
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 25 February 2021
Sec. Stroke
Volume 12 - 2021 | https://doi.org/10.3389/fneur.2021.586366
This article is part of the Research Topic Update on Vascular Contributions to Age-Related Neurodegenerative Diseases and Cognitive Impairment - Research of ISNVD 2020 Meeting View all 16 articles
 Cindy W. Yoon1
Cindy W. Yoon1 Young-Eun Kim2
Young-Eun Kim2 Hee Jin Kim3
Hee Jin Kim3 Chang-Seok Ki4
Chang-Seok Ki4 Hyejoo Lee3
Hyejoo Lee3 Joung-Ho Rha1
Joung-Ho Rha1 Duk L. Na3
Duk L. Na3 Sang Won Seo3*
Sang Won Seo3*No study yet has compared the longitudinal course and prognosis between subcortical vascular cognitive impairment patients with and without genetic component. In this study, we compared the longitudinal changes in cerebral small vessel disease markers and cognitive function between subcortical vascular mild cognitive impairment (svMCI) patients with and without NOTCH3 variant [NOTCH3(+) svMCI vs. NOTCH3(–) svMCI]. We prospectively recruited patients with svMCI and screened for NOTCH3 variants by sequence analysis for mutational hotspots in the NOTCH3 gene. Patients were annually followed-up for 5 years through clinical interviews, neuropsychological tests, and brain magnetic resonance imaging. Among 63 svMCI patients, 9 (14.3%) had either known mutations or possible pathogenic variants. The linear mixed effect models showed that the NOTCH3(+) svMCI group had much greater increases in the lacune and cerebral microbleed counts than the NOTCH3(–) svMCI group. However, there were no significant differences between the two groups regarding dementia conversion rate and neuropsychological score changes over 5 years.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an autosomal dominant disorder of cerebral small vessels caused by mutations in the NOTCH3 gene on chromosome 19 (1). CADASIL is characterized by cerebral small vessel disease (CSVD) and cognitive impairment, and is therefore considered as a genetic form of subcortical vascular cognitive impairment (SVCI). On the other hand, sporadic SVCI is mostly caused by vascular risk factors. Advanced age, hypertension (HTN), diabetes mellitus (DM), and other vascular risk factors can lead to CSVD characterized by white matter hyperintensities (WMHs), lacunes, and cerebral microbleeds (CMBs) on magnetic resonance imaging (MRI) (2).
Typically, patients with CADASIL are young at onset and have no vascular risk factors (3). However, we previously reported that approximately 13% of consecutive patients with SVCI had NOTCH3 variants, although they were of advanced age and frequently had a history of HTN (4). In our previous cross-sectional studies, these atypical SVCI patients with NOTCH3 variant have shown no significant differences in clinical and imaging features compared to patients without NOTCH3 variant (4, 5). However, cross-sectional comparison, a snapshot of a single moment in time, has interpretative limitations.
To our best knowledge, no study yet has compared the longitudinal course and prognosis between SVCI patients with and without genetic component. Therefore, in this study, we compared the longitudinal changes in CSVD markers and cognitive function in subcortical vascular mild cognitive impairment (svMCI) patients with [NOTCH3(+) svMCI] and without [NOTCH3(–) svMCI] NOTCH3 variant for a better understanding of the impact of NOTCH3 variant.
We prospectively recruited 72 patients with svMCI between September 2008 and September 2011 at Samsung Medical Center in Seoul, Korea. All recruited patients met the modified Petersen's criteria for MCI as previously described (6) and had evidence of significant ischemia on their MRI scans, seen as a cap or band ≥ 10 mm and a deep white matter lesion ≥ 25 mm (modified from Fazekas ischemia criteria) (7, 8). Of the 72 svMCI patients, nine were excluded because they or their caregivers chose not to participate in the study. After a complete description of the study, written informed consent was obtained from each patient (or legally authorized representatives). The Institutional Review Board of Samsung Medical Center approved the study protocol. This study was planned and conducted in accordance with the Declaration of Helsinki. This study has been registered in the Korean Clinical Trial Registry (registration number: KCT0005516).
Genetic analysis was performed according to the protocols previously described (4). Peripheral blood specimens were collected after obtaining informed consent. Genomic DNA was extracted using the Wizard Genomic DNA purification kit according to the manufacturer's instructions. Mutational hotspots of the NOTCH3 gene including exons 2–6, 8, 11, 18, 19, and 22 were sequenced. All tested exons and their exon-intron boundaries in the NOTCH3 gene were amplified by polymerase chain reaction, as described previously (9). Cycle sequencing was performed using a BigDye Terminator Cycle Sequencing Ready Reaction kit on an ABI 3130xl Genetic Analyzer (Applied Biosystems). The nucleotides of NOTCH3 complementary DNA were numbered according to a reference sequence (GenBank accession number: NM_000435.2). Sorting Intolerant From Tolerant (10) and Polymorphism Phenotyping (PolyPhen-2 v2.1) (11) servers were used to predict the effect of non-synonymous variants of unknown significance (VUS) on protein structure, function, phenotype, sequence conservation, and/or protein structure. In addition, 358 age- and sex-matched healthy Korean controls were screened for novel VUS in the NOTCH3 gene using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, as described previously (12). Written informed consent was obtained from all participants including healthy controls.
Among 63 svMCI patients, nine (14.3%) had either known mutations or variants of unknown significance (VUS). Three known mutations were found in six patients: p.R544C (n = 4), p.R587C (n = 1), and p.V237M (n = 1). In addition, four VUS were identified in three patients: three missense variants (p.P572L, p.S947I, and p.R1175W) and one frameshift variant (p.Glu990Argfs*282). Two VUS (p.P572L and p.R1175W) were found in one patient. A control study of 716 chromosomes identified one variant (p.R1175W) (Supplementary Table 1). This variant might be a polymorphism rather than a pathogenic variant. These results were included in our previous report (4).
T1, T2, 3-dimensional fluid-attenuated inversion recovery (FLAIR), and T2 Fast Field Echo-MR images were acquired using the same 3.0T MRI scanner (Philips 3.0T Achieva).
Lacunes were defined as small lesions (≤15 and ≥3 mm in diameter) with low signal on T1-weighted images, high signal on T2-weighted images, and a perilesional halo on 80 axial slices from FLAIR images.
CMBs were defined as lesions ≤ 10 mm in diameter by using the criteria proposed by Greenberg et al. (13) on 20 axial slices of T2*gradient echo-MR images. CMBs were counted in four lobar regions (frontal, temporal, parietal, and occipital) and deep brain regions. The lobar regions were defined as regions ≤ 10 mm from the brain surface.
All patients underwent neuropsychological testing using the Seoul Neuropsychological Screening Battery (14, 15).
All patients underwent a standardized [11C]-PiB-PET scan at the Samsung or Asan Medical Center on a Discovery STE PET/CT scanner (GE Medical Systems, Milwaukee, WI, USA) to minimize any variance due to scanner differences. The detailed radiochemistry profiles, scanning protocol, and data analysis method were described in a previous study (8). Briefly, we calculated the PiB-uptake ratio of each voxel using the cerebellum as a reference region in the analysis. The global cortical PiB-uptake ratio was determined by combining the bilateral frontal, parietal, and temporal cortices, and the posterior cingulate gyrus. Patients were considered PiB-positive if their global PiB uptake ratio was more than 2 standard deviations (PiB retention ratio ≥ 1.5) from the mean of the normal controls.
All patients underwent clinical interviews, a neurological examination, neuropsychological tests, brain MRI, and PiB-PET imaging at baseline. Patients were annually evaluated for 5 years through clinical interviews, neuropsychological tests, and brain MRI.
Completeness of follow-up was 63/63 (100%) at 1 year, 60/63 (95.2%) at 2 years, 55/63 (87.3%) at 3 years, 51/63 (81.0%) at 4 years, and 43/63 (68.3%) at 5 years; 7/9 (77.8%) among NOTCH3(+) svMCI and 36/54 (66.7%) among NOTCH3(–) svMCI (Supplementary Figure 1). The comparison between patients with and without complete 5-year follow-up is shown in Supplementary Table 2. There were no significant differences between the two groups except for the female ratio. The female ratio was higher in patients with complete 5-year follow-up than in those without complete follow-up.
To analyze the baseline differences between svMCI patients with and without NOTCH3 variant, we used the Chi-square test or Fisher's exact test for categorical variables, and the Mann–Whitney U-test or Student t-test for continuous variables.
We used the linear mixed-effects model to estimate changes in CSVD markers and cognitive measures over the follow-up period. In the linear mixed-effects model, the interactions between the presence of NOTCH3 variant and time interval (presence of NOTCH3 variant × time) were explored to determine the influence of the presence of NOTCH3 variant on the rate of change in CSVD markers. We controlled for age, HTN, baseline number of lacunes or CMBs, and time interval from baseline tests. To determine the trend of changes in each group, linear mixed-model analyses were separately performed in each group using age, HTN, and baseline number of lacunes or CMBs as covariates, and time interval from baseline evaluation as a predictor.
Longitudinal changes of cognitive scores in two svMCI groups were compared with linear mixed-effect models using the presence of NOTCH3 variant and time interval (presence of NOTCH3 variant × time) as a predictor; age, sex, education, and time interval from baseline tests were used as covariates. Because multiple cognitive scores were used for comparison, correction for multiple comparisons was performed by false discovery rate correction.
Cox regression models were used to compare the risks of progression to dementia between NOTCH3(+) and NOTCH3(–) svMCI groups after controlling for age, sex, education, and PiB positivity. Patients who did not progress to dementia were treated as censored observations from the time of their final follow-up visit.
Baseline characteristics of the subjects are shown in Table 1. There were no significant differences between the two groups with respect to age, sex ratio, education years, and prevalence of vascular risk factors. No significant group differences were seen in the baseline number of lacunes and CMBs, PiB SUVR, and mini-mental state exam (MMSE) and clinical dementia rating scale sum of boxes (CDR-SOB) scores.
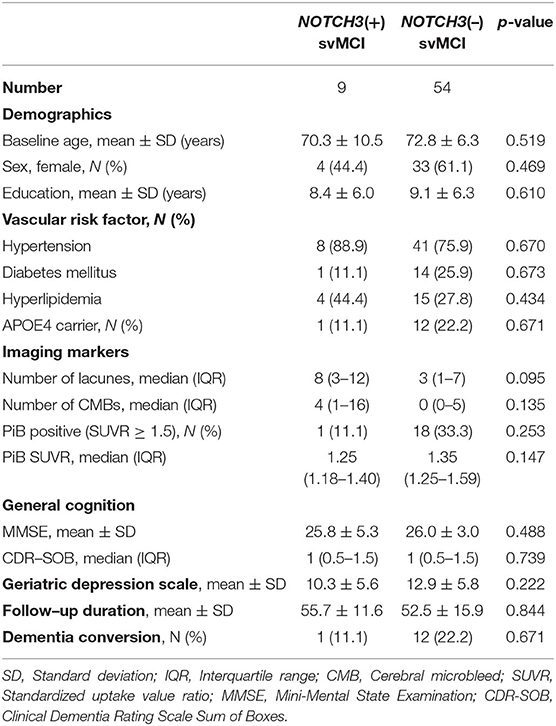
Table 1. Baseline characteristics of patients with subcortical vascular mild cognitive impairment (svMCI), with and without NOTCH3 variant.
The mean (standard deviation) duration of follow-up were 55.7 (11.6) months in nine NOTCH3(+) svMCI patients and 52.5 (15.9) months in 54 NOTCH3(–) svMCI patients. Thirteen of 63 patients (20.6%) showed conversion to dementia on follow-up: 1/9 (11.1%) among the NOTCH3(+) svMCI group and 12/54 (22.2%) among the NOTCH3(–) svMCI group. The time to dementia diagnosis was 11 months in one NOTCH3(+) svMCI patient. The mean (range) time to dementia diagnosis was 31.1 (11–54) months in 12 NOTCH3(–) svMCI patients.
Linear mixed-effect model analysis separately performed in each svMCI group showed that there were increases in lacune and CMB counts in both svMCI groups according to the time interval from baseline evaluation (Table 2). The linear mixed-effect models that tested the interactive effect of NOTCH3 variant and time showed that the NOTCH3(+) svMCI group had much greater increases in lacune and CMB (total and deep) counts than NOTCH3(–) svMCI group after controlling for age, HTN, and baseline number of lacunes or CMBs (Table 2). Figure 1 shows the estimated effect of NOTCH3 variant on the longitudinal changes of the number of lacunes and CMBs over a 5-year follow-up period.
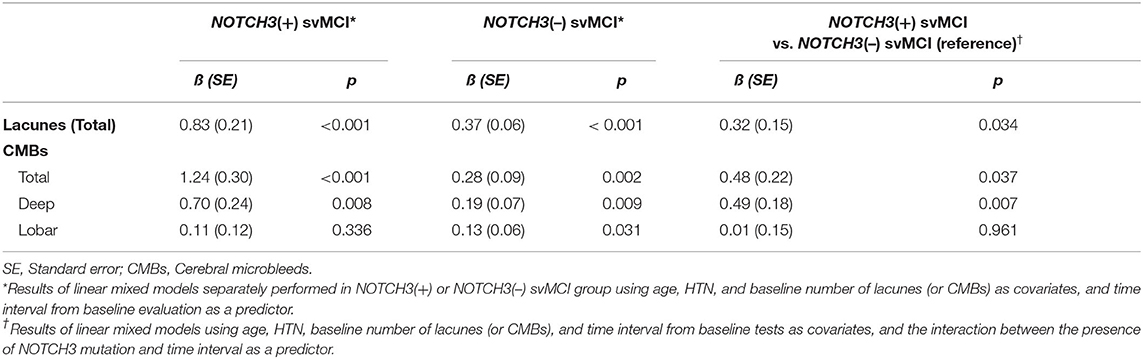
Table 2. Comparison of longitudinal changes in the number of lacunes and cerebral microbleeds in patients with subcortical vascular mild cognitive impairment (svMCI), with and without NOTCH3 variant.
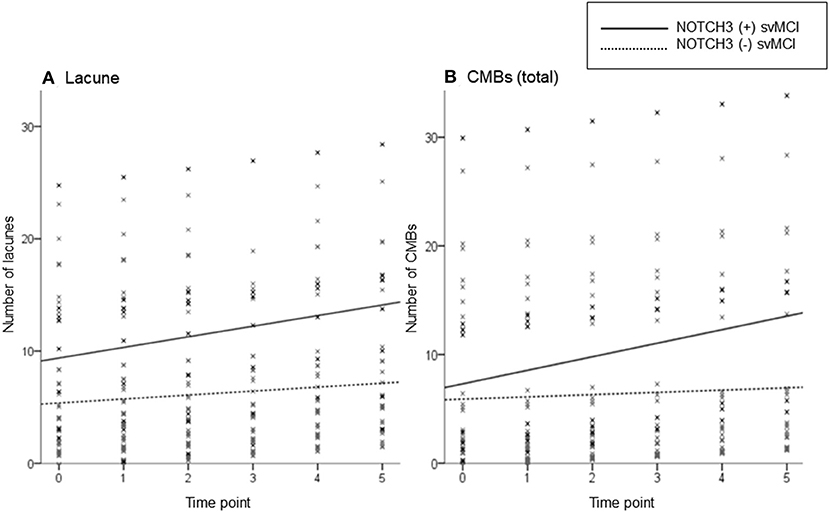
Figure 1. Scatterplot of the predicted number of lacunes (A) and cerebral microbleeds (CMBs) (B) in subcortical vascular mild cognitive impairment (svMCI) patients with and without NOTCH3 variant. The solid and dotted lines show the linear regression model of patients with and without NOTCH3 variant, respectively. Analysis controlled for age, HTN, baseline number of lacunes or CMBs, and time interval from baseline test.
In the linear mixed-effect model that tested the interactive effect of NOTCH3 variant and time on changes of neuropsychological test scores, no neuropsychological tests showed significant differences in longitudinal change according to the presence of NOTCH3 variant (Table 3).
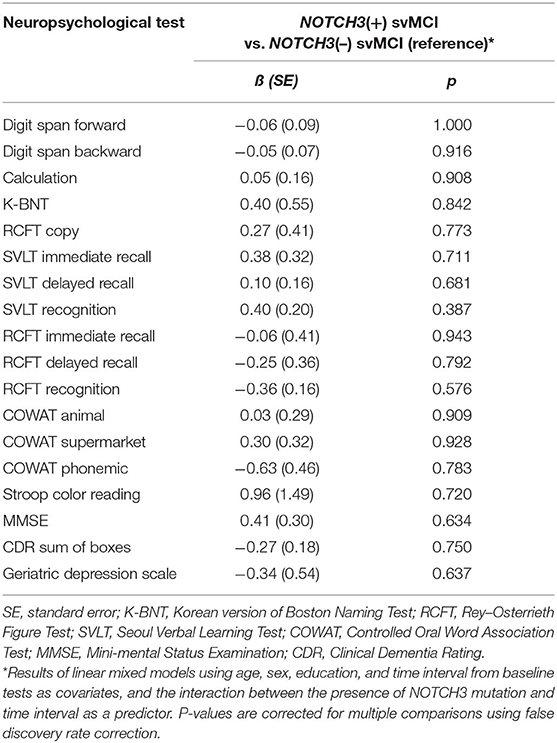
Table 3. Comparison of longitudinal changes in neuropsychological test scores between subcortical vascular mild cognitive impairment (svMCI) patients with and without NOTCH3 variant.
Cox regression model showed that dementia risk was not significantly different between NOTCH3(+) and NOTCH3(–) svMCI patients after controlling for age, sex, education, and PiB positivity (p = 0.763; adjusted hazard ratio, 0.723; 95% confidence interval, 0.088–5.926) (Figure 2).
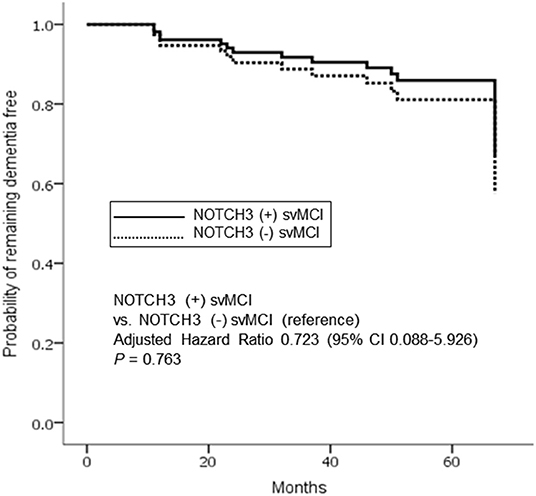
Figure 2. Cox proportional hazards model for progression to dementia in subcortical vascular mild cognitive impairment (svMCI) according to the presence of NOTCH3 variant. Analysis controlled for age, sex, education, and PiB positivity. The solid and dotted lines indicate patients with and without NOTCH3 mutation, respectively.
In this prospective study, we compared the longitudinal course of svMCI patients according to the presence of NOTCH3 variant in a well-defined svMCI cohort based on standardized imaging protocols, detailed clinical evaluation, and genetic analysis. Regarding the longitudinal changes of CSVD markers, NOTCH3(+) svMCI patients showed much greater increase in lacune and CMB counts than the NOTCH3(–) svMCI patients. However, there were no significant differences between the two groups regarding longitudinal cognitive changes including dementia conversion rate and neuropsychological score changes over 5 years. We believe this is the first study to compare the longitudinal changes of CSVD markers and cognition between SVCI patients with and without NOTCH3 variant.
Our major finding was that the rate of increase in lacune and CMB counts was much greater in patients with NOTCH3 variant than in those without NOTCH3 variant. Because previous longitudinal studies have suggested that the baseline burden of CSVD is associated with the rate of change in CSVD markers (16, 17), we adjusted for baseline lacune and CMB counts. We also controlled for age and HTN as well-known risk factors for progression of CSVD. The presence of NOTCH3 variant still remained an independent predictor for change in lacune and CMB counts.
Mutations in the NOTCH3 gene cause degeneration of smooth muscle cells in the tunica media and thickening of the walls of cerebral small vessels (18, 19). Lacunes in CADASIL are caused by reduced microvascular perfusion due to small arteriopathy affected by NOTCH3 mutation. Considering the similar burden of conventional vascular risk factors and additional damage to cerebral small vessels by NOTCH3 mutation, it is understandable that NOTCH3(+) svMCI patients in our study showed a markedly greater increase in lacune count than NOTCH3(–) svMCI patients.
Both total and deep CMBs tended to increase much faster in NOTCH3(+) than NOTCH3(–) patients; however, this association was not found in lobar CMBs. In previous CADASIL studies, the distribution of CMBs is predominantly in deep location including the bilateral thalami and basal ganglia and less commonly at the gray–white junction (20–22). NOTCH3 mutations cause arteriopathy affecting the small cerebral (deep) and leptomeningeal (superficial) penetrating arteries (23), and CMBs might result from vascular leakage of these fragile small vessels (24). Although superficial small perforating arteries associated with lobar CMBs might be also affected by NOTCH3 mutation, the majority are thought to be deep small perforating arteries responsible for deep CMBs.
Lacunes and CMBs have been associated with cognitive impairment and decline in many previous cross-sectional (25–28) and longitudinal studies (29–32). A previous 7-year follow-up study investigating longitudinal associations between radiologic changes and cognitive decline in CADASIL patients have also shown that cognitive decline might be associated with increases in lacune and CMB counts (33). However, unexpectedly, despite a much greater increase in lacune and CMB counts in NOTCH3(+) svMCI patients than NOTCH3(–) svMCI patients, there were no significant differences in dementia conversion rate or neuropsychological score changes over 5 years between the two groups. This is likely because of similar baseline CSVD burden and inadequate differences in radiological changes to make a significant difference to cognitive decline between the two groups. Baseline CSVD burden including the number of lacunes or CMBs has been a significant predictor of cognitive decline in previous longitudinal studies (29–32). In our study, both svMCI groups with and without NOTCH3 variant had relatively severe CSVD burden at baseline with a similar degree between the two groups. It is also possible that a much longer follow-up is required to demonstrate significant differences in cognitive decline between the two groups. Another possible explanation is because of amyloid burden. In our study, NOTCH3(–) svMCI group tended to have more amyloid burden than NOTCH3(+) svMCI group, although it was not statistically significant. In previous studies from our group, higher amyloid uptake in svMCI was associated with more severe cognitive impairment and faster cognitive decline (34, 35). A relatively small number of NOTCH3(+) patients could have also attributed to these negative findings.
The strengths of our study are its prospective setting, standardized imaging protocols, and detailed clinical evaluation during follow-up. However, our results should be interpreted with caution because our NOTCH3(+) patients were not representative of the typical CADASIL patients (4). This was a single-center study examining a small cohort of patients and the sample size of the NOTCH3(+) svMCI group was particularly small. The rate of follow-up loss at 5 years was relatively high. Finally, the possibility of polymorphisms rather than pathogenic variants remains in three VUS, although these VUS were not found in 716 control chromosomes. Thus, we performed additional analyses excluding three patients with VUS and obtained similar results (Supplementary Tables 3, 4).
NOTCH3(+) svMCI group had much greater increases in the lacune and cerebral microbleed counts than the NOTCH3(–) svMCI group. However, there were no significant differences between the two groups regarding dementia conversion rate and neuropsychological score changes over 5 years.
The datasets generated for this study will be made available on request to the corresponding author.
The studies involving human participants were reviewed and approved by The Institutional Review Board of Samsung Medical Center. The patients/participants provided their written informed consent to participate in this study.
CY: conceptualization of the study, methodology, formal analysis, and writing - original draft. Y-EK and C-SK: methodology, validation, and formal analysis. HK: methodology and data curation. HL: methodology and formal analysis. J-HR: methodology, writing - review and editing. DN: conceptualization of the study, methodology, investigation, and supervision. SS: conceptualization of the study, methodology, formal analysis, writing-review and editing, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1913844), the grant funded by Research of Korea Centers for Disease Control and Prevention (2018-ER6203-02), the NRF grant funded by the Korea government (MSIT) (NRF-2019R1A5A2027340), and the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and Ministry of science and ICT, Republic of Korea (HU20C0111).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
A preliminary version of this paper was presented at the 6th congress of the European Academy of Neurology (36).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.586366/full#supplementary-material
1. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. (1996) 383:707. doi: 10.1038/383707a0
2. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
3. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M-G. Cadasil. Lancet Neurol. (2009) 8:643–53. doi: 10.1016/S1474-4422(09)70127-9
4. Yoon CW, Kim Y-E, Seo SW, Ki C-S, Choi SH, Kim J-W, et al. NOTCH3 variants in patients with subcortical vascular cognitive impairment: a comparison with typical CADASIL patients. Neurobiol Aging. (2015) 36:2443.e1–7. doi: 10.1016/j.neurobiolaging.2015.04.009
5. Kim KW, Kwon H, Kim Y-E, Yoon CW, Kim YJ, Kim YB, et al. Multimodal imaging analyses in patients with genetic and sporadic forms of small vessel disease. Sci Rep. (2019) 9:787 doi: 10.1038/s41598-018-36580-0
6. Seo SW, Cho SS, Park A, Chin J, Na DL. Subcortical vascular versus amnestic mild cognitive impairment: comparison of cerebral glucose metabolism. J Neuroimaging. (2009) 19:213–9. doi: 10.1111/j.1552-6569.2008.00292.x
7. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
8. Lee J, Kim S, Kim G, Seo S, Park H, Oh S, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. (2011) 77:18–25. doi: 10.1212/WNL.0b013e318221acee
9. Kim Y-E, Yoon CW, Seo SW, Ki C-S, Kim YB, Kim J-W, et al. Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging. (2014) 35:726.e1–6. doi: 10.1016/j.neurobiolaging.2013.09.004
10. Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. (2001) 11:863–74. doi: 10.1101/gr.176601
11. Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. (2002) 30:3894–900. doi: 10.1093/nar/gkf493
12. Song M-J, Lee S-T, Lee M-K, Ji Y, Kim J-W, Ki C-S. Estimation of carrier frequencies of six autosomal-recessive Mendelian disorders in the Korean population. J Hum Genet. (2012) 57:139. doi: 10.1038/jhg.2011.144
13. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Salman RA-S, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. (2009) 8:165–74. doi: 10.1016/S1474-4422(09)70013-4
14. Kang Y. Samsung Neuropsychological Screening Battery. Current Research in Dementia. Seoul: The Korean Dementia Association (1998). p. 99–107.
15. Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer's continuum. Dementia Neurocogn Disord. (2019) 18:77–95. doi: 10.12779/dnd.2019.18.3.77
16. Lee S-H, Lee S-T, Kim BJ, Park H-K, Kim C-K, Jung K-H, et al. Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study. PLoS ONE. (2011) 6:e25930. doi: 10.1371/journal.pone.0025930
17. Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. (2007) 68:214–22. doi: 10.1212/01.wnl.0000251302.55202.73
18. Miao Q, Paloneva T, Tuominen S, Pöyhönen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. (2004) 14:358–64. doi: 10.1111/j.1750-3639.2004.tb00078.x
19. Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case. Stroke. (2002) 33:2565–9. doi: 10.1161/01.STR.0000032620.91848.1C
20. Oberstein SL, Van den Boom R, Van Buchem M, Van Houwelingen H, Bakker E, Vollebregt E, et al. Cerebral microbleeds in CADASIL. Neurology. (2001) 57:1066–70. doi: 10.1212/WNL.57.6.1066
21. Lee JS, Kang C-h, Park SQ, Choi HA, Sim K-B. Clinical significance of cerebral microbleeds locations in CADASIL with R544C NOTCH3 mutation. PLoS ONE. (2015) 10:e0118163. doi: 10.1371/journal.pone.0118163
22. Schrag M, Greer DM. Clinical associations of cerebral microbleeds on magnetic resonance neuroimaging. J Stroke Cerebrovasc Dis. (2014) 23:2489–97. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.006
23. Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. (2002) 12:371–84. doi: 10.1111/j.1750-3639.2002.tb00451.x
24. Braun H, Schreiber S. Microbleeds in cerebral small vessel disease. Lancet Neurol. (2013) 12:735–6. doi: 10.1016/S1474-4422(13)70148-0
25. Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. (2012) 78:326–33. doi: 10.1212/WNL.0b013e3182452928
26. Yakushiji Y, Noguchi T, Hara M, Nishihara M, Eriguchi M, Nanri Y, et al. Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke. (2012) 43:1800–5. doi: 10.1161/STROKEAHA.111.647065
27. Gold G, Kövari E, Herrmann FR, Canuto A, Hof PR, Michel J-P, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. (2005) 36:1184–8. doi: 10.1161/01.STR.0000166052.89772.b5
28. Benjamin P, Lawrence AJ, Lambert C, Patel B, Chung AW, MacKinnon AD, et al. Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. Neuroimage Clin. (2014) 4:828–37. doi: 10.1016/j.nicl.2014.05.009
29. Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. (2016) 73:934–43. doi: 10.1001/jamaneurol.2016.1017
30. Benjamin P, Trippier S, Lawrence AJ, Lambert C, Zeestraten E, Williams OA, et al. Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke. (2018) 49:586–93. doi: 10.1161/STROKEAHA.117.017526
31. Jokinen H, Gouw A, Madureira S, Ylikoski R, Van Straaten E, Van Der Flier W, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology. (2011) 76:1872–8. doi: 10.1212/WNL.0b013e31821d752f
32. Gregoire S, Smith K, Jäger H, Benjamin M, Kallis C, Brown M, et al. Cerebral microbleeds and long-term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis. (2012) 33:430–5. doi: 10.1159/000336237
33. Liem M, Oberstein SL, Haan J, Van der Neut I, Ferrari M, Van Buchem M, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. (2009) 72:143–8. doi: 10.1212/01.wnl.0000339038.65508.96
34. Lee MJ, Seo SW, Na DL, Kim C, Park JH, Kim GH, et al. Synergistic effects of ischemia and β-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiatry. (2014) 71:412–22. doi: 10.1001/jamapsychiatry.2013.4506
35. Kim HJ, Yang JJ, Kwon H, Kim C, Lee JM, Chun P, et al. Relative impact of amyloid-β, lacunes, and downstream imaging markers on cognitive trajectories. Brain. (2016) 139:2516–27. doi: 10.1093/brain/aww148
Keywords: NOTCH3, CADASIL, lacune, cerebral microbleed, subcortical vascular cognitive impairment
Citation: Yoon CW, Kim Y-E, Kim HJ, Ki C-S, Lee H, Rha J-H, Na DL and Seo SW (2021) Comparison of Longitudinal Changes of Cerebral Small Vessel Disease Markers and Cognitive Function Between Subcortical Vascular Mild Cognitive Impairment With and Without NOTCH3 Variant: A 5-Year Follow-Up Study. Front. Neurol. 12:586366. doi: 10.3389/fneur.2021.586366
Received: 23 July 2020; Accepted: 05 February 2021;
Published: 25 February 2021.
Edited by:
Mark Haacke, Wayne State University, United StatesReviewed by:
Michele Romoli, University of Perugia, ItalyCopyright © 2021 Yoon, Kim, Kim, Ki, Lee, Rha, Na and Seo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Won Seo, c2FuZ3dvbnNlb0BlbXBhbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.