- 1Department of Neurology, Zhuhai City Hospital of Integrated Traditional Chinese and Western Medicine, Zhuhai, China
- 2Department of Nephrology, Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, China
Objective: To investigate the roles of serum uric acid (UA), bilirubin (BIL), albumin (ALB), and creatinine (CRE) as major intravascular antioxidants, in benign paroxysmal positional vertigo (BPPV).
Methods: The serum levels of UA, BIL, ALB, and CRE were retrospectively analyzed in 70 patients with new-onset idiopathic BPPV and 140 age- and sex-matched healthy controls (HCs).
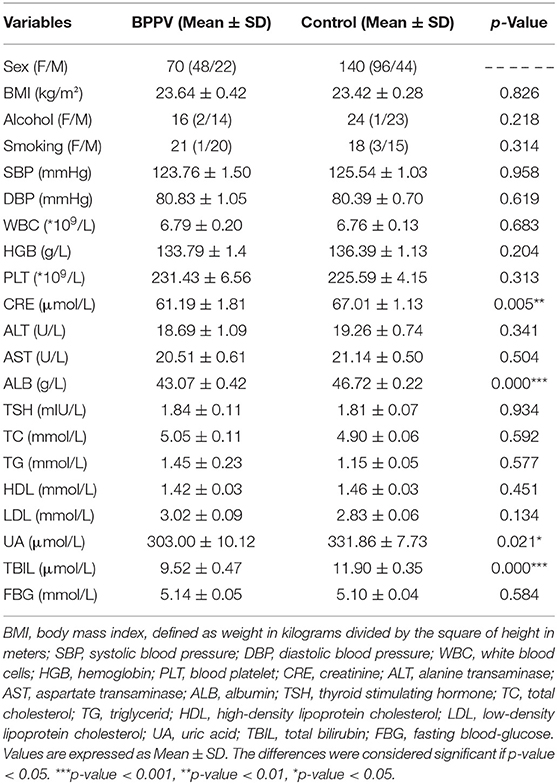
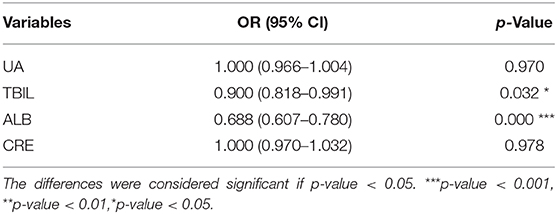
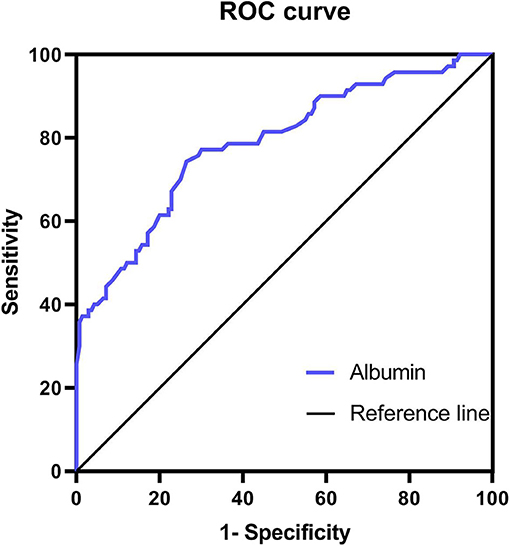
Results: Serum UA, BIL, ALB, and CRE levels were significantly lower in the BPPV group than the HC group. Furthermore, serum levels of BIL and ALB were significantly lower in the BPPV group when compared by sex. Multiple stepwise logistic regression revealed that a reduction in serum ALB was independently related to BPPV (odds ratio = 0.688; 95% confidence interval = 0.607– 0.780). Receiver operating characteristic analyses revealed a cut-off value of 45.15 g/L for ALB with a sensitivity of 74.29% (62.97– 83.07%) and specificity of 73.57% (65.71– 80.18%).
Conclusions: Serum levels of UA, BIL, ALB, and CRE were lower in BPPV patients, indicating a lower antioxidant status. Furthermore, a reduction in serum ALB was independently associated with BPPV. These results provide insights into the possible roles of oxidative stress in the pathogenesis of BPPV.
Introduction
Benign paroxysmal positional vertigo (BPPV), characterized by dizziness and vertigo, has a lifetime prevalence of more than 2.4% (1). The cause of BPPV is the detachment of otoconia (calcium carbonate crystals) that either float in the semicircular canal or attach to the cupule (2). However, at present, there are limited methods and techniques available to evaluate the condition of semicircular canals and vestibules, and the physiopathological explanations of BPPV are mainly speculative. Although most recover after positional maneuvers, up to 2/3 of patients may experience chronic, obstructive instability, dizziness, and discomfort, also known as residual dizziness (3). Meanwhile, the relapse rate of BPPV in the elderly is reportedly 23.5–50% (4). Hence, further studies of the mechanism underlying the onset of BPPV could provide new and efficient treatment regimens for residual dizziness and to decrease the recurrence rate.
It has been reported that Meniere's disease (MD) may be a systemic oxidative disorder, in which excessive free radicals and oxidative stress promote microvascular damage and participate in the development of endolymphedema (5). Recent studies have shown that some of the symptoms of BPPV are similar to those of MD and can occur at any stage, including the age of onset (6). Although it is attractive to speculate that oxidative stress plays a major role in pathogenesis of BPPV, there are few validated blood markers of the antioxidant status of BPPV.
Current evidence suggests that serum uric acid (UA), bilirubin (BIL), albumin (ALB), and creatinine (CRE) are the main non-enzymatic antioxidants in human plasma, accounting for 85% of the total antioxidant capacity (7). UA is a naturally occurring product of purine metabolism and is known as a strong scavenger of peroxynitrite. UA can exert protective antioxidant effects, inhibit inflammatory cascades, reduce blood–brain barrier permeability, and protect central nervous tissue (8). BIL was previously considered as a fat-soluble metabolite with only slight cytotoxicity, but recent studies have found that BIL is a natural antioxidant with strong antioxidant activity and an endogenous scavenger of reactive oxygen species (ROS) (8). CRE is a metabolite of creatine phosphate, which exists in skeletal muscle and is one of the components of total antioxidant determination (9). ALB is the main antioxidant molecule in extracellular fluid and can remove ROS and reactive nitrogen species (RNS) produced by various reactions (10). The serum levels of these markers can be used to predict whether oxidative stress is involved in the pathology of BPPV.
ALB and CRE levels are lower in patients with BPPV as compared to healthy controls (HCs) (11, 12). However, in BPPV, the exact relationship between UA and BPPV remains controversial (13), while that between BIL and BPPV remains unknown. Overall, previous studies have failed to comprehensively investigate the significance of these four markers (UA, BIL, ALB, and CRE) of antioxidant status in BPPV.
Therefore, the aims of the present study were to (1) test the hypothesis that serum levels of UA, BIL, ALB, and CRE are low in patients with BPPV, and (2) determine if any of these markers are protective factors in BPPV.
Materials and Methods
Participants
The study cohort consisted of 210 people, including 70 patients with idiopathic BPPV recruited from the Neurology Department and 140 sex- and age-matched HCs recruited from the Physical Examination Center at our hospital from January 1, 2015 to December 31, 2018.
Inclusion and Exclusion Criteria
The diagnosis of BPPV was based on a typical history of recurrent, brief attacks of positional vertigo and positioning tests, such as the Dix-Hallpike test or roll test (2). Only patients with new-onset idiopathic BPPV were include in this study. All patients with BPPV underwent canalith repositioning maneuvers of the affected semicircular canal. Moreover, for all BPPV patients, a thorough medical history was obtained and neurological testing was performed.
The exclusion criteria were as follows: (1) hospitalization for more than 48 h after vertigo; (2) secondary factors, such as a history of head trauma and vestibular neuritis; (3) pre-existing chronic instability at the onset of BPPV; (4) severe cardiovascular disease, central nervous system disease, parathyroid dysfunction, or thyroid disease; (5) severe renal and/or hepatic impairment; (6) hypertension and/or diabetes; and (7) current infection.
Measures
Serum levels of UA, ALB, CRE, and total BIL (TBIL) were measured using a Hitachi LST008 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan) after 8–10 h of fasting during the first 48 h after the onset of BPPV. The concentrations of alanine transaminase (ALT), aspartate transaminase (AST), fasting blood-glucose (FBG), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), and thyroid stimulating hormone (TSH) were measured by the same method.
Ethical Approval
The study protocol was approved by the local Ethics Review Board and conducted in accordance with the ethical standards of the Declaration of Helsinki.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 24.0. (IBM Corporation, Armonk, NY, USA). All data are presented as the mean ± standard deviation (SD). To evaluate the significance of the difference between the groups, the independent-samples t-test was used for normally distributed data, while the Mann–Whitney U test was used for non-normally distributed data. Analysis of covariance was used to compare serum levels of UA, TBIL, ALB, and CRE between the BPPV and HC groups. Multiple stepwise logistic regression analysis was performed to identify predictive indicators of BPPV. The odds ratio (OR) and corresponding 95% confidence interval (CI) were calculated. On-parameter receiver operating characteristic (ROC) analysis was conducted and the area under curve (AUC) was calculated using 95% CIs. Cut-off values were calculated for each factor. A probability (p) value of < 0.05 was considered statistically significant.
Results
General Statistics
The clinical characteristics of the study participants are summarized in Table 1. As compared with the HC group, serum levels of UA (p = 0.021) and CRE (p = 0.005) were significant lower, while ALB and TBIL levels were very significantly lower (p < 0.001) in the BPPV group (Table 1).
Subgroup Analysis by Sex
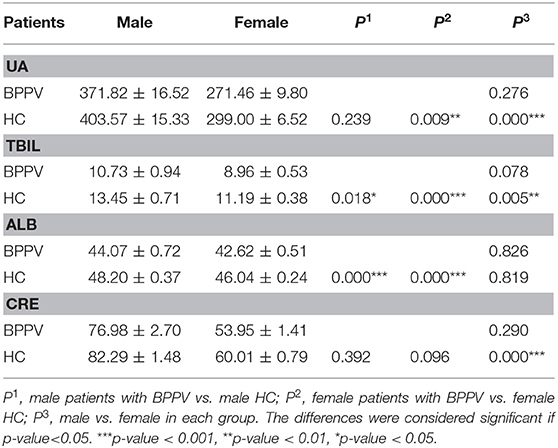
The effects of sex on serum levels of UA, TBIL, ALB, and CRE between the BPPV and HC groups were determined (Table 2, Figure 1). As expected, the results showed highly significant differences in serum levels of UA, TBIL, and CRE between males and females in the HC group, but not ALB (p = 0.819). In contrast, there were no significant differences in the serum levels of UA, TBIL, ALB, and CRE between males and females in the BPPV group (p = 0.276, 0.078, 0.826, and 0.290, respectively). Thus, there was no sex-specific differences in the BPPV group, as in the HC group. Notably, there was no sex-specific difference in serum ALB between males and females in the BPPV and HC groups.
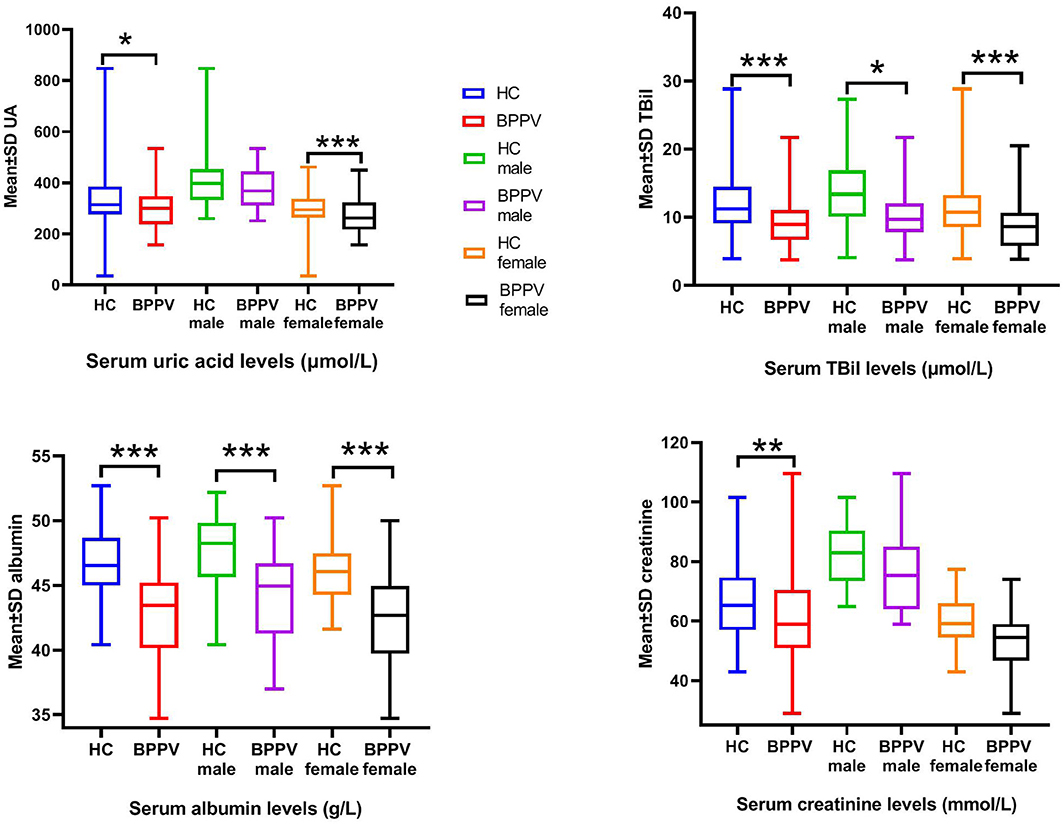
Figure 1. Serum levels of UA, BIL, ALB, and CRE between males and females in the BPPV and HC groups. ***p-value < 0.001, ** p-value < 0.01, *p–value < 0.05.
Sex-specific comparisons showed that there were no significant differences in serum levels of UA and CRE among males between the BPPV and HC groups (p = 0.239 and 0.392, respectively). On the other hand, with the exception of CRE (p = 0.096), there were highly significant differences in serum levels of UA, TBIL, and ALB among females between the BPPV and HC groups. In summary, there were no significant differences in serum CRE levels between males and females in the BPPV and HC groups, but highly significant differences in serum levels of TBIL (p = 0.018) and ALB (p = 0.000) among females between the BPPV and HC groups. The high significance between males and females in the BPPV group as compared to the HC group also explains why there was no significant difference between males and females in the BPPV group.
Multiple Stepwise Logistic Regression Analysis
Multiple stepwise logistic regression analysis unexpectedly revealed that a reduction in serum ALB, but not UA, TBIL, or CRE, was associated with BPPV (p < 0.05; OR = 0.688; 95% CI = 0.607–0.780) (Table 3).
ROC Analyses
ROC analyses were performed to assess the levels of ALB. The AUC for ALB was 0.787 (0.720 – 0.855). The cut-off value of ALB was 45.15 g/L with a sensitivity of 74.29% (62.97– 83.07%) and specificity of 73.57% (65.71– 80.18%) (Figure 2).
Discussion
The results of the present study showed that serum levels of UA, BIL, ALB, and CRE were significantly lower in the BPPV group than the HC group. In addition, subgroup analysis based on sex confirmed these results for BIL and ALB. Moreover, ALB was associated with BPPV. To the best of our knowledge, this is the first report of the relationship between BIL and BPPV, and the first report of UA, BIL, ALB, and CRE as indicators of oxidative stress to evaluate the antioxidant status of BPPV.
BPPV is induced by the detachment of otoconia, which are composed of inorganic calcium carbonate crystals and proteins (14). Many studies have shown that otolith shedding is associated with calcium homeostasis in the inner ear. Evidence supports a role of oxidative stress in calcium homeostasis (15). Calcium metabolism is closely related to oxidative stress (16). The endoplasmic reticulum is the main organelle for calcium storage. Under oxidative stress conditions, the endoplasmic reticulum can increase the inflow of calcium, thus, triggering ROS production and accumulation in the mitochondria (17). Subsequently, in response to reperfusion injury, calcium ions flowing into the mitochondria causes rupture of the mitochondrial membrane and subsequent apoptosis (18). Tsai et al. found that malondialdehyde levels were higher in the BPPV group before the relocation of calcium. The higher level of antioxidant superoxide dismutase in the post-treatment group suggests that oxidative stress might play a role in the pathology of BPPV (15).
In this study, serum levels of UA, BIL, ALB, and CRE were lower in the BPPV group as compared to the HC group, suggesting an association between oxidative stress and BPPV. Epidemiological studies have shown that the prevalence of BPPV is relatively high in those aged 50–60 years and the ratio of female to male is 2–3:1 (14). As shown in Figure 1, all blood markers were lower in women than men by both inter-group and intra-group comparisons (although not all findings were statistically significant), indicating weaker antioxidant capacity in women, which could explain the higher incidence of BPPV in women. Therefore, further studies are warranted to elucidate the mechanism underlying changes in serum levels of UA, BIL, ALB, and CRE associated with BPPV.
UA and BPPV
UA is a naturally occurring product of purine metabolism and is known as a strong scavenger of peroxynitrite. In addition, UA acts as a protective antioxidant in neurodegenerative diseases, such as Parkinson's disease (19), Guillain–Barre syndrome (20), multiple sclerosis (21), and amyotrophic lateral sclerosis (22). However, there is no consensus whether UA is a protective or risk factor for BPPV. Some studies have reported that UA levels are reduced in patients with BPPV (12), while other have not replicated these findings (11). A meta-analysis reported that the relationship between UA and BPPV is complex, but may not be an independent risk factor for BPPV (13). The results of the present study indicated that UA levels were reduced in BPPV. However, there were significant differences in UA levels among females, but not males, between the BPPV and HC groups. The differences between the sexes may be caused by differences in the levels of protective estrogen in females as well as differences in dietary and lifestyle habits (23). Moreover, these differences may have something to do with the duality of UA, which is considered a natural antioxidant, but also has pro-oxidation properties, leading to increased expression of ROS, lipid peroxidation, DNA damage, and the production of inflammatory cytokines (24).
Creatine and BPPV
CRE is a waste product produced by muscles from the breakdown of creatine and is known as an effective scavenger of free radicals (9). In this study, CRE levels were lower in the BPPV group that the HC group, which may be due to the depletion of free radicals. However, there were no significant difference in CRE levels among males and females between the BPPV groups, but not in HC groups. Actual CRE levels are easily affected by blood volume and physical condition (25). However, the lower CRE levels in the healthy females reflected lower antioxidant levels and a higher risk for BPPV.
BIL and BPPV
Some studies have shown that BIL is an effective antioxidant even at physiological concentrations by increasing antioxidant enzyme activities and decreasing ROS levels (26). Many studies have reported a negative association between BIL levels and a range of diseases associated with oxidative stress, such as diabetes (27), asthma (28), and inflammatory bowel disease (29). Yao et al. found that short-term preservation of BIL could prevent cell damage and maintain the viability and function of transplanted islets (30). The results of the present study also revealed lower BIL levels in the BPPV group with statistical differences between males and females.
ALB and BPPV
ALB is the major antioxidant molecule in extracellular fluid and can remove ROS and RNS. The antioxidant capacity of ALB is stronger than that of UA, BIL, ALB, and CRE (31). Redox modification changes the physiological properties of serum ALB, which can serve as a biomarker of oxidative stress (32). Actually, the oxidative state of ALB is reportedly modulated in metabolic syndrome (33), inflammation (34), and immunoglobulin A nephropathy (35). The results of the present study also revealed lower ALB levels in the BPPV group with statistical differences between males and females. Yuan et al. speculated that repeating dizziness and vomiting could lead to malnutrition and hypoproteinemia in patients with BPPV (12). We disagree with this view because relatively few patients with BPPV reported vomiting. Moreover, short-term vomiting or anorexia did not lead to a decrease in serum ALB. We believe that a low concentration of ALB represents a strong state of oxidative stress in BPPV. Furthermore, the serum ALB redox status in patients with vestibular neuritis was significantly lower than in HCs (36), as was the incidence of MD (37). Kim et al. studied the differences of protein profiles between patients with recent hearing loss and a HC group, and found that the concentrations of ALB-like proteins in the plasma and inner ear were higher in the HC group (38). In summary, ALB may be a protective factor for BPPV, although further studies are needed to clarify the underlying mechanism.
Is BPPV an Autoimmune Disease?
Here, serum levels of BIL and ALB were decreased in the BPPV group. Multiple stepwise logistic regression analysis revealed that serum concentrations of BIL (although not statistically significant) and ALB were related to BPPV. Of note, previous studies have shown that low serum BIL and ALB levels are associated with various autoimmune diseases, such as neuromyelitis optica (39), multiple sclerosis (40), myasthenia gravis (41), and anti-N-methyl-D-aspartate receptor encephalitis (42). Hence, to determine whether BPPV is an autoimmune disease, further studies are needed to reveal the association between serum levels of BIL and ALB, and the pathogenesis of BPPV.
As discussed in previous sections, the results of the present study revealed lower serum levels of UA, BIL, ALB, and CRE in the BPPV group, suggesting higher levels of oxidative stress in BPPV patients, although it is uncertain whether low antioxidant status led to or was the result of disease. Nonetheless, the low antioxidant status in BPPV patients could not reverse damage to the vestibular system due to free radical toxicity. As another possibility, serum levels of UA, BIL, ALB, and CRE were reduced in the BPPV group because of the removal of excessive free radicals. However, future studies are needed to elucidate the pathological mechanisms underlying these associations in the inner ear.
Three were three major limitations to this study. First, this was a preliminary descriptive study, which lacked evidence of biological and pathological mechanisms. Second, only patients hospitalized for idiopathic BPPV were included in this study. Third, serum levels of UA, BIL, ALB, and CRE were not followed-up in acute and remission patients. Since data are scarce, the evidence to support the conclusion remains weak, thus further studies with larger populations are needed to define these relationships.
Conclusions
Serum levels of UA, BIL, ALB, and CRE were reduced in patients with BPPV. In addition, reduced ALB was independently associated with BPPV, although further studies are required to clarify the underlying mechanism. This finding may be attributed to an active oxidative process in BPPV patients with low antioxidant status.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Statement
The study protocol was approved by the Ethics Committee of Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine and conducted in accordance with the ethical standards of the Declaration of Helsinki.
Author Contributions
K-HX and L-LL: conceptualization. Q-QC and HL: data curation. C-YS, X-FH, and J-SH: software. B-XW, R-NL, and Y-KR: validation. K-HX and L-LL: writing original draft and writing review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. (2007) 78:710–5. doi: 10.1136/jnnp.2006.100420
2. Balatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clin Interv Aging. (2018) 13:2251–66. doi: 10.2147/CIA.S144134
3. Casani AP, Navari E, Albera R, Agus G, Asprella Libonati G, Chiarella G, et al. Approach to residual dizziness after successfully treated benign paroxysmal positional vertigo: effect of a polyphenol compound supplementation. Clin Pharmacol. (2019) 11:117–25. doi: 10.2147/CPAA.S210763
4. Plodpai Y, Atchariyasathian V, Khaimook W. The characteristic differences of benign paroxysmal positional vertigo among the elderly and the younger patients: a 10-year retrospective review. J Med Assoc Thai. (2014) 97:850–5.
5. Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK. Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope. (2012) 122:624–35. doi: 10.1002/lary.22480
6. Balatsouras DG, Ganelis P, Aspris A, Economou NC, Moukos A, Koukoutsis G. Benign paroxysmal positional vertigo associated with Meniere's disease: epidemiological, pathophysiologic, clinical, and therapeutic aspects. Ann Otol Rhinol Laryngol. (2012) 121:682–8. doi: 10.1177/000348941212101011
7. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond). (1993) 84:407–12. doi: 10.1042/cs0840407
8. Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. (2010) 49:186–95. doi: 10.1016/j.yjmcc.2010.04.011
9. Fumagalli S, Fattirolli F, Guarducci L, Cellai T, Baldasseroni S, Tarantini F, et al. Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol. (2011) 34:211–7. doi: 10.1002/clc.20846
10. Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. (2013) 3:4. doi: 10.1186/2110-5820-3-4
11. Celikbilek A, Gencer ZK, Saydam L, Zararsiz G, Tanik N, Ozkiris M. Serum uric acid levels correlate with benign paroxysmal positional vertigo. Eur J Neurol. (2014) 21:79–85. doi: 10.1111/ene.12248
12. Yuan J, Dai J, Li WA, Hu W. Factors associated with benign paroxysmal positional vertigo: a Chinese case-control study. Med Sci Monit. (2017) 23:3885–9. doi: 10.12659/MSM.905716
13. Yang X, Yang B, Wu M, Wang F, Huang X, Li K, et al. Association between serum uric acid levels and benign paroxysmal positional vertigo: a systematic review and meta-analysis of observational studies. Front Neurol. (2019) 10:91. doi: 10.3389/fneur.2019.00091
14. Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. (2014) 370:1138–47. doi: 10.1056/NEJMcp1309481
15. Tsai KL, Cheng YY, Leu HB, Lee YY, Chen TJ, Liu DH, et al. Investigating the role of Sirt1-modulated oxidative stress in relation to benign paroxysmal positional vertigo and Parkinson's disease. Neurobiol Aging. (2015) 36:2607–16. doi: 10.1016/j.neurobiolaging.2015.05.012
16. Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. (2002) 38:713–21. doi: 10.1016/S0161-5890(01)00108-0
17. Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. (2012) 14:434–56. doi: 10.3390/ijms14010434
18. Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. (2002) 84:143–52. doi: 10.1016/S0300-9084(02)01368-8
19. Wen M, Zhou B, Chen YH, Ma ZL, Gou Y, Zhang CL, et al. Serum uric acid levels in patients with Parkinson's disease: a meta-analysis. PLoS ONE. (2017) 12:e0173731. doi: 10.1371/journal.pone.0173731
20. Su Z, Chen Z, Xiang Y, Wang B, Huang Y, Yang D, et al. Low serum levels of uric acid and albumin in patients with Guillain–Barre syndrome. Medicine (Baltimore). (2017) 96:e6618. doi: 10.1097/MD.0000000000006618
21. Moccia M, Capacchione A, Lanzillo R, Carbone F, Micillo T, Matarese G, et al. Sample size for oxidative stress and inflammation when treating multiple sclerosis with interferon-beta1a and coenzyme Q10. Brain Sci. (2019) 9:259. doi: 10.3390/brainsci9100259
22. Wang Z, Bai Z, Qin X, Cheng Y. Aberrations in oxidative stress markers in amyotrophic lateral sclerosis: a systematic review and meta-analysis. Oxid Med Cell Longev. (2019) 2019:1712323. doi: 10.1155/2019/1712323
23. Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. (1986) 35:343–8. doi: 10.1016/0026-0495(86)90152-6
24. Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. (2010) 28:1234–42. doi: 10.1097/HJH.0b013e328337da1d
25. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. (2009) 20:672–9. doi: 10.1681/ASN.2008070669
26. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. (1987) 235:1043–6. doi: 10.1126/science.3029864
27. Benton MC, Lea RA, Macartney-Coxson D, Bellis C, Carless MA, Curran JE, et al. Serum bilirubin concentration is modified by UGT1A1 haplotypes and influences risk of type-2 diabetes in the Norfolk Island genetic isolate. BMC Genet. (2015) 16:136. doi: 10.1186/s12863-015-0291-z
28. Kim DE, Lee Y, Kim M, Lee S, Jon S, Lee SH. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials. (2017) 140:37–44. doi: 10.1016/j.biomaterials.2017.06.014
29. Lenicek M, Duricova D, Hradsky O, Dusatkova P, Jiraskova A, Lukas M, et al. The relationship between serum bilirubin and Crohn's disease. Inflamm Bowel Dis. (2014) 20:481–7. doi: 10.1097/01.MIB.0000440817.84251.98
30. Yao Q, Jiang X, Huang ZW, Lan QH, Wang LF, Chen R, et al. Bilirubin improves the quality and function of hypothermic preserved islets by its antioxidative and anti-inflammatory effect. Transplantation. (2019) 103:2486–96. doi: 10.1097/TP.0000000000002882
31. Kim KJ, Lee BW. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J. (2012) 36:98–107. doi: 10.4093/dmj.2012.36.2.98
32. Oettl K, Birner-Gruenberger R, Spindelboeck W, Stueger HP, Dorn L, Stadlbauer V, et al. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J Hepatol. (2013) 59:978–83. doi: 10.1016/j.jhep.2013.06.013
33. Jin SM, Hong YJ, Jee JH, Bae JC, Hur KY, Lee MK, et al. Change in serum albumin concentration is inversely and independently associated with risk of incident metabolic syndrome. Metabolism. (2016) 65:1629–35. doi: 10.1016/j.metabol.2016.08.006
34. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
35. Kawai Y, Masutani K, Torisu K, Katafuchi R, Tanaka S, Tsuchimoto A, et al. Association between serum albumin level and incidence of end-stage renal disease in patients with Immunoglobulin A nephropathy: a possible role of albumin as an antioxidant agent. PLoS ONE. (2018) 13:e0196655. doi: 10.1371/journal.pone.0196655
36. Han W, Wang D, Wu Y, Fan Z, Guo X, Guan Q. Correlation between vestibular neuritis and cerebrovascular risk factors. Am J Otolaryngol. (2018) 39:751–3. doi: 10.1016/j.amjoto.2018.08.006
37. Chiarella G, Saccomanno M, Scumaci D, Gaspari M, Faniello MC, Quaresima B, et al. Proteomics in Meniere disease. J Cell Physiol. (2012) 227:308–12. doi: 10.1002/jcp.22737
38. Kim SH, Kim UK, Lee WS, Bok J, Song JW, Seong JK, et al. Albumin-like protein is the major protein constituent of luminal fluid in the human endolymphatic sac. PLoS ONE. (2011) 6:e21656. doi: 10.1371/journal.pone.0021656
39. Peng F, Yang Y, Liu J, Jiang Y, Zhu C, Deng X, et al. Low antioxidant status of serum uric acid, bilirubin and albumin in patients with neuromyelitis optica. Eur J Neurol. (2012) 19:277–83. doi: 10.1111/j.1468-1331.2011.03488.x
40. Ljubisavljevic S, Stojanovic I, Vojinovic S, Milojkovic M, Dunjic O, Stojanov D, et al. Association of serum bilirubin and uric acid levels changes during neuroinflammation in patients with initial and relapsed demyelination attacks. Metab Brain Dis. (2013) 28:629–38. doi: 10.1007/s11011-013-9409-z
41. Yang D, Su Z, Wu S, Bi Y, Li X, Li J, et al. Low antioxidant status of serum bilirubin, uric acid, albumin and creatinine in patients with myasthenia gravis. Int J Neurosci. (2016) 126:1120–6. doi: 10.3109/00207454.2015.1134526
Keywords: benign paroxysmal positional vertigo, antioxidant status, uric acid, bilirubin, albumin, creatinine
Citation: Xie K-H, Liu L-L, Su C-Y, Huang X-F, Wu B-X, Liu R-N, Li H, Chen Q-Q, He J-S and Ruan Y-K (2020) Low Antioxidant Status of Serum Uric Acid, Bilirubin, Albumin, and Creatinine in Patients With Benign Paroxysmal Positional Vertigo. Front. Neurol. 11:601695. doi: 10.3389/fneur.2020.601695
Received: 01 September 2020; Accepted: 12 October 2020;
Published: 20 November 2020.
Edited by:
Marco Mandalà, Siena University Hospital, ItalyReviewed by:
Vincenzo Marcelli, Local Health Authority Naples 1 Center, ItalyTjasse Bruintjes, Gelre Hospitals, Netherlands
Copyright © 2020 Xie, Liu, Su, Huang, Wu, Liu, Li, Chen, He and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke-Hang Xie, a2VoYW44MjZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Ke-Hang Xie
Ke-Hang Xie Ling-Ling Liu2†
Ling-Ling Liu2†