CPP responses during exam must be handled in a case-by-case basis with appropriate risk-benefit profiles appraised, with more careful appraisal using concurrent multimodality monitoring.
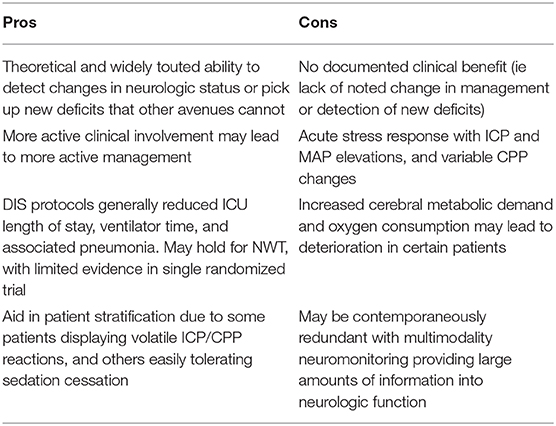
The reliability of the neurologic exam, its cost, and its ability to detect subtle deficits not picked up via multimodality monitoring make it indispensable. Multimodality monitoring can assist in ensuring cerebral metabolism and perfusion/oxygenation are adequate against the background of elevated ICP or a stable exam. This monitoring may be essential in those patients in whom NWTs are contraindicated. It may assist in individualizing treatment, and detecting metabolic distress before injury has completed. In one randomized controlled trial, ICP-guided treatment did not have better outcomes than clinical examination and neuroimaging alone (27). Overall, multimodality neuromonitoring should be seen as a complement to the NWT, and vice versa (41). More investigations are required to tease out the absolute clinical utility of the NWT, with specific regard to patient outcomes and management guidance (73). Current pros vs. cons are listed in Table 2.
Is There an Optimal Frequency of Performing NWTs?
Another central question pertaining to NWTs is their optimal frequency of utilization. Unfortunately, there are a paucity of studies examining this issue, and no clear guidelines have been set. Furthermore, due to the NWT not being recommended in established TBI care guidelines (20), there is great variability in their utilization and frequency. However, these guidelines have come under scrutiny due to their reliance on absolute thresholds (103). The recent SIBICC recommends a sedation holiday (NWT) to facilitate an accurate neurologic examination in severe TBI patients with ongoing ICP monitoring, but do not mention frequency (79). One report suggests that upwards of 50% of NCCU centers in Scandinavian countries do not utilize NWTs (104), which may partly be explained by differences in sedative use. In those centers utilizing NWTs, the majority use daily, with sometimes twice daily checks (7, 72), and one center utilizes NWTs between 4 and 6 times per day (7).
This demonstrates the enormous variability in both use and frequency of NWTs, necessitating the need for more data and guidelines to be established to guide clinical practice. Indeed, only one official recommendation has been offered, from the European Society for Intensive Care Medicine (ESICM) NeuroIntensive Care Section (NIC) (14). They convened an expert panel and state that a daily, brief interruption of sedation is recommended to facilitate an accurate neurologic examination (an NWT) and improve outcomes, giving it moderate evidence and a strong recommendation. Additionally, they state that brain injured patients, ICU patients, and in general all critically ill patients, shoulder undergo a neurological examination on initial ICU admission, and at least once daily, giving it moderative evidence, and a best practice recommendation. They also note the clear contraindication, as previously stated, with DIS and NWTs not recommended in patients with preexisting intracranial hypertension, assigning it moderate evidence and a strong recommendation (14). Lastly, in their concluding remarks, they note that despite technological advances, the neurologic examination remains a foundation of accurate evaluation and prognostic assessment of neurologic function, pointing out the robust prognostic value of GCS scores and pupillary light responses. Therefore, the only concrete recommendation that has been put forth pertaining to the optimal frequency of NWTs is at minimum once daily. Randomized controlled trials will need to be undertaken utilizing differential NWT frequencies to gain insight into what the optimal frequency is, if different patient populations have an impact on this, and what influence injury type has. Thus, much more research will need to be carried out before concrete recommendations about NWT frequency can be given and guidelines administered.
Choice of Sedative
Another consideration is sedative selection. There are myriad sedating agents utilized in the ICU and NCCU, namely propofol, benzodiazepines, dexmedetomidine, opioids, and barbiturates. The most common agents utilized in ICUs and NCCUs are propofol and midazolam (7), and these may be used in conjunction with opioids for additional analgesia. Recently, dexmedetomidine has begun to see increasing use, and it represents another attractive option for sedation. They each possess their own risk vs. benefit profiles.
Propofol
Propofol enjoys ubiquitous ICU and NCCU use owing to several factors, including its neuroprotective effects. It is recommended to use for ICP control in current TBI-care guidelines (20), and it dampens cerebral metabolic oxygen demand (96). It possesses both rapid onset of action, and rapid plasma clearance, which facilitates reliable recovery of consciousness even after prolonged administration and thereby a consistent NWT. In higher doses it can induce burst suppression, which can effectively treat status epilepticus (96). It can abate oxidative stress, making it especially useful to combat the free radical generation in head injury (105). An MD study in TBI patients comparing propofol to midazolam found no significant differences in measures of LPR, glutamate, glycerol, or glucose between the two agents over a 72-h period (106). The doses utilized in that study may have been inadequate, and it was a short-term, small study, therefore more investigations are required to determine if propofol can improve outcomes via mitigating oxidative stress. Two retrospective studies have shown that sedation with propofol decreased mortality in TBI (107) and hemorrhagic stroke patients (108). It also has risks, including depressing effects on myocardial contractility, reductions in MAP, elevation in pancreatic enzymes and pancreatitis, and the development of propofol infusion syndrome (PRIS) (105). Though ICP reductions occur which mediate elevations in CPP, occasionally, MAP can fall to such an extent that CPP decreases, which requires judicious fluid resuscitation and vasopressor use. PRIS is a rare but extremely dangerous adverse effect of propofol infusion. It can lead to multi-organ failure and when suspected requires immediate cessation of propofol. Early warning signs include unexplained lactic acidosis, increasing need for inotropic agents, lipemia, and Brugada-like ECG changes (109). Risk factors for PRIS include large cumulative doses of propofol, young age, innate mitochondrial impairments, low carbohydrate intake, high fat intake (which propofol itself possesses, owing to the lipid formulation), critical illness, and catecholamine infusion (109). Importantly, PRIS is believed to be more common in TBI patients, largely owing to the larger doses often used for ICP control (105). For this reason, continuous propofol infusion should not be infused at a rate > 4 mg/kg/h for > 48 h (105). Propofol is an ideal agent for the NWT, and is widely recommended and utilized. Although rare, PRIS may limit its utility for long-term use in brain injured patients.
Midazolam
Midazolam has rapid onset with a rapid recovery. It decreases cerebral oxygen demand, although to a lesser extent than propofol, and has only slight influences on ICP levels (96). Despite possessing a short half-life, when infused continuously, its half-life increases due to both high lipid-solubility with associated tissue accumulation, and the presence of active metabolites that may be deleterious (7, 96). Consistently, midazolam use is associated with higher mortality rates in ICUs (110). Its persistent use leads to protracted sedation and prolonged time to awakening, which will confound a consistent NWT (96, 111). It also causes significant respiratory depression and inhibition of the cough reflex, along with issues of tolerance development and significant withdrawal symptoms upon cessation. Its use is also a risk factor for ICU delirium, which is itself associated with worse outcomes (105). Accordingly, its use increases ICU length of stay, and either propofol or dexmedetomidine sedation is preferred to improve clinical outcomes in intubated ICU patients (112). Midazolam is not recommended for use when serial NWTs are desired.
Dexmedetomidine
Dexmedetomidine is a sympatholytic agent that can achieve excellent sedation without respiratory depression, while possessing anxiolytic and analgesic properties. It has rapid onset of action and elimination, does not accumulate in tissues, has a half-life of 6 min, and a terminal elimination half-life of 2–2.5 h, making it ideal for a reliable NWT (96, 105, 113). It also decreases ICP through reducing CBF, increases CPP, and reduces incidence of delirium (105). Though it often leads to a slight decrement in blood pressure, there may be an initial vasoconstrictive effect due to peripheral smooth muscle α-2 adrenergic receptors that occurs more rapidly than its sedative and sympatholytic effects (114). One report of all patients undergoing mechanical ventilation demonstrated that dexmedetomidine was associated with fewer ventilator-associated events and increased chance of extubation in comparison to midazolam and propofol (115). However, as the patient cohort did not comprise strictly brain-injured, care must be taken to extrapolate those findings to the NCCU. A meta-analysis of eight studies concluded that dexmedetomidine is a safe and efficacious agent in the NCCU (116). In a TBI murine model, dexmedetomidine showed significant neuroprotective effects (117), although human studies are required to corroborate these findings. Given its known role of impeding SNS discharge (118), it may decrease the injury-promoting catecholaminergic influence in TBI (119), and has been shown to decrease plasma cortisol after administration (120). This could make this choice of sedative especially useful in the NWT for lessening the NE and E excursions. In one report of 198 severe TBI patients, dexmedetomidine facilitated the highest mean daily time in target Richmond Agitation-Sedation Scale (RASS) compared to propofol, or dexmedetomidine plus propofol (121). Moreover, in another report of 85 severe TBI patients it was demonstrated that in comparison to propofol or midazolam, dexmedetomidine patients were better able to be aroused and cooperate, suggesting it may facilitate a more appropriate level of sedation, better enabling serial NWTs (122). Overall, this agent represents an extremely attractive option to utilize for long-term sedation and to facilitate an NWT. However, more studies are required, and given its limited clinical data, some authors do not currently recommend its use for sedation in brain-injured patients (96).
Sedative Conclusion
Most recommendations call for continuous propofol sedation, allowing for more frequent cessation of sedation for NWTs (3, 7, 73, 105). There has been some consideration of maintaining a low-dose of analgesics during NWTs (73). Thus, the few recommendations on the subject suggest using propofol to facilitate a smoother transition toward the NWT, with careful attention paid to the development of PRIS, and ensuring infusion rates remain <4 mg/kg/h unless for bolus ICP control. Dexmedetomidine has not yet received strong recommendations, especially in the NCCU, due to an absence of large-scale data. However, the existing literature indicates that dexmedetomidine can be safely and efficaciously used for brain-injured patients, and its rapid onset and short half-life devoid of residual tissue accumulation make it a very attractive choice to facilitate serial NWTs. It is already utilized in NCCUs that employ NWTs and has established feasibility and tolerability for the process (92). Moreover, a recent retrospective observational study found that dexmedetomidine was more commonly prescribed than propofol to facilitate frequent neurologic assessments (123). It is strongly recommended as the sedative agent of choice in the non-intubated patient or with development of PRIS (105). The primary choice of sedative should be individualized, and further based on comfort levels and experience.
Midazolam use is not recommended for sedation because it precludes the ability to perform reliable, serial NWTs (105); it is associated with greater ICU length of stay, duration of mechanical ventilation, and delirium compared to propofol or dexmedetomidine (124); and is associated with dramatically prolonged time to awakening compared to propofol (125–128) and dexmedetomidine (129, 130). Despite the numerous pitfalls of midazolam use, it remains one of the most common sedatives utilized in ICUs (3). Therefore, in centers that primarily use midazolam, it must be recognized that NWTs may lack consistency due to bioaccumulation and residual sedation, with much greater time to awakening. Notwithstanding, when multimodality monitoring is not utilized, the NWT becomes the only source of information about neurologic function. This is already a reality in many lower-income countries and areas, with assessment coming from neurologic exams and serial CT imaging (131). In those cases, the NWT should still be done despite midazolam use, with acknowledgment that time to awakening is increased and day to day reliability of the exam diminished.
Conclusion
It is widely recognized that the NWT is considered the gold-standard for continued evaluation of brain-injured patients. Hard contraindications exist for patients with preexisting intracranial hypertension, hyperthermia, in status-epilepticus, or on barbiturate therapy. The NWT also induces a significant systemic stress response with ICP elevations, but there has yet to be evidence of secondary deterioration, plus MD and PbtiO2 metrics have shown no alterations in the few reports on the subject. The advent of multimodality neuromonitoring has added a tremendous amount of data pertaining to neurologic function into the physician's armamentarium, but have yet to demonstrate clearly improved patient outcomes, and uncertainties exist about their ability to change and guide clinical decisions. NWT frequency is an additional point of uncertainty; the only available expert recommendation endorsed a frequency of at least once per day. Propofol is the most widely recommended sedative to facilitate serial NWTs, but dexmedetomidine represents another viable choice, especially in the non-intubated patient or should PRIS develop. More research is required to elucidate the clinical utility of the NWT, its ability to detect neurologic insults and guide appropriate management, and establish guidelines about the optimal frequency of its utilization with stratification based on injury type and patient population.
Author Contributions
SM undertook all of the literature search and task of writing the manuscript. AA, as the senior faculty, provided the idea for the review topic and assisted in editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACTH, adrenocorticotrophic hormone; BTF, Brain trauma foundation; CBF, cerebral blood flow; CMRO2, cerebral metabolic demand for O2; CPP, Cerebral perfusion pressure; CSF, Cerebrospinal fluid; CT, computed tomography; DIS, daily interruption of continuous sedation; E, epinephrine; ED, emergency department; ESICM, European Society for Intensive Care Medicine; EVD, External ventricular drain; FOUR, Full Outline of UnResponsiveness; GCS, Glasgow coma scale; GCS-M, Glasgow coma scale – motor component; GFAP, glial fibrillary acidic protein; ICP, Intracranial pressure; ICU, Intensive care unit; IPM, Intraparenchymal monitors; ISS, injury severity score; LPR, Lactate to pyruvate ratio; MAP, Mean arterial pressure; MCA, middle cerebral artery; MD, Intracerebral microdialysis; NCCU, Neurocritical care unit; NE, norepinephrine; NFL, Neurofilament light chain; NIC, NeuroIntensive Care Section; NSE, neuron-specific enolase; NWT, Neurological wake-up test; O2 sat, oxygen saturation; ONSD, Optic nerve sheath diameter; PbtiO2, brain tissue oxygen tension; PDH, pyruvate dehydrogenase; PET, positron emission tomography; PRIS, Propofol infusion syndrome; rCBF, regional cerebral blood flow; SAH, subarachnoid hemorrhage; SAPS 2, Simplified acute physiology score; SIBICC, Seattle International Brain Injury Consensus Conference; SjvO2, jugular venous oxygen saturation; SNS, Sympathetic nervous system; TBI, Traumatic brain injury; TCD, Transcranial doppler; TDF, Thermal diffusion flowmetry; UCH-L1, ubiquitin carboxyl-terminal hydrolase isozyme L1.
References
1. Lele A, Souter M. Sedation practices in the neurocritical care unit. J Neuroanaesthesiol Crit Care. (2016) 3:81–7. doi: 10.4103/2348-0548.174743
2. Bruder N, Lassegue D, Pelissier D, Graziani N, Francois G. Energy expenditure and withdrawal of sedation in severe head-injured patients. Crit Care Med. (1994) 22:1114–9. doi: 10.1097/00003246-199407000-00011
3. Marklund N. The neurological wake-up test-a role in neurocritical care monitoring of traumatic brain injury patients? Front Neurol. (2017) 8:540. doi: 10.3389/fneur.2017.00540
4. Rhoney DH, Parker Jr D. Use of sedative and analgesic agents in neurotrauma patients: effects on cerebral physiology. Neurol Res. (2001) 23:237–59. doi: 10.1179/016164101101198398
5. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled Trial): a randomised controlled trial. Lancet. (2008) 371:126–34. doi: 10.1016/S0140-6736(08)60105-1
6. Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. (1998) 114:541–8. doi: 10.1378/chest.114.2.541
7. Skoglund K. The Neurological Wake-up Test in Neurocritical Care [Doctoral thesis, comprehensive summary]. Uppsala: Acta Universitatis Upsaliensis (2012).
8. Maas AI, Murray G, Henney H 3rd, Kassem N, Legrand V, Mangelus M, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. (2006) 5:38–45. doi: 10.1016/S1474-4422(05)70253-2
9. Vidgeon SD, Strong AJ. Multimodal cerebral monitoring in traumatic brain injury. Sage J. (2011) 12:126–33. doi: 10.1177/175114371101200208
10. Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB, Smielewski P, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. (2017) 16:452–64. doi: 10.1016/S1474-4422(17)30118-7
11. Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care : a statement for healthcare professionals from the neurocritical care society and the european society of intensive care medicine. Intensive Care Med. (2014) 40:1189–209. doi: 10.1007/s00134-014-3369-6
12. Gill M, Windemuth R, Steele R, Green SM. A comparison of the glasgow coma scale score to simplified alternative scores for the prediction of traumatic brain injury outcomes. Ann Emerg Med. (2005) 45:37–42. doi: 10.1016/j.annemergmed.2004.07.429
13. Majdan M, Steyerberg EW, Nieboer D, Mauritz W, Rusnak M, Lingsma HF. Glasgow coma scale motor score and pupillary reaction to predict six-month mortality in patients with traumatic brain injury: comparison of field and admission assessment. J Neurotrauma. (2015) 32:101–8. doi: 10.1089/neu.2014.3438
14. Sharshar T, Citerio G, Andrews PJ, Chieregato A, Latronico N, Menon DK, et al. Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive Care Med. (2014) 40:484–95. doi: 10.1007/s00134-014-3214-y
15. Bouzat P, Oddo M, Payen JF. Transcranial doppler after traumatic brain injury: is there a role? Curr Opin Crit Care. (2014) 20:153–60. doi: 10.1097/MCC.0000000000000071
16. Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. (2008) 26:521–41. doi: 10.1016/j.ncl.2008.02.003
17. Olson DM, Ortega Perez S, Ramsay J, Venkatasubba Rao CP, Suarez JI, McNett M, et al. Differentiate the source and site of intracranial pressure measurements using more precise nomenclature. Neurocrit Care. (2019) 30:239–43. doi: 10.1007/s12028-018-0613-x
18. Hockel K, Schuhmann MU. ICP monitoring by open extraventricular drainage: common practice but not suitable for advanced neuromonitoring and prone to false negativity. Acta Neurochir Suppl. (2018) 126:281–6. doi: 10.1007/978-3-319-65798-1_55
19. Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, et al. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. (2015) 83:794–800. doi: 10.1016/j.wneu.2014.12.040
20. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
21. Stocchetti N, Penny KI, Dearden M, Braakman R, Cohadon F, Iannotti F, et al. Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med. (2001) 27:400–6. doi: 10.1007/s001340000825
22. The Brain Trauma Foundation. The American association of neurological surgeons. The joint section on neurotrauma and critical care. Indications for intracranial pressure monitoring. J Neurotrauma. (2000) 17:479–91. doi: 10.1089/neu.2000.17.479
23. Shen L, Wang Z, Su Z, Qiu S, Xu J, Zhou Y, et al. Effects of intracranial pressure monitoring on mortality in patients with severe traumatic brain injury: a meta-analysis. PLoS ONE. (2016) 11:e0168901. doi: 10.1371/journal.pone.0168901
24. Citerio G, Oddo M, Taccone FS. Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care. (2015) 21:113–9. doi: 10.1097/MCC.0000000000000179
25. Alberico AM, Ward JD, Choi SC, Marmarou A, Young HF. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. (1987) 67:648–56. doi: 10.3171/jns.1987.67.5.0648
26. Piccinini A, Lewis M, Benjamin E, Aiolfi A, Inaba K, Demetriades D. Intracranial pressure monitoring in severe traumatic brain injuries: a closer look at level 1 trauma centers in the United States. Injury. (2017) 48:1944–50. doi: 10.1016/j.injury.2017.04.033
27. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. (2012) 367:2471–81. doi: 10.1056/NEJMoa1207363
28. Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American heart association/american stroke association. Stroke. (2014) 45:1222–38. doi: 10.1161/01.str.0000441965.15164.d6
29. Roh D, Park S. Brain multimodality monitoring: updated perspectives. Curr Neurol Neurosci Rep. (2016) 16:56. doi: 10.1007/s11910-016-0659-0
30. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. (2011) 37:1059–68. doi: 10.1007/s00134-011-2224-2
31. Jeon JP, Lee SU, Kim SE, Kang SH, Yang JS, Choi HJ, et al. Correlation of optic nerve sheath diameter with directly measured intracranial pressure in Korean adults using bedside ultrasonography. PLoS ONE. (2017) 12:e0183170. doi: 10.1371/journal.pone.0183170
32. Nag DS, Sahu S, Swain A, Kant S. Intracranial pressure monitoring: gold standard and recent innovations. World J Clin Cases. (2019) 7:1535–53. doi: 10.12998/wjcc.v7.i13.1535
33. Chen LM, Wang LJ, Hu Y, Jiang XH, Wang YZ, Xing YQ. Ultrasonic measurement of optic nerve sheath diameter: a non-invasive surrogate approach for dynamic, real-time evaluation of intracranial pressure. Br J Ophthalmol. (2019) 103:437–41. doi: 10.1136/bjophthalmol-2018-312934
34. Rao V, Klepstad P, Losvik OK, Solheim O. Confusion with cerebral perfusion pressure in a literature review of current guidelines and survey of clinical practice. Scand J Trauma Resusc Emerg Med. (2013) 21:78. doi: 10.1186/1757-7241-21-78
35. Kosty JA, Leroux PD, Levine J, Park S, Kumar MA, Frangos S, et al. Brief report: a comparison of clinical and research practices in measuring cerebral perfusion pressure: a literature review and practitioner survey. Anesth Analg. (2013) 117:694–8. doi: 10.1213/ANE.0b013e31829cc765
37. Thomas E, Naccs, Czosnyka M, Hutchinson P, Sbns. Calculation of cerebral perfusion pressure in the management of traumatic brain injury: joint position statement by the councils of the neuroanaesthesia and critical care society of great britain and ireland (NACCS) and the society of british neurological surgeons (SBNS). Br J Anaesth. (2015) 115:487–8. doi: 10.1093/bja/aev233
38. Andrews PJ, Sleeman DH, Statham PF, McQuatt A, Corruble V, Jones PA, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. (2002) 97:326–36. doi: 10.3171/jns.2002.97.2.0326
39. Elf K, Nilsson P, Ronne-Engstrom E, Howells T, Enblad P. Cerebral perfusion pressure between 50 and 60 mm Hg may be beneficial in head-injured patients: a computerized secondary insult monitoring study. Neurosurgery. (2005) 56:962–71; discussion −71. doi: 10.1227/01.NEU.0000207977.64009.F9
40. Kirkman MA, Smith M. Intracranial pressure monitoring, cerebral perfusion pressure estimation, and ICP/CPP-guided therapy: a standard of care or optional extra after brain injury? Br J Anaesth. (2014) 112:35–46. doi: 10.1093/bja/aet418
41. Smith M. Multimodality neuromonitoring in adult traumatic brain injury: a narrative review. Anesthesiology. (2018) 128:401–15. doi: 10.1097/ALN.0000000000001885
42. Czosnyka M Miller C Participants in the International Multidisciplinary Consensus Conference on Multimodality M. Monitoring of cerebral autoregulation. Neurocrit Care. (2014) 21(Suppl.2):S95–102. doi: 10.1007/s12028-014-0046-0
43. Gopinath SP, Valadka AB, Uzura M, Robertson CS. Comparison of jugular venous oxygen saturation and brain tissue Po2 as monitors of cerebral ischemia after head injury. Crit Care Med. (1999) 27:2337–45. doi: 10.1097/00003246-199911000-00003
44. Shaaban Ali M, Harmer M, Latto I. Jugular bulb oximetry during cardiac surgery. Anaesthesia. (2001) 56:24–37. doi: 10.1046/j.1365-2044.2001.01707.x
45. Dash HH, Chavali S. Management of traumatic brain injury patients. Korean J Anesthesiol. (2018) 71:12–21. doi: 10.4097/kjae.2018.71.1.12
46. Gupta AK, Hutchinson PJ, Al-Rawi P, Gupta S, Swart M, Kirkpatrick PJ, et al. Measuring brain tissue oxygenation compared with jugular venous oxygen saturation for monitoring cerebral oxygenation after traumatic brain injury. Anesth Analg. (1999) 88:549–53. doi: 10.1213/00000539-199903000-00016
47. Artru F, Dailler F, Burel E, Bodonian C, Grousson S, Convert J, et al. Assessment of jugular blood oxygen and lactate indices for detection of cerebral ischemia and prognosis. J Neurosurg Anesthesiol. (2004) 16:226–31. doi: 10.1097/00008506-200407000-00007
48. Rao GS, Durga P. Changing trends in monitoring brain ischemia: from intracranial pressure to cerebral oximetry. Curr Opin Anaesthesiol. (2011) 24:487–94. doi: 10.1097/ACO.0b013e32834a8965
49. van den Brink WA, van Santbrink H, Steyerberg EW, Avezaat CJ, Suazo JA, Hogesteeger C, et al. Brain oxygen tension in severe head injury. Neurosurgery. (2000) 46:868–76; discussion 76–8. doi: 10.1227/00006123-200004000-00018
50. Maloney-Wilensky E, Gracias V, Itkin A, Hoffman K, Bloom S, Yang W, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. (2009) 37:2057–63. doi: 10.1097/CCM.0b013e3181a009f8
51. Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, et al. Brain Oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. (2017) 45:1907–14. doi: 10.1097/CCM.0000000000002619
52. Nangunoori R, Maloney-Wilensky E, Stiefel M, Park S, Andrew Kofke W, Levine JM, et al. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: a systematic literature review. Neurocrit Care. (2012) 17:131–8. doi: 10.1007/s12028-011-9621-9
53. Martini RP, Deem S, Treggiari MM. Targeting brain tissue oxygenation in traumatic brain injury. Respir Care. (2013) 58:162–72. doi: 10.4187/respcare.01942
54. Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. (2004) 30:2166–9. doi: 10.1007/s00134-004-2461-8
55. Tisdall MM, Smith M. Cerebral microdialysis: research technique or clinical tool. Br J Anaesth. (2006) 97:18–25. doi: 10.1093/bja/ael109
56. Kett-White R, Hutchinson PJ, Al-Rawi PG, Gupta AK, Pickard JD, Kirkpatrick PJ. Adverse cerebral events detected after subarachnoid hemorrhage using brain oxygen and microdialysis probes. Neurosurgery. (2002) 50:1213–21; discussion 21–2. doi: 10.1227/00006123-200206000-00008
57. Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. (2008) 36:2871–7. doi: 10.1097/CCM.0b013e318186a4a0
58. Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr Opin Crit Care. (2006) 12:112–8. doi: 10.1097/01.ccx.0000216576.11439.df
59. Mathieu F, Khellaf A, Thelin EP, Zeiler FA. Continuous thermal diffusion-based cerebral blood flow monitoring in adult traumatic brain injury: a scoping systematic review. J Neurotrauma. (2019) 36:1707–23. doi: 10.1089/neu.2018.6309
60. Ruhatiya RS, Adukia SA, Manjunath RB, Maheshwarappa HM. Current status and recommendations in multimodal neuromonitoring. Indian J Crit Care Med. (2020) 24:353–60. doi: 10.5005/jp-journals-10071-23431
61. Rivera Lara L, Puttgen HA. Multimodality monitoring in the neurocritical care unit. Continuum. (2018) 24:1776–88. doi: 10.1212/CON.0000000000000671
62. Czeiter E, Amrein K, Gravesteijn BY, Lecky F, Menon DK, Mondello S, et al. Blood biomarkers on admission in acute traumatic brain injury: relations to severity, CT findings and care path in the CENTER-TBI study. Ebio Med. (2020) 56:102785. doi: 10.1016/j.ebiom.2020.102785
63. Minkkinen M, Iverson GL, Kotilainen AK, Pauniaho SL, Mattila VM, Lehtimaki T, et al. Prospective validation of the scandinavian guidelines for initial management of minimal, mild, and moderate head injuries in adults. J Neurotrauma. (2019) 36:2904–12. doi: 10.1089/neu.2018.6351
64. Martinez BI, Stabenfeldt SE. Current trends in biomarker discovery and analysis tools for traumatic brain injury. J Biol Eng. (2019) 13:16. doi: 10.1186/s13036-019-0145-8
65. Menon DK, Ercole A. Critical care management of traumatic brain injury. Handb Clin Neurol. (2017) 140:239–74. doi: 10.1016/B978-0-444-63600-3.00014-3
66. Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. (2018) 17:782–9. doi: 10.1016/S1474-4422(18)30231-X
67. Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: what are we measuring? Neurosci Biobehav Rev. (2016) 68:460–73. doi: 10.1016/j.neubiorev.2016.05.009
68. Dadas A, Washington J, Diaz-Arrastia R, Janigro D. Biomarkers in traumatic brain injury (TBI): a review. Neuropsychiatr Dis Treat. (2018) 14:2989–3000. doi: 10.2147/NDT.S125620
69. Muizelaar JP. Multimodal monitoring after traumatic brain injury: useless or useful? Crit Care Med. (2015) 43:506–7. doi: 10.1097/CCM.0000000000000792
70. Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. (2020) 46:919–29. doi: 10.1007/s00134-019-05900-x
71. Burry L, Rose L, McCullagh IJ, Fergusson DA, Ferguson ND, Mehta S. Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. Cochrane Database Syst Rev. (2014) 2014:CD009176. doi: 10.1002/14651858.CD009176.pub2
72. Helbok R, Badjatia N. Is daily awakening always safe in severely brain injured patients? Neurocrit Care. (2009) 11:133–4. doi: 10.1007/s12028-009-9262-4
73. Stover JF. Arousal from sedation in wake-up tests requires careful risk stratification. Crit Care Med. (2012) 40:338–40. doi: 10.1097/CCM.0b013e31823291bf
74. Jeon SB, Koh Y, Choi HA, Lee K. Critical care for patients with massive ischemic stroke. J Stroke. (2014) 16:146–60. doi: 10.5853/jos.2014.16.3.146
75. Payen JF, Francony G, Canet C, Coppo F, Fauvage B. [Sedation in neurointensive care unit]. Ann Fr Anesth Reanim. (2009) 28:1015–9. doi: 10.1016/j.annfar.2009.10.003
76. Skoglund K, Enblad P, Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care. (2009) 11:135–42. doi: 10.1007/s12028-009-9255-3
77. Petridis AK, Beseoglu K, Steiger HJ. The clinical examination in the patient with subarachnoid hemorrhage is still the most reliable parameter for predicting pathophysiological changes. Surg Neurol Int. (2017) 8:294. doi: 10.4103/sni.sni_332_17
78. Oddo M, Bracard S, Cariou A, Chanques G, Citerio G, Clerckx B, et al. Update in neurocritical care: a summary of the 2018 paris international conference of the french society of intensive care. Ann Intensive Care. (2019) 9:47. doi: 10.1186/s13613-019-0523-x
79. Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for patients with intracranial pressure monitoring: the seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. (2019) 45:1783–94. doi: 10.1007/s00134-019-05805-9
80. Lussier BL, Olson DM, Aiyagari V. Automated pupillometry in neurocritical care: research and practice. Curr Neurol Neurosci Rep. (2019) 19:71. doi: 10.1007/s11910-019-0994-z
81. Couret D, Boumaza D, Grisotto C, Triglia T, Pellegrini L, Ocquidant P, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care. (2016) 20:99. doi: 10.1186/s13054-016-1239-z
82. Suys T, Bouzat P, Marques-Vidal P, Sala N, Payen JF, Rossetti AO, et al. Automated quantitative pupillometry for the prognostication of coma after cardiac arrest. Neurocrit Care. (2014) 21:300–8. doi: 10.1007/s12028-014-9981-z
83. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. (2008) 7:728–41. doi: 10.1016/S1474-4422(08)70164-9
84. Stevens RD, Shoykhet M, Cadena R. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. (2015) 23(Suppl. 2):S76–82. doi: 10.1007/s12028-015-0168-z
85. Poca MA, Benejam B, Sahuquillo J, Riveiro M, Frascheri L, Merino MA, et al. Monitoring intracranial pressure in patients with malignant middle cerebral artery infarction: is it useful? J Neurosurg. (2010) 112:648–57. doi: 10.3171/2009.7.JNS081677
86. Hawryluk GWJ, Rubiano AM, Totten AM, O'Reilly C, Ullman JS, Bratton SL, et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. (2020) 87:427–34. doi: 10.1093/neuros/nyaa278
87. Prisco L, Citerio G. To wake-up, or not to wake-up: that is the hamletic neurocritical care question! Crit Care. (2012) 16:190. doi: 10.1186/cc11891
88. Skoglund K, Enblad P, Hillered L, Marklund N. The neurological wake-up test increases stress hormone levels in patients with severe traumatic brain injury. Crit Care Med. (2012) 40:216–22. doi: 10.1097/CCM.0b013e31822d7dbd
89. Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Harter L, Keel M, et al. In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care. (2008) 12:R80. doi: 10.1186/cc6931
90. Daley ML, Leffler CW, Czosnyka M, Pickard JD. Plateau waves: changes of cerebrovascular pressure transmission. Acta Neurochir Suppl. (2005) 95:327–32. doi: 10.1007/3-211-32318-X_67
91. Skoglund K, Hillered L, Purins K, Tsitsopoulos PP, Flygt J, Engquist H, et al. The neurological wake-up test does not alter cerebral energy metabolism and oxygenation in patients with severe traumatic brain injury. Neurocrit Care. (2014) 20:413–26. doi: 10.1007/s12028-013-9876-4
92. Helbok R, Kurtz P, Schmidt MJ, Stuart MR, Fernandez L, Connolly SE, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care. (2012) 16:R226. doi: 10.1186/cc11880
93. Esnault P, Montcriol A, D'Aranda E, Bordes J, Goutorbe P, Boret H, et al. Early neurological wake-up test in intubated brain-injured patients: a long-term, single-centre experience. Aust Crit Care. (2017) 30:273–8. doi: 10.1016/j.aucc.2016.10.002
94. Stocchetti N, Pagan F, Calappi E, Canavesi K, Beretta L, Citerio G, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma. (2004) 21:1131–40. doi: 10.1089/neu.2004.21.1131
95. Anifantaki S, Prinianakis G, Vitsaksaki E, Katsouli V, Mari S, Symianakis A, et al. Daily interruption of sedative infusions in an adult medical-surgical intensive care unit: randomized controlled trial. J Adv Nurs. (2009) 65:1054–60. doi: 10.1111/j.1365-2648.2009.04967.x
96. Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. (2016) 20:128. doi: 10.1186/s13054-016-1294-5
97. Ryttlefors M, Howells T, Nilsson P, Ronne-Engstrom E, Enblad P. Secondary insults in subarachnoid hemorrhage: occurrence and impact on outcome and clinical deterioration. Neurosurgery. (2007) 61:704–14; discussion 14–5. doi: 10.1227/01.NEU.0000298898.38979.E3
98. Myburgh JA. Intracranial pressure thresholds in severe traumatic brain injury: pro. Intensive Care Med. (2018) 44:1315–7. doi: 10.1007/s00134-018-5264-z
99. Helbok R, Meyfroidt G, Beer R. Intracranial pressure thresholds in severe traumatic brain injury: con : the injured brain is not aware of ICP thresholds!. Intensive Care Med. (2018) 44:1318–20. doi: 10.1007/s00134-018-5249-y
100. Kahraman S, Dutton RP, Hu P, Xiao Y, Aarabi B, Stein DM, et al. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. J Trauma. (2010) 69:110–8. doi: 10.1097/TA.0b013e3181c99853
101. Sheth KN, Stein DM, Aarabi B, Hu P, Kufera JA, Scalea TM, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care. (2013) 18:26–32. doi: 10.1007/s12028-012-9780-3
102. Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. (2008) 109:678–84. doi: 10.3171/JNS/2008/109/10/0678
103. Meyfroidt G, Citerio G. Letter: guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 81:E1. doi: 10.1093/neuros/nyx144
104. Skoglund K, Enblad P, Marklund N. Monitoring and sedation differences in the management of severe head injury and subarachnoid hemorrhage among neurocritical care centers. J Neurosci Nurs. (2013) 45:360–8. doi: 10.1097/JNN.0b013e3182a3cf4f
105. Flower O, Hellings S. Sedation in traumatic brain injury. Emerg Med Int. (2012) 2012:637171. doi: 10.1155/2012/637171
106. Tanguy M, Seguin P, Laviolle B, Bleichner JP, Morandi X, Malledant Y. Cerebral microdialysis effects of propofol versus midazolam in severe traumatic brain injury. J Neurotrauma. (2012) 29:1105–10. doi: 10.1089/neu.2011.1817
107. Chiu WT, Lin TJ, Lin JW, Huang SJ, Chang CK, Chen HY. Multicenter evaluation of propofol for head-injured patients in Taiwan. Surg Neurol. (2006) 66(Suppl. 2):S37–42. doi: 10.1016/j.surneu.2006.08.028
108. Hung YC, Lee EJ, Chen HY, Ko SW, Shyr MH, Chen TY. Effects of propofol sedation during the early postoperative period in hemorrhagic stroke patients. Acta Anaesthesiol Taiwan. (2009) 47:128–33. doi: 10.1016/S1875-4597(09)60039-4
109. Otterspoor LC, Kalkman CJ, Cremer OL. Update on the propofol infusion syndrome in ICU management of patients with head injury. Curr Opin Anaesthesiol. (2008) 21:544–51. doi: 10.1097/ACO.0b013e32830f44fb
110. Song Y, Gao S, Tan W, Qiu Z, Zhou H, Zhao Y. Dexmedetomidine versus midazolam and propofol for sedation in critically ill patients: mining the medical information mart for intensive care data. Ann Transl Med. (2019) 7:197. doi: 10.21037/atm.2019.04.14
111. McKenzie CA, McKinnon W, Naughton DP, Treacher D, Davies G, Phillips GJ, et al. Differentiating midazolam over-sedation from neurological damage in the intensive care unit. Crit Care. (2005) 9:R32–6. doi: 10.1186/cc3010
112. Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182783b72
113. Paul BS, Paul G. Sedation in neurological intensive care unit. Ann Indian Acad Neurol. (2013) 16:194–202. doi: 10.4103/0972-2327.112465
114. Talke P, Anderson BJ. Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol. (2018) 84:1364–72. doi: 10.1111/bcp.13571
115. Moreira FT, Serpa Neto A. Sedation in mechanically ventilated patients-time to stay awake? Ann Transl Med. (2016) 4:382. doi: 10.21037/atm.2016.09.37
116. Tsaousi GG, Lamperti M, Bilotta F. Role of dexmedetomidine for sedation in neurocritical care patients: a qualitative systematic review and meta-analysis of current evidence. Clin Neuropharmacol. (2016) 39:144–51. doi: 10.1097/WNF.0000000000000151
117. Wu J, Vogel T, Gao X, Lin B, Kulwin C, Chen J. Neuroprotective effect of dexmedetomidine in a murine model of traumatic brain injury. Sci Rep. (2018) 8:4935. doi: 10.1038/s41598-018-23003-3
118. Kenney MJ, Larsen BT, McMurphy RM, Mason D, Fels RJ. Dexmedetomidine and regulation of splenic sympathetic nerve discharge. Auton Neurosci. (2014) 183:111–5. doi: 10.1016/j.autneu.2014.02.009
119. Elmansoury Michael R. Dexmedetomidine in traumatic brain injury, why not? J Anesth Intens Care Med. (2017) 1. doi: 10.19080/JAICM.2017.01.555571
120. Luo J HJ, He Y, Weng Q, Liu J, Yang M, Bian G, et al. Role of dexmedetomidine in stress control in traumatic brain injury and its influence on neuroendocrine system. Med J Chin. (2013) 38. doi: 10.11855/j.issn.0577-7402.2013.11.011
121. Pajoumand M, Kufera JA, Bonds BW, Devabhakthuni S, Boswell S, Hesselton K, et al. Dexmedetomidine as an adjunct for sedation in patients with traumatic brain injury. J Trauma Acute Care Surg. (2016) 81:345–51. doi: 10.1097/TA.0000000000001069
122. Humble SS, Wilson LD, Leath TC, Marshall MD, Sun DZ, Pandharipande PP, et al. ICU sedation with dexmedetomidine after severe traumatic brain injury. Brain Inj. (2016) 30:1266–70. doi: 10.1080/02699052.2016.1187289
123. Owusu KA, Kurczewski L, Armahizer MJ, Zichichi A, Maciel CB, Heavner MS. DEXmedetomidine compared to PROpofol in NEurocritical care [DEXPRONE]: a multicenter retrospective evaluation of clinical utility and safety. J Crit Care. (2020) 60:79–83. doi: 10.1016/j.jcrc.2020.07.021
124. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
125. Byrne MF. “Wake me up before you go-go”. Drug, “wham”, scope, then snooze. Can't we do better with conscious sedation for endoscopy? Can J Gastroenterol. (2006) 20:767–9. doi: 10.1155/2006/670754
126. Canbay O, Altiparmak B, Celebi N, Karagoz H, Saricaoglu F. Comparison of propofol and midazolam on patients undergoing spinal surgery with intraoperative wake-up test: randomized clinical trial. Braz J Anesthesiol. (2015) 65:470–5. doi: 10.1016/j.bjan.2013.10.001
127. Sandiumenge Camps A, Sanchez-Izquierdo Riera JA, Toral Vazquez D, Sa Borges M, Peinado Rodriguez J, Alted Lopez E. Midazolam and 2% propofol in long-term sedation of traumatized critically ill patients: efficacy and safety comparison. Crit Care Med. (2000) 28:3612–9. doi: 10.1097/00003246-200011000-00009
128. Weinbroum AA, Halpern P, Rudick V, Sorkine P, Freedman M, Geller E. Midazolam versus propofol for long-term sedation in the ICU: a randomized prospective comparison. Intensive Care Med. (1997) 23:1258–63. doi: 10.1007/s001340050495
129. Fu X, Huang F, Chen Y, Deng Y, Wang Z. Application of dexmedetomidine-remifentanil in high-intensity ultrasound ablation of uterine fibroids: a randomised study. BJOG. (2017) 124(Suppl. 3):23–9. doi: 10.1111/1471-0528.14740
130. Tripathi M, Kumar V, Kalashetty MB, Malviya D, Bais PS, Sanjeev OP. Comparison of dexmedetomidine and midazolam for sedation in mechanically ventilated patients guided by bispectral index and sedation-agitation scale. Anesth Essays Res. (2017) 11:828–33. doi: 10.4103/aer.AER_48_17
Keywords: neurological wake-up test, multimodality monitoring, neurologic examination, daily-interruption of sedation, traumatic brain injury, sedation cessation
Citation: Musick S and Alberico A (2021) Neurologic Assessment of the Neurocritical Care Patient. Front. Neurol. 12:588989. doi: 10.3389/fneur.2021.588989
Received: 20 August 2020; Accepted: 02 March 2021;
Published: 22 March 2021.
Edited by:
Niklas Marklund, Lund University, SwedenReviewed by:
Raimund Helbok, Innsbruck Medical University, AustriaBo-Michael Bellander, Karolinska Institutet (KI), Sweden
Copyright © 2021 Musick and Alberico. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shane Musick, TXVzaWNrMjJAbWFyc2hhbGwuZWR1
 Shane Musick
Shane Musick Anthony Alberico
Anthony Alberico