- 1Department of Neurology, Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Geriatrics, Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Neurology, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 4Department of Radiology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Objective: The aims of this study were to compare the characteristics of three motor subtype classifications in patients with de novo Parkinson's disease (PD) and to find the most suitable motor subtype classification for identifying non-motor symptoms (NMSs).
Methods: According to previous studies, a total of 256 patients with de novo PD were classified using the tremor-dominant/mixed/akinetic-rigid (TD/mixed/AR), TD/indeterminate/postural instability and gait disturbance (PIGD), and predominantly TD/predominantly PIGD (p-TD/p-PIGD) classification systems.
Results: Among the TD/mixed/AR subgroups, the patients with the AR subtype obtained more severe motor scores than the patients with the TD subtype. Among the TD/indeterminate/PIGD subgroups and between the p-TD and p-PIGD subgroups, the patients with the PIGD/p-PIGD subtype obtained more severe scores related to activities of daily living (ADL), motor and non-motor symptoms, including depression, anxiety, and sleep impairment, than the patients with the TD/p-TD subtype. Furthermore, symptoms in the cardiovascular, gastrointestinal, and miscellaneous domains of the Non-motor Questionnaire (NMSQuest) were more prevalent in the patients with the PIGD/p-PIGD subtypes than the patients with the TD/p-TD subtypes.
Conclusions: The PIGD/p-PIGD subtypes had more severe ADL, motor and non-motor symptoms than the TD/p-TD subtypes. We disclosed for the first time that the TD/indeterminate/PIGD classification seems to be the most suitable classification among the three motor subtype classifications for identifying NMSs in PD.
Introduction
Parkinson's disease (PD) is a highly clinically heterogeneous neurodegenerative disorder with wide variations in motor and non-motor manifestations (1). It is still unclear whether such heterogeneity merely reflects a diverse spectrum of clinical manifestations of a unitary disease or indicates the existence of disease phenotypes with distinctive clinical patterns and different pathophysiological abnormalities (2). Due to the lack of specific biomarkers in the diagnosis and progression of PD, an in-depth and comprehensive understanding of disease subtypes may be crucial to delineate disease mechanisms and ultimately improve tailored therapeutic strategies (3).
Among numerous empirical subtype systems based on clinical observation, including categories of age at onset, classifications of main motor phenotypes, and patterns of cognitive impairment and other non-motor symptoms (NMSs) (4), motor subtype classifications based on the prominent motor symptoms are the most commonly used (5). The clinical utility of three motor subtype classifications in PD, namely, tremor-dominant (TD)/mixed/akinetic-rigid (AR) (6, 7), TD/indeterminate/postural instability and gait difficulty (PIGD) (8, 9) and predominantly TD (p-TD)/predominantly PIGD (p-PIGD) subtype classifications (10, 11) has been extensively investigated. However, the terminology utilized to describe motor phenotypes of PD is equivocal and overlapping, and defining subtypes based on the ratio of two Unified Parkinson's Disease Rating Scale (UPDRS) subscores is arbitrary and intuitive, which contributes to an inaccurate body of published literature on the clinical roles of motor subtypes (12, 13). Therefore, the choice of motor subtype classifications is a key issue in the progression of clinical research on PD subtypes.
A recent study compared two motor subtype classifications of de novo PD patients and found that the TD/indeterminate/PIGD subtype classification appeared to be more likely to detect NMSs than the TD/mixed/AR phenotypes (14). Nevertheless, comparisons of three motor subtype classifications with de novo PD patients have not been examined previously. Furthermore, it is not known which of the three motor subtype classifications is the most suitable for identifying NMSs in patients with PD. Therefore, the purposes of this study were to compare demographic and clinical characteristics of the patients categorized using the three motor subtype classifications and to find the most suitable motor subtype classification for identifying NMSs.
Methods
Participants
A total of 256 newly-diagnosed untreated idiopathic PD patients from the Department of Neurology at the Affiliated Brain Hospital of Nanjing Medical University were recruited to participate in the study between January 2012 and June 2020. All participants were examined by a movement disorder specialist and diagnosed according to the United Kingdom Parkinson's Disease Society Brain Bank clinical diagnostic criteria (15). Patients showed positive responsiveness to levodopa treatment through the standardized acute levodopa challenge test and had at least one follow-up. Exclusion criteria comprised the following: (1) treated; (2) late-stage PD (modified Hoehn and Yahr (H-Y) stage >3); (3) atypical or secondary parkinsonism; (4) clinically significant lesions visible on brain magnetic resonance imaging (MRI) scans; (5) serious chronic diseases such as renal failure, heart failure, diabetes and its complications; and (6) difficulty in completing the clinical evaluation.
This study was approved by the Medical Ethics Committee of Affiliated Brain Hospital of Nanjing Medical University before study initiation and carried out in accordance with the Declaration of Helsinki. All individuals participating in the study provided written informed consent prior to participation in the experiment.
Clinical Assessments
Demographic and clinical characteristics of PD patients were collected before starting dopaminergic treatment. Demographic characteristics specifically included age at assessment, sex, formal education, age at onset, and disease duration in years. The states of activities of daily living (ADL) were evaluated using part II subscales of the UPDRS. The UPDRS part III and modified H-Y stages were used to assess motor disability and disease severity, respectively. Global cognition was assessed with the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Mood was measured with the Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA). Sleep was rated with the Parkinson Disease Sleep Scale (PDSS). NMSs were assessed by the Non-motor Questionnaire (NMSQuest) (16). The NMSQuest comprises 30 items, divided into nine domains, namely cardiovascular, sleep, mood/cognitive, perception/hallucinations, attention/memory, gastrointestinal, urinary, sexual function and miscellaneous (17).
Motor Subtype Classifications
According to the three different methods described by Kang et al. (7), Stebbins et al. (18), and Herman et al. (10), the PD patients were categorized into the TD/mixed/AR, TD/indeterminate/PIGD and p-TD/p-PIGD classifications. In Kang's method, the ratio of the mean UPDRS tremor scores (UPDRS III items 20–21 divided by 4) to the mean UPDRS AR scores (UPDRS III items 22–27 and 31 divided by 15) was used to identify TD (ratio >1), mixed (0.8 ≤ ratios ≤ 1.0) and AR (ratio <0.8) PD patients (7). In Stebbins's method, the ratio of the mean UPDRS tremor scores (UPDRS II item 16 and UPDRS III items 20–21 divided by 8) to the mean UPDRS PIGD scores (UPDRS II items 13–15 and UPDRS III items 29–30 divided by 5) was used to identify TD (ratio ≥1.5), indeterminate (1.0 < ratios <1.5) and PIGD (ratio ≤ 1) PD patients (18). In Herman's method, a more stringent criteria was applied on the basis of the Stebbins method to identify the two representative subtypes with minimal symptom overlap, namely the p-TD and p-PIGD subtypes (10). If the PIGD score was higher than 3 or the tremor score was lower than 4, the patients were excluded from the TD group. Similarly, if the tremor score was higher than 3 or the PIGD score was lower than 4, the patients were excluded from the PIGD group.
Statistical Analysis
Statistical analyses were performed using IBM SPSS software version 25.0. For demographic and clinical characteristics, the Kolmogorov-Smirnov test was used to check whether the data followed a normal distribution. The mean and standard deviation (SD) is presented for parametric variables, and the median and interquartile range (IQR) is presented for non-parametric variables. Categorical variables are presented as frequencies together with proportions. Among TD/mixed/AR and TD/indeterminate/PIGD subtypes, continuous data were compared using parametric (one-way analysis of variance, ANOVA) or non-parametric (Kruskal-Wallis H-test) tests, while categorical data were compared with Chi-square tests, followed by the Bonferroni correction for multiple comparisons. Between the p-TD and p-PIGD subtypes, continuous data were compared using parametric (two-sample t-test) or non-parametric (Mann-Whitney U-test) tests, while categorical data were compared with Chi-square tests. P < 0.05 was deemed to be significant.
Results
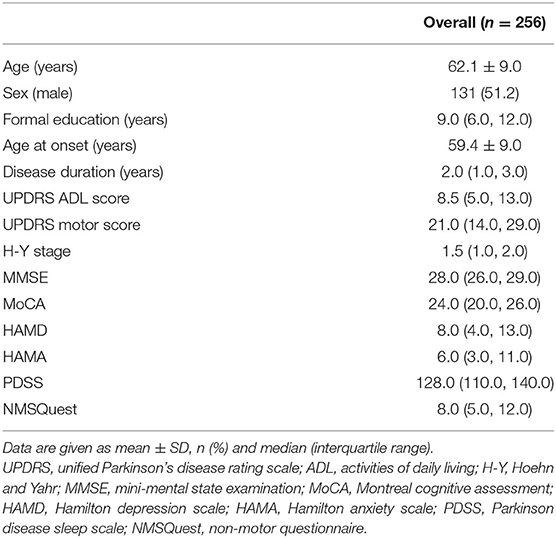
The demographic and clinical characteristics of 256 de novo PD patients are summarized in Table 1. The PD patients had a mean age of 62.1 years old and a median PD duration of 2.0 years from the PD diagnosis.
Based on Kang's method of motor subtype classification, we identified 119 (46.5%) patients belonging to the TD subtype, 115 (44.9%) to the AR subtype, and only 22 (8.6%) to the mixed subtype. Among the TD/mixed/AR subgroups, there were no significant differences with respect to the demographic and clinical characteristics other than motor scores. In addition, in post-hoc analyses, the differences in the motor scores remained significant between the TD and AR subtypes, and the patients with the AR subtype obtained more severe motor scores than the patients with the TD subtype (Table 2).
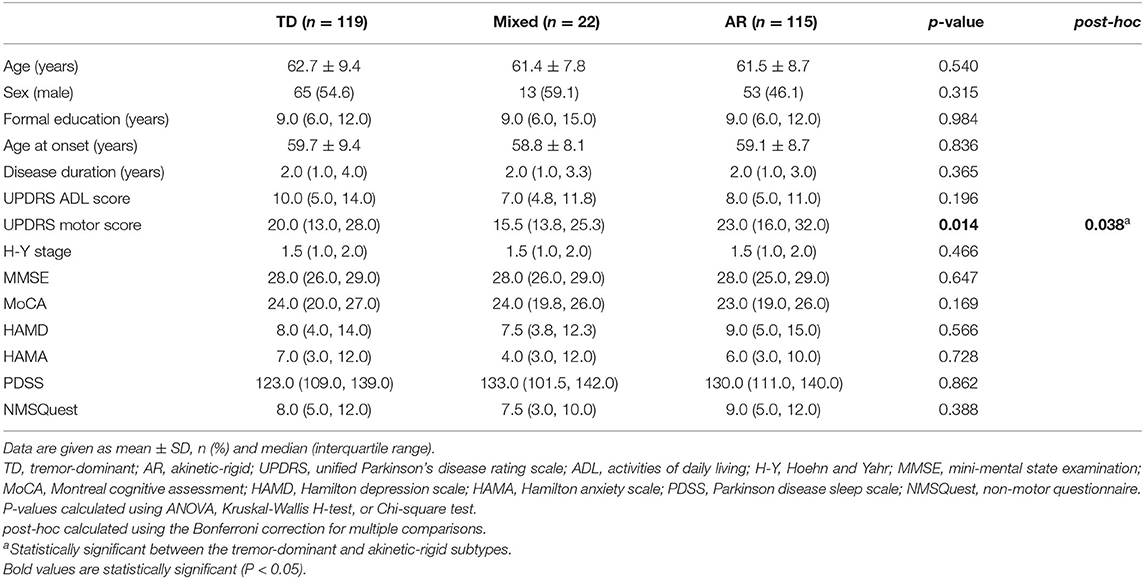
Table 2. Comparison of the demographic and clinical characteristics of de novo Parkinson's disease patients among the tremor-dominant/mixed/akinetic-rigid subgroups.
Based on Stebbins's method of motor subtype classification, we identified 140 (54.7%) patients belonging to the PIGD subtype, 78 (30.5%) to the TD subtype, and only 38 (14.8%) to the indeterminate subtype. Among the TD/indeterminate/PIGD subgroups, there were no significant differences with respect to the demographic or clinical characteristics, including age at assessment, sex, formal education, age at onset, disease duration in years, MMSE scores and MoCA scores. However, scores associated with ADL, motor and non-motor symptoms, including UPDRS ADL and motor scores, the modified H-Y stage, and HAMD, HAMA, PDSS, and NMSQuest scores significantly differed among the three groups. In addition, in post-hoc analyses, these differences remained significant between the TD and PIGD (or indeterminate) subtypes, and the patients with the PIGD subtype obtained more severe scores related to ADL, motor, and non-motor symptoms, including depression, anxiety, and sleep impairment, than patients with the TD subtype (Table 3).
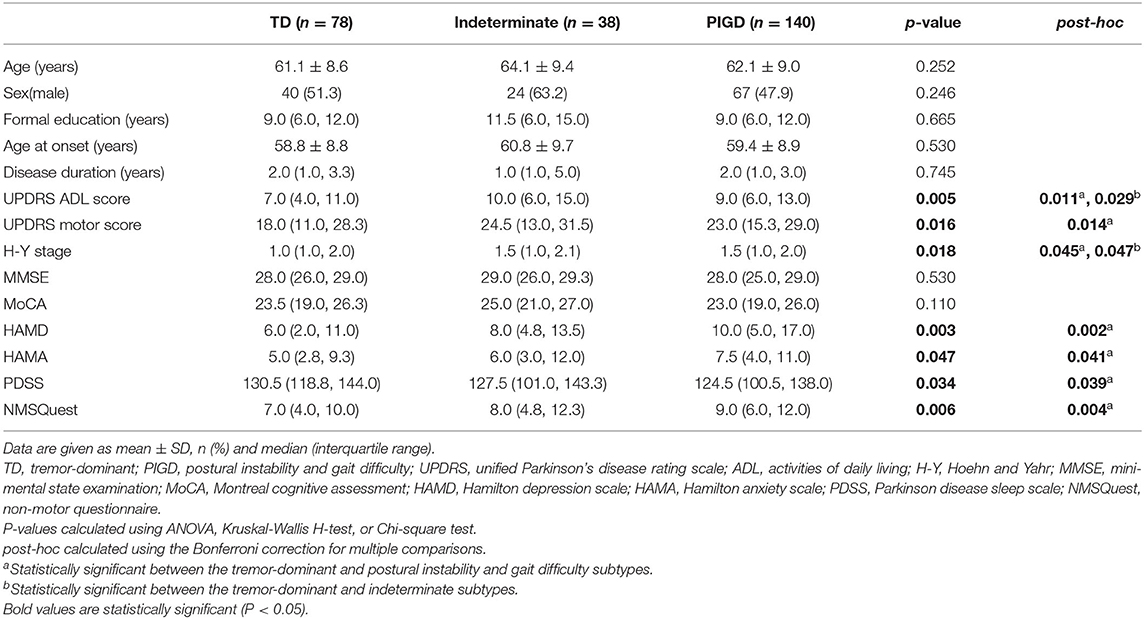
Table 3. Comparison of the demographic and clinical characteristics of de novo Parkinson's disease patients among the tremor-dominant/indeterminate/postural instability and gait difficulty subgroups.
Based on Herman's method of motor subtype classification, we identified 52 (20.3%) patients belonging to the p-TD subtype and 42 (16.4%) to the p-PIGD subtype. Between the p-TD and p-PIGD subtypes, there were no significant differences with respect to the demographic or clinical characteristics, including age at assessment, sex, formal education, age at onset, disease duration in years, MMSE scores and MoCA scores. However, scores associated with ADL, motor symptoms and non-motor symptoms, including UPDRS ADL and motor scores, the modified H-Y stage, and HAMD, HAMA, PDSS, and NMSQuest scores significantly differed between the two groups, and the patients with the p-PIGD subtype obtained more severe scores related to ADL, motor and non-motor symptoms, including depression, anxiety and sleep impairment, than patients with the p-TD subtype (Table 4).
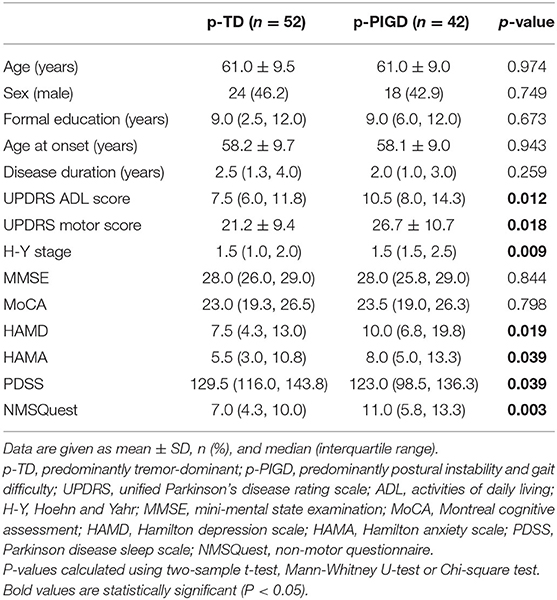
Table 4. Comparison of the demographic and clinical characteristics of de novo Parkinson's disease patients between the predominantly tremor-dominant and predominantly postural instability and gait difficulty subgroups.
Since the NMSQuest scores were significantly different among the TD/indeterminate/PIGD groups and between the p-TD and p-PIGD groups, the nine domains of the NMSQuest were further compared across the two classifications. Differences in cardiovascular, gastrointestinal, and miscellaneous domains of the NMSQuest in the TD/indeterminate/PIGD classification were similar to the p-TD/p-PIGD classification, and the symptoms in these three domains were more prevalent in the patients with the PIGD/p-PIGD subtypes than the patients with the TD/p-TD subtypes (Table 5).
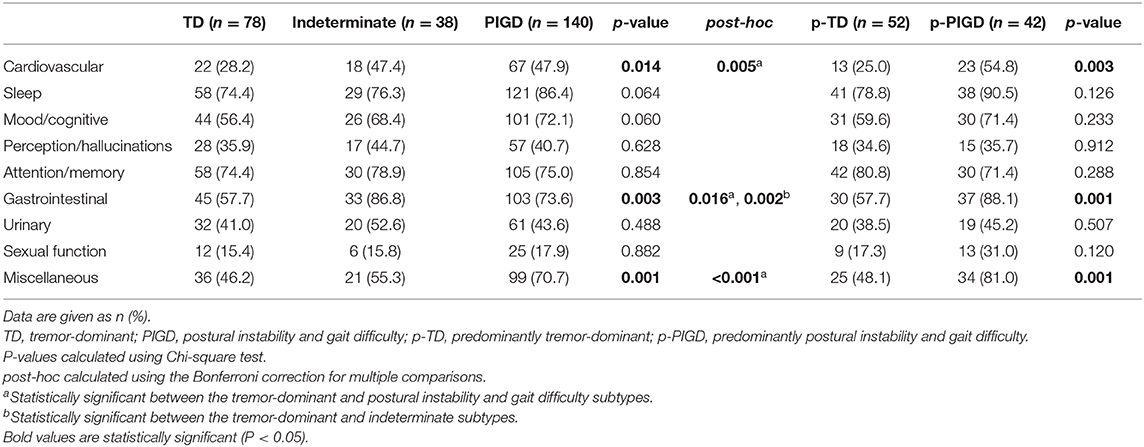
Table 5. Comparison of the non-motor symptoms in two motor subtype classifications of de novo Parkinson's disease patients.
Discussion
To the best of our knowledge, this is the first paper exploring the distinction of the demographic and clinical characteristics of de novo PD patients among the three most commonly used motor subtype classifications. Among the three classification methods, patients with the non-tremor-dominant (AR or PIGD or p-PIGD) subtypes obtained more severe motor scores than the patients with the TD/p-TD subtypes. However, only patients with the PIGD/p-PIGD subtypes showed more severe NMSs than the patients with the TD/p-TD subtypes. Hence, compared with the other two classifications, the TD/mixed/AR subgroups may be unsuitable for identifying NMSs of PD patients, which is consistent with previous research results (14). Furthermore, differences in the NMSQuest domains between patients in the p-TD/p-PIGD classification were similar to those in the patients in the TD/indeterminate/PIGD classification. The p-TD/p-PIGD classification removes a large number of people, resulting in the loss of potentially significant information, but no more differences in non-motor features were found than with the TD/indeterminate/PIGD classification. Therefore, the TD/indeterminate/PIGD classification seems to be the most suitable classification among the three motor subtype classifications of PD for identifying NMSs.
The terms describing the PD motor subtypes overlap and are frequently puzzling. Jankovic et al. (8) first proposed that the PIGD subtype refers to a more representative subtype of axial symptoms presented in medical histories and observed in motor examinations compared with the TD subtype. Subsequently, Rajput et al. used AR terminology to represent a group of patients with PD whose axial and appendicular rigidity was more predominant relative to the TD subtype (19). Recently, Herman et al. used the p-PIGD/p-TD term to describe, with what appears to be the main goal, two more homogenous groups with minimal overlap of symptoms (20). In addition, the PD motor subtypes discussed above are based on clinical judgment and intuition, and the cutoffs used to define subtypes are arbitrarily selected (13). Although subtypes are determined according to the same principle by calculating the ratio between two UPDRS subscores, the UPDRS items and cutoffs for defining the subtypes are different in each study, so patients with PD may be categorized as a particular subtype according to one method and into another subtype with a different algorithm. Considering the vagueness of terminology and the arbitrariness of cutoffs, literature reports on the clinical role of PD motor subtype classification are inaccurate.
Clinically, however, almost all movement disorder experts still believe that these subtypes are credible, mainly because there is substantial evidence to support the link between these motor subtype classifications and relevant clinical features. In the TD/mixed/AR classification, patients with the AR phenotype were older at onset, had faster disease progression and had a higher cumulative incidence of cognitive impairment than patients with the TD subtype (19). In the TD/indeterminate/PIGD classification, patients categorized into the PIGD subtype had more severe motor symptoms, more NMSs including depression, fatigue, sleep impairment, urinary symptoms and sexual dysfunction, greater occupational disability, more aggressive disease progression and poorer prognoses than patients with the TD phenotype (8, 9, 21–23). In the p-TD/p-PIGD classification, patients with the p-PIGD subtype experienced more NMSs, greater autonomic function and poorer quality of life than patients with the p-TD subtype (11). Taken together, the PIGD/p-PIGD subtypes seemed to have more severe ADL, motor and non-motor symptoms than the TD/p-TD subtypes, which is consistent with our findings.
In this context, it is crucial to choose the appropriate motor subtype classification for further clinical research with PD patients. Our study compared the three motor subtype classifications of newly diagnosed PD patients, which eliminated the possible effects of drugs, and found that the TD/indeterminate/PIGD classification was the most suitable for PD clinical studies on NMSs. Because the presence of certain NMSs, such as hyposmia, constipation, depression and idiopathic REM sleep behavior disorder, has been recognized to significantly increase the risk of PD (24), the emerging concept of the prodromal phase of PD is based on not only motor symptoms but also NMSs (25, 26). Furthermore, there have been studies on the relationships between specific clusters of prodromal NMSs and the subsequent development of the PD motor phenotype (27). With the increasing clinical significance of NMSs, identifying subtypes of PD patients with unique motor and non-motor characteristics may provide a better understanding of the pathogenesis and help clinicians predict these symptoms and implement personalized, stratified treatment (28, 29).
In addition to motor subtypes empirically based on clinical observation, a data-driven approach with cluster analysis based on the co-occurrence of characteristics can also be used to define subtypes (30–32). Relative to motor subtypes, there are a priori hypotheses about how the characteristics of the disease are interrelated, but the data-driven approach does not involve any of these hypotheses, so it seems more unbiased. However, this novel approach still depends on certain choices, such as the variables selected to enter the analysis, the techniques of clustering, and the number of clusters found. Additionally, with these two methods, the exploratory results of motor subgrouping have not been consistent. Data-driven methods have provided support for the determination of TD subtypes, but they have failed to distinguish PIGD subtypes (33, 34). Therefore, when comparing subgroups of PD patients, clinicians should consider the characteristics of each classification.
When interpreting our results, several limitations need to be taken into consideration. First, most of the study participants were recruited from a single center in a clinical study, so it may not reflect the general population of PD and cannot be used for universalization. Nevertheless, the sample size of the study was sufficient for analysis. Second, the motor symptoms and non-motor abnormalities of de novo PD patients are generally mild, and a certain percentage of de novo PD patients show great variability in these motor subtype classifications (35, 36). Future studies can compare the clinical characteristics of different motor subtypes in the late stages of the disease. Third, the data we obtained are not comprehensive and may ignore potential factors that may have effect on ADL, motor and non-motor symptoms of PD patients, such as genetic and environmental attributes. Fourth, although de novo PD patients have at least one follow-up, they may still be mixed with patients with atypical parkinsonisms, so longer longitudinal follow-up is needed to distinguish atypical parkinsonisms from PD to improve the accuracy of PD diagnosis.
Conclusion
In conclusion, the PIGD/p-PIGD subtypes had more severe ADL, motor and non-motor symptoms than the TD/p-TD subtypes. In addition, we demonstrated for the first time that the TD/indeterminate/PIGD classification seems to be the most suitable classification among the three motor subtype classifications of PD patients for identifying NMSs that is conducive to identifying subtypes with specific motor and non-motor symptoms relevant to the etiology, prognosis and response to subtype-specific treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Affiliated Brain Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WL organized the research project and critically revised for important academic content. JR collected data, designed, and performed statistical analysis, and drafted the preliminary manuscript. PH, YL, and CP helped in acquiring data. LY and CY critiqued the statistical analysis. LZ, PX, and MZ critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key Research and Development Program of China (Grant/Award Number: 2017YFC1310302 and 2016YFC1306600); General Program of National Natural Science Foundation of China (Grant/Award Number: 81571348); Science and Technology Program of Jiangsu Province (Grant/Award Number: BE2019611); General Program of Jiangsu Provincial Natural Science Foundation of China (Grant/Award Number: BK20151077).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the patients and their families for their support.
References
1. Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
2. Calne DB. Is “Parkinson's disease” one disease? J. Neurol. Neurosurg. Psychiatry. (1989) 52(Suppl.):18-21. doi: 10.1136/jnnp.52.Suppl18
3. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. (2014) 71:499–504. doi: 10.1001/jamaneurol.20136233
4. Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson's disease. Parkinsonism Relat. Disord. (2016) 22(Suppl. 1):S41–6. doi: 10.1016/j.parkreldis.2015.09027
5. Marras C, Chaudhuri KR. Nonmotor features of Parkinson's disease subtypes. Mov. Disord. (2016) 31:1095–102. doi: 10.1002/mds26510
6. Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J. Neuropathol. Exp. Neurol. (1991) 50:743–55. doi: 10.1097/00005072-199111000-00006
7. Kang GA, Bronstein JM, Masterman DL, Redelings M, Crum JA, Ritz B. Clinical characteristics in early Parkinson's disease in a central California population-based study. Mov. Disord. (2005) 20:1133–42. doi: 10.1002/mds20513
8. Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. Neurology. (1990) 40:1529–34. doi: 10.1212/WNL.40.101529
9. Huang X, Ng SY, Chia NS, Setiawan F, Tay KY, Au WL, et al. Non-motor symptoms in early Parkinson's disease with different motor subtypes and their associations with quality of life. Eur. J. Neurol. (2019) 26:400–6. doi: 10.1111/ene13803
10. Herman T, Weiss A, Brozgol M, Giladi N, Hausdorff JM. Gait and balance in Parkinson's disease subtypes: objective measures and classification considerations. J. Neurol. (2014) 261:2401–10. doi: 10.1007/s00415-014-7513-6
11. Herman T, Weiss A, Brozgol M, Wilf-Yarkoni A, Giladi N, Hausdorff JM. Cognitive function and other non-motor features in non-demented Parkinson's disease motor subtypes. J. Neural Transm. (2015) 122:1115–24. doi: 10.1007/s00702-014-1349-1
12. Kotagal V. Is PIGD a legitimate motor subtype in Parkinson disease? Ann. Clin. Transl. Neurol. (2016) 3:473–7. doi: 10.1002/acn3312
13. von Coelln R, Shulman LM. Clinical subtypes and genetic heterogeneity: of lumping and splitting in Parkinson disease. Curr. Opin. Neurol. (2016) 29:727–34. doi: 10.1097/WCO0000000000000384
14. Choi SM, Kim BC, Cho BH, Kang KW, Choi KH, Kim JT., et al. Comparison of two motor subtype classifications in de novo Parkinson's disease. Parkinsonism Relat. Disord. (2018) 54:74–8. doi: 10.1016/j.parkreldis.2018.04021
15. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. (1988) 51:745–52. doi: 10.1136/jnnp.51.6745
16. Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov. Disord. (2006) 21:916–23. doi: 10.1002/mds20844
17. Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov. Disord. (2007) 22:1901–11. doi: 10.1002/mds21596
18. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov. Disord. (2013) 28:668–70. doi: 10.1002/mds25383
19. Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. (2009) 73:206–12. doi: 10.1212/WNL0b013e3181ae7af1
20. Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. (2013) 80:1476–84. doi: 10.1212/WNL0b013e31828cfaa4
21. Deng X, Xiao B, HH Li, Lo YL, Chew LM, Prakash KM., et al. Sexual dysfunction is associated with postural instability gait difficulty subtype of Parkinson's disease. J. Neurol. (2015) 262:2433–9. doi: 10.1007/s00415-015-7855-8
22. van der Heeden JF, Marinus J, Martinez-Martin P, Rodriguez-Blazquez C, Geraedts VJ, van Hilten JJ. Postural instability and gait are associated with severity and prognosis of Parkinson disease. Neurology. (2016) 86:2243–50. doi: 10.1212/WNL0000000000002768
23. Ren J, Hua P, Pan C, Li Y, Zhang L, Zhang W, et al. Non-motor symptoms of the postural instability and gait difficulty subtype in de novo Parkinson's disease patients: a cross-sectional study in a single center. Neuropsychiatr. Dis. Treat. (2020) 16:2605–12. doi: 10.2147/NDTS280960
24. Mantri S, Morley JF, Siderowf AD. The importance of preclinical diagnostics in Parkinson disease. Parkinsonism Relat. Disord. (2019) 64:20–8. doi: 10.1016/j.parkreldis.2018.09011
25. Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T., et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Mov Disord. (2014) 29:454–62. doi: 10.1002/mds25844
26. Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B., et al. MDS research criteria for prodromal Parkinson's disease. Mov. Disord. (2015) 30:1600–11. doi: 10.1002/mds26431
27. Durcan R, Wiblin L, Lawson RA, Khoo TK, Yarnall AJ, Duncan GW., et al. Prevalence and duration of non-motor symptoms in prodromal Parkinson's disease. Eur. J. Neurol. (2019) 26:979–85. doi: 10.1111/ene13919
28. Titova N, Chaudhuri KR. Personalized medicine in Parkinson's disease: time to be precise. Mov. Disord. (2017) 32:1147–54. doi: 10.1002/mds27027
29. Latourelle JC, Beste MT, Hadzi TC, Miller RE, Oppenheim JN, Valko MP, et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson's disease: a longitudinal cohort study and validation. Lancet Neurol. (2017) 16:908–16. doi: 10.1016/S1474-4422(17)30328-9
30. van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, Marinus J. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov. Disord. (2010) 25:969–78. doi: 10.1002/mds23116
31. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. (2015) 72:863–73. doi: 10.1001/jamaneurol.20150703
32. Graham JM, Sagar HJ. A data-driven approach to the study of heterogeneity in idiopathic Parkinson's disease: identification of three distinct subtypes. Mov. Disord. (1999) 14:10–20. doi: 10.1002/1531-8257(199901)14:1<10::AID-MDS1005>3.0.CO;2-4
33. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J. Neurol. Neurosurg. Psychiatry. (2005) 76:343–8. doi: 10.1136/jnnp.2003033530
34. Reijnders JS, Ehrt U, Lousberg R, Aarsland D, Leentjens AF. The association between motor subtypes and psychopathology in Parkinson's disease. Parkinsonism Relat. Disord. (2009) 15:379–82. doi: 10.1016/j.parkreldis.2008.09003
35. Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K. How stable are Parkinson's disease subtypes in de novo patients: analysis of the PPMI cohort? Parkinsonism Relat. Disord. (2016) 28:62–7. doi: 10.1016/j.parkreldis.2016.04027
Keywords: de novo Parkinson's disease, motor subtype classifications, tremor-dominant, postural instability and gait difficulty, akinetic-rigid
Citation: Ren J, Hua P, Li Y, Pan C, Yan L, Yu C, Zhang L, Xu P, Zhang M and Liu W (2020) Comparison of Three Motor Subtype Classifications in de novo Parkinson's Disease Patients. Front. Neurol. 11:601225. doi: 10.3389/fneur.2020.601225
Received: 31 August 2020; Accepted: 04 December 2020;
Published: 23 December 2020.
Edited by:
Maria Teresa Pellecchia, University of Salerno, ItalyReviewed by:
Matteo Bologna, Sapienza University of Rome, ItalyMarcello Moccia, University of Naples Federico II, Italy
Copyright © 2020 Ren, Hua, Li, Pan, Yan, Yu, Zhang, Xu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiguo Liu, d2dsaXVuYmgmI3gwMDA0MDtzaW5hLmNvbQ==
†ORCID: Jingru Ren orcid.org/0000-0002-9186-6621
Weiguo Liu orcid.org/0000-0001-5916-9837
 Jingru Ren1†
Jingru Ren1† Yuqian Li
Yuqian Li Li Zhang
Li Zhang Pingyi Xu
Pingyi Xu Minming Zhang
Minming Zhang Weiguo Liu
Weiguo Liu