- 1Veneto Institute of Oncology IOV–Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Padua, Italy
- 2Department of Biomedical and NeuroMotor Sciences (DIBINEM), Alma Mater Studiorum–University of Bologna, Bologna, Italy
- 3Regional Center for Biomarkers, Department of Clinical Pathology and Transfusion Medicine, Venice, Italy
- 4U.O. Neurologia, Santa Chiara Hospital, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy
- 5U.O. Neurologia, Azienda ULS Imola, Imola, Italy
Purpose: Algorithms for the detection of a malignancy in patients with unclear neurologic symptoms of suspicious paraneoplastic origins are not universally applied. Frequently, circulating tumor markers (TMs) are considered a valuable tool for cancer diagnosis in patients with paraneoplastic neurologic syndromes (PNS). Our aim was to extract the recommendations on the use of TMs and onconeural antibodies (Abs) for the diagnosis of malignancies in PNS from clinical practice guidelines and put them forward as evidence in a common framework to facilitate diffusion, dissemination, and implementation.
Methods: Systematic literature searches were performed for guidelines on both oncology and PNS published since 2007. Guidelines containing information and recommendations for clinical practice pertaining to the screening and diagnosis of PNS were selected. Information on circulating TMs and onconeural Abs was extracted and synthesized in consecutive steps of increasing simplification.
Results: We retrieved 799 eligible guidelines on oncology for the potential presence of information on PNS but only six covered treated diagnosis or the screening of cancer in PNS, which were then selected. Seventy-nine potentially relevant guidelines on PNS were identified as eligible and 15 were selected. Synoptic tables were prepared showing that classical TMs are not recommended for the screening or the diagnosis of a malignancy in patients with a suspected PNS. Neither should onconeural Abs be considered to screen for the presence of a malignancy, although they could be helpful to define the probability of the paraneoplastic origin of a neurologic disorder.
Conclusion: The present work of synthesis may be a useful tool in the diffusion, dissemination, and implementation of guideline recommendations, potentially facilitating the decrease of the inappropriate use of circulating biomarkers for cancer screening in the presence of PNS.
Introduction
Paraneoplastic neurologic syndromes (PNS) are neurologic immune-mediated disorders occurring as a remote effect of a tumor, frequently associated with antibodies (1, 2). Antibodies (Abs) associated with PNS can recognize intracellular antigens (onconeural Abs) or bind to cell surface antigens on neuronal cells (3). While neuronal surface Abs often occur in the absence of tumors in non-paraneoplastic autoimmune diseases such as autoimmune encephalitis, onconeural Abs are closely related to the presence of a malignancy (4).
Neurologic symptoms suggestive of a PNS necessitate the proper diagnosis of PNS and the identification of a malignancy. Interestingly, evidence shows that PNS may occur in most cases before the clinical appearance of the malignancy, thus increasing the need for tools for early cancer detection in these patients (5, 6).
Laboratory tests are expected to play a key role in this diagnostic workflow due to the availability of screening tests for both antibodies and classical circulating tumor markers (TMs), e.g., CEA or PSA.
The presence of onconeural Abs increases the probability of a paraneoplastic neurologic syndrome (7) and several studies support clinicians on the appropriate interpretation of antibody tests results. Conversely, the detection of an underlying malignancy is more challenging, as the presence of antibodies is associated with risk rate, which is hardly applicable for diagnostic purposes for an individual patient (8).
Circulating TMs have an established clinical role to detect the relapse of malignancies during follow-up and to monitor the response to therapy in advanced disease, whereas they are not recommended for the early diagnosis of cancer due to their low sensitivity and specificity (9–11). Nonetheless, circulating TMs are widely requested for diagnostic purposes in clinical practice (12–18), being also ordered for patients with unclear neurological symptoms. Clinical practice guidelines are designed, disseminated, and implemented to help clinicians make appropriate diagnostic and therapeutic decisions. Thus, available guidelines should provide proper recommendations on the use of TMs and onconeural Abs for the diagnosis of occult malignances in PNS. However, the provided recommendations may not cover all clinical questions or may not be consistent throughout the guidelines produced in different contexts (19). Moreover, physician adherence is critical for translating guideline recommendations into improved healthcare, but a variety of barriers can affect the physician knowledge of guideline recommendations, undermining this process. These barriers include a lack of awareness and familiarity, caused by a high volume of information, scarce guideline accessibility, and the amount of time needed to stay informed (20).
Therefore, although numerous guidelines on PNS exist, this may not be sufficient to ensure evidence-based decision making. In fact, it was shown that the uptake of knowledge does not occur with simple dissemination (21), mainly in cases such as that of the use of TMs in PNS, in which a specific issue may be difficult to individuate in the body of comprehensive but multifaceted guidelines. The aim of this study was to apply a previously validated method (9) to systematically and critically review guidelines, extracting, synthesizing, and comparing, in a synoptic manner, the recommendations on the use of TMs and onconeural Abs for the early diagnosis of malignancies in PNS. The ultimate aim of this study was to document only recommendations on circulating biomarkers by structuring them in a common framework to facilitate their diffusion, dissemination, and implementation.
Materials and Methods
Following a protocol designed by the authors and adherent to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22), two different independent systematic reviews of guidelines were sequentially undertaken to seek out recommendations or information on the detection of a tumor in patients with PNS. One review was focused on oncology guidelines and derived from a previous project, that lasted 5 years which had the objective of collecting and synthesizing recommendations from guidelines related to 18 different neoplasms [hereinafter referred to as the “Guide project” (9)]. The second review was focused on PNS guidelines.
Search Strategy for Guidelines on Oncology
In the first phase of the study, 1,181 guidelines published from January 2009 to July 2015 were selected from the 8,266 documents retrieved from the bibliographic databases generated through the Guide project (9). The 1,181 full-texts were screened for the presence of recommendations and information for clinical practice related to PNS. The details for the guidelines selection have been previously published (9). In brief, a systematic search for guidelines was performed in bibliographic databases (PubMed, National Guidelines Clearinghouse, and GIN library). This first broader systematic search included key terms, their synonyms, and associated MeSH terms related to cancer and guidelines (see Pubmed strategy in Supplementary Table 1, Section A). Moreover, guidelines were searched in websites of organizations and scientific societies producing guidelines (Supplementary Table 2).
Documents containing recommendations for clinical practice were included. Reviews, technology assessments, commentaries to guidelines, and guidelines limited to sarcomas, hematological malignancies, pediatric population, and pregnant women, were excluded.
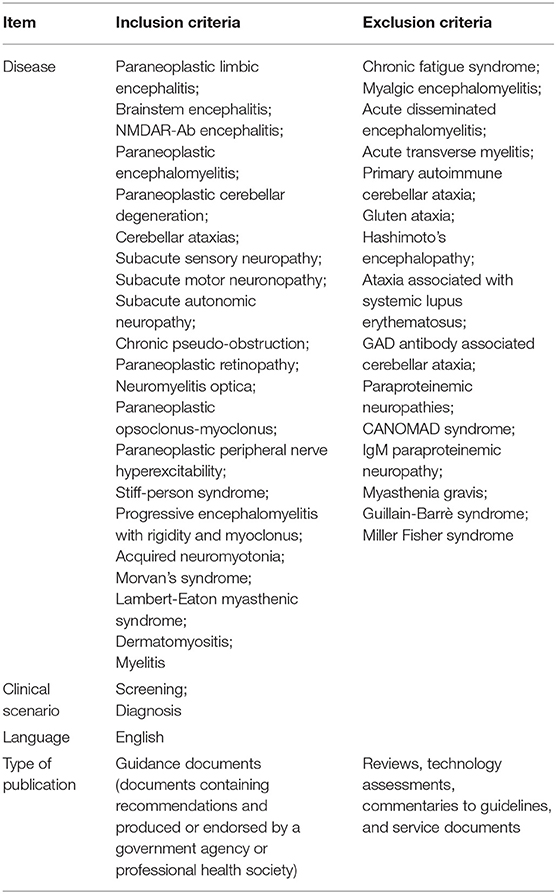
A standardized set of selection criteria was used to identify eligible publications addressing PNS (details are reported in Table 1).
Search Strategy for Guidelines on PNS
A subsequent narrower search with MeSH terms and more specific keywords was conducted for identifying existing guidelines on PNS. Bibliographic databases were consulted including PubMed, National Guideline Clearinghouse, and websites of organizations and scientific societies producing guidelines (Supplementary Table 2). The full PubMed search strategy is shown in Supplementary Table 1, Section B. Any guideline containing information and recommendations for clinical practice related to PNS were eligible for inclusion (details in Table 1). Documents were included if they were published or updated between 2007 and 2017. A search for updated versions of the identified guidance documents was performed in January 2019 and the most up-to-date documents were included in the study.
Two authors (CT and IC) independently reviewed the two literature search results using the predefined eligibility criteria. Conflicts were resolved by discussion until a consensus was reached.
Synthesis and Presentation of Recommendations
In the present study, we applied a novel approach to summarize and compare recommendations and other information on TMs published in guidelines that had been recently developed (9). During this previous project (9), guidelines on solid tumors were identified through systematic search, and their quality appraised; information on TMs were then extracted and summarized using the method summed up below, which has been designed to be explicit, verifiable, and reproducible.
The information related to the following clinical question was then searched in the selected guidelines: “In a patient with a suspected PNS, are circulating TMs and/or circulating antibodies recommended for the screening or diagnosis of any possible associated malignancy and its site of origin?”
The clinical information on diagnostic laboratory tests was searched, and data on circulating TMs and/or antibodies were extracted from every guideline and synthesized in a multistep process according to the previously published method (9). For each guideline, recommendations, implicit advices for clinical practice (not recognizable as explicit recommendations), and additional information concerning; (i) circulating TMs, (ii) antibodies, and (iii) the most commonly associated malignancies to a given syndrome were verbatim transferred in an (Excel) electronic sheet by one author (IC). Information extracted from the different guidelines was grouped with reference to the type of syndrome, according to the classification into “classical,” “non-classical,” and “others” subtypes (7). All the relevant information was summarized by one author (MG) and clustered in a single entry when different guidelines provided similar messages. The tabulated results were then appraised by three other authors (CT, IC, and PD) with methodological skills or specific clinical expertise on PNS. Every effort was made to avoid any interpretation of the content of guidelines and verbatim reporting of the original sentences were used whenever possible throughout the synthesis and clustering process. Comments and suggestions were discussed and resolved by consensus.
Results
Guidelines Focused on Oncology
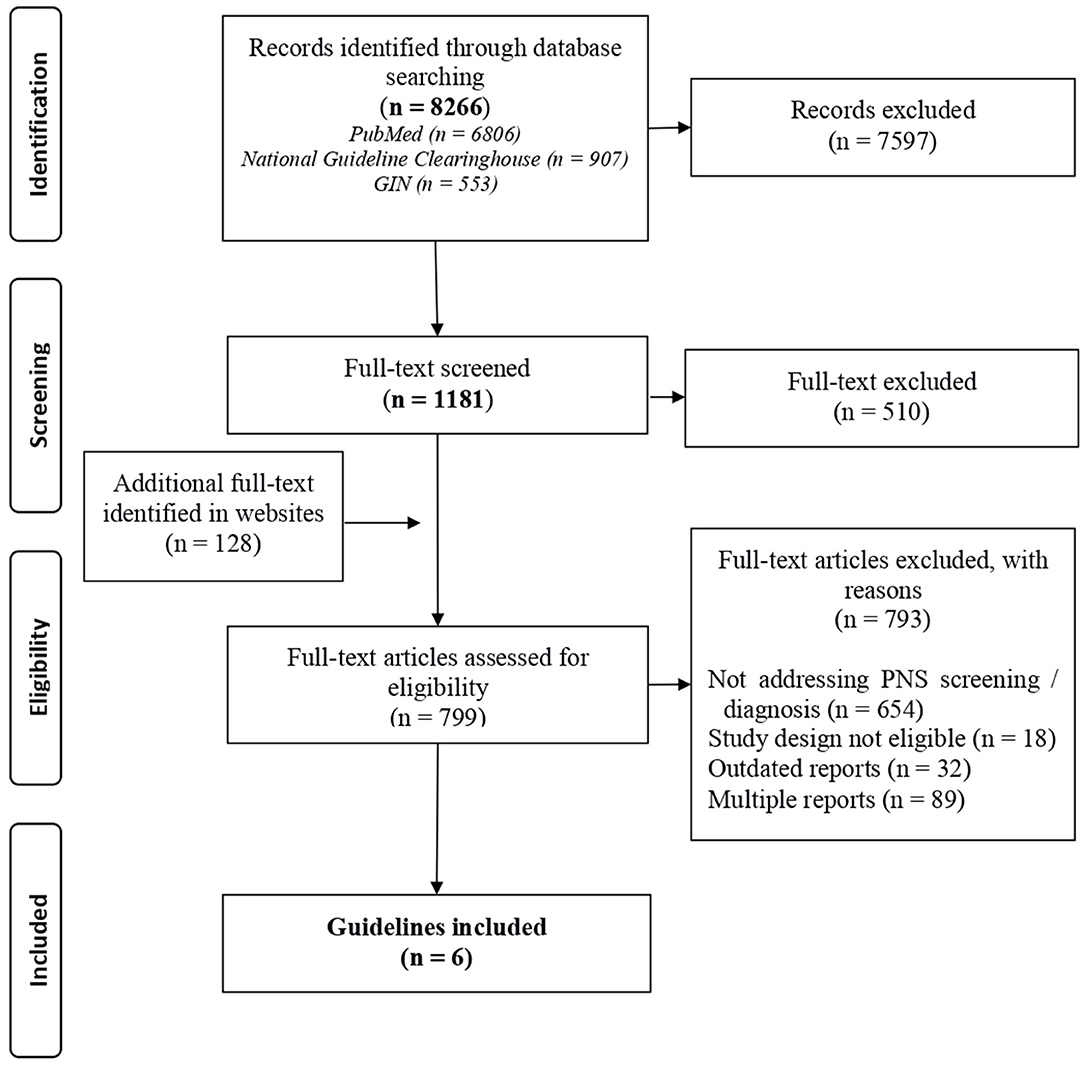
Of the identified 1,181 guidelines focused in oncology in the Guide project, 671 were selected as they were eligible and contained the potential presence of information on PNS. In addition, 128 documents were identified in the website search. Of these 799 guidelines only 6 treated diagnosis or the screening of PNS and were selected (details in Figure 1, Supplementary Table 3). Three documents concerned lung cancer (23–25), one renal cell carcinoma (26), one pulmonary neuroendocrine tumor (27), and 1 was dedicated to screening for tumors in patients with paraneoplastic syndromes (28). Details on the recommendations and supplementary information on circulating TMs or antibodies for the screening or diagnosis of any possible concomitant malignancy are reported in Table 2. In summary, the guideline on neuroendocrine tumors supports the determination of 5-hydroxy-indole-acetic acid in 24-h urine and circulating adrenocorticotropic hormone (ACTH) and growth hormone-releasing hormone (GHRH) in the diagnostic work-up of the tumor, irrespectively, of the presence of PNS (27); two guidelines considered a diagnostic workflow in PNS cases but did not mention TMs (24, 26) one guideline considered the diagnostic workflow and mentioned TMs to underline their low diagnostic sensitivity and specificity (28); one guideline recommended against the use of TMs as a diagnostic tool (25); and one guideline reported the association of anti-Hu onconeural Abs in small cell lung cancer (SCLC), but did not provide clinical practice recommendations and did not address TMs (23).
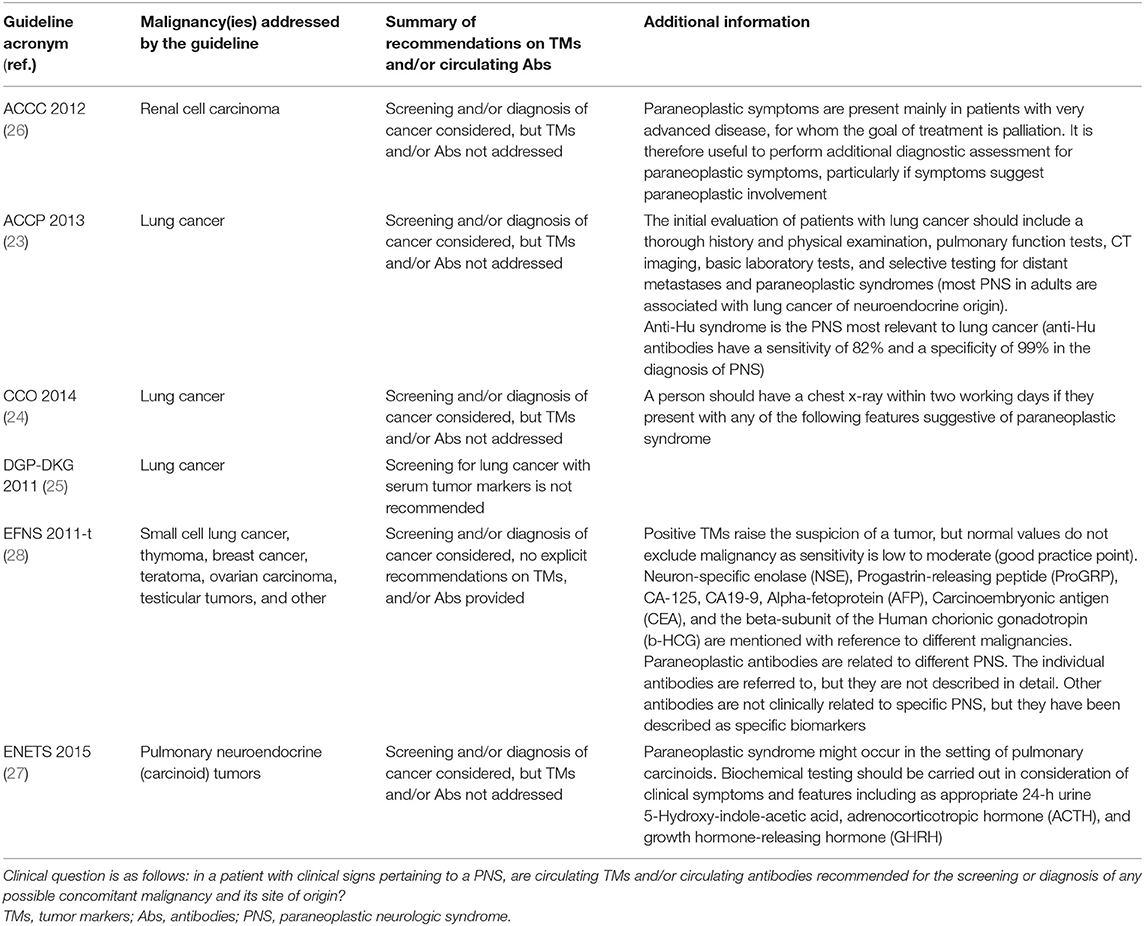
Table 2. Summary of recommendations and supplementary information concerning circulating TMs and/or antibodies for the screening or diagnosis of the malignancies in patients with PNS from guidelines focused on oncology.
Guidelines Focused on PNS
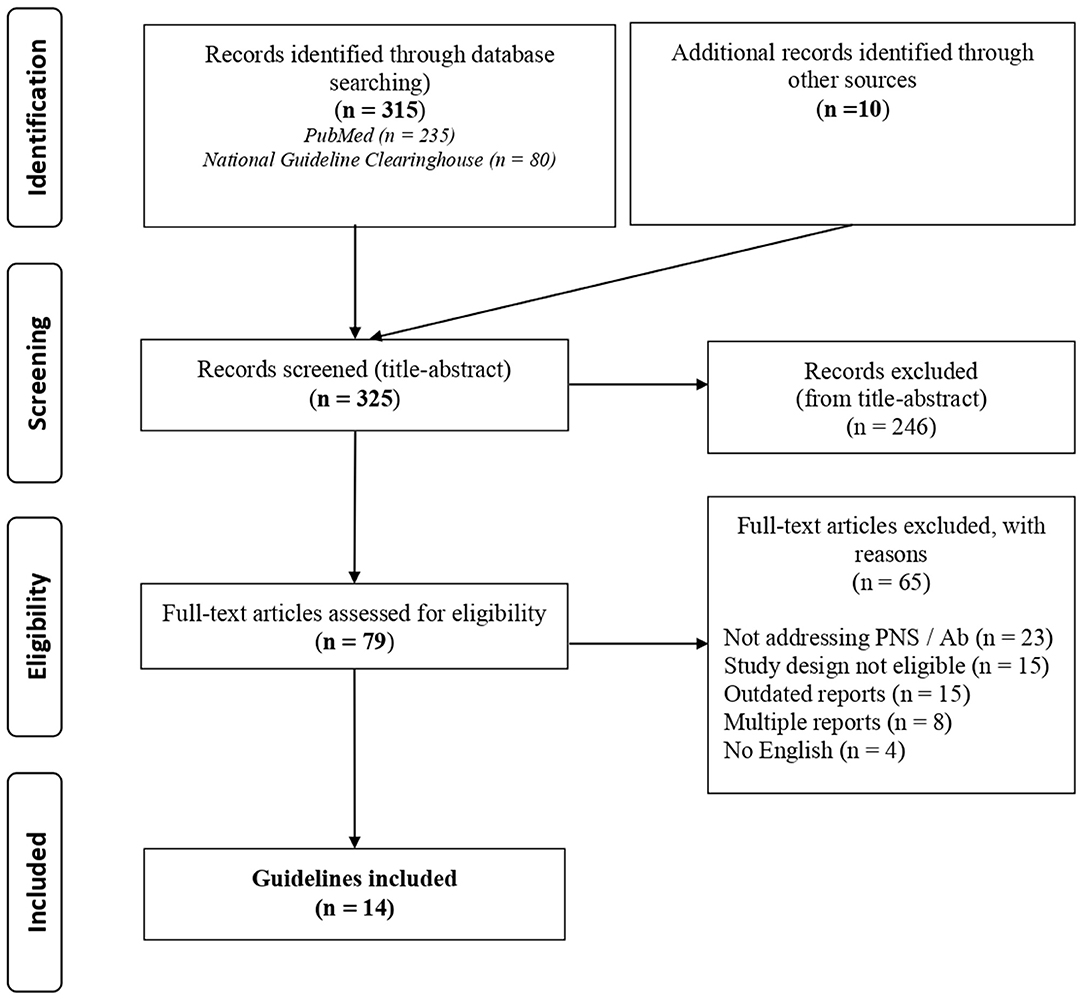
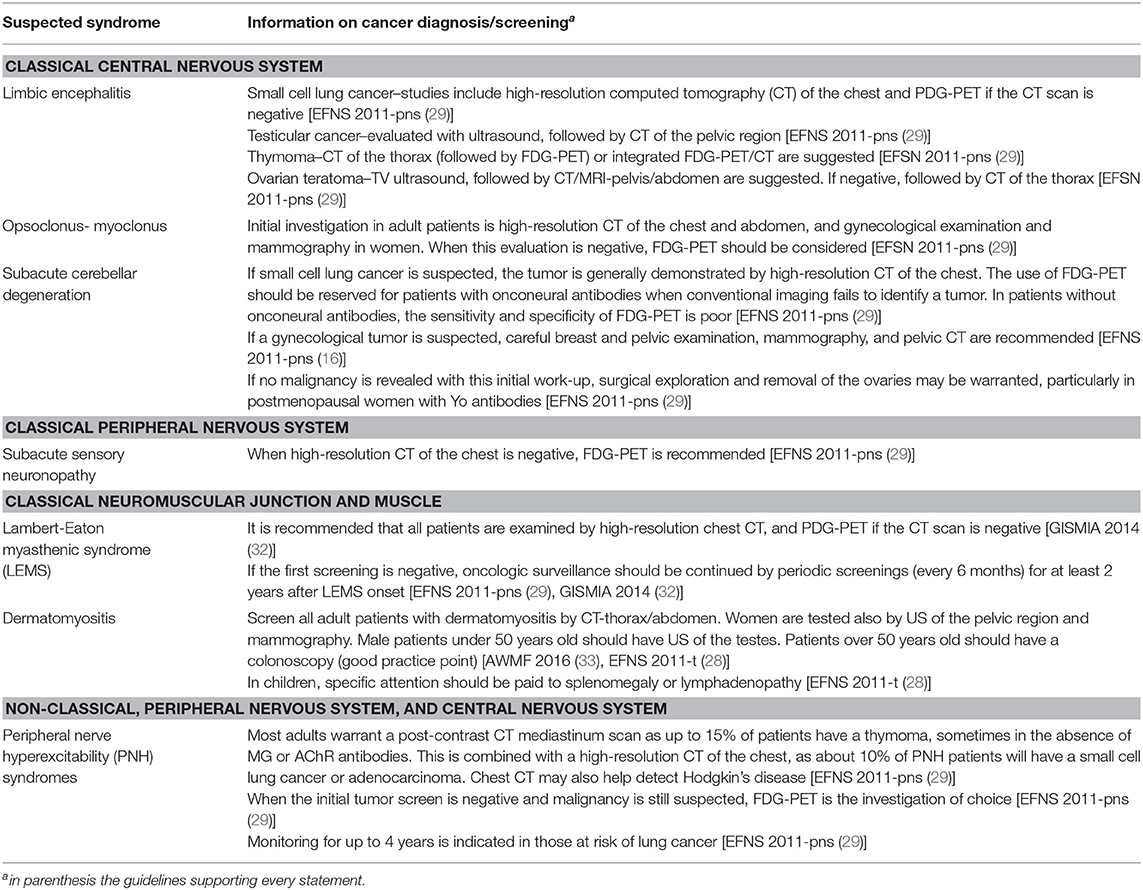
A total of 325 records were identified with the search strategy regarding PNS; from these, 79 were selected by title and abstract and 14 were included on the basis of the assessment of the full text (details in Figure 2, Supplementary Table 4). Data concerning circulating antibodies and/or TMs suggested for the diagnosis of the malignancies and the corresponding summary of recommendations are reported in detail in Table 3. The recommended strategies for the detection of possibly associated tumors in each PNS type are summarized in Table 4. Additional information regarding antibodies recommended to characterize each syndrome and the most commonly associated malignancies are reported in Supplementary Table 5.
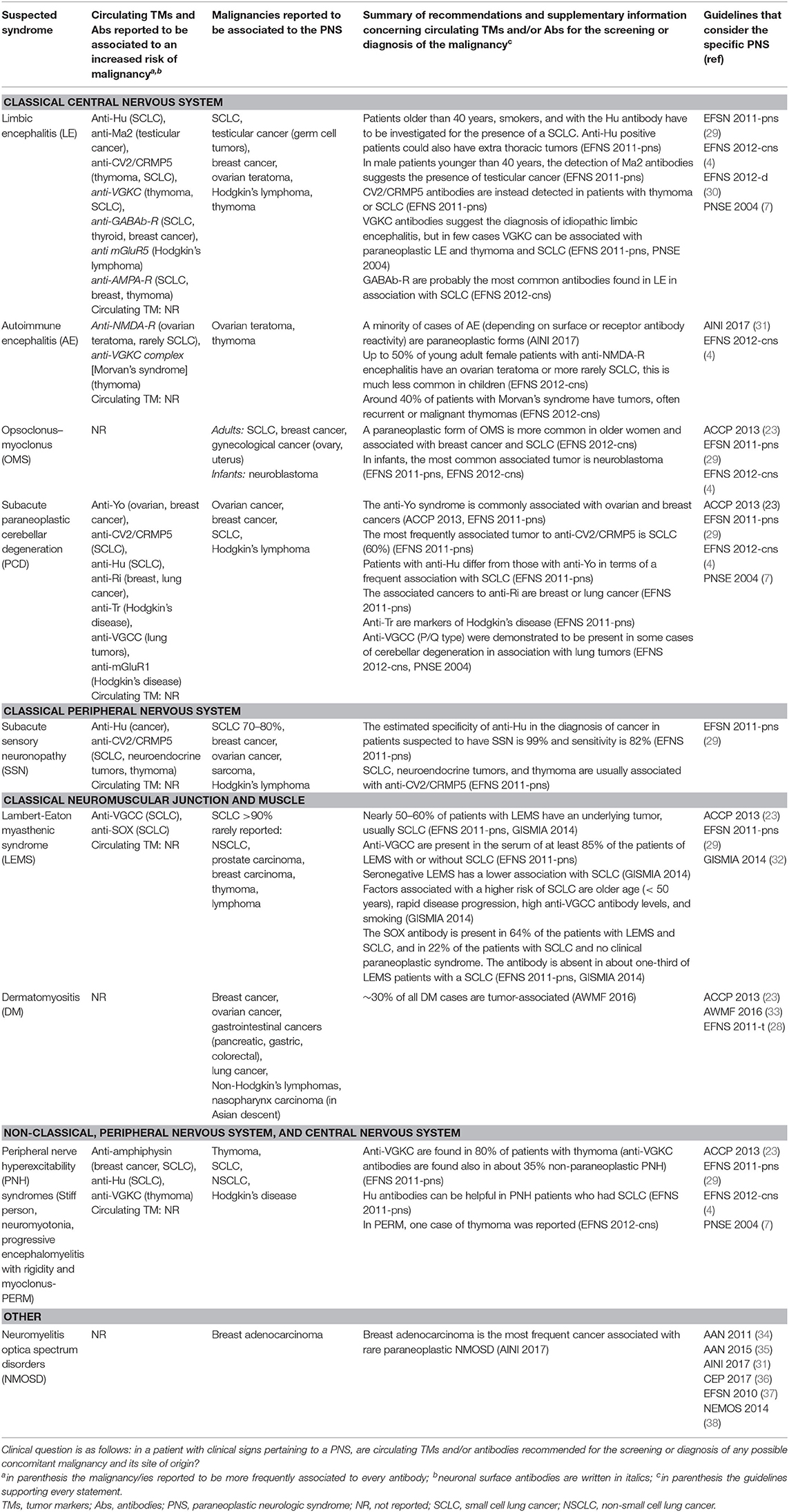
Table 3. Summary of recommendations and supplementary information concerning circulating TMs and/or antibodies for the screening or diagnosis of the malignancies in patients with PNS from guidelines focused on PNS.
Classical PNS of the central nervous system such as limbic encephalitis, autoimmune encephalitis, opsoclonus-myoclonus syndrome, and subacute cerebellar degeneration were considered by six guidelines (4, 7, 23, 29–31). The most commonly associated malignancy in adults is SCLC, though other malignancy types have been described to occur with minor frequency. Five guidelines did not consider a strategy for cancer diagnosis (4, 7, 23, 30, 31), whereas one provided recommendations on the screening of cancer in patients with classical PNS without mentioning TMs (29). Onconeural Abs positivity such as anti-Hu and anti-CV2/CRMP5 have been reported to be associated with an increased risk of SCLC occurrence whereas positive anti-Yo were associated with the risk of breast or ovarian cancer. Neuronal surface Abs anti-VGCC have been reported to be associated with an increased risk of SCLC.
As concerns classical PNS of the peripheral nervous system, one guideline referred to subacute sensory neuronopathy (29). SCLC is the malignancy most frequently associated with subacute sensory neuronopathy, but breast cancer, ovarian cancer, sarcoma, or Hodgkin's disease may occasionally occur as well. This guideline recommended a strategy for screening underlying cancer, but did not consider TMs (29). The positivity for anti-Hu is a strong predictor of the presence of cancer especially for SCLC. The positivity of anti-CV2/CRMP5 is reported to be frequently associated with SCLC, neuroendocrine tumor, and thymoma; however, the absence of antibodies cannot be considered a reliable criterion to exclude the presence of an underlying cancer.
Five guidelines considered classical PNS affecting the neuromuscular junction and muscle like Lambert-Eaton myasthenic syndrome and dermatomyositis (23, 28, 29, 32, 33). SCLC is described as the most frequently underlying tumor in patients with Lambert-Eaton myasthenic syndrome; breast, ovarian, pancreatic, stomach, colorectal, and lung cancer, as well as lymphomas have been reported in patients with dermatomyositis. Three guidelines (28, 32, 33) proposed a strategy for cancer screening which did not recommend TMs; one guideline considered NSE in SCLC patients, but underlined the low diagnostic sensitivity of the marker (32). Onconeural Abs anti-SOX are associated with an increased risk of cancer in Lambert-Eaton myasthenic syndrome, anti-P155/140 and anti TIF1y in dermatomyositis, but their absence is not sufficient to rule out the presence of a tumor (33).
Peripheral nerve hyperexcitability disorders included in non-classical PNS were considered by four guidelines (4, 7, 23, 29). Associated malignancies more frequently reported are thymoma, SCLC, lung adenocarcinoma, and Hodgkin's disease. One guideline recommended to screening for cancer, but did not mention TMs (29). Only onconeural Abs anti-amphiphysin were reported to occur more frequently in paraneoplastic cases of Stiff person syndrome (4).
Neuromyelitis optica spectrum disorders were considered by six guidelines (31, 34–38). We have classified them in the “other types” category because it is rarely associated with cancer. One guideline reported that breast carcinoma was the most frequently associated malignancy (31). Guidelines did not provide recommendations for cancer screening in patients with this type of presentation.
Discussion
PNS clinical manifestations are often indistinguishable from other more common neurologic diseases. Therefore, in clinical practice differential diagnosis between PNS and other neurologic conditions occur more frequently than should be expected, considering the low incidence of PNS, in general hospitals in particular.
Numerous guidelines for PNS management exist, but despite the general agreement among the recommendations, their implementation in clinical practice remains poor. In fact, when a patient has symptoms pertaining to a PNS, circulating TMs tests are frequently ordered. In a survey performed in three general hospitals in Italy as part of an internal quality assurance program, TMs requests were registered in recovery charts of 10.4–21.9% of consecutive patients admitted in neurological wards. In addition, from 61.6 to 84% of requested TMs were ordered in patients discharged from neurological wards without any diagnosis of malignancy (M. Gion, T. Trenti, personal communication). This empirical use of TMs in clinical practice is probably based on the anecdotal finding in sporadic patients with PNS, of increased levels of a TM even months before the clinical appearance of the malignancy. However, TMs have poor specificity (9–11), as circulating TM levels may be increased in several benign diseases, including systemic autoimmune and inflammatory diseases also involving the nervous system. In addition, TMs have a poor diagnostic sensitivity as their circulating levels are approximately proportional to tumor bulk (9–11, 39). In patients with PNS, this conflicts with the fact that TMs are usually considered for the screening of a malignancy when the tumor is not clinically apparent nor already detected by imaging techniques. A study (40) discussing strategies to avoid overuse as the next frontier to a health care of high quality, stated that “quality refers to the degree of match between health products and services, on the one hand, and the need they are intended to meet on the other” and that “health care that meets the needs is high quality, health care that does not meet the needs, is low quality.” In light of this statement, we can say that the request of a TM for the early diagnosis of cancer simply does not meet the need, and can be classified as overuse (41, 42). The overuse of TMs has several negative effects, including the risk of underestimating the presence of a cancer in the case of a false negative TM result, or the anxiety for the patients and the unnecessary workload of healthcare professionals for the additional tests needed to confirm or exclude the presence of a malignancy in the case of a false positive TM result. Therefore, the easy availability of a laboratory test does not justify its inappropriate request (43–45).
The present study provides a summary of the existing information reported in guidelines regarding the use of TMs for the detection of a tumor in patients with PNS. This is the most comprehensive review of guidelines on the use of circulating biomarkers in PNS diagnosis, looking at 18 guidelines from 13 different medical societies/associations. The systematic review highlights the consensus between recommended practices on this issue. Classical TMs are not recommended by any of the examined guidelines for the screening or the diagnosis of a malignancy in patients with a suspected PNS. In most cases, guidelines recommend searching for a tumor by clinical signs and traditional imaging techniques (i.e., CT scan of the chest, abdomen, and pelvis or by mammogram or ultrasound of the pelvis and testes). According to some guidelines (28, 29, 31), a combination of whole body FDG-PET and CT can also be considered to detect a tumor in some specific clinical situations. For example, a recently published guideline recommended performing PET scans on patients with suspected PNS (with/without onconeural Abs) and negative conventional imaging (46).
The detection of paraneoplastic antibodies, above all onconeural Abs, should be sought in patients with suspected PNS (28, 29, 31). Onconeural Abs are useful in defining the probability that a neurologic syndrome has a paraneoplastic origin leading clinicians toward the most appropriate work up (7).
Conversely, antibodies are only partially helpful in leading to the identification of a given malignancy, with the exception of onconeural Abs which are frequently associated with some specific malignancies. Paradigmatic examples are the association of anti-Hu Abs with SCLC and anti-Yo Abs with gynecological malignancies (23, 29).
In spite of the established role of antibodies in the differential diagnosis of PNS, all guidelines are in agreement with the limited value of antibodies as a tool to screen for the underlying malignancy. Accordingly, no guideline recommends the use of antibodies to detect a tumor and classical PNS presentation should prompt investigations of occult tumors regardless of the antibody status.
A study on the diagnostic accuracy for the cancer screening of panels of PNS antibody test results was recently published (47). The authors retrospectively reviewed 384 panels sent to two reference laboratories and found that diagnostic utility was poor, with a positive predictive value of 3.6% (1/28). In addition, they found that 15 patients negative for the PNS antibody, developed a malignancy on follow-up. The findings of this pragmatic study agree with the guidelines found in our systematic revision.
The present study has some limitations. First, the search for guidelines focused on PNS was systematic between 2007 and 2017, while the search for guidelines focused on oncology was updated in 2015 using documents identified in the Guide project (9). The explanation for this apparent temporal discrepancy is that these two searches were conducted as two independent sequential literature searches. At the time of completion of the oncological guidelines broader search and successive analysis, recommendations for the use of biomarkers in PNS were not found. The second literature search therefore included key terms to more specifically identify guidelines on PNS published or updated between 2007 and 2017. Also, in this second narrower search, a very small amount of information related to PNS was identified in the retrieved documents. Moreover, a successive scrutiny of guidelines published up to July 2019 confirmed the paucity of recommendations concerning the use of TM in PNS, attesting that no new relevant information emerged to affect the conclusions reported here. The second limitation of the present study relates to the absence of a quality evaluation for the selected guidelines. However, as a remarkable degree of unanimity was observed among the recommendations from the identified documents, one can presume that the quality appraisal of the guidelines would not have a real effect on the conclusions of the present study.
Notwithstanding these limitations, this study presents a novel tool which allows for the improvement of the implementation of recommendations on the role of circulating biomarkers for the screening of underlying malignancies in patients with PNS. We did not propose novel recommendations, but we summarized and compared all the recommendations produced by the existing guidelines. Considering the barriers to physician adherence to guidelines (20), we believe that the present work of synthesis may provide a more comprehensive, transparent, evidence-based, and theoretically informed rationale. The synthesized information tool may assist practitioners and stakeholders in the diffusion, dissemination, and implementation of guideline recommendations, decreasing inappropriate use of biomarkers for cancer screening in PNS, and ultimately improve the quality of health care.
Conclusions
A consistent agreement was found among recommendations on the use of circulating biomarkers in PNS management across multiple guidelines from various societies, reflecting the strength of the evidence. Guidelines assert that classical TMs do not have any evidence of clinical utility in this context and that they should not be ordered before the diagnosis of the malignancy is confirmed. Only after the diagnosis of a malignancy can TMs be requested for the initial work-up, treatment monitoring, and follow-up when recommended by clinical practice guidelines (9–11).
Guidelines confirm that circulating onconeural Abs remain valuable markers of neurologic autoimmune reactions and are useful in the differential diagnosis of PNS. Conversely, onconeural Abs can only be partially helpful in the identification of a tumor, being useful in defining the probability that a neurologic disorder has a paraneoplastic origin. In addition, they are not useful in the follow-up of the malignancy as they do not behave as classical markers associated to tumor bulk.
Guidelines agree on recommending that the search of the tumor has to be performed by clinical signs and traditional imaging techniques.
The information synthesized here may potentially help clinicians in choosing the appropriate diagnostic tools for malignancies, also leading to a reduction of the work-up costs.
Future efforts should focus on optimizing the implementation of the recommendations in health care settings where the inappropriate use of circulating TMs and antibodies for cancer screening and PNS is still occurring.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
PD and MG conceived the idea. AF, MG, and CT designed the study. IC and CT performed the literature review, selected articles, and extracted data. AF and CT contributed to the quality control of data and algorithms. IC, AF, MG, and CT drafted and edited the manuscript. PD, IC, BG, MG, AF, and CT contributed to the data analysis and interpretation. All authors contributed to the article, critically revised the manuscript draft, and approved the submitted version.
Funding
This study was supported by Institutional funding of the Veneto Institute of Oncology IOV-IRCCS and the publication fee of this work was supported by Ricerca Corrente 2020 from the Italian Ministry of Health (IT-MOH). Additional support was provided by Veneto Region (IT) through the Programma Regionale per i Biomarcatori Diagnostici, Prognostici e Predittivi assigned to Azienda ULSS3 Serenissima and by the Interreg V-A Italia-Slovenia Program 2014–2020, project TRANS-GLIOM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Tommaso Trenti (Head of Laboratory Medicine and Pathology Department, University and Healthcare Trust, Università degli Studi di Modena e Reggio Emilia, Italy), for communicating data from a survey performed in collaboration with one of the authors (MG) on the appropriateness of TM orders in inpatients as part of an internal quality assurance program. We would like to thank Dr. Antonette E. Leon (Regional Center for Biomarkers, Department of Clinical Pathology and Transfusion Medicine, Venice, Italy) for editorial assistance in the manuscript preparation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.607553/full#supplementary-material
Abbreviations
Abs, antibodies; PNS, paraneoplastic neurologic syndromes; SCLC, small cell lung cancer; TMs, tumor markers.
References
1. Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. (1996) 93:4529–36.
2. Giometto B, Taraloto B, Graus F. Autoimmunity in paraneoplastic neurological syndromes. Brain Pathol. (1999) 9:261–73.
3. Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. (2010) 257:509–17. doi: 10.1007/s00415-009-5431-9
4. Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. (2012) 83:638–45. doi: 10.1136/jnnp-2011-301237
5. Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet J Rare Dis. (2007) 2:22. doi: 10.1186/1750-1172-2-22
6. Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol. (2010) 67:330–5. doi: 10.1001/archneurol.2009.341
7. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. (2004) 75:1135–40. doi: 10.1136/jnnp.2003.034447
8. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. (2004) 159:882–90. doi: 10.1093/aje/kwh101
9. Gion M, Trevisiol C, Rutjes AW, Rainato G, Fabricio AS. Circulating tumor markers: a guide to their appropriate clinical use | comparative summary of recommendations from clinical practice guidelines (PART 1). Int J Biol Markers. (2016) 31:e332–67. doi: 10.5301/jbm.5000251
10. Gion M, Trevisiol C, Rutjes AWS, Rainato G, Fabricio ASC. Circulating tumor markers: a guide to their appropriate clinical use | comparative summary of recommendations from clinical practice guidelines (PART 2). Int J Biol Markers. (2017) 32:e1–52. doi: 10.5301/ijbm.5000259
11. Gion M, Trevisiol C, Rutjes AWS, Rainato G, Fabricio ASC. Circulating tumor markers: a guide to their appropriate clinical use | comparative summary of recommendations from clinical practice guidelines (PART 3). Int J Biol Markers. (2017) 32:e147–81. doi: 10.5301/ijbm.5000272
12. Loi S, Haydon AM, Shapiro J, Schwarz MA, Schneider HG. Towards evidence-based use of serum tumour marker requests: an audit of use in a tertiary hospital. Intern Med J. (2004) 34:545–50. doi: 10.1111/j.1445-5994.2004.00671.x
13. Al-Mughales JA, Alahwal MS. Inappropriate practice in tumor marker requests at a university hospital in Western Saudi Arabia: a 3-year retrospective study. Int J Biol Markers. (2020) 6:1724600820971305. doi: 10.1177/1724600820971305
14. Zhang H, Song Y, Zhang X, Hu J, Yuan S, Ma J. Extent and cost of inappropriate use of tumour markers in patients with pulmonary disease: a multicentre retrospective study in Shanghai, China. BMJ Open. (2018) 8:e019051. doi: 10.1136/bmjopen-2017-019051
15. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS ONE. (2013) 8:e78962. doi: 10.1371/journal.pone.0078962
16. Gion M, Trevisiol C, Fabricio ASC. Appropriateness of tumor marker request: a case of study. Ann Transl Med. (2017) 5:274. doi: 10.21037/atm.2017.06.19
17. Gion M, Cardinali G, Trevisiol C, Zappa M, Rainato G, Fabricio ASC. Indicators of inappropriate tumour marker use through the mining of electronic health records. J Eval Clin Pract. (2017) 23:895–902. doi: 10.1111/jep.12754
18. Mrazek C, Simundic AM, Salinas M, von Meyer A, Cornes M, Bauçà JM, et al. Inappropriate use of laboratory tests: how availability triggers demand - examples across Europe. Clin Chim Acta. (2020) 505:100–7. doi: 10.1016/j.cca.2020.02.017
19. Trevisiol C, Cinquini M, Fabricio ASC, Gion M, Rutjes AWS. Insufficient uptake of systematic search methods in oncological clinical practice guideline: a systematic review. BMC Med Res Methodol. (2019) 19:180. doi: 10.1186/s12874-019-0818-5
20. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? a framework for improvement. JAMA. (1999) 282:1458–65. doi: 10.1001/jama.282.15.1458
21. Harrison MB, Légaré F, Graham ID, Fervers B. Adapting clinical practice guidelines to local context and assessing barriers to their use. CMAJ. (2010) 182:E78–84. doi: 10.1503/cmaj.081232
22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
23. American College of Chest Physicians. Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e1S−512S. Available online at: https://journal.chestnet.org/issue/S0012-3692(13)X6006-4
24. Del Giudice L, Young S, Vella E, Ash M, Bansal P, Robinson A, et al. Referral of Suspected Lung Cancer by Family Physicians and other Primary Care Providers. Toronto, ON: Cancer Care Ontario (2011).
25. Goeckenjan G, Sitter H, Thomas M, Branscheid D, Flentje M, Griesinger F, et al. Prevention, diagnosis, therapy, and follow-up of lung cancer: interdisciplinary guideline of the German respiratory society and the German cancer society. Pneumologie. (2011) 65:39–59. doi: 10.1055/s-0030-1255961
26. Urological Tumours National Working Group. Renal Cell Carcinoma, Version: 2.0. Utrecht, Netherlands: Association of Comprehensive Cancer Centres (2010).
27. Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. (2015) 26:1604–20. doi: 10.1093/annonc/mdv041
28. Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. (2011) 18:19–27e3. doi: 10.1111/j.1468-1331.2010.03220.x
29. Vedeler CA, Antoine JC, Giometto B, Graus F, Grisold W, Honnorat J, et al. Paraneoplastic neurological syndromes. In: Gilhus NE, Barnes MP, Brainin M, editors. European Handbook of Neurological Management. Vol 1. 2nd Edn. Hoboken, NJ: Wiley (2010). p. 447–57.
30. Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, et al. EFNS-ENS guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. (2012) 19:1159–79. doi: 10.1111/j.1468-1331.2012.03784.x
31. Zoccarato M, Gastaldi M, Zuliani L, Biagioli T, Brogi M, Bernardi G, et al. Diagnostics of paraneoplastic neurological syndromes. Neurol Sci. (2017) 38:237–42. doi: 10.1007/s10072-017-3031-5
32. Evoli A, Liguori R, Romani A, Mantegazza R, Di Muzio A, Giometto B, et al. Italian recommendations for Lambert-Eaton myasthenic syndrome (LEMS) management. Neurol Sci. (2014) 35:515–20. doi: 10.1007/s10072-014-1637-4
33. Sunderkötter C, Nast A, Worm M, Dengler R, Dörner T, Ganter H, et al. Guidelines on dermatomyositis - excerpt from the interdisciplinary S2k guidelines on myositis syndromes by the German society of neurology. J Dtsch Dermatol Ges. (2016) 14:321–38. doi: 10.1111/ddg.12909
34. Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG, Therapeutics and Technology Assessment Subcommittee of American Academy of Neurology. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. (2011) 77:2128–34. doi: 10.1212/WNL.0b013e31823dc535
35. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
36. Sahraian MA, Moghadasi AN, Azimi AR, Asgari N H, Akhoundi F, Abolfazli R, et al. Diagnosis and management of neuromyelitis optica spectrum disorder (NMOSD) in Iran: a consensus guideline and recommendations. Mult Scler Relat Disord. (2017) 18:144–51. doi: 10.1016/j.msard.2017.09.015
37. Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. (2010) 17:1019–32. doi: 10.1111/j.1468-1331.2010.03066.x
38. Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the neuromyelitis optica study group (NEMOS). J Neurol. (2014) 261:1–16. doi: 10.1007/s00415-013-7169-7
39. Hayes DF. Biomarker validation and testing. Mol Oncol. (2015) 9:960–6. doi: 10.1016/j.molonc.2014.10.004
40. Berwick DM. Avoiding overuse-the next quality frontier. Lancet. (2017) 390:102–4. doi: 10.1016/S0140-6736(16)32570-3
41. Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, et al. Evidence for overuse of medical services around the world. Lancet. (2017) 390:156–68. doi: 10.1016/S0140-6736(16)32585-5
42. Korenstein D, Chimonas S, Barrow B, Keyhani S, Troy A, Lipitz-Snyderman A. Development of a conceptual map of negative consequences for patients of overuse of medical tests and treatments. JAMA Intern Med. (2018) 178:1401–7. doi: 10.1001/jamainternmed.2018.3573
43. Smellie WS. Demand management and test request rationalization. Ann Clin Biochem. (2012) 49:323–36. doi: 10.1258/acb.2011.011149
44. Moynihan R, Henry D, Moons KG. Using evidence to combat overdiagnosis and overtreatment: evaluating treatments, tests, and disease definitions in the time of too much. PLoS Med. (2014) 11:e1001655. doi: 10.1371/journal.pmed.1001655
45. Cadamuro J, Gaksch M, Wiedemann H, Lippi G, von Meyer A, Pertersmann A, et al. Are laboratory tests always needed? frequency and causes of laboratory overuse in a hospital setting. Clin Biochem. (2018) 54:85–91. doi: 10.1016/j.clinbiochem.2018.01.024
46. Harlos C, Poon R. PET Imaging in Paraneoplastic Neurological Syndromes. Toronto, ON: Cancer Care Ontario (2017).
Keywords: circulating tumor markers, paraneoplastic neurologic syndromes, practice guidelines, cancer diagnosis, quality of health care
Citation: Trevisiol C, Cani I, Fabricio ASC, Gion M, Giometto B and De Massis P (2021) Serum Tumor Markers in Paraneoplastic Neurologic Syndromes: A Systematic Review of Guidelines. Front. Neurol. 11:607553. doi: 10.3389/fneur.2020.607553
Received: 17 September 2020; Accepted: 09 December 2020;
Published: 18 January 2021.
Edited by:
Leonora Balaj, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Dusten Unruh, Northwestern Medicine, United StatesSimon Hanft, Westchester Medical Center, United States
Copyright © 2021 Trevisiol, Cani, Fabricio, Gion, Giometto and De Massis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Trevisiol, Y2hpYXJhLnRyZXZpc2lvbEBpb3YudmVuZXRvLml0
†These authors have contributed equally to this work
 Chiara Trevisiol
Chiara Trevisiol Ilaria Cani
Ilaria Cani Aline S. C. Fabricio
Aline S. C. Fabricio Massimo Gion
Massimo Gion Bruno Giometto
Bruno Giometto Patrizia De Massis
Patrizia De Massis