
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 20 January 2021
Sec. Neuroinfectious Diseases
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.613552
This article is part of the Research TopicConsequences of the COVID-19 Pandemic on Care for Neurological ConditionsView all 77 articles
The recent outbreak of coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2, has become a global threat. Due to neurological manifestations presented throughout the coronavirus disease process, the potential involvement of COVID-19 in central nervous system has attracted considerable attention. Notably, the neurologic system could be widely affected, with various complications such as acute cerebrovascular events, encephalitis, Guillain-Barré syndrome, and acute necrotizing hemorrhagic encephalopathy. However, the risk assessment of exposure to potential biohazards in the context of the COVID-19 pandemic has not been clearly clarified regarding the sampling, preparation, and processing neurological specimens. Further risk managements and implantations are seldom discussed either. This article aims to provide current recommendations and evidence-based reviews on biosafety issues of preparation and processing of cerebrospinal fluid and neurological specimens with potential coronavirus infection from the bedside to the laboratory.
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has caused the coronavirus disease 2019 (COVID-19) pandemic, an illness with the high transmissibility and a broad spectrum of clinical manifestation. As of December 15, 2020, the World Health Organization (WHO) reported more than 70 million cases and over 1.6 million deaths globally in the COVID-19 pandemic (1). COVID-19 is the third epidemic of human coronavirus diseases after the severe acute respiratory syndrome (SARS) in November 2002, and the Middle East respiratory syndrome (MERS) in September 2012 (2). In comparison with other epidemic coronaviruses, SARS-CoV-2 is less lethal but far more transmissible than MERS coronavirus (MERS-CoV) and SARS coronavirus (SAR-CoV) (3, 4). It is believed that SARS–CoV-2 can spread by respiratory droplets, unprotected direct contact with patients, and touching contaminated objects (5, 6). Since symptoms of COVID-19 can be in a wide variety of severity, medical professionals are in particular at risk of exposure to SARS-CoV-2 through close contact via respiratory droplets and contaminated surface and direct handling of contagious materials from patients with COVID-19 (7). With regard to the safe collecting and handling clinical specimens in the pandemic, a few reports have emphasized the need for the worldwide standardization of biosafety protocols (5, 8, 9). Notably, the neurological manifestation and morbidities of COVID-19 have been widely reported (10–16). Mao et al. (15) reported that neurologic symptoms were present in 36.4% of all patients with COVID-19, especially more frequent in patients with severe illness. Moreover, in a patient with acute cerebellitis, the viral RNA of SARS-CoV-2 was detected in his oropharynx, nasopharynx, and cerebrospinal fluid (CSF) (17). However, the biosafety and risk assessment in preparation and processing of CSF and other neurological specimens were seldom discussed. This mini-review aims to provide an integrative, evidence-based review to guide the preparation and processing of neurological specimens with potential coronavirus infection and therefore to prevent nosocomial infection.
Although COVID-19 primarily presents as a respiratory disease, SARS-CoV-2 affects multiple organs or systems, including the central nervous system (CNS), peripheral nervous system (PNS), and neuromuscular system (15, 18–20). In a large case cohort of COVID-19, 24.8% had CNS symptoms (e.g., dizziness, headache, and impaired consciousness), 8.9% had PNS symptoms, and 10.7% had skeletal muscle injury (15). In a nationwide surveillance of 125 patients with COVID-19 and neurological or psychiatric disease, 62% of them had a cerebrovascular event, while 31% of them presented with altered mental status (19). Similarly, the epidemic of SARS was reported with various neurological complications including encephalopathy, seizures, stroke, cranial nerve palsies, peripheral neuropathy, and myopathy (20–24). Also, patients with MERS were occasionally presented to have neurological symptoms and neuromuscular complications (24–27). The prevalence of CNS complications reached 0.04% for SARS and 0.20% for MERS, and besides the prevalence of PNS complications was 0.05% for SARS and 0.16% for MERS (14).
Although the mechanism of CNS involvement of COVID-19 remains unclear, there is a three-step model which refers to viral neuroinvasion, CNS clearance, and immune response (28). In the first stage, SARS-CoV-2 may enter the brain via the bloodstream and/or transcribriform route along the olfactory nerve, and the viral load in CSF should increase (28). The respiratory symptoms are minimal in the early stage. With the interaction between the spike protein S1 of SARS-CoV-2 and the host angiotensin-converting enzyme 2 receptor (ACE2), SARS-CoV-2 may enter both glial and neuronal cells (29). In some cases, the neuroinvasion may cause a direct neuronal damage and subsequently result in neurological symptoms. Moreover, the consumption and downregulation of ACE2 by SARS-CoV-2 virus may lead to imbalance of the renin angiotensin system resulting in endothelial dysfunction, vasoconstriction, and subsequently ischemic events (30). In the second stage, SARS-CoV-2 may infect the brainstem affecting the respiratory drive. The viral load in respiratory secretions would increase predominantly, but the viral load in CSF significantly decreases. The CSF clearance of SARS-CoV-2 may greatly contribute to a low virus detection rate in CSF samples from patients with COVID-19 and CNS involvement. In the third stage, an immuno-mediated CNS damage may form, since SARS-CoV-2 can trigger the production of antibodies against glial cells, as a para-infective or post-infective phenomenon (28). In consequence, the respiratory system would be severely affected and cause neurotoxic hypoxia with subsequent brain damage (28).
With regard to neuromuscular involvement of SARS-CoV-2, myositis, acute myelitis, Guillain Barre syndrome, Miller Fisher syndrome, polyneuritis cranialis, oculomotor paralysis and Bell's Palsy have been discussed to be associated with COVID-19 (18, 30–34). On electrodiagnostic testing, most of the abovementioned patients had demyelinating pattern, some had acute sensory motor axonal neuropathy, and few had acute motor axonal neuropathy (18). In a patient with COVID-19 and myositis, the muscle biopsy revealed inflammatory infiltration around vessels and endomysial extension, regeneration of muscular fibers, and elevated HLA Class ABC expression (33). The exact mechanism remains unknown, although a few hypothetic theories were proposed, including ACE2 mediated pathway, olfactory pathway, trans-synaptic pathway, and immune mediated pathway (18). Since the muscle cells express ACE2, the direct invasion by the SARS-CoV-2 entering the muscle cells via the ACE2 may be possible (30). In addition, cytokine storms in the advanced phase of COVID-19 could lead to immune-mediated muscle damages (30).
Lumbar puncture (LP) is a medical procedure at the level of L2 to L5 vertebrae to collect CSF for examining infectious, inflammatory, and neoplastic diseases involving the CNS. In viral encephalitis, there is usually a mild to moderate CSF pleocytosis with predominant lymphocytes, normal glucose ratio, and slightly elevated protein (14, 35). The standard of diagnosing a CNS viral infection is to demonstrate the virus in the CNS, either from culture or polymerase chain reaction (PCR) of brain tissue or CSF.
Muscle biopsy (MB) is important for the evaluation and diagnosis of patients who are suspected of having an underlying neuromuscular disorder (36). With an open biopsy or needle biopsy technique under local anesthesia, bundles of skeletal muscles are taken for the required tests, including frozen sections for enzyme histochemistry, paraffin embedding for muscle fiber morphology and inflammatory patterns, electron microscopy for ultrastructural analysis, and biochemical testing for assessing storage and mitochondrial diseases (36).
In the pandemic, to perform a LP or a MB might be at risk to expose coronaviruses, since direct contact or respiratory droplets might be infectious. Since both LP and MB are time-consuming, the performer and all teammates would expose to patients' droplet aerosols in a poorly ventilated room. In closed rooms, the SARS-CoV-2 can be detectable in aerosols for 3 h and persists on surfaces (such as cardboard, stainless steel, and plastic surfaces) from 24 to 72 h (6). Thus, the sampling or collecting biological materials from patients should be careful and need to follow the recommendations or guidelines in the pandemic (5, 37–39). First, a site-specific and activity-specific risk assessment should be regularly performed to ensure the competency level of the healthcare workers, the equipment and facility, and the resources that are available. Meanwhile, clinical triage should be ensured by assessing all patients for early detection of COVID-19, and immediate isolation of patients with suspected COVID-19 in an area separate from others (37). Regarding the environment, LP and MB should be performed in an adequately ventilated room with at least of 60 liters/s/patient air flow (37). The environmental cleaning and disinfection procedures should be consistently and correctly performed. Notably, coronaviruses can be inactivated by surface disinfectants with 62–71% ethanol (C2H6O), 0.5% hydrogen peroxide (H2O2) or 0.1% sodium hypochlorite (NaClO) within 1 min (40).
Although LP and MB are not aerosol-generating procedures, the neurological professionals should wear a medical mask, eye protection (goggles) or facial protection (face shield), a clean long-sleeved gown, and gloves (37). After procedures, personal protective equipment and wastes should be properly disposed, and hand hygiene should be performed before and after contact with each patient. Lastly, it is important to clean and disinfect the surfaces that the patient was in contact with. With regard to the transportation, CSF or muscle for virus detection can be shipped at 2–8°C and delivered promptly to the laboratory (41). Notably, patient specimens from suspected or confirmed SARS-CoV-2 infection should be transported as UN3373, “Biological Substance Category B” (42, 43). All the biosafety recommendations are summarized in Table 1.
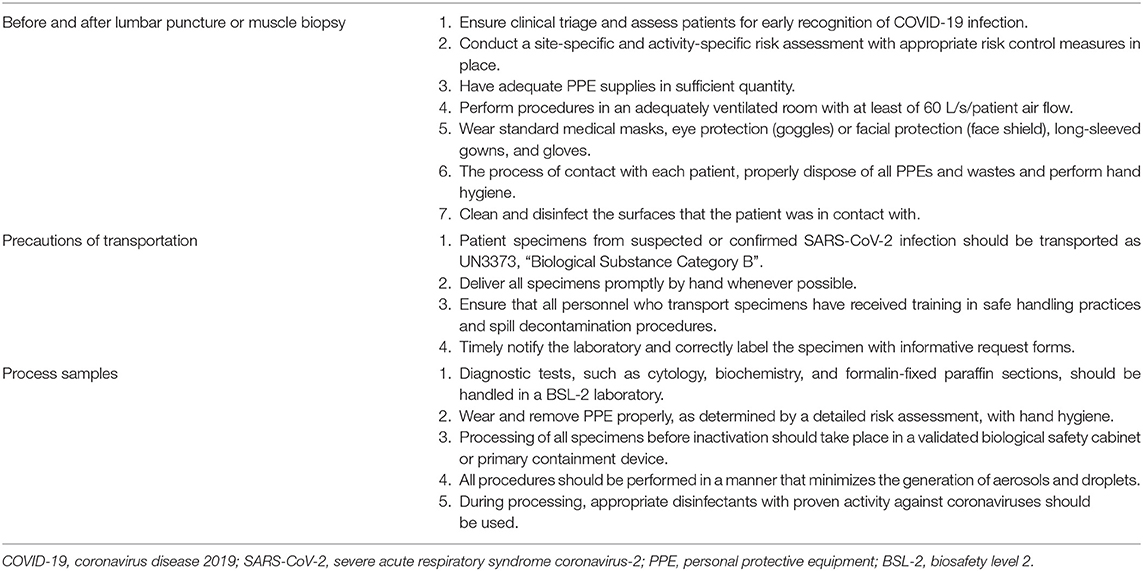
Table 1. A summary of biosafety recommendations to prevent coronavirus (COVID-19) for lumbar puncture and muscle biopsy.
Since all specimens collected for laboratory investigations should be considered potentially infectious, all procedures must be performed according to risk assessment and strategies for biosafety (42, 43). Before inactivation of all specimens, the initial processing should be performed in a validated biological safety cabinet or primary containment device (42). In addition to detecting viruses by sequencing or PCR, all diagnostic laboratory works for neurological specimens, including biochemistry, cytology, and special stains should be performedat a facility using procedures equivalent to Biosafety Level 2 (BSL-2) (42, 43). In the light of inactivation of coronaviruses, fixatives with ethanol concentrations of 78%-95% for at least 30 s could inactivate SARS-CoV, and either 10% formalin or 4% paraformaldehyde for at least 30 min would efficiently inactivate MERS-CoV–infected cells (9). Alcohol fixed preparation also lyses red blood cells, reducing the risk of viremia. The abovementioned fixations are the reasons why specimens with Papanicolaou staining or formalin fixation can be taken as inactivated (9). Moreover, the external lysis buffer of common RNA extraction kits for viral detection is effective to inactivate SARS-CoV-2 without heat or other additional methods (42).
Currently, the identification of viral RNA through nucleic acid amplification technologies, such as reverse-transcription polymerase chain reaction (RT-PCR) in a patient's biological samples, remains the gold standard for identifying infections with coronaviruses. Notably, SARS-CoV was detected in CSF by RT-PCR in two cases of encephalopathy (44, 45) and was cultured from brain tissues of an autopsy case (46). In the COVID-19 pandemic, although the neurological manifestations were not uncommon, SARS-CoV-2 RNA was rarely detected in CSF by RT-PCR (Table 2) (17, 47–65). And, to the best of our knowledge, there is no MB specimen demonstrating the evidence of SARS-CoV-2 infection via culture or RT-PCR. Based on the relative frequencies of detectable SARS-CoV-2 RNA in different samples from published reports, Chen and Chi (5) suggested to categorize the cytological and pathological samples into the high risk, intermediate risk, and low risk groups. Accordingly, CSF and MB specimens can be categorized into the low risk group with limited evidence of SARS-CoV-2 RNA detection and should subsequently follow the principles of good microbiological practices and procedures to be handled (5). Although the presence of viral RNA is not equivalent to live infectious viruses, RT-PCR is an important method to identify infectious agents (66).
With the growing number of confirmed COVID-19 cases, it is essential that the neurological experts and clinical laboratories implement clinical triage, drastic measures, and appropriate procedures and facilities for ensuring the safety and interests of valuable healthcare workers in times of the pandemic. The lessons learned from SARS and MERS could give us more insights to conduct efficient preventive measures in healthcare settings. Although LP and MB are important diagnostic procedures for CNS and neuromuscular diseases, neurological practitioners must be well-prepared and avoid of non-emergent procedures to prevent potential exposures to COVID-19. The collection, transportation, and processing of neurological specimens should warrant the use of WHO guidelines, academic recommendations, and BSL-2 procedures. Herein, although the biological safety and security issues were rarely discussed in neurology, we hope that both neurologists and laboratory professionals can benefit from this integrative mini-review in dealing with the COVID-19 crisis.
C-CC: study concept and design, acquisition of data, manuscript writing, critical revision of the manuscript for important intellectual content, and study supervision. P-CC: acquisition of data, analysis and interpretation, manuscript writing, and critical revision of the manuscript for important intellectual content. T-HC: critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. World Health Organization. Weekly Epidemiological Update-15 December 2020. (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201215_weekly_epi_update_18.pdf?sfvrsn=9c9aac11_5&download=true (accessed December 16, 2020).
2. Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. (2020) 153:420–1. doi: 10.1093/ajcp/aqaa029
3. Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. (2020) 20:e238–44. doi: 10.1016/S1473-3099(20)30484-9
4. Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. (2020) 26:729–34. doi: 10.1016/j.cmi.2020.03.026
5. Chen CC, Chi CY. Biosafety in the preparation and processing of cytology specimens with potential coronavirus (COVID-19) infection: perspectives from Taiwan. Cancer Cytopathol. (2020) 128:309–16. doi: 10.1002/cncy.22280
6. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382:1564–7. doi: 10.1056/NEJMc2004973
7. Zhou P, Huang Z, Xiao Y, Huang X, Fan XG. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. (2020) 41:745–6. doi: 10.1017/ice.2020.60
8. Wang YH, Bychkov A, Chakrabarti I, Jain D, Liu Z, He S, et al. Impact of the COVID-19 pandemic on cytology practice: an international survey in the Asia-Pacific region. Cancer Cytopathol. (2020). 128:895-904. doi: 10.1002/cncy.22354
9. Chen CC, Chi CY. Reply to Rapid on-site evaluation and the COVID-19 pandemic. Cancer Cytopathol. (2020) 128:910–2. doi: 10.1002/cncy.22296
10. Román GC, Spencer PS, Reis J, Buguet A, Faris MEA, Katrak SM, et al. The neurology of COVID-19 revisited: a proposal from the environmental neurology specialty group of the world federation of neurology to implement international neurological registries. J Neurol Sci. (2020) 414:116884. doi: 10.1016/j.jns.2020.116884
11. Zhou Y, Li W, Wang D, Mao L, Jin H, Li Y, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. (2020) 5:177–9. doi: 10.1136/svn-2020-000398
12. Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. (2020) 24:1–9. doi: 10.1038/s41582-020-0398-3
13. Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. (2020) 183:16–27.e1. doi: 10.1016/j.cell.2020.08.028
14. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. (2020) 19:767–83. doi: 10.1016/S1474-4422(20)30221-0
15. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. lan with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
16. Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. (2020) 11:806. doi: 10.3389/fneur.2020.00806
17. Fadakar N, Ghaemmaghami S, Masoompour SM, Shirazi Yeganeh B, Akbari A, Hooshmandi S, et al. A first case of acute cerebellitis associated with Coronavirus Disease (COVID-19): a case report and literature review. Cerebellum. (2020) 19:911–4. doi: 10.1007/s12311-020-01177-9
18. Katyal N, Narula N, Acharya S, Govindarajan R. Neuromuscular complications with SARS-COV-2 infection: a review. Front Neurol. (2020) 11:1052. doi: 10.3389/fneur.2020.01052
19. Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. (2020) 7:875–82. doi: 10.2139/ssrn.3601761
20. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. (2020) 87:18–22. doi: 10.1016/j.bbi.2020.03.031
21. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. (2005) 14:113–9. doi: 10.29819/ANT.200509.0002
22. Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. (2004) 61:1669–73. doi: 10.1001/archneur.61.11.1669
23. Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. (2014) 194:145–58. doi: 10.1016/j.virusres.2014.09.011
24. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. (2016) 14:523–34. doi: 10.1038/nrmicro.2016.81
25. Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. (2015) 43:495–501. doi: 10.1007/s15010-015-0720-y
26. Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. (2016) 2016:3502683. doi: 10.1155/2016/3502683
27. Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. (2017) 13:227–33. doi: 10.3988/jcn.2017.13.3.227
28. Panciani PP, Saraceno G, Zanin L, Renisi G, Signorini L, Battaglia L, et al. SARS-CoV-2: “Three-steps” infection model and CSF diagnostic implication. Brain Behav Immun. (2020) 87:128–9. doi: 10.1016/j.bbi.2020.05.002
29. Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci. (2020) 21:5475. doi: 10.3390/ijms21155475
30. Sharifian-Dorche M, Huot P, Osherov M, Wen D, Saveriano A, Giacomini PS, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. (2020) 417:117085. doi: 10.1016/j.jns.2020.117085
31. Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. (2020) 13:e236720. doi: 10.1136/bcr-2020-236720
32. Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R, Krämer S, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. (2020) 267:2196–7. doi: 10.1007/s00415-020-09934-w
33. Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. (2020) 62:E57–60. doi: 10.1002/mus.27003
34. Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. (2020) 41:3039–56. doi: 10.1007/s10072-020-04708-8
35. Solomon T, Hart IJ, Beeching NJ. Viral encephalitis: a clinician's guide. Pract Neurol. (2007) 7:288–305. doi: 10.1136/jnnp.2007.129098
36. Joyce NC, Oskarsson B, Jin LW. Muscle biopsy evaluation in neuromuscular disorders. Phys Med Rehabil Clin N Am. (2012) 23:609–31. doi: 10.1016/j.pmr.2012.06.006
37. World Health Organization. Infection Prevention and Control During Health Care When Novel Coronavirus (nCoV) Infection Is Suspected. Interim Guidance, 19 March 2020. (2020). Available online at: https://www.who.int/publications/i/item/10665-331495 (accessed September 27, 2020).
38. Ozoner B, Gungor A, Hasanov T, Toktas ZO, Kilic T. Neurosurgical practice during coronavirus disease 2019 (COVID-19) pandemic. World Neurosurg. (2020) 140:198–207. doi: 10.1016/j.wneu.2020.05.195
39. Fraser JF, Arthur AS, Chen M, Levitt M, Mocco J, Albuquerque FC, et al. Society of neurointerventional surgery recommendations for the care of emergent neurointerventional patients in the setting of COVID-19. J Neurointerv Surg. (2020) 12:539–41. doi: 10.1136/neurintsurg-2020-016098
40. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. (2020) 104:246–51. doi: 10.1016/j.jhin.2020.01.022
41. World Health Organization. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases. Interim Guidance, 13 May 2020. (2020). Available online at: https://www.who.int/publications/i/item/10665-331501 (accessed September 27, 2020).
42. World Health Organization. Laboratory Biosafety Guidance Related to Coronavirus Disease (COVID-19). Interim Guidance, 13 May 2020. (2020). Available online at: https://www.who.int/publications/i/item/laboratory-biosafety-guidance-related-to-coronavirus-disease-(covid-19) (accessed September 27, 2020).
43. Centers for Disease Control and Prevention. Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19). (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html (accessed September 27, 2020).
44. Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. (2003) 49:2108–09. doi: 10.1373/clinchem.2003.025437
45. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. (2004)10:342–44. doi: 10.3201/eid1002.030638
46. Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. (2005) 41:1089–96. doi: 10.1086/444461
47. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
48. Cebrián J, Gonzalez-Martinez A, García-Blanco MJ, Celdrán-Vivancos D, Palacios EL, Reig-Roselló G, et al. Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: a case report. Neurology. (2020) 95:266–8. doi: 10.1212/WNL.0000000000010213
49. Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. (2020) 20:1–3. doi: 10.21203/rs.3.rs-31801/v1
50. El-Zein RS, Cardinali S, Murphy C, Keeling T. COVID-19-associated meningoencephalitis treated with intravenous immunoglobulin. BMJ Case Rep. (2020) 13:e237364. doi: 10.1136/bcr-2020-237364
51. Lu S, Wei N, Jiang J, Wu L, Sheng J, Zhou J, et al. First report of manic-like symptoms in a COVID-19 patient with no previous history of a psychiatric disorder. J Affect Disord. (2020) 277:337–40. doi: 10.1016/j.jad.2020.08.031
52. Vandervorst F, Guldolf K, Peeters I, Vanderhasselt T, Michiels K, Berends KJ, et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip Neurosurg. (2020) 22:100821. doi: 10.1016/j.inat.2020.100821
53. Sun T, Guan J. Novel coronavirus and the central nervous system. Eur J Neurol. (2020) 27:e52. doi: 10.1111/ene.14227
54. Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. (2020) 91:846–8. doi: 10.1136/jnnp-2020-323522
55. Bodro M, Compta Y, Llansó L, Esteller D, Doncel-Moriano A, Mesa A, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e821. doi: 10.1212/NXI.0000000000000821
56. Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. (2020) 26:2016–21. doi: 10.3201/eid2609.202122
57. Delorme C, Paccoud O, Kas A, Hesters A, Bombois S, Shambrook P, et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. (2020) 27:2651–7. doi: 10.1111/ene.14478
58. Neumann B, Schmidbauer ML, Dimitriadis K, Otto S, Knier B, Niesen WD, et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci. (2020) 418:117090. doi: 10.1016/j.jns.2020.117090
59. Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. (2020). doi: 10.1007/s00415-020-10108-x. [Epub ahead of print].
60. Uncini A, Vallat JM, Jacobs BC. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. (2020) 91:1105–10. doi: 10.1136/jnnp-2020-324491
61. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. (2020). doi: 10.1007/s00415-020-10124-x. [Epub ahead of print].
62. Espíndola OM, Siqueira M, Soares CN, Lima MASD, Leite ACCB, Araujo AQC, et al. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. (2020) 96:567–9. doi: 10.1016/j.ijid.2020.05.123
63. Miller EH, Namale VS, Kim C, Dugue R, Waldrop G, Ciryam P, et al. Cerebrospinal analysis in patients with COVID-19. Open Forum Infect Dis. (2020) 7:ofaa501. doi: 10.1093/ofid/ofaa501
64. Edén A, Kanberg N, Gostner J, Fuchs D, Hagberg L, Andersson LM, et al. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. (2020). doi: 10.1212/WNL.0000000000010977. [Epub ahead of print].
65. Khodamoradi Z, Hosseini SA, Gholampoor Saadi MH, Mehrabi Z, Sasani MR, Yaghoubi S. COVID-19 meningitis without pulmonary involvement with positive cerebrospinal fluid PCR. Eur J Neurol. (2020) 27:2668–9. doi: 10.1111/ene.14536
Keywords: COVID-19, CSF, neurology, coronavirus, cytology, biosafety, myopathy, neuromuscular
Citation: Chen C-C, Chiang P-C and Chen T-H (2021) The Biosafety and Risk Management in Preparation and Processing of Cerebrospinal Fluid and Other Neurological Specimens With Potential Coronavirus Infection. Front. Neurol. 11:613552. doi: 10.3389/fneur.2020.613552
Received: 05 October 2020; Accepted: 24 December 2020;
Published: 20 January 2021.
Edited by:
Cheng-Yang Hsieh, Sin-Lau Christian Hospital, TaiwanReviewed by:
Junpei Koge, National Cerebral and Cardiovascular Center, JapanCopyright © 2021 Chen, Chiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Chin Chen, aGxtYXJrY0BnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.