
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 15 January 2021
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.608000
This article is part of the Research TopicMoyamoya DiseaseView all 12 articles
 Fei Ye1,2†
Fei Ye1,2† Jiaoxing Li1†
Jiaoxing Li1† Tianzhu Wang3,4†
Tianzhu Wang3,4† Kai Lan5,6†
Kai Lan5,6† Haiyan Li7
Haiyan Li7 Haoyuan Yin8
Haoyuan Yin8 Tongli Guo9
Tongli Guo9 Xiong Zhang10
Xiong Zhang10 Tingting Yang11
Tingting Yang11 Jie Liang1,2
Jie Liang1,2 Xiaoxin Wu1
Xiaoxin Wu1 Qi Li3,4*
Qi Li3,4* Wenli Sheng1,2*
Wenli Sheng1,2*Background: The use of antiplatelet agents in ischemic moyamoya disease (MMD) is controversial. This study aimed to investigate the effectiveness and safety of antiplatelet therapy compared with conservative treatment and surgical revascularization in ischemic MMD patients.
Methods: Ischemic MMD patients were retrospectively enrolled from eight clinical sites from January 2013 to December 2018. Follow-up was performed through clinical visits and/or telephone interviews from first discharge to December 2019. The primary outcome was the episodes of further ischemic attacks, and the secondary outcome was the individual functional status. Risk factors for future stroke were identified by the LASSO-Cox regression model. Propensity score matching was applied to assemble a cohort of patients with similar baseline characteristics using the TriMatch package.
Results: Among 217 eligible patients, 159 patients were included in the analyses after a 1:1:1 propensity score matching. At a mean follow-up of 33 months, 12 patients (7.5%) developed further incident cerebral ischemic events (surgical:antiplatelet:conservative = 1:3:8; p = 0.030), 26 patients (16.4%) developed a poor functional status (surgical:antiplatelet:conservative = 7:12:7; p = 0.317), and 3 patients (1.8%) died of cerebral hemorrhage (surgical:antiplatelet:conservative = 1:2:0; p = 0.361). The survival curve showed that the risk of further cerebral ischemic attacks was lowest with surgical revascularization, while antiplatelet therapy was statistically significant for preventing recurrent risks compared with conservative treatment (χ2 = 8.987; p = 0.011). No significant difference was found in the functional status and bleeding events. The LASSO-Cox regression model revealed that a family history of MMD (HR = 6.93; 95% CI: 1.28–37.52; p = 0.025), a past history of stroke or transient ischemic attack (HR = 4.35; 95% CI: 1.09–17.33; p = 0.037), and treatment (HR = 0.05; 95% CI: 0.01–0.32; p = 0.001) were significantly related to the risk of recurrent strokes.
Conclusions: Antiplatelet agents were effective and safe in preventing further cerebral ischemic attacks in adult patients with ischemic MMD. They may be a replacement therapy for patients with surgical contraindications and for patients prior to revascularization.
Moyamoya disease (MMD) is an unusual cerebrovascular disease characterized by progressive steno-occlusive changes in the distal internal carotid artery (ICA) and its major branches with the development of an abnormal vascular network at the base of the brain visible on angiography (1). The Asian population has a bimodal age of presentation and is classified into two major categories: ischemic type and hemorrhagic type (2). Although current evidence has demonstrated the epidemiological and clinical characteristics of MMD, the cause of this disease is not well-known (3–8). Currently, revascularization surgery is recommended as a standard treatment for MMD patients to prevent future strokes (9). However, there are still many MMD patients who are not willing to undergo revascularization or have surgical contraindications. Consequently, it is necessary to identify a replacement therapy.
Antiplatelet agents are considered to be a vital tool in acute ischemic stroke and transient ischemic attack (TIA) management for reducing and preventing recurrent stroke risks (10, 11). To date, limited reports have focused on antiplatelet therapy for MMD, and no randomized controlled trial has suggested a lack of strongly supporting evidence indicating the benefits of this therapy. In addition, there is still controversy regarding the use of antiplatelet agents among ischemic MMD patients. Many non-Asian experts advise their use for improving microcirculation and preventing recurrent strokes, while most Asian physicians hold the opposite view, namely, that they are useless in improving blood supply and carry the potential risk of hemorrhage (12, 13). Thus, we conducted this study to evaluate the effectiveness and safety outcomes of treatment with antiplatelet agents compared with conservative treatment and surgical revascularization in ischemic MMD patients.
This study was a multicenter retrospective cohort study involving adult patients with ischemic MMD who took an oral antiplatelet agent, underwent surgical revascularization, and received conservative management between January 2013 and December 2018 in eight Chinese teaching hospitals. The study was approved by the independent ethics committee of each participating teaching hospital [(2020)137]. Patient informed consent was waived due to the use of deidentified data by the clinical research review board. This study was carried out on the basis of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (14).
Patients with a clinical diagnosis of MMD were identified from the national health care system using the diagnosis-specific codes by the department of medical records in each participating hospital, including International Classification of Disease Ninth revision (ICD-9) code 437.5 and ICD-10 code I67.5. Cerebral angiography was carried out for all the patients. The diagnostic criteria for definitive or probable MMD were based on the 2012 Tokyo guideline (9, 15): (1) stenosis and/or occlusion of the terminal portion of the ICA and the proximal portion of the anterior cerebral artery (ACA) and/or middle cerebral artery (MCA), (2) abnormal vascular networks near the lesion, and (3) unilateral or bilateral involvement. Patients were eligible for inclusion in this study if they met the following criteria: (1) aged 18 or older, (2) clinical presentations of TIA or cerebral infarction, (3) no history of prior antiplatelet agents or neurosurgery, and (4) complete clinical data available. Patients who were diagnosed with moyamoya syndrome, were lost to follow-up, refused to participate, died before discharge, or had malignant tumors were excluded.
Electronic medical records were carefully reviewed, including hospital charts, clinic notes, operative notes, radiographic data, and therapeutic medications. All the clinical data were retrospectively collected by the data coordinators at each participating hospital in December 2019. The baseline characteristics mainly included age at symptom onset, age at diagnosis, sex, vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, active smoking, and alcohol consumption), a past history of stroke or TIA, a family history of MMD, a modified Rankin Scale (mRS) score (16), angiography findings (bilateral steno-occlusive change, Suzuki classification, and intracranial aneurysm), and clinical manifestation (TIA, lacunar infarction, and cerebral infarction). In this study, if a patient presented with an initial symptom of TIA, we did not add it to the past history of TIA. Although the RNF213 gene polymorphism and cerebral perfusion changes can influence the prognosis, these factors were excluded because they are not routine examinations in clinical practice (17, 18).
There are currently no clear guidelines for surgical revascularization in the treatment of MMD, but it holds a comparatively higher level of evidence than non-surgical treatment (9). Thus, randomized assignment is not applicable and unethical in clinical practice. In this study, surgical revascularization was recommended to patients with a non-emergency status, markedly ischemic symptoms, and no surgical contraindications, while non-surgical treatment was recommended to patients with mild ischemic symptoms and/or surgical contraindications. The treatment choice was independently determined by patients themselves based on their obtained medical information about the benefits and risks of different treatments. Treatments were classified into antiplatelet therapy, conservative treatment, and surgical revascularization. The treatment for patients who received antiplatelet therapy included oral aspirin 100 mg daily, oral clopidogrel 75 mg daily, and combined aspirin and clopidogrel for the first 3 weeks, followed by aspirin daily. The treatment for patients who underwent surgical revascularization procedures mainly includes direct bypass, indirect bypass, and combined bypass. Patients who had undergone surgical treatment but not surgical revascularization were excluded in this study. The conservative treatment group also included untreated participants and participants who received other medical management.
The follow-up was performed through clinical visits and/or telephone interviews from first discharge to December 2019. If patients could not be contacted in December 2019, the follow-up was determined from their last clinical visit. In this study, the primary outcome was episodes of further ischemic attacks. It was defined as an acute focal infarction of the brain or retina, including sudden onset of a new focal neurological deficit lasting 24 h or more with clinical and/or imaging evidence of infarction, sudden onset of a new focal neurological deficit lasting <24 h, or rapid worsening of an existing focal neurological deficit lasting more than 24 h but accompanied by new ischemic changes on MRI or CT of the brain (19). The secondary outcome was the individual functional status assessed by the mRS score. mRS scores ≤ 2 were regarded as a good outcome, and scores >2 were regarded as a poor outcome. The safety outcome was evaluated according to episodes of serious bleeding events based on the definition of the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) (20). The follow-up period was defined as the time from initial discharge to the main outcome or December 2019. All reported clinical outcomes were confirmed by data coordinators who were unaware of the study-group assignments.
The G*Power software (version 3.1.9.7) was applied to determine the sample size for the current study (21). Calculations were performed for three groups according to the initial treatment choice. The statistical program was set to the F family of tests, to a one-way analysis of variance (ANOVA), and to the “A Prior” power analysis necessary to identify sample size (22). The effect size, type I error (α), and statistical power (1 – β) were set at 0.25, 0.05, and 0.80, respectively. Based on the setup above, the total sample size was calculated to be at least 159 patients with an actual power of 0.805 (Figure 1).
Propensity-score matching (PSM) was widely used to adjust the between-group variations and remove the biases for non-randomized trials with two treatment alternatives (23). The propensity score (PS) is a conditional probability of receiving a particular treatment, which is calculated by a non-parsimonious multivariable logistic regression model with the treatment choice as the dependent variable and all baseline characteristics as covariates (23). In this study, a 1:1:1 matching was performed with an optimal matching algorithm and a caliper width equal to 0.25 of the standard deviation of the logit of the PS using TriMatch package (23, 24). In detail, the PS is estimated by logistic regression separately for all possible pairs of three groups. Each member of the antiplatelet group is matched to all surgical groups within a pre-specified caliper, and each member of the antiplatelet group is also matched to all conservative groups within the caliper. Those patients who do not have any match are removed from the sample. The PS distance is also calculated for all possible pairs between the remaining conservative group and surgical group members. The pairs with a PS distance greater than the caliper value are further eliminated. Only the unique triplets with the smallest total PS distance are finally retained in the match sample. Additionally, the standardized differences were estimated for all the baseline covariates before and after matching to assess the pre-match imbalance and post-match balance, which indicated a relatively good balance with an acceptable range of <25.0% (23).
In the matched cohort, continuous variables were reported as the median ± standard deviation. Proportions were calculated for categorical variables. ANOVA was used to analyze continuous variables. Pearson's chi-square test was used to analyze categorical variables. Univariate Cox regression analyses were performed to estimate the comparative risks of primary and secondary outcomes. Pre-specified subgroup analysis was performed on the basis of type of antiplatelet therapy (aspirin, clopidogrel, and combined antiplatelet agents). Tests for interaction were performed to assess for heterogeneity of treatment effect among subgroups. Additionally, the least absolute shrinkage and selection operator (LASSO) method was used to identify major covariates associated with future strokes from all the covariates. The major covariates were filtered via non-zero coefficients. Risk factors were then selected by multivariate Cox regression analysis using a forward stepwise method. These selected risk factors were reported as hazard ratios (HRs) with 95% confidence intervals (CIs) and p-values.
All statistical analyses were performed with the R Project for Statistical Computing (version 4.0.2) and Statistical Program for Social Science (SPSS) Statistics (version 26). The significance level was set at 0.05 in all tests.
This study was carried out according to the flow chart shown in Figure 2. A total of 667 MMD patients were identified at the eight Chinese teaching hospitals from January 2013 to December 2018. After applying the inclusion and exclusion criteria, 217 patients with ischemic MMD were enrolled in this study. All the baseline characteristics of these patients are summarized in Table 1. The average ages at symptom onset and diagnosis were 46 ± 12 and 47 ± 11 years, respectively. The female-to-male ratio was 0.9 (100:117). Vascular risk factors were noted in 143 patients (65.9%), including 45.6% with hypertension, 19.8% with diabetes mellitus, 7.8% with hyperlipidemia, 33.6% with active smoking, and 27.2% with alcohol consumption. The family history of MMD was approximately 5.5%. A past history of stroke or TIA was identified in 27 (12.4%) patients. The most common initial symptoms were cerebral infarction (61.3%) and TIA (32.7%). On angiography, most patients had bilateral steno-occlusive changes with Suzuki stage III–VI, but nearly 10% of them had intracranial aneurysms. Among them, 85 (39.2%) patients received antiplatelet therapy: 47 were treated with oral aspirin 100 mg daily, 18 with oral clopidogrel 75 mg daily, and 20 with combined aspirin and clopidogrel for the first 3 weeks, followed by aspirin daily. Fifty-three (24.4%) patients had undergone surgical revascularization procedures. The remaining 79 patients (36.4%) were conservatively treated.
Of the enrolled patients, seven patients (3.2%) could not be contacted in December 2019, so the follow-up was determined from their last clinical visits. During a follow-up of 34 ± 18 months, 18 patients (8.3%) had acute ischemic strokes, 37 patients (17.1%) developed a poor functional status, and 4 patients (1.8%) died of cerebral hemorrhage (Figure 2). The median interval from first discharge to subsequent ischemic stroke was 36 ± 25 months. Eleven of the 79 (13.9%) conservatively treated patients, 6 of the 85 (7.1%) antiplatelet-treated patients, and 1 of the 53 (1.9%) surgically treated patients had future strokes (Table 2).
There were significant differences between the three groups in several of the baseline characteristics before PSM (Table 3). With the use of 1:1:1 PSM via the TriMatch package, 53 patients with antiplatelet agents were matched with 53 conservative patients and 53 patients underwent surgical revascularization (Table 3). After matching, the between-group variations were successfully removed using the optimal matching algorithm and a caliper width equal to 0.25 (Figure 3). The standardized differences were <25.0% for almost all potential risk factors, indicating only small differences between these groups (Supplementary Tables 1, 2).
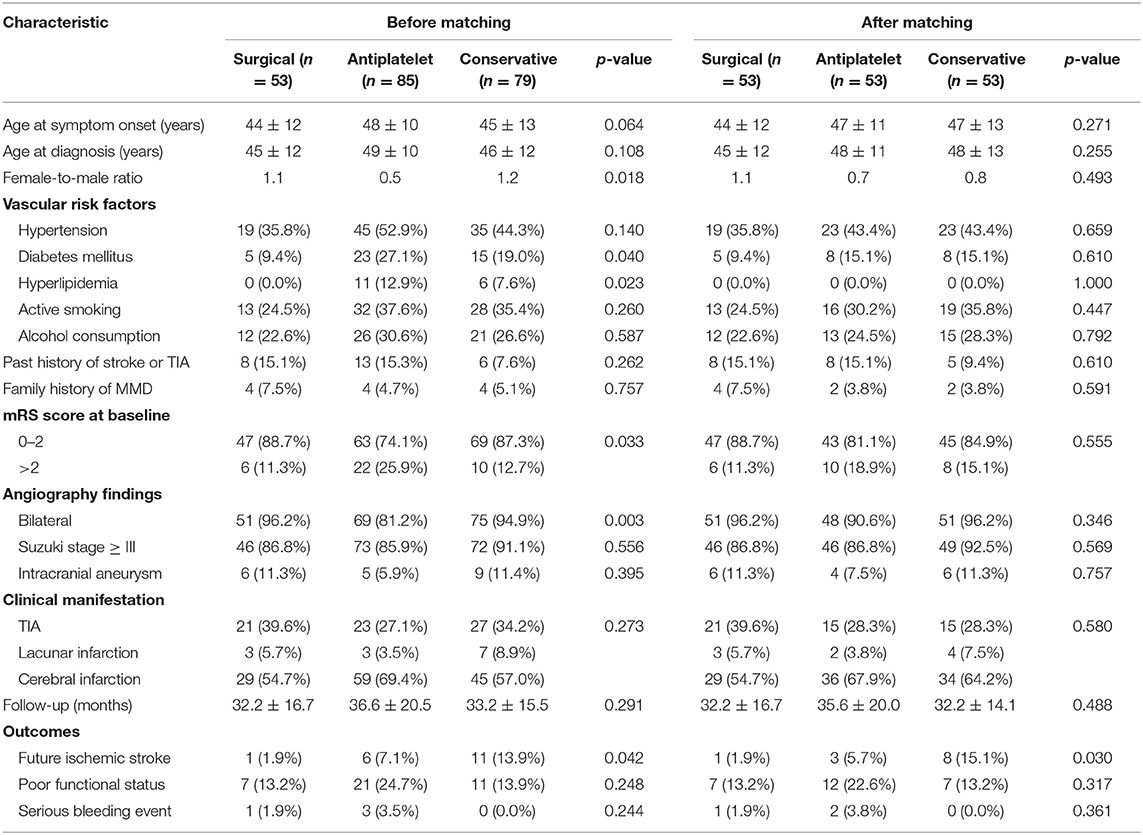
Table 3. Comparison of clinical features and outcomes between different groups before and after matching.
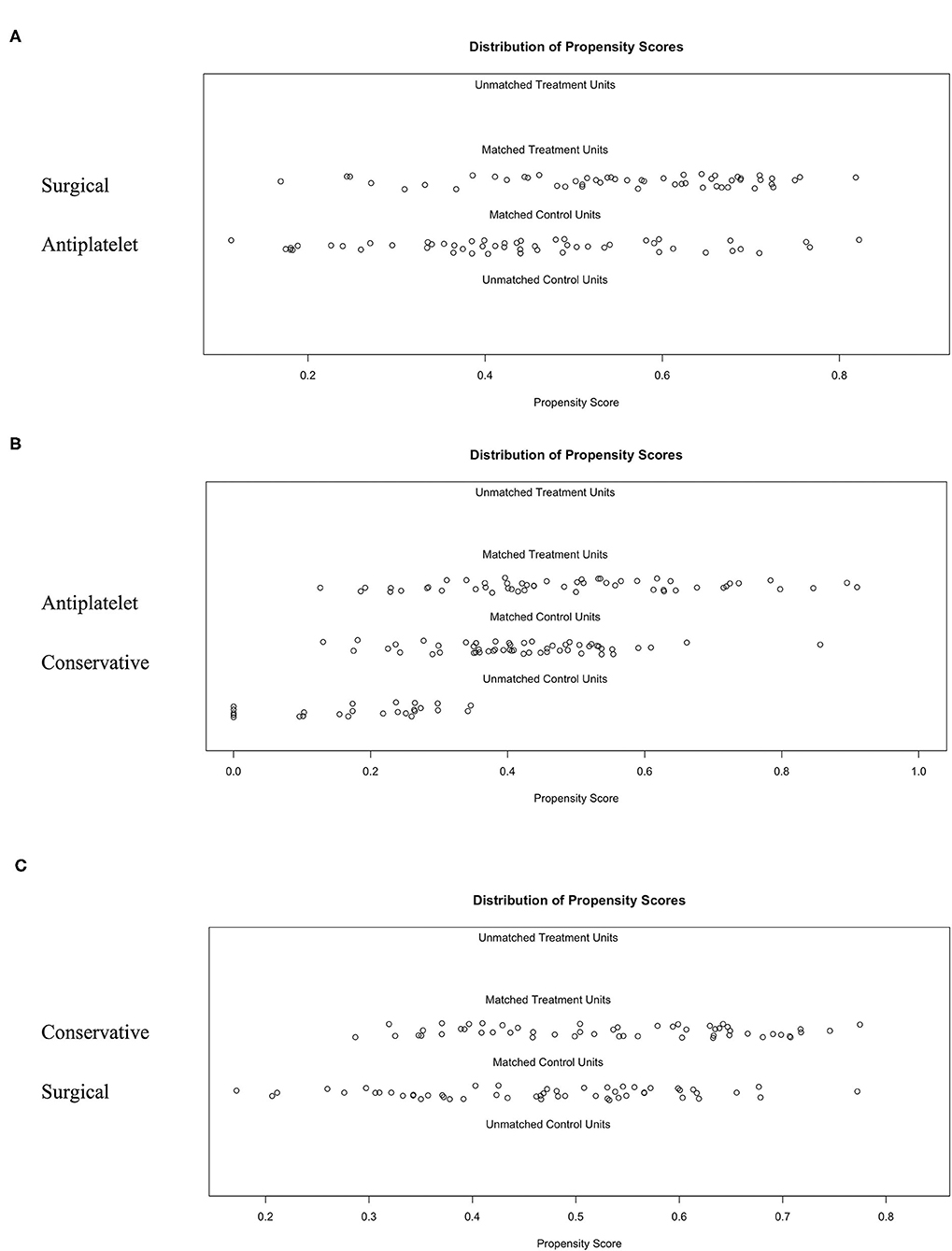
Figure 3. The 1:1:1 PSM results for different groups using the optimal matching algorithm and a caliper width equal to 0.25. (A) Surgical group matched to the antiplatelet group. (B) Antiplatelet group matched to the conservative group. (C) Identification of the accuracy between the matched conservative group and surgical group.
Among the matched groups, 12 patients (7.5%) developed incident ischemic stroke after an average follow-up of 33 ± 17 months. All of them had an initial symptom of cerebral infarction with a median interval period of 29 ± 25 months. The shortest interval from first discharge to the episode of cerebral infarction was 5 months, and the longest was 72 months. Of these patients, one who took antiplatelet agents died due to severe cerebral hemorrhage, and eight patients had a poor functional status. Additionally, further cerebral ischemic attack occurred in three patients (5.7%) in the antiplatelet group, compared with one patient (1.9%) in the surgical group and eight patients (15.1%) in the conservative group (p = 0.030). The univariate Cox regression analysis of recurrent ischemic events is shown in Figure 4, demonstrating that the probability of further cerebral ischemic attacks was lowest with surgical revascularization, while antiplatelet therapy was also statistically significant for preventing and reducing recurrent risks compared with the effects of conservative treatment. The omnibus tests of model coefficients revealed that there was a significant difference between the three risk curves (χ2 = 8.987; p = 0.011). No significant difference in recurrence risk was found between subgroups of different antiplatelet agents (p > 0.05; Table 4).
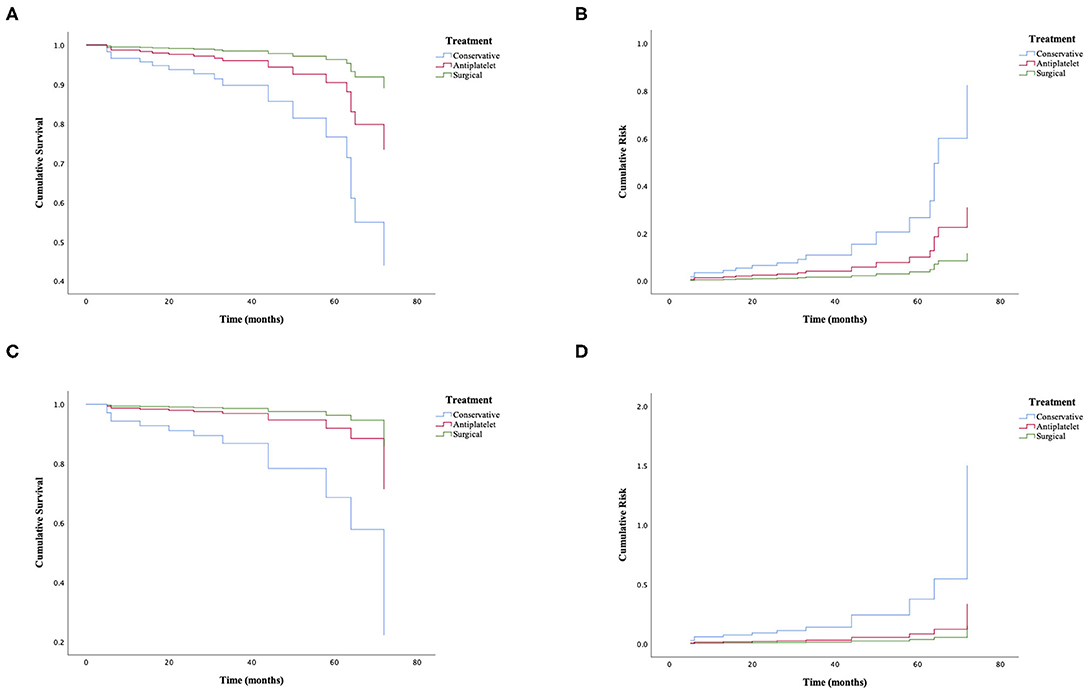
Figure 4. Cox regression analysis of further cerebral ischemic attacks in adult patients with ischemic moyamoya disease treated by different methods. (A,B) The cumulative survival before (χ2 = 7.188; p = 0.027) and after matching (χ2 = 8.987; p = 0.011). (C,D) The cumulative risk before (χ2 = 7.188; p = 0.027) and after matching (χ2 = 8.987; p = 0.011).
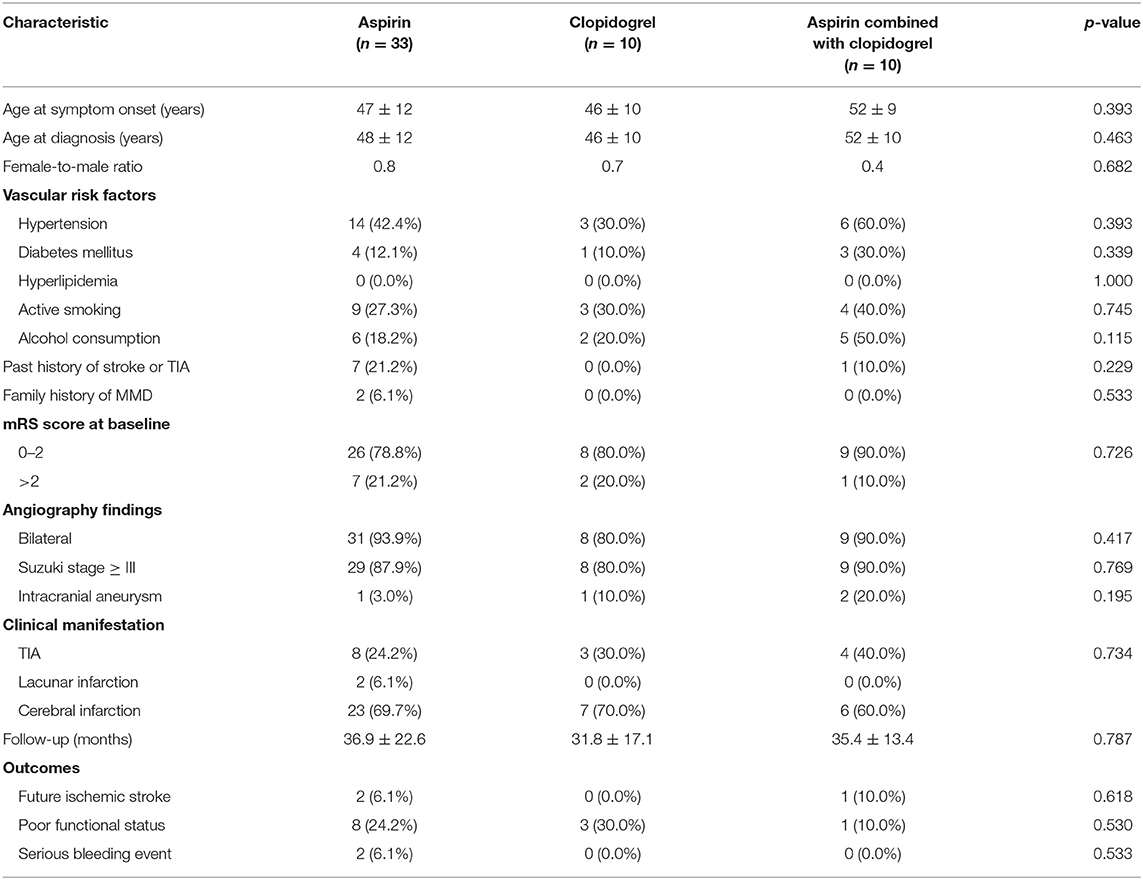
Table 4. Comparison of the clinical features and outcomes between subgroups with different antiplatelet agents.
In the matched groups, 26 patients (16.4%) had a poor functional status at follow-up. Of these patients, 12 (46.2%) had moderate-to-severe neurological dysfunction at baseline, and these patients accounted for a greater proportion than the 24 patients (15.1%) among all subjects. In the present study, a poor functional status occurred in 12 patients (22.6%) in the antiplatelet group, compared with 7 patients (13.2%) in the surgical group and 7 patients (13.2%) in the conservative group (Table 3). Although antiplatelet therapy was found to be associated with a comparatively higher risk of a poor outcome, the univariate Cox regression analysis showed no significant difference between groups (χ2 = 0.400; p = 0.819).
In the matched groups, three deaths (1.8%) occurred because of severe cerebral hemorrhages, including two deaths among patients who were treated with antiplatelet agents and one patient who was treated with surgical revascularization (Table 3). No hemorrhagic events were observed in the conservative groups. No significant difference was observed among groups and subgroups, but antiplatelet therapy showed a relatively higher risk of bleeding (3.8%) in two patients treated with aspirin (Table 4).
All the baseline characteristics and treatment choices were entered as covariates. Five potential major factors with non-zero coefficients were selected from the 16 clinical features in terms of the 159 patients via the LASSO regression model (1:3 ratio, Figure 5), including a family history of MMD, a past history of stroke or TIA, an mRS score at baseline, clinical manifestation, and treatment choice. A family history of MMD (HR = 6.93; 95% CI: 1.28–37.52; p = 0.025), a past history of stroke or TIA (HR = 4.35; 95% CI: 1.09–17.33; p = 0.037), and treatment (HR = 0.05; 95% CI: 0.01–0.32; p = 0.001) were identified as risk factors in multivariate Cox regression analysis using the forward stepwise method. No significant correlations were observed between further cerebral ischemic attacks and other factors (p > 0.05).
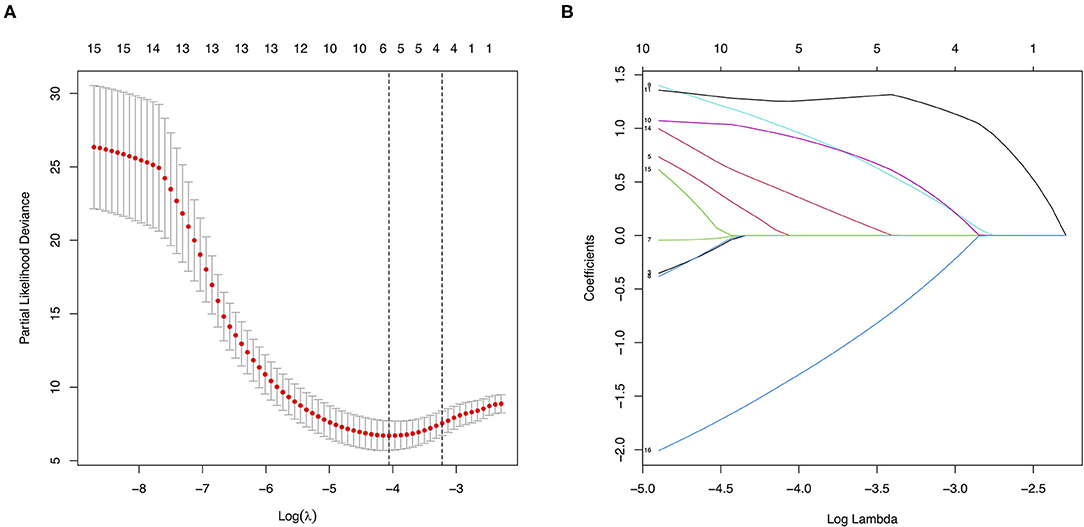
Figure 5. Features selection using the least absolute shrinkage and selection operator (LASSO) method. (A) Tuning parameter (λ) selection in the LASSO model used five-fold cross-validation via minimum criteria. The partial likelihood deviance (binomial deviance) curve was plotted vs. log(λ). Dotted vertical lines were drawn at the optimal values by using the minimum criteria and the 1 standard error of the minimum criteria (the 1-SE criteria). (B) LASSO coefficient profiles of the 16 covariates. A coefficient profile plot was produced against the log (λ) sequence. Vertical line was drawn at the value selected using five-fold cross-validation, where optimal λ resulted in 5 non-zero coefficients.
In the present study, we evaluated whether antiplatelet agents showed a potential effect in adult patients with ischemic MMD. To clarify the efficacy and safety of antiplatelet agents in ischemic MMD, we performed this retrospective cohort study and assessed the outcomes of 217 patients during a follow-up of 34 ± 18 months; the participants included 79 conservatively treated patients, 85 patients treated with antiplatelet agents, and 53 patients treated with surgical revascularization. Our results showed that antiplatelet agents were effective in preventing and reducing further cerebral ischemic attacks in these patients.
The baseline characteristics were similar to those of samples in previous studies, and the ratio of women to men (0.9) and family history of MMD (5.5%) in this study appeared to be similar to the respective parameters in the study by Duan et al. (6), which indicated an important influence of heredity. Most of the patients were diagnosed with bilateral steno-occlusive changes (89.9%), and a few were diagnosed with intracranial aneurysms (9.2%) on angiography, a finding that was consistent with a previous study by Kraemer et al. (25) and remained unclear. In addition, as Hervé et al. (26) found, the most common symptom was cerebral infarction (61.3%), followed by TIA (32.7%). However, the average ages at symptom onset (46 ± 12 years) and diagnosis (47 ± 11 years) in our study were comparably older than those in previous studies (3–8) because our strict criteria excluded a large portion of pediatric patients and adult patients with hemorrhagic MMD, which may explain the different results. Furthermore, our study revealed that a family history of MMD, a past history of stroke or TIA, and treatment choice could be independent risk factors for additional cerebral ischemic events based on a LASSO-Cox regression model.
Similarly, the univariate Cox regression analysis showed that revascularization surgery performed best for the treatment of ischemic MMD, with the lowest rate of further cerebral ischemic events (1.9%) and longest interval period between strokes (72 months) in this study, which was generally employed as the first choice to treat MMD patients to prevent future strokes with strong evidence (9, 27, 28). Interestingly, our study also showed that compared with conservative treatment, antiplatelet therapy was beneficial for reducing further cerebral ischemic events (5.7 vs. 15.1%) and extending the interval period (45 months vs. 18 months) among these patients. It was obvious that conservative treatment was inferior to surgical revascularization with regard to the prognosis of MMD patients. In the past, observational studies categorized antiplatelet therapy as conservative treatment for statistical analysis. Thus, the effectiveness of antiplatelet agents could be weakened due to the mixture of no treatment and treatment with other medications. In addition, current studies suggested that aspirin was effective in the postoperative management of ischemic MMD (29, 30). However, the use of antiplatelet agents in the treatment of MMD is controversial, and few studies have reported the efficacy of antiplatelet agents among non-surgical patients or before surgery. The majority of non-Asian experts advised using them for improving microcirculation and preventing recurrent strokes, while most Asian physicians held the opposite view that they were useless in improving blood supply and carried the potential risk of hemorrhages (12, 13). A recent study in Japan demonstrated that antiplatelet agents were effective in improving cerebral perfusion in adult patients with symptomatically ischemic MMD (31, 32). Likewise, our study focused on the efficacy and safety of antiplatelet agents used for adult MMD patients with the initial symptom of ischemia and compared their efficacy and safety with those of conservative treatment and surgical revascularization. Regarding the primary outcome, the recurrence rate of cerebral ischemic attack in the antiplatelet group was 5.7%, 1.9% in the surgical group, and 15.1% in the conservative group (p = 0.030), indicating that antiplatelet treatment could be effective for preventing ischemic stroke or TIA. However, regarding the secondary outcome, the patients in the antiplatelet group account for a higher proportion of poor neurological function than the other two groups, although statistical significance was absent among them. We noticed that the number of patients with mRS > 2 at baseline in the antiplatelet group was also higher than that in the other two groups. Therefore, we could not conclude that antiplatelet agents are responsible for a poorer functional status. We re-analyzed these 12 patients with recurrent strokes. Among these patients, four patients presented with a baseline mRS of 0–2 (surgical:antiplatelet:conservative = 0:1:3), suggesting a trend to benefit these patients with mild symptoms at baseline from both surgical revascularization and antiplatelet therapy could be compared with conservative treatment. However, because of our small sample size, we still needed a large sample size randomized control study to prove this finding. Thus, these differences may influence the secondary outcome. Because of the limitation of the retrospective study, some covariates could not be adjusted by the propensity score matching method. Moreover, the insufficient follow-up period weakened the statistical power in the secondary outcome comparison. Although the mechanism of MMD is still unclear, pathological studies have demonstrated that endovascular thrombosis and hyperplasia of smooth muscle cells lead to the progressive stenosis or occlusion of the distal ICA, which could explain the cause of ischemic symptoms (1). In addition, antiplatelet agents are widely used for the primary or secondary preventive management of acute ischemic stroke and TIA patients (10, 11). Aspirin inhibits the metabolism of arachidonic acid to prostacyclin 2 (PGI2) and serum thromboxane A2 (TXA2) by blocking cyclooxygenase-1 (COX-1) to reduce thrombosis. Clopidogrel is an adenosine diphosphate (ADP) receptor inhibitor that selectively interacts with P2Y12 receptor to depress platelet aggregation. Indeed, antiplatelet agents have been effectively applied to prevent further cerebral ischemic events because of their ability to improve the development of luminal thrombosis.
There were also several limitations of this study. First, this study is a non-randomized observational study with a small sample size. Second, although PSM is used to correct baseline differences, selection biases may not be removed because our patients were enrolled from eight clinical sites. Professional performances at different clinical centers could also affect the validity of the conclusions. Third, our subjects were all Asians; thus, whether our results are applicable to Western populations is unknown. Finally, genetic factors were not assessed in our study. Thus, a large randomized controlled trial is necessary.
In brief, we found that surgical revascularization was the first treatment choice, but antiplatelet therapy was a relatively significant management strategy for adult patients with ischemic MMD for reducing and preventing future strokes. Therefore, antiplatelet therapy may be a replacement therapy for patients with surgical contraindications and for patients before revascularization. We believe that this information about actual therapeutic practices in China will be helpful for the management of ischemic MMD.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the independent ethics committe of the First Affiliated Hospital of Sun Yat-sen University and each participating medical center's ethics committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
FY designed the study, drafted the manuscript, and contributed to the discussion. JLi, TW, and KL designed the study, collected the data, and analyzed the data. HL, TG, XZ, and TY collected the data and contributed to the discussion. JLia and XW collected the data. QL and WS designed the study, reviewed the manuscript, and contributed to the discussion. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81671132 and 81471180) and the National Natural Science Foundation of China for Young Scientists Fund (Grant No. 81701142).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Weidong Li Ph.D. (Department of Statistics, School of Public Health, Sun Yat-sen University), for statistical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.608000/full#supplementary-material
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
2. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatr. (2008) 79:900–4. doi: 10.1136/jnnp.2007.130666
3. Chiu D, Shedden P, Bratina P, Grotta JC. Clinical features of moyamoya disease in the United States. Stroke. (1998) 29:1347–51. doi: 10.1161/01.STR.29.7.1347
4. Kraemer M, Heienbrok W, Berlit P. Moyamoya disease in Europeans. Stroke. (2008) 39:3193–200. doi: 10.1161/STROKEAHA.107.513408
5. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. (2008) 39:42–7. doi: 10.1161/STROKEAHA.107.490714
6. Duan L, Bao XY, Yang WZ, Shi WC, Li DS, Zhang ZS, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke. (2012) 43:56–60. doi: 10.1161/STROKEAHA.111.621300
7. Ahn IM, Park DH, Hann HJ, Kim KH, Kim HJ, Ahn HS. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. (2014) 45:1090–5. doi: 10.1161/STROKEAHA.113.004273
8. Chen PC, Yang SH, Chien KL, Tsai IJ, Kuo MF. Epidemiology of moyamoya disease in Taiwan: a nationwide population-based study. Stroke. (2014) 45:1258–63. doi: 10.1161/STROKEAHA.113.004160
9. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, and Health Labour Sciences Research Grant for Research on Measures for Infractable, Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir. (2012) 52:245–66. doi: 10.2176/nmc.52.245
10. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
11. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000158
12. Kraemer M, Berlit P, Diesner F, Khan N. What is the expert's option on antiplatelet therapy in moyamoya disease? Results of a worldwide Survey. Euro J Neurol. (2012) 19:163–7. doi: 10.1111/j.1468-1331.2011.03481.x
13. Yamada S, Oki K, Itoh Y, Kuroda S, Houkin K, Tominaga T, et al. Effects of surgery and antiplatelet therapy in ten-year follow-up from the registry study of research committee on moyamoya disease in Japan. J Stroke Cerebrovasc Dis. (2016) 25:340–9. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.003
14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
15. Fujimura M, Tominaga T. Diagnosis of moyamoya disease: international standard and regional differences. Neurol Med Chir. (2015) 55:189–93. doi: 10.2176/nmc.ra.2014-0307
16. Savio K, Pietra GL, Oddone E, Reggiani M, Leone MA. Reliability of the modified rankin scale applied by telephone. Neurol Int. (2013) 5:e2. doi: 10.4081/ni.2013.e2
17. Liu XJ, Zhang D, Wang S, Zhao YL, Teo M, Wang R, et al. Clinical features and long-term outcomes of moyamoya disease: a single-center experience with 528 cases in China. J Neurosurg. (2015) 122:392–9. doi: 10.3171/2014.10.JNS132369
18. Wang Y, Zhang Z, Wei L, Zhang Q, Zou Z, Yang L, et al. Predictive role of heterozygous p.R4810K of RNF213 in the phenotype of Chinese moyamoya disease. Neurology. (2020) 94:e678–86. doi: 10.1212/WNL.0000000000008901
19. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
20. GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. (1993) 329:673–82. doi: 10.1056/NEJM199309023291001
21. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
22. Dupont WD, Plummer WDJr. Power and sample size calculations. A review and computer program. Control Clin Trials. (1990) 11:116–28. doi: 10.1016/0197-2456(90)90005-M
23. Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. (2017) 98:76–90. doi: 10.1016/j.brat.2017.01.005
24. Antonelli A, Minervini A, Mari A, Bertolo R, Bianchi G, Lapini A, et al. TriMatch comparison of the efficacy of FloSeal versus TachoSil versus no hemostatic agents for partial nephrectomy: results from a large multicenter dataset. Int J Urol. (2015) 22:47–52. doi: 10.1111/iju.12603
25. Kraemer M, Schwitalla JC, Diesner F, Aktas O, Hartung HP, Berlit P. Clinical presentation of moyamoya angiopathy in Europeans: experiences from Germany with 200 patients. J Neurol. (2019) 266:1421–8. doi: 10.1007/s00415-019-09277-1
26. Hervé D, Ibos-Augé N, Calvière L, Rogan C, Labeyrie MA, Guichard JP, et al. Predictors of clinical or cerebral lesion progression in adult moyamoya angiopathy. Neurology. (2019) 93:e388–97. doi: 10.1212/WNL.0000000000007819
27. Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. (2014) 45:3025–31. doi: 10.1161/STROKEAHA.114.005624
28. Kuroda S, Nakayama N, Yamamoto S, Kashiwazaki D, Uchino H, Saito H, et al. Late (5-20 years) outcomes after STA-MCA anastomosis and encephalo-duro-myo-arterio-pericranial synangiosis in patients with moyamoya disease. J Neurosurg. (2020) 1–8. doi: 10.3171/2019.12.JNS192938. [Epub ahead of print].
29. Rashad S, Fujimura M, Niizuma K, Endo H, Tominaga T. Long-term follow-up of pediatric moyamoya disease treated by combined direct-indirect revascularization surgery: single institute experience with surgical and perioperative management. Neurosurg Rev. (2016) 39:615–23. doi: 10.1007/s10143-016-0734-7
30. Zhao Y, Zhang Q, Zhang D, Zhao Y. Effect of aspirin in postoperative management of adult ischemic Moyamoya disease. World Neurosurg. (2017) 105:728–31. doi: 10.1016/j.wneu.2017.06.057
31. Chiba T, Setta K, Shimada Y, Yoshida J, Fujimoto K, Tsutsui S, et al. Comparison of effects between clopidogrel and cilostazol on cerebral perfusion in nonsurgical adult patients with symptomatically ischemic moyamoya disease: subanalysis of a prospective cohort. J Stroke Cerebrovasc Dis. (2018) 27:3373–9. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.041
32. Miyoshi K, Chida K, Kobayashi M, Kubo Y, Yoshida K, Terasaki K, et al. Two-year clinical, cerebral hemodynamic, and cognitive outcomes of adult patients undergoing medication alone for symptomatically ischemic moyamoya disease without cerebral misery perfusion: a prospective cohort study. Neurosurgery. (2019) 84:1233–41. doi: 10.1093/neuros/nyy234
Keywords: Moyamoya disease, antiplatelet agents, efficacy, safety, recurrent stroke prevention
Citation: Ye F, Li J, Wang T, Lan K, Li H, Yin H, Guo T, Zhang X, Yang T, Liang J, Wu X, Li Q and Sheng W (2021) Efficacy and Safety of Antiplatelet Agents for Adult Patients With Ischemic Moyamoya Disease. Front. Neurol. 11:608000. doi: 10.3389/fneur.2020.608000
Received: 18 September 2020; Accepted: 30 November 2020;
Published: 15 January 2021.
Edited by:
Sepideh Amin-Hanjani, University of Illinois at Chicago, United StatesReviewed by:
Marialuisa Zedde, Local Health Authority of Reggio Emilia, ItalyCopyright © 2021 Ye, Li, Wang, Lan, Li, Yin, Guo, Zhang, Yang, Liang, Wu, Li and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Li, cWlsaV9tZEAxMjYuY29t; Wenli Sheng, c2hlbmd3bEBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.