- 1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2College of Health, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3The First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4School of Energy and Power Engineering, Shandong University, Jinan, China
- 5Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
Introduction: Despite surgical and chemotherapeutical treatment options, the prognosis for glioblastoma (GBM) remains poor. Some studies have found that using lomustine plus bevacizumab to treat GBM can prolong overall survival (OS) and progression-free survival (PFS). The aim of this study was to explore the efficacy of the two drugs in combination treatment of GBM using a meta-analysis of the existing literature to help settle the ongoing debate.
Materials and Methods: PubMed, EMBASE, and the Cochrane Library were searched for the effectiveness of lomustine plus bevacizumab in GBM literature, updated on June 6, 2020. The main outcomes analyzed included PFS and OS; the effects of this drug combination on the 6-month PFS, which represents the percentage of patients who had PFS for 6 months, were also analyzed. All the data were pooled: OS and PFS with the mean difference (MD) and 6-month PFS with the risk ratio (RR). Because there were different control groups and dose groups, two subgroup analyses were run to ensure they were comparable. All statistical analyses were performed using the Review Manager Version 5.3 software.
Results: Six clinical trials were identified which included 1,095 patients (treatment group: 516; control group: 579). The group treated with lomustine and bevacizumab showed an improvement in OS (MD =1.37; 95% CI, 0.49–2.25; p = 0.002), PFS (MD = 0.23; 95% CI, 0.13–0.34; p < 0.00001), and 6-month PFS (RR = 2.29; 95% CI, 1.43–3.65; p = 0.0005). Two subgroup analyses of the main outcome, OS, show that the results of Control group A (p = 0.01) and Dose group 2 (p = 0.003) are significantly different from those of the other control or dose groups.
Conclusion: This study shows that lomustine and bevacizumab can effectively increase OS, PFS, and 6-month PFS in patients with GBM. The encouraging results of the lomustine and bevacizumab combination therapy for GBM should be studied in more clinical trials in the future.
Introduction
Glioblastoma is the most aggressive glioma (WHO Grade IV) and is associated with a uniformly poor prognosis (1). The current standard treatment for glioblastoma (GBM) consists of a multimodality approach which includes maximal surgical resection and radiotherapy with concurrent temozolomide, followed by cycles of adjuvant chemotherapy (2). Despite multimodality treatments, recent clinical trials have reported a median survival of only 14–16 months with a 2-year survival rate of 26–33% (2, 3). There is therefore an urgent need to explore new therapeutic strategies to improve patient prognosis.
Glioblastoma multiforme is a highly vascularized tumor where the vascular endothelial growth factor (VEGF) pathway is up-regulated, and it has been hypothesized that GBM would respond well to antiangiogenic treatments (4). Bevacizumab (BEV) is an antibody against the vascular endothelial growth factor receptor (VEGF) and a common therapy used for colorectal, lung, breast, kidney, and ovarian cancers (5–7). In 2009, bevacizumab was approved by the Food and Drug Administration for use as a treatment of recurrent glioblastoma (8). Despite obvious radiographic responses and an observed increase in progression-free survival (PFS), some clinical studies which investigated BEV reported that treatment has not resulted in a durable overall survival (OS) benefit in either recurrent or newly diagnosed GBM (9–12).
In a phase III study, enzastaurin was compared with lomustine, an alkylating agent of the nitrosourea family that is widely used as a salvage treatment drug, and lomustine was found to be more effective in treating GBM, suggesting that nitrosourea plays an important role in the treatment of recurrent GBM (13). A previous meta-analysis (14) study explored the efficacy of bevacizumab plus lomustine treatment in progressive glioblastoma, but the studies included were so few that the results were controversial, and the correlation subgroup analysis was incomplete. Thus, a further statistical analysis is needed to increase the credibility of this treatment combination.
Materials and Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and is presented based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. The protocol for this meta-analysis is available in PROSPERO (CRD42020190739).
Inclusion Criteria
Studies that met the following criteria were included in the meta-analysis: Population: recurrent GBM adult patients (≥18 years old), with a Karnofsky Performance Status score ≥50 or a WHO Performance Status score between 0 and 2, were used. Intervention: lomustine plus bevacizumab; Comparison: monotherapy of bevacizumab, lomustine, or bevacizumab plus irinotecan. Outcome: the outcomes of interest were OS and PFS or 6-month PFS, and their corresponding 95% confidence intervals (CIs) were provided; Non-English language literature was excluded. In addition, when we found duplicated or overlapping data in multiple reports, we included the one with the most complete information.
Search Strategy
Two investigators independently searched the electronic databases PubMed, EMBASE, and the Cochrane Library for relevant literature published up until June 2020. The search syntax included the following text words: “glioblastoma,” “bevacizumab,” “lomustine,” and “CCNU.” The detailed search strategy is available in the Supplementary Material.
Data Extraction
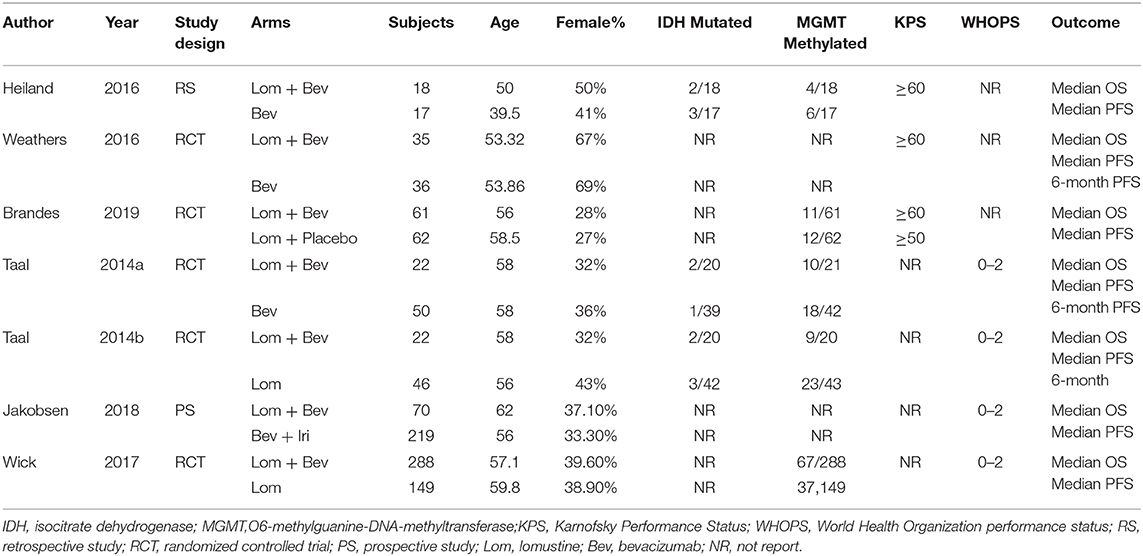
All data were reviewed and separately computed by two independent investigators. The following information was extracted from each trial: median OS, median PFS, 6-month PFS, study design, control group measures, isocitrate dehydrogenase (IDH) status, promoter of O6-methylguanine-DNA-methyltransferase (MGMT) status, Karnofsky Performance Status or WHO Performance Status score, drug dose, and the number and age of the patients in the experimental and control arms.
Quality Assessment
Two investigators separately rated the quality of the retrieved studies. We chose the risk-of-bias items recommended by The Cochrane Handbook for Systematic Reviews of Interventions for randomized controlled trials (RCTs). Items were evaluated in three categories: low risk of bias, unclear bias, and high risk of bias. In addition, we used the Methodological Index for Non-Randomized Studies (MINORS) for other clinically controlled trials.
Statistical Analysis
The statistical analyses were performed using the Review Manager Version 5.3 software (RevMan; The Cochrane Collaboration, Oxford, UK). The end points of interest in the pooled analysis were OS, PFS, and 6-month PFS. Because the outcome index is a continuous variable, the mean difference (MD) was used as the effect index. Heterogeneity across studies was examined using the I2 statistic (15). Studies with an I2 of 25–50%, 50–75%, or >75% were considered to have low, moderate, or high heterogeneity, respectively (16). We used the random-effects model of statistical analysis and a value of p < 0.05 indicated statistical significance. In addition, we did a subgroup analysis based on dose and measures in the control groups in order to analyze the factors that influence disease response and a sensitivity analysis to find the sources of heterogeneity.
Results
Overview of the Literature Search
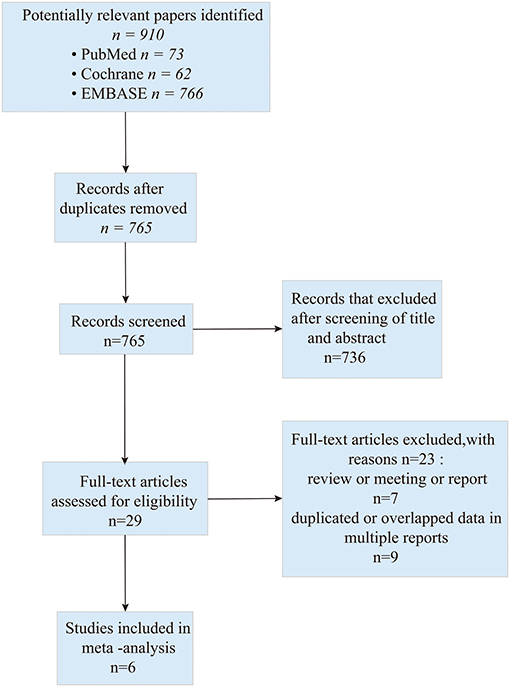
A total of 901 studies were retrieved initially for the evaluation. We did the initial screening based on the title and abstract, and 29 publications were chosen for further analysis. Eventually, six studies (one of the studies was split into two groups) (17–22) which addressed the combination of bevacizumab and lomustine in treating GBM were included in this study. The search process is described in Figure 1.
Study Characteristics and Bias Risk Assessment Results
All included studies in this study were based on moderate to high-quality evidence. The primary characteristics of the six studies are detailed in Table 1. Of the six studies included, four were RCTs and two were non-RCTs. According to the type of study, we used The Cochrane Handbook for Systematic Reviews of Interventions and MINORS to assess the risk of bias, respectively. The results of the quality assessment results are shown in the Supplementary Tables 1, 2.
Clinical Effect With the Combination of Bevacizumab and Lomustine in GBM
Pooled Analysis of OS
Pooling the OS data from the six studies (17–22) showed that bevacizumab combined with lomustine did prolong OS (MD =1.37; 95% CI, 0.49–2.25; p = 0.002) when compared to OS in the bevacizumab or lomustine monotherapy groups and the bevacizumab plus irinotecan group (Figure 2).
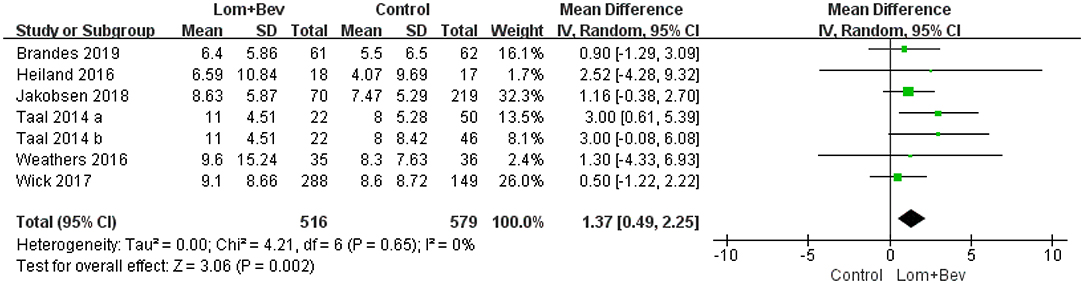
Figure 2. Forest plot of OS in glioblastoma between lomustine plus bevacizumab and control groups. OS, overall survival; Lom, lomustine; Bev, bevacizumab.
Pooled Analysis of PFS and Sensitivity Analysis
A random effects model was used to pool the PFS data (17–22). Despite the high degree of heterogeneity (I2 = 83%), the pooled data showed that the combination of bevacizumab and lomustine resulted in longer PFS (MD = 1.46; 95% CI, 0.27–2.65; p = 0.02) than in the control groups. We then looked for heterogeneous sources based on the sensitivity analysis. The results showed a significant decrease in heterogeneity (I2 = 11%) when the study of Wick (20) was removed and still had statistical significance (p = 0.02). This data indicates the results were robust (Figure 3).
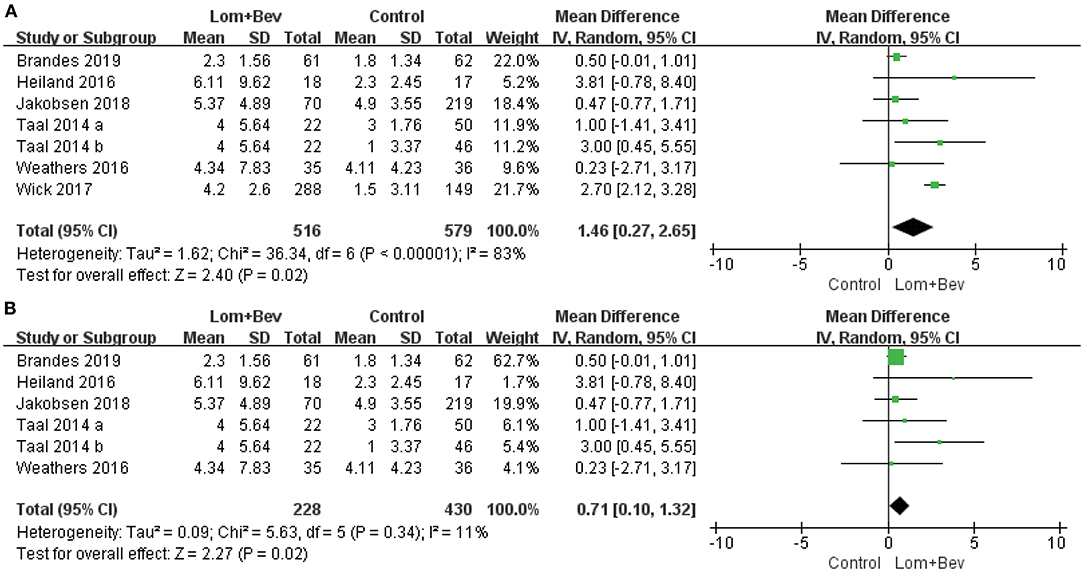
Figure 3. Forest plot of PFS in glioblastoma between lomustine plus bevacizumab and control groups. (A) All of the studies were included. (B) The study of Wick was removed. PFS, progression-free survival; Lom, lomustine; Bev, bevacizumab.
Pooled Analysis of 6-Month PFS
Pooling the 6-month PFS data from two of the studies (17, 19) showed that bevacizumab plus lomustine did improve the 6-month PFS (RR = 2.29; 95% CI, 1.43–3.65; p < 0.0005) compared with that in the bevacizumab or lomustine monotherapy groups (Figure 4).
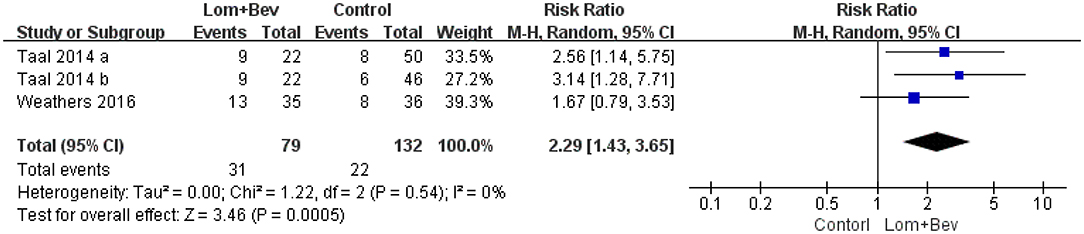
Figure 4. Forest plot of 6-month PFS in glioblastoma between lomustine plus bevacizumab and control groups. Six-month PFS, the percentage of patients who could progression-free survival for 6 months; Lom, lomustine; Bev, bevacizumab.
Subgroup Analysis of OS
First, we divided the patient data into Control group A (bevacizumab monotherapy; MD = 2.72; 95% CI, 0.63–4.81; p = 0.01), Control group B (lomustine monotherapy or lomustine plus placebo; MD = 1.03; 95% CI, −0.21 to 2.27; p = 0.10), and Control group C (bevacizumab plus irinotecan; MD = 1.16; 95% CI, −0.38 to 2.7; p = 0.14). Only Control group A showed statistical significance. Based on the different doses used in each study, we then divided the patients into Dose group 1 [Bev (5 mg/kg every 3 weeks) + Lom (90 mg/m2 every 6 weeks)]; MD = 1.80; 95% CI, −2.54 to 6.13; p = 0.42, Dose group 2 [Bev (10 mg/kg every 2 weeks) + Lom (90 mg/m2 every 6 weeks)]; MD = 1.95; 95% CI, 0.67−3.23; p = 0.003, and Dose group 3 [Bev (10 mg/kg every 2 weeks) + Lom (90–200 mg/m2 every 6 weeks)]; MD = 0.65; 95% CI, −0.70 to 2.01; p = 0.34. The results show that only the results of Dose group 2 had statistical significance. A forest plot of all subgroup analyses is shown in Figure 5.
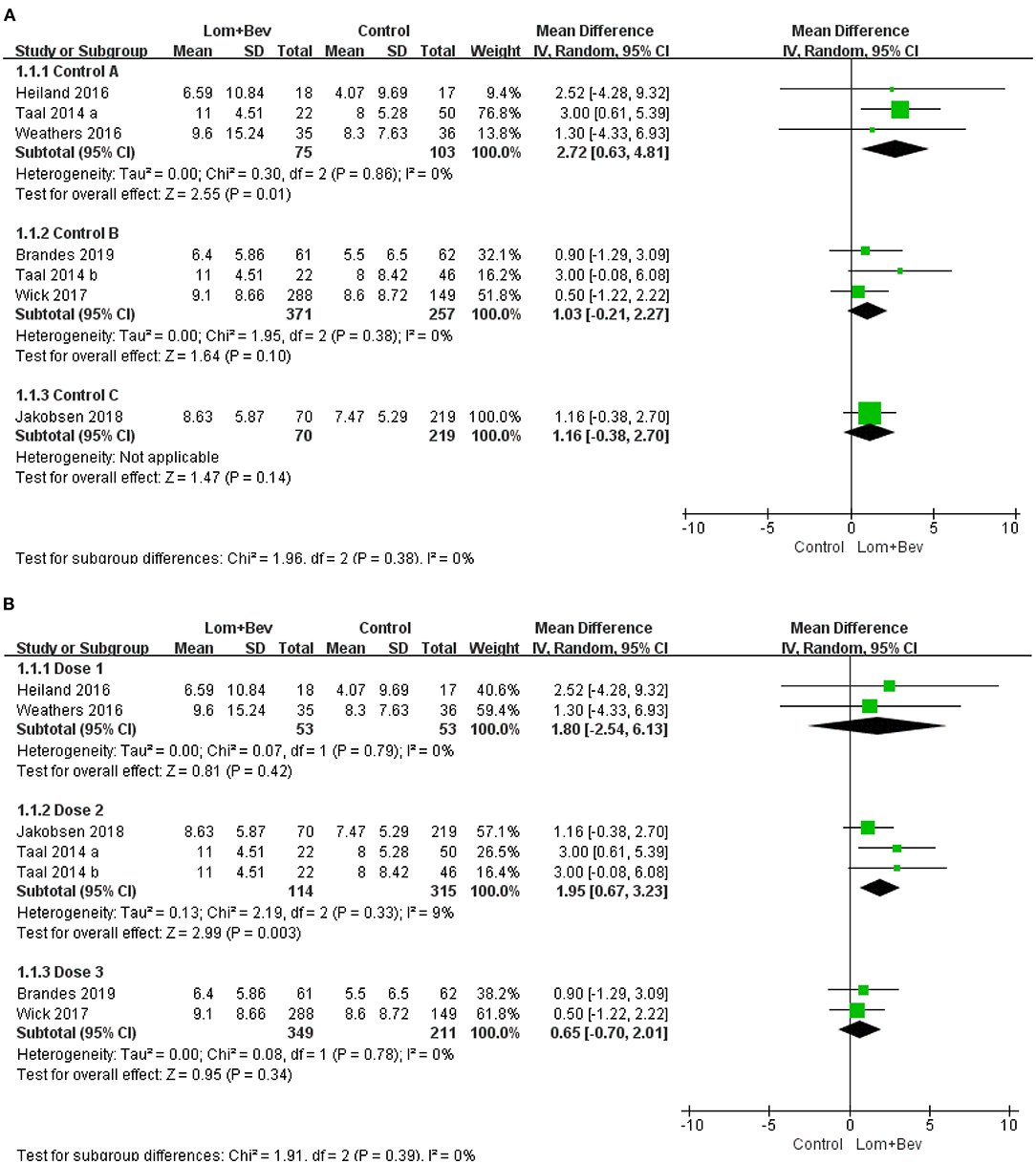
Figure 5. Subgroup analysis of OS in glioblastoma according to (A) different control groups, (B) different dose groups. Control group A, bevacizumab monotherapy; Control group B, lomustine monotherapy or lomustine plus placebo; Control group C, bevacizumab plus irinotecan; Dose1, Bev(5 mg/kg every 3 weeks) + Lom(90 mg/m2 every 6 weeks); Dose2, Bev (10 mg/kg every 2 weeks) + Lom (90 mg/m2 every 6 weeks); Dose3, Bev(10 mg/kg every 2 weeks) + Lom (90–200mg/m2 every 6 weeks).
Discussion
Glioblastoma (GBM) is one of the most aggressive brain cancers in adults (2). Despite surgical treatment and chemotherapy options, the prognosis for patients remains poor (23). Bevacizumab, a monoclonal antibody against VEGF-A, used alone or in combination with cytotoxic drugs, showed interesting results in terms of radiographic response rates and PFS in initial phase 2 studies of GBM (11, 24). However, these studies lacked a non-bevacizumab control group and OS did not increase with the experimental group compared to the control group. Another study showed that early treatment with bevacizumab improved PFS, but not OS, again suggesting that the treatment with bevacizumab alone might not be sufficient in improving GBM patient prognosis (25). Thus, we looked for another drug to combine with bevacizumab to improve OS and chose lomustine, a DNA alkylating agent. Lomustine is an approved treatment option for recurrent GBM and has also been frequently administered in clinical trials as the standard treatment (26).
In our study, lomustine plus bevacizumab showed a positive effect not only on PFS but also on OS in GBM patients. In addition, we found that the combination significantly improved 6-month PFS in GBM patients. These results are different to a previous meta-analysis (14) study which had explored the efficacy of lomustine plus bevacizumab in progressive GBM and which showed that treating patients with bevacizumab and lomustine could improve PFS significantly compared to control groups. However, there was no significant difference on OS. Because the previous study (14) did not provide a subgroup analysis, we performed some subgroup analyses based on the different control groups and drug doses to determine whether the results from the six studies included in our study, were comparable.
It is a remarkable fact that the dose selection we obtained through the subgroup analysis is different from previous study (27) results. The previous study (27) emphasized that the potential negative consequences of higher doses of bevacizumab are related to the promotion of tumor hypoxia, a well-known therapeutic tolerant medium, and the promotion of the aggressive phenotype of GBM. Furthermore, in a retrospective analysis, bevacizumab at low dose strength (<5 mg/kg/week) can improve PFS and OS better than bevacizumab at normal dose strength, and for patients with high-grade glioma, there was an inverse relationship between the dose intensity of bevacizumab and OS (r = 0.48, p > 0.00001) (28). It was hypothesized that lower doses of anti-angiogenic therapy may potentially improve the delivery of chemotherapeutic drugs and ultimately improve the prognosis of patients. However, in our study, the improvement in survival outcomes was more pronounced in Dose group 2 than in Dose group 1, which suggests that the bevacizumab dose may not be inversely related to OS. Moreover, based on the results of Dose group 3, we concluded that at the same dose of bevacizumab, an excessive dose of lomustine may be detrimental to OS. Although this study shows that Dose group 2 resulted in the best outcomes, the grouping is still not detailed enough. In future trials, more doses should be tested to determine the best dose combination, and the corresponding adverse reactions.
The mechanism behind the observed increase in OS, PFS, and 6-month PFS, when patients are treated with both lomustine and bevacizumab, is still not completely clear. It has been proposed that the normalization of vessels around the tumor, improvement of regional cerebral blood flow, augmentation of the antitumor effects of chemotherapy and radiotherapy are key components of the antiangiogenic activity (29–31), but more studies are needed.
Since there are not enough clinical studies on lomustine combined with bevacizumab in the treatment of GBM, only six studies were included. There may be some bias in these conclusions and a more systematic and theoretical analysis is required to determine the effectiveness of this drug combination in GBM. At present, the results of lomustine combined with bevacizumab are still encouraging for clinical treatment of GBM, and Dose group 2 is a potential option that provides a good starting point for discussions and further clinical trials.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XR: the main author of this article and the thought provider of this meta-analysis. DA: provided technical guidance for the meta-analysis. TL: assisting in writing articles, searching literature, and proofreading. LX: mainly responsible for the editing of figures and tables. LS: responsible for overall supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.603947/full#supplementary-material
References
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
3. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. (2013) 31:4085–91. doi: 10.1200/JCO.2013.49.6968
4. Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y, Naz-Khan H, Stubbs A, van der Spek P, et al. Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res. (2016) 76:525–34. doi: 10.1158/0008-5472.CAN-15-0776
5. Reinmuth N, Heigener D, Reck M. Novel angiogenesis inhibitors in nonsmall cell lung cancer. Curr Opin Oncol. (2015) 27:79–86. doi: 10.1097/CCO.0000000000000166
6. Komiyama S, Kugimiya T, Takeya C, Takahashi R, Kubushiro K. Platinum-resistant recurrent ovarian cancer with long survival on bevacizumab and gemcitabine. J Obstetr Gynaecol Res. (2018) 44:1330–4. doi: 10.1111/jog.13664
7. Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. (2012) 22:21–35. doi: 10.1016/j.ccr.2012.05.037
8. Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. (2009) 14:1131–8. doi: 10.1634/theoncologist.2009-0121
9. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. New Engl J Med. (2014) 370:709–22. doi: 10.1056/NEJMoa1308345
10. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. New Engl J Med. (2014) 370:699–708. doi: 10.1056/NEJMoa1308573
11. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. (2009) 27:4733–40. doi: 10.1200/JCO.2008.19.8721
12. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. (2007) 25:4722–9. doi: 10.1200/JCO.2007.12.2440
13. Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. (2010) 28:1168–74. doi: 10.1200/JCO.2009.23.2595
14. Song J, Xue YQ, Zhao MM, Xu P. Effectiveness of lomustine and bevacizumab in progressive glioblastoma: a meta-analysis. OncoTargets Therapy. (2018) 11:3435–9. doi: 10.2147/OTT.S160685
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Taal W, Oosterkamp HM, Walenkamp AME, Dubbink HJ, Beerepoot LV, Hanse MCJ, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. (2014) 15:943–53. doi: 10.1016/S1470-2045(14)70314-6
18. Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A. Progression-free and overall survival in patients with recurrent Glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol. (2016) 126:567–75. doi: 10.1007/s11060-015-2002-z
19. Weathers SP, Han X, Liu DD, Conrad CA, Gilbert MR, Loghin ME, et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neuro Oncol. (2016) 129:487–94. doi: 10.1007/s11060-016-2195-9
20. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. New Engl J Med. (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
21. Jakobsen JN, Urup T, Grunnet K, Toft A, Johansen MD, Poulsen SH, et al. Toxicity and efficacy of lomustine and bevacizumab in recurrent glioblastoma patients. J Neurooncol. (2018) 137:439–46. doi: 10.1007/s11060-017-2736-x
22. Brandes AA, Gil-Gil M, Saran F, Carpentier AF, Nowak AK, Mason W, et al. A randomized phase II trial (TAMIGA) evaluating the efficacy and safety of continuous bevacizumab through multiple lines of treatment for recurrent glioblastoma. Oncologist. (2019) 24:521–8. doi: 10.1634/theoncologist.2018-0290
23. Lombardi G, Pambuku A, Bellu L, Farina M, Della Puppa A, Denaro L, et al. Effectiveness of antiangiogenic drugs in glioblastoma patients: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. (2017) 111:94–102. doi: 10.1016/j.critrevonc.2017.01.018
24. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. (2009) 27:740–5. doi: 10.1200/JCO.2008.16.3055
25. Schaub C, Schäfer N, Mack F, Stuplich M, Kebir S, Niessen M, et al. The earlier the better? Bevacizumab in the treatment of recurrent MGMT-non-methylated glioblastoma. J Cancer Res Clin Oncol. (2016) 142:1825–9. doi: 10.1007/s00432-016-2187-3
26. Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. (2013) 31:3212–8. doi: 10.1200/JCO.2012.47.2464
27. de Groot JF. High-dose antiangiogenic therapy for glioblastoma: less may be more? Clin Cancer Res. (2011) 17:6109–11. doi: 10.1158/1078-0432.CCR-11-1853
28. Lorgis V, Maura G, Coppa G, Hassani K, Taillandier L, Chauffert B, et al. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol. (2012) 107:351–8. doi: 10.1007/s11060-011-0748-5
29. Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. (2007) 11:83–95. doi: 10.1016/j.ccr.2006.11.021
30. Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA. Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. (2015) 121:997–1007. doi: 10.1002/cncr.28935
Keywords: glioblastoma, lomustine, bevacizumab, meta-analysis, dose
Citation: Ren X, Ai D, Li T, Xia L and Sun L (2021) Effectiveness of Lomustine Combined With Bevacizumab in Glioblastoma: A Meta-Analysis. Front. Neurol. 11:603947. doi: 10.3389/fneur.2020.603947
Received: 08 September 2020; Accepted: 18 December 2020;
Published: 20 January 2021.
Edited by:
Maria Caffo, University of Messina, ItalyReviewed by:
Antonio Silvani, Fondazione IRCCS Istituto Neurologio Carlo Besta, ItalyFrancesco Pasqualetti, Azienda Ospedaliero Universitaria Pisana, Italy
Copyright © 2021 Ren, Ai, Li, Xia and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingzhi Sun, MjAxOTE0MTE0QG1haWwuc2R1LmVkdS5jbg==
 Xing Ren
Xing Ren Di Ai2
Di Ai2