
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 12 January 2021
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.610318
 Mengke Tian1,2
Mengke Tian1,2 Youfeng Li1,2
Youfeng Li1,2 Xiao Wang1,2
Xiao Wang1,2 Xuan Tian1,2
Xuan Tian1,2 Lu-lu Pei1,2
Lu-lu Pei1,2 Xin Wang1,2
Xin Wang1,2 Luyang Zhang1,2
Luyang Zhang1,2 Wenxian Sun1,2
Wenxian Sun1,2 Jun Wu1,2
Jun Wu1,2 Shilei Sun1,2
Shilei Sun1,2 Mingming Ning3
Mingming Ning3 Ferdinando Buonanno3
Ferdinando Buonanno3 Yuming Xu1,2
Yuming Xu1,2 Bo Song1,2*
Bo Song1,2*Background: The combined index of hemoglobin, albumin, lymphocyte, and platelet (HALP) is considered a novel score to reflect systemic inflammation and nutritional status. This study aimed to investigate the association between HALP score and poor outcome in patients with acute ischemic stroke (AIS).
Methods: Consecutive AIS patients within 24 h after onset were prospectively enrolled. Poor outcome was a combination of a new stroke event (ischemic and hemorrhagic) and all-cause death within 90 days and 1 year. The association between HALP score and poor outcome was analyzed using Cox proportional hazards.
Results: A total of 1,337 patients were included. Overall, 60 (4.5%) and 118 (8.8%) patients experienced poor outcome within 90 days and 1 year, respectively. Patients in the highest tertile of HALP score had a lower risk of poor outcome within 90 days and 1 year (hazard ratio: 0.25 and 0.42; 95% confidence intervals: 0.11–0.57 and 0.25–0.69, P for trend <0.01 for all) compared with those in the lowest tertile after adjusting relevant confounding factors. Adding HALP score to the conventional risk factors improved prediction of poor outcome in patients with AIS within 90 days and 1 year (net reclassification index, 48.38 and 28.95%; integrated discrimination improvement, 1.51 and 1.51%; P < 0.05 for all).
Conclusions: Increased HALP score was associated with a decreased risk of recurrent stroke and death within 90 days and 1 year after stroke onset, suggesting that HALP score may serve as a powerful indicator for AIS.
Stroke is a leading cause of death and long-term disability worldwide, with ischemic stroke as the most common subtype (1). Exploring new predictors of stroke prognosis may help in early identification of high-risk patients with poor outcome and contribute to more effective secondary prevention. Inflammation, abnormal blood coagulation, and poor nutritional status are associated with poor prognosis of acute ischemic stroke (AIS) (2–4). Lymphocytes have key regulatory functions in post-stroke inflammation (5). Platelet hyperactivity increases the risk of thromboembolism and atherosclerotic lesions and may lead to abnormal thrombosis, which exacerbates inflammation response (6). Anemia and hypoalbuminemia are manifestations of poor nutritional status and have been identified as risk factors for cerebrovascular events that may lead to poor prognosis in patients with AIS (7, 8). In this study, we explored the association between the combination of these four common indicators and the prognosis of AIS.
The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is considered to be an easily calculated marker of systemic inflammation and nutritional status (9, 10) and has been found as a significant prognostic factor in patients with multiple tumors (11–13). However, it is unknown whether HALP score is associated with the poor outcome of AIS. Therefore, our study aimed to investigate the predictive value of HALP score in a prospective cohort of patients with AIS.
The study was a prospective consecutive hospital-based cohort study. We enrolled patients with AIS within 24 h of onset from the Ischemic Cerebrovascular Disease Database of the First Affiliated Hospital of Zhengzhou University from January 2015 to June 2018, which has been published previously (14, 15). The diagnosis of AIS was based on the criteria of the World Health Organization (WHO) (16). The database was approved by the Ethics Committee, and informed consent forms were obtained from all patients or their relatives.
The exclusion criteria were as follows: (i) patients with active or chronic inflammatory disease; (ii) neoplastic hematologic disorders or using immunosuppressant drugs; (iii) major trauma or surgery within 3 months; (iv) patients without blood cell count data; and (v) severe liver and kidney dysfunction.
Baseline clinical information was obtained from case report forms at admission, including demographic characteristics, medical histories, imaging features, and medication use. Clinical factors, including baseline stroke severity, the use of intravenous thrombolysis therapy, and stroke subtype, were also evaluated. The severity of AIS was assessed according to the National Institutes of Health Stroke Scale (NIHSS) at admission. Stroke subtypes were classified according to the Trial of Org 10172 in the Acute Stroke Treatment classification.
Laboratory tests were obtained within 24 h of admission after stroke onset, including the serum albumin, hemoglobin, lymphocyte, and platelet levels. The blood counts were analyzed using an autoanalyzer (Beckman Coulter Hematology Analyzer LH750, USA). All the serum biochemical parameters were assayed using an automatic biochemical analyzer (Roche COBAS 8000 automatic biochemical analyzer). The HALP score was calculated according to the following formula: hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)/platelets (/L).
The poor outcome was a combination of a new stroke event (ischemic and hemorrhagic) and all-cause death within 90 days and 1 year. Patients enrolled were followed up by face-to-face or telephone interviews at 90 days and 1 year after stroke onset.
Continuous variables with normal distribution were expressed as mean ± standard deviation (SD), and skewed distribution were expressed as medians with interquartile ranges (IQRs). Categorical variables were expressed as frequencies (n) and percentages (%). Patients were classified into three groups based on the HALP score tertiles. Tests for linear trend of baseline characteristics across HALP score tertiles were performed using ANOVA for continuous variables and χ2 trend analysis for categorical variables. The cumulative incidence risks of poor outcome across baseline HALP score were calculated with Kaplan–Meier curves and compared by log-rank test. Multivariate Cox proportional hazards models were used to assess the risk of poor outcome with HALP score tertiles. Unadjusted and adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated for the two higher HALP score groups (with the lowest group serving as a reference) and for a 1-SD increment of HALP score. Optimal HALP score cutoff values were obtained using receiver operating characteristic (ROC) curve analysis. C statistics, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were used to evaluate the incremental predictive ability of HALP score beyond the conventional model. Some conventional risk factors were selected as potential covariates for poor outcome of ischemic stroke based on prior knowledge. In addition, we used restricted cubic splines to evaluate the shape of the association between HALP score and outcomes with four knots defined at the fifth, 35, 65, and 95th percentiles of HALP score. Statistical analysis was performed using IBM SPSS software version 24.0 (SPSS, Inc., Chicago, IL, USA) and R (version 3.5.0). A two-tailed value of P < 0.05 was considered statistically significant.
A total of 1,337 patients were included in our analysis (Figure 1). The mean age of the patients was 60.55 years, and 30.5% of them were female. The study population was divided into three tertiles based on the HALP score value (Table 1). Compared with the higher HALP score patients, those with a lower HALP score were more likely to be older; be female; have atrial fibrillation; have lower lymphocyte, hemoglobin, and albumin levels; and have higher platelet counts, frequencies of cardioembolic stroke, and baseline NIHSS scores.
After 90 days of follow-up, 60 (4.5%) patients were lost to follow-up and 60 (4.5%) patients experienced poor outcome. At the 1-year follow-up, 92 (6.9%) patients were lost and 118 (8.8%) patients experienced poor outcome (Figure 1). All Kaplan–Meier curves showed that patients in the lowest tertile of HALP score (<36.65) had the highest cumulative incidence of poor outcome within 90 days and at 1 year (log-rank P < 0.05 for all; Figure 2).
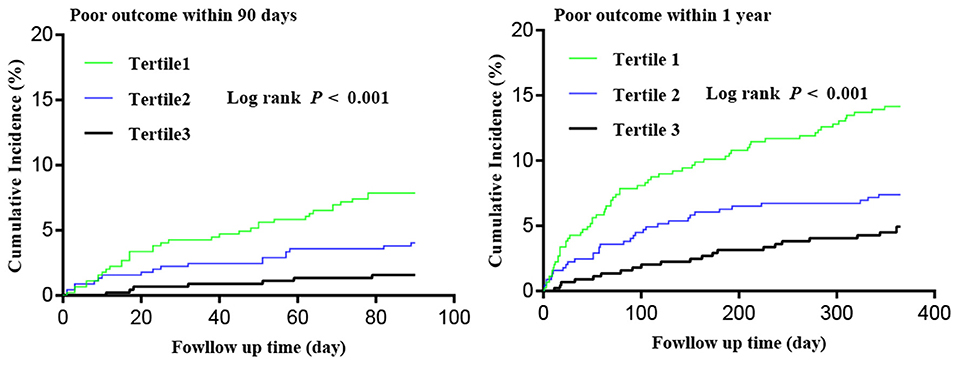
Figure 2. Kaplan–Meier curves of cumulative incidence (%) of poor outcome by tertiles of hemoglobin, albumin, lymphocyte, and platelet (HALP) score at 90-day and 1-year follow-up.
After adjustment for age, sex, history of hypertension, diabetes, ischemic stroke, coronary heart disease, atrial fibrillation, smoking, time from onset to blood sampling, IV thrombolysis treatment and baseline NIHSS score, higher levels of HALP score were associated with decreased risk of poor outcome within 90 days and 1 year (Table 2). The adjusted HRs (95% confidence intervals) for the highest vs. lowest tertile of HALP score were 0.25 (0.11–0.57) for poor outcome at 90 days and 0.42 (0.25–0.69) at 1 year. Similarly, on continuous analyses, each 1-SD increase of the HALP score was associated with 90-day and 1-year poor outcome (Table 2). ROC curve analysis of HALP score and poor outcome at 90-day and 1-year follow-up indicated that optimal cutoff values for HALP score were 33.82 and 32.77, respectively. The sensitivity and specificity were 53.3 and 75.9% (C statistic: 0.69, 95% CI: 0.66–0.71, P < 0.001) for 90-day poor outcome, and for 1-year poor outcome, they were 49.2 and 74.3% (C statistic: 0.65, 95% CI: 0.62–0.67, P < 0.001).
In addition, multivariable-adjusted spline regression models showed J-shaped associations between HALP score and the risk of poor outcome within 90 days and 1 year (Figure 3).
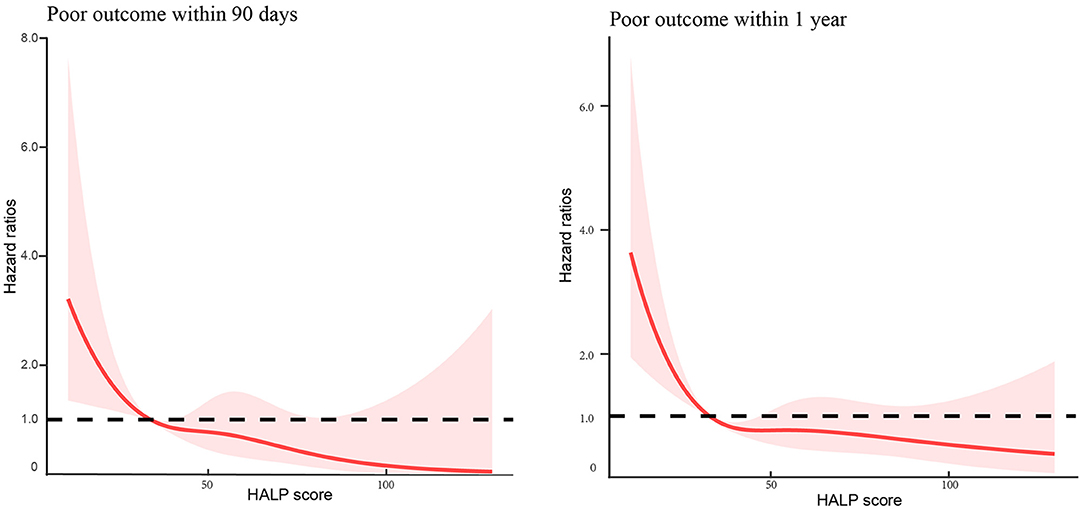
Figure 3. Association of hemoglobin, albumin, lymphocyte, and platelet (HALP) score with the risk of poor outcome among patients with acute ischemic stroke at 90-day and 1-year follow-up.
Adding HALP score to a multivariable model, which included age, sex, history of hypertension, diabetes, ischemic stroke, coronary heart disease, atrial fibrillation, smoking, intravenous (IV) thrombolysis treatment, and baseline NIHSS scores, significantly improved its predictive ability (Table 3). The continuous net reclassification improvement was 48.38% (P < 0.001) and 28.95% (P = 0.002) for 90-day and 1-year poor outcome, respectively. IDI was 1.51% (P = 0.027) and 1.51% (P < 0.001) for 90-day and 1-year poor outcome, respectively.
We explored the prognostic value of the novel index HALP score in patients with AIS in this prospective cohort study and found that higher levels of HALP score at admission were highly associated with a decreased risk of recurrent stroke and death within 90 days and 1 year. Furthermore, we demonstrated that the addition of HALP score to the conventional risk factors could increase the discriminatory power and risk reclassification for recurrent stroke and death. These results suggested that decreased HALP score could be an independent risk factor of recurrent stroke and death.
Ischemic stroke initiates with gradual or sudden cerebral hypoperfusion, including oxidative stress, hemostatic activation, and inflammation, and eventually leads to a corresponding loss of neurological function (17). Ischemic brain tissue activates leukocytes and promotes their migration to the ischemic site by releasing pro-inflammatory chemokines (18). The inflammation triggers the process of thrombosis, in which platelets participate in adhesion, release reaction, and aggregation (6). Studies have shown that platelet count may be a qualified predictor for long-term recurrent stroke, mortality, and poor functional outcome (19). Lymphocytes play an important role in the elimination and repair of inflammation; lower lymphocyte counts were associated with increased infarct volume, neurological deterioration, and poor prognosis after ischemic stroke (20). Albumin has a neuroprotective effect because of its antagonism of oxidation, stagnant, thrombosis, and leukocyte adhesion (21, 22). Serum albumin levels can predict the prognosis of ischemic stroke (23). Hemoglobin has oxygen-carrying capacity and can affect the energy balance in the penumbra (24). There is a U-shaped association between hemoglobin and poor prognosis of ischemic stroke (25). Studies have shown that a high hemoglobin concentration is associated with carotid atherosclerosis and may be a risk factor for ischemic stroke (26). Similarly, low hemoglobin and hematocrit levels are strongly associated with poor outcome and mortality after AIS (27). Further, serum albumin and hemoglobin concentrations have a predictive value for stroke recurrence and combined events (28, 29). The new indicator HALP score is based on the combination of the four hematological parameters mentioned above.
Recent studies have shown that the HALP score can reflect the inflammation–nutritional status of patients (9, 30) and has been proved to be an important prognostic indicator for patients with multiple tumors (10–12). Anemia and thrombosis could exacerbate inflammation, while lymphocytes reduce inflammation (26). Serum albumin has been considered an indicator of nutritional status. Some studies have also suggested that albumin reflects the severity of inflammation and illness in acute diseases (31). It is widely accepted that the inflammatory response and nutritional status are correlated with the prognosis of patients with stroke (4, 32, 33). HALP score is obtained by hemoglobin (g/L) × albumin (g/L) × lymphocytes (/L)/platelets (/L), which makes it a cost-effective, simple parameter to easily assess the inflammation–nutritional status. This finding may be significant because instant inflammation–nutritional status assessment can help clinicians assess prognosis and formulate appropriate treatment plans.
There were some limitations to this study. First, this was a single-center prospective study. Although we included patients in three branches, there was still a possibility of selection bias. Second, the potential influence of concomitant medications, such as thrombolytic therapy, oral anti-platelet, and statins, were not considered. Third, we only obtained the hemoglobin count, albumin levels, lymphocyte count, and platelet count at admission but did not present the dynamic change of HALP score at different stages. Therefore, multicenter cohort studies are still needed to validate the findings.
Our study indicated an association between HALP score and the risk of recurrent stroke and death within 90 days and 1 year, suggesting that HALP score at admission may act as a powerful indicator of recurrent stroke and death in patients with AIS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
BS and YX designed the overall study with contributions from MN, FB, JW, and SS. MT designed and carried out experiments and collected data with YL, XiaW, XT, L-lP, XinW, LZ, and WS. MT wrote the manuscript. BS supervised this study, designed experiments, and edited the paper. All authors critically reviewed the manuscript and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81571158) and the Education Department of Henan Province (16A320054 and 172102310086).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the study participants and the clinical staff for their support and contribution to this study. This manuscript has been released as a preprint at Research Square (34).
1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38-360. doi: 10.1161/CIR.0000000000000350
2. Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. (2016) 12:594–604. doi: 10.1038/nrneurol.2016.125
3. de Lau LM, Leebeek FW, de Maat MP, Koudstaal PJ, Dippel DW. A review of hereditary and acquired coagulation disorders in the aetiology of ischaemic stroke. Int J Stroke. (2010) 5:385–94. doi: 10.1111/j.1747-4949.2010.00468.x
4. Collaboration. FT. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. (2003) 34:1450–6. doi: 10.1161/01.STR.0000074037.49197.8C
5. Baird AE. The forgotten lymphocyte: immunity and stroke. Circulation. (2006) 113:2035–6. doi: 10.1161/CIRCULATIONAHA.105.620732
6. Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, Farndale RW, et al. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol. (2010) 55:1147–58. doi: 10.1016/j.jacc.2009.11.051
7. Milionis H, Papavasileiou V, Eskandari A, D'Ambrogio-Remillard S, Ntaios G, Michel P. Anemia on admission predicts short- and long-term outcomes in patients with acute ischemic stroke. Int J Stroke. (2015) 10:224–30. doi: 10.1111/ijs.12397
8. Tanne D, Molshatzki N, Merzeliak O, Tsabari R, Toashi M, Schwammenthal Y. Anemia status, hemoglobin concentration and outcome after acute stroke: a cohort study. BMC Neurol. (2010) 10:22. doi: 10.1186/1471-2377-10-22
9. Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. (2020) 26:828–38. doi: 10.3748/wjg.v26.i8.828
10. Shen XB, Zhang YX, Wang W, Pan YY. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. (2019) 25:5630–9. doi: 10.12659/MSM.917968
11. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2017) 46:75–9. doi: 10.1016/j.intimp.2017.02.027
12. Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. (2018) 8:794. doi: 10.1038/s41598-018-19146-y
13. Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. (2019) 10:81–91. doi: 10.7150/jca.27210
14. Song B, Hu R, Pei L, Cao Y, Chen P, Sun S, et al. Dual antiplatelet therapy reduced stroke risk in high-risk patients with transient ischaemic attack assessed by ABCD3-I score. Eur J Neurol. (2019) 26:610–6. doi: 10.1111/ene.13864
15. Zhao L, Wang R, Song B, Tan S, Gao Y, Fang H, et al. Association between atherogenic dyslipidemia and recurrent stroke risk in patients with different subtypes of ischemic stroke. Int J Stroke. (2015) 10:752–8. doi: 10.1111/ijs.12471
16. Stroke−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
17. Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. (2009) 111:483–95. doi: 10.1016/j.clineuro.2009.04.001
18. Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. (2016) 25:241–51. doi: 10.5607/en.2016.25.5.241
19. Yang M, Pan Y, Li Z, Yan H, Zhao X, Liu L, et al. Platelet count predicts adverse clinical outcomes after ischemic stroke or TIA: subgroup analysis of CNSR II. Front Neurol. (2019) 10:370. doi: 10.3389/fneur.2019.00370
20. Kim J, Song TJ, Park JH, Lee HS, Nam CM, Nam HS, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
21. Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. (2013) 3:4. doi: 10.1186/2110-5820-3-4
22. Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. (2013) 132:69–76. doi: 10.1016/j.thromres.2013.04.018
23. Dziedzic T, Slowik A, Szczudlik A. Serum albumin level as a predictor of ischemic stroke outcome. Stroke. (2004) 35:e156–8. doi: 10.1161/01.STR.0000126609.18735.be
24. Kimberly WT, Lima FO, O'Connor S, Furie KL. Sex differences and hemoglobin levels in relation to stroke outcomes. Neurology. (2013) 80:719–24. doi: 10.1212/WNL.0b013e31828250ff
25. Sacco S, Marini C, Olivieri L, Pistoia F, Carolei A. Contribution of hematocrit to early mortality after ischemic stroke. European neurology. (2007) 58:233–8. doi: 10.1159/000107946
26. Barlas RS, Honney K, Loke YK, McCall SJ, Bettencourt-Silva JH, Clark AB, et al. Impact of hemoglobin levels and anemia on mortality in acute stroke: analysis of UK Regional Registry Data, Systematic Review, and Meta-Analysis. J Am Heart Assoc. (2016) 5:e003019. doi: 10.1161/JAHA.115.003019
27. Kellert L, Martin E, Sykora M, Bauer H, Gussmann P, Diedler J, et al. Cerebral oxygen transport failure?: decreasing hemoglobin and hematocrit levels after ischemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of hemoGlobin, Hematocrit and Transfusion (STRAIGHT)–an observational study. Stroke. (2011) 42:2832–7. doi: 10.1161/STROKEAHA.110.606665
28. Zhang Q, Lei YX, Wang Q, Jin YP, Fu RL, Geng HH, et al. Serum albumin level is associated with the recurrence of acute ischemic stroke. Am J Emerg Med. (2016) 34:1812–6. doi: 10.1016/j.ajem.2016.06.049
29. Chang JY, Lee JS, Kim BJ, Kim JT, Lee J, Cha JK, et al. Influence of hemoglobin concentration on stroke recurrence and composite vascular events. Stroke. (2020) 51:1309–12. doi: 10.1161/STROKEAHA.119.028058
30. Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. (2018) 18:20. doi: 10.1186/s12894-018-0333-8
31. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. (2019) 133:713–22.e7. doi: 10.1016/j.amjmed.2019.10.031
32. Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nat Rev Neurol. (2015) 11:524–35. doi: 10.1038/nrneurol.2015.144
33. Martineau J, Bauer JD, Isenring E, Cohen S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin Nutr. (2005) 24:1073–7. doi: 10.1016/j.clnu.2005.08.010
Keywords: acute ischemic stroke, cohort study, HALP score, outcome, risk factors
Citation: Tian M, Li Y, Wang X, Tian X, Pei L-l, Wang X, Zhang L, Sun W, Wu J, Sun S, Ning M, Buonanno F, Xu Y and Song B (2021) The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score Is Associated With Poor Outcome of Acute Ischemic Stroke. Front. Neurol. 11:610318. doi: 10.3389/fneur.2020.610318
Received: 25 September 2020; Accepted: 23 November 2020;
Published: 12 January 2021.
Edited by:
Mira Katan, University Hospital Zürich, SwitzerlandReviewed by:
Simona Sacco, University of L'Aquila, ItalyCopyright © 2021 Tian, Li, Wang, Tian, Pei, Wang, Zhang, Sun, Wu, Sun, Ning, Buonanno, Xu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Song, ZmNjc29uZ2JAenp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.