- 1National Engineering Laboratory for Neuromodulation, Tsinghua University, Beijing, China
- 2Charité, Department of Neurology, Movement Disorders and Neuromodulation Unit, University Medicine Berlin, Berlin, Germany
- 3Section of Neurosurgery, Department of Surgical Sciences, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand
- 4Department of Psychiatry and Behavioural Science, Emory University, Atlanta, GA, United States
- 5Department of Radiology, Mount Sinai School of Medicine, New York, NY, United States
- 6Department of Neurosurgery, Mount Sinai School of Medicine, New York, NY, United States
- 7Department of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, United States
- 8Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
- 9Harquail Centre for Neuromodulation, Sunnybrook Research Institute, Toronto, ON, Canada
- 10Department of Neurosciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, United States
- 11Neurological Institute, Cleveland Clinic, Cleveland, OH, United States
- 12Department of Neurosurgery, John Radcliffe Hospital, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
- 13Department of Neurology, University of São Paulo Medical School, São Paulo, Brazil
- 14Hospital Sírio-Libanês and Hospital Albert Einstein, São Paulo, Brazil
- 15Department of Medical Neurobiology (Physiology), Institute of Medical Research–Israel-Canada (IMRIC), Faculty of Medicine, Jerusalem, Israel
- 16The Edmond and Lily Safra Center for Brain Research (ELSC), The Hebrew University and Department of Neurosurgery, Hadassah Medical Center, Hebrew University, Jerusalem, Israel
- 17University of Southern California, Children's Hospital Los Angeles, Los Angeles, CA, United States
- 18Department of Neuroscience, College of Medicine, Medical University of South Carolina, Charleston, SC, United States
- 19Department of Pharmacology and Physiology, University of Rochester School of Medicine & Dentistry, Rochester, NY, United States
- 20McLean Hospital and Harvard Medical School, Belmont, MA, United States
Deep brain stimulation (DBS) is one of the most important clinical therapies for neurological disorders. DBS also has great potential to become a great tool for clinical neuroscience research. Recently, the National Engineering Laboratory for Neuromodulation at Tsinghua University held an international Deep Brain Stimulation Initiative workshop to discuss the cutting-edge technological achievements and clinical applications of DBS. We specifically addressed new clinical approaches and challenges in DBS for movement disorders (Parkinson's disease and dystonia), clinical application toward neurorehabilitation for stroke, and the progress and challenges toward DBS for neuropsychiatric disorders. This review highlighted key developments in (1) neuroimaging, with advancements in 3-Tesla magnetic resonance imaging DBS compatibility for exploration of brain network mechanisms; (2) novel DBS recording capabilities for uncovering disease pathophysiology; and (3) overcoming global healthcare burdens with online-based DBS programming technology for connecting patient communities. The successful event marks a milestone for global collaborative opportunities in clinical development of neuromodulation to treat major neurological disorders.
Introduction
The National Engineering Laboratory for Neuromodulation (NELN) at Tsinghua University organized its first deep brain stimulation (DBS) initiative meeting in Beijing on October 11–12, 2018. Leading experts in neuromodulation, specifically in the field of DBS, were in attendance for discussions on the latest research in neuromodulation technologies and applications, clinical indications, as well as current and foreseeable challenges in DBS therapy. Participants from multidisciplinary backgrounds that included neural engineers, neurosurgeons, neurologists, neuroscientists, and industry professionals engaged in round-table discussions following the thematic sessions and presentations. With expert updates and reports on the latest clinical approaches, there were open discussions on the opportunities in neuromodulation with recent technological advancements. This included an exchange of ideas on the connectome approach to DBS, novel developments of 3-Tesla magnetic resonance imaging (3T MRI)-compatible DBS devices and the use of neuroimaging to understand the neurocircuitry of effective DBS, including demonstrations of the latest DBS neural recording technology in real patients. This meeting came to provide reports of recent DBS application for unmet clinical needs, such as gait disability in Parkinson's disease (PD) and stroke rehabilitation, and the challenges in the current transition of DBS therapy toward neuropsychiatric disorders, including depression and memory disorders.
With the current rapid and widespread rise of neuromodulation therapies in China and across the globe, NELN's first DBS initiative meeting set out to stimulate collaborations between leaders in clinical, engineering, and basic science research for the rapid translation of therapies using state-of-the-art technologies. Marking a unique milestone in fostering international collaborations in DBS research, we report here a summary of the meeting that covers topic overviews, presentations, and follow-up discussions aiding to uncover expert perspectives and support advancements in the field of neuromodulation as we progress into the future.
Some Recent Deep Brain Stimulation Technology Advancements
MRI Compatibility
Among patients with an active implantable medical device, it is estimated that ~50–75% will require an MRI scan during the time course of treatment (1). In 2016, it was reported that 66–75% of DBS patients treated for movement disorders required an MRI scan within 10 years of device implantation (2). However, clear dangers exist for DBS patients under MRI, specifically the potential for permanent neurological damage due to radiofrequency (RF) lesioning caused by heating of DBS electrodes (3). Indeed, the main risk in MRI comes from wires enclosed in DBS extensions and leads, which can receive RF energy from the MRI magnetic field, inducing current discharge through contacts that align at the tip of the lead. This can cause thermal damage to the surrounding brain tissue. Therefore, inhibiting MRI RF-induced heat remains key for addressing the safety of patients implanted with DBS devices under MRI.
While some DBS device manufacturers claim safety under 1.5T MRI, DBS devices available to patients across global markets are still considered to be unsafe under 3T MRI, limiting patients from imaging and diagnostic benefits. Currently, patients can only use head/body coils for scanning under 1.5T, with the head specific absorption rate (SAR) value being <0.1 W/kg or the B1RMS value remaining below 2.0 uT (4). This is far lower than the upper limit of 2.0 W/kg of patients without medical devices for MRI (5), as set by the International Electrotechnical Commission (IEC) standard.
With the limitations of low-quality imaging using 1.5T MRI, clinical research in DBS patients would greatly benefit from advances in MRI compatibility. Following laboratory evaluations and preclinical testing, a study conducted by the NELN was reported for testing the safety and efficacy of high-field 3T MRI-compatible DBS system in PD patients. The clinical trial was initiated in November 2016, and the final follow-up was completed in June 2018. A total of 24 PD patients were screened, and 14 subjects were eligible for the study. Follow-ups were successfully completed at 1, 3, 6, and 12 months, with an average time of 4.12 h taken per patient for anatomical and brain function 3T MRI scan. No adverse events were found that related to MRI (6) (Figure 1).
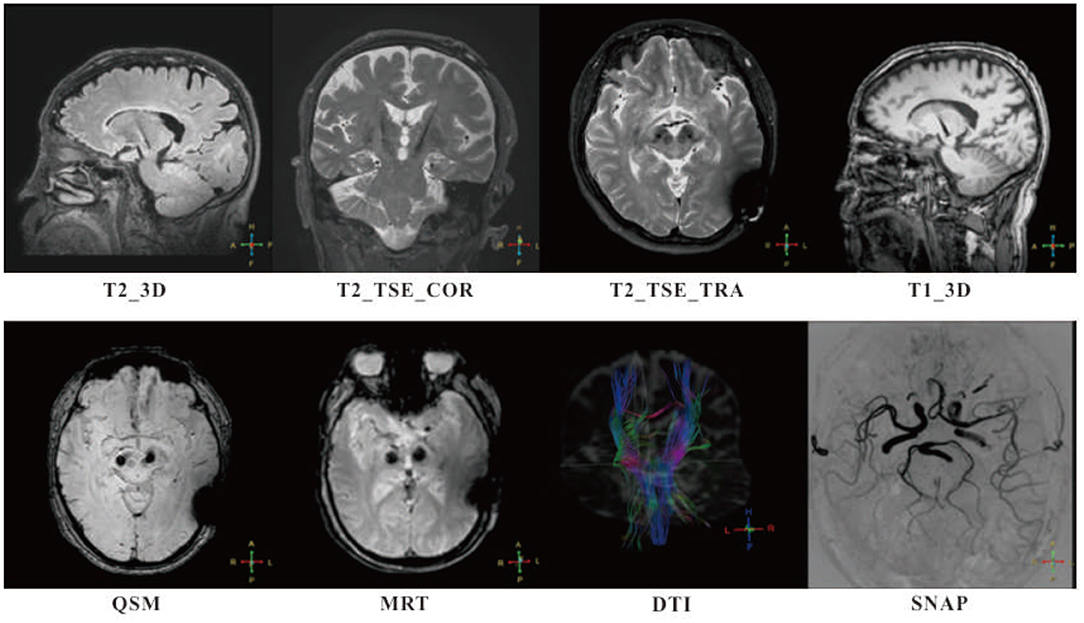
Figure 1. Follow-ups of patients with MR-compatible deep brain stimulation (DBS) implanted were successfully completed at 1, 3, 6, and 12 months, with intensive 3T MR scan. High in-plan resolution T2-weighted fast spin echo sequences (T2_TSE_COR and T2_TSE_TRA) and high specific absorption rate (SAR) isotropic sequences (T1_3D, T2_3D, and QSM) were adopted for anatomy analysis. Simultaneous non-contrast angiographies (SNAP) and diffusion tensor imaging (DTI) were taken for monitoring potential lesion on blood vessels and edema occurrence. A special sequence, magnetic resonance thermometry (MRT) used for tissue temperature online assessment. No adverse events were found that related to MRI.
Recording Neural Signals in Deep Brain Stimulation
Electrophysiological recordings of oscillatory neural networks remain an important tool for advancing brain research. In PD, β-band oscillations detected from the basal ganglia correspond to the degree of motor symptoms, such as rigidity and L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia, representing a pathophysiological marker of movement disorders that are beyond parkinsonism alone (7–10). Previous clinical studies have recorded neural activity intraoperatively during DBS surgery, with local field potentials (LFPs) being captured from external cables connected to DBS leads. With limited data recording and subsequent processing being completed after DBS implantation, along with potential complications from microlesion effect and edema along lead trajectories, studies of disease pathophysiology have been limited with such methods. Current implantable LFP-recording DBS systems are available but generally have a non-rechargeable battery lasting 3–5 years. Notably, these devices have high power consumption that results from LFP acquisition and readouts, limiting the time of use and requires earlier replacement of the implantable pulse generator (IPG), a factor that is not favored by patients. The need for extending the longevity of the DBS recording device remains at the forefront of neural engineering research. This would allow for efficient long-term LFP recordings, for example, to assess the changes of β-band oscillations in response to motor symptoms over time. Hopefully, more research on θ-band relating to tremor and prokinetic γ-band can help us develop robust algorithms for closed-loop control.
While the implantable DBS device PC+S produced by Medtronic has been used in previous research, its storage capacity in the IPG has limitations for long-term continuous recordings that often require massive LFP data storage. To resolve such issues, streaming of data to an external storage provides a solution for unlimited data collection and simultaneous assessment of physiological signals, such as movement behaviors, in a freely moving environment that allows for sophisticated clinical experimentation. The latest DBS devices for LFP recordings would be expected to include (1) large-capacity battery or wireless charging technology that fulfills long-term implantation acceptable to patients receiving treatment, (2) continuous high-precision data acquisition capability, (3) real-time external transmission that is wireless and therefore can be applied in freely moving conditions, and (4) multiple differential signal acquisition channels that can function simultaneously with DBS-ON, with sampling rates exceeding 500 Hz.
Here, the NELN demonstrated and reported PD patients implanted with DBS device G102RS from PINS Medical Ltd., a device engineered with rechargeable LFP-sensing and data streaming capacity. The first clinical trial has been completed in PD patients (n = 13) with a successful post-surgery follow-up through 12 months. Preliminary data have been used to characterize components of β-band oscillations during sleep states (11). Furthermore, it is reported that the response of β-band oscillations to high-frequency DBS is changed over time, which is a likely result from changes in neural network plasticity.
Remote Online Deep Brain Stimulation Programming
Following implantation of a DBS device, postoperative DBS programming conducted by specialists is a vital part of achieving optimal clinical efficacy in patients (12). However, practical burdens in the clinical setting exist such as limited specialists available, time constraints, patient travel to specialist centers, and additional care costs (13). In recent years, the NELN has developed a remote online DBS programming system that operates with hardware-level protection for remote communication security (14). This has been specifically aimed at alleviating common healthcare burdens in the field of DBS worldwide. To date, the number of patients who have successfully used the remote DBS program control in China has exceeded 3,000 and has also been implemented between different countries, such as the UK, Spain, and Singapore, for patient management.
The development of the remote programming technology has notably reduced the burden of patient visits. In a recent survey with approximately 200 patients, costs and time-spent related to follow-up have both been reduced by >90%. Remote programming allows for clinical evaluations to be conducted through video and audio streams with Unified Parkinson's Disease Rating Scale (UPDRS)-III scoring and physical examination. As we look to the future, there is a natural evolution toward machine learning algorithm applications for automatic movement evaluation and objective output readings. Such applications allow for significantly increasing the amount of data collected on disease progression and enriching data pools for diagnoses and potential use toward future closed-loop systems.
Variable Frequency Stimulation
High-frequency stimulation of the subthalamic nucleus (STN) through DBS in PD patients is a well-established application for alleviating parkinsonism. However, the application of high-frequency stimulation fails to alleviate axial disabilities in PD patients, which may occur due to disease progression, surgical injury, and side effects of electrical stimulation (15). Previous reports of low-frequency stimulation in PD have demonstrated the alleviation of axial disability but may compromise improvements in parkinsonism (16, 17). A recent pilot study completed has shown the promising effects of variable frequency stimulation (VFS), which applies alternating high- and low-frequency stimulations for freezing of gait (FOG) in PD patients (18). The long-term stability of VFS application is now being evaluated in a large clinical trial. In addition, video data collected from previous trials have been assessed with automatic classification and scoring based on machine learning methods to evaluate typical Timed Up and Go (TUG) tasks. This is now being utilized for objective classifications of FOG under separate analyses.
Perspective of Artificial Intelligence for Deep Brain Stimulation
Artificial intelligence (AI) has great potential in medicine. Being broadly defined as the development of intelligent machines, the field of AI focuses on capabilities, such as understanding human languages and natural scenes, and development methods, such as machine learning (19–21). Machine learning entails building knowledge from patterns in data rather than being specified by human programmers. Much of the recent success in AI has come from the aggregation of massive training data and new computing systems for large-scale learning. New algorithms and systems have accelerated the widespread experimentation for prediction problem as supervised learning. In real-world situations, there is the desire to take strategies based on predictions. A next target for learning systems is data-driven decision-making.
Recent achievements in AI (including machine learning, computer vision, natural language processing) have the potential to improve our understanding of neurological disorders and corresponding treatments. In specific relation to DBS, there can be difficulty and time expenditure in finding optimal parameters for each patient. AI may help shape effective treatment for some of the most prevalent neurological disorders, such as PD, based on previous data (Figure 2). Future developments toward robust online learning techniques that explore large decision spaces and adapt to feedback in real time are essential to online learning problems. The application of AI techniques may allow us to uncover the mechanisms of DBS and the understanding of how DBS influences brain networks (11). It is noteworthy that recent advances in MRI-compatible DBS devices are allowing for acquisition of neuroimages during stimulation. It can be envisioned that a combination of advanced imaging techniques and AI techniques can facilitate the identification of DBS surgical targets in individual patients. Indeed, personalized implantation and automatic stimulation strategies can be aimed to maximize safety and efficacy of optimal treatment benefits and improve patient care.
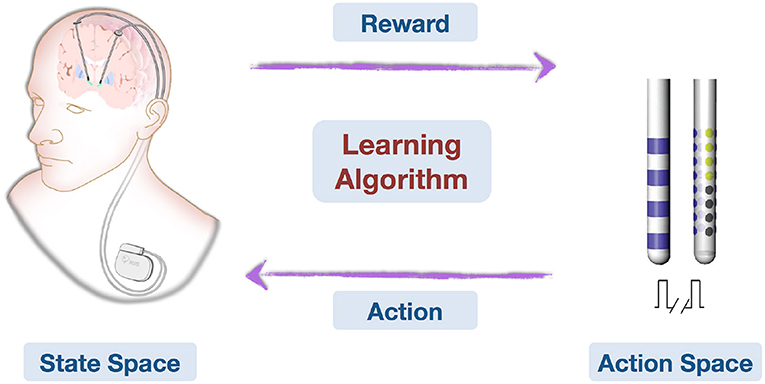
Figure 2. This is an illustration on how deep brain stimulation parameters can be learned and optimized online with feedback. Patient conditions are represented in the State Space. Action Space contains all possible stimulating patterns. The Learning Algorithm learns from domain knowledge and history data, then observes treatment effects (Reward) and optimizes stimulating pattern (Action) online.
Overall, advances in machine learning and robotics have the potential to improve health care delivery, from scheduling treatment plans to guiding surgical procedures, that are beyond current clinical capabilities. While these technologies shed light on the way toward better treatment, they also pose new challenges in terms of scale, complexity, safety, robustness, and efficacy.
Innovations That Aim At Uncovering Deep Brain Stimulation Mechanisms of Action
Toward Connectomic Deep Brain Stimulation
It was long thought that DBS exerts its function by local modulation of the target region itself, and a large number of studies have focused on such local effects by delineating optimal “sweet spots” for effective DBS. However, accumulating evidence suggests that DBS modulates fiber tracts or distributed brain networks and that such effects may be equally important for optimal treatment outcome (22–27). This has given way to a paradigm shift in the field of DBS, away from localized targeting and toward modulation of whole-brain networks by invasive neuromodulation.
In a parallel development, in the field of neuroimaging, the concept of the connectome, a formal mathematical description of brain regions and their interconnections was introduced in 2005 (28). Given the strong impact of the connectomics concept on the neuroimaging field, it is somewhat surprising that, so far, only a handful of studies have applied it to DBS (22, 29–31). One reason for this may be that patient-specific connectivity data [resting-state functional MRI (rs-fMRI) or diffusion MRI (dMRI)] are usually not acquired within clinical routine and are hard, if not impossible, to acquire postoperatively.
To overcome this limitation, Horn et al. (23, 32–34) established a method that combines normative connectomes, i.e., average brain connectomes that are estimated on large cohorts of subjects, with DBS electrode reconstructions from a single patient. This concept has been successfully applied to other areas of clinical neuroimaging, for instance, to map stroke symptoms to brain regions (26, 28, 35) or to explain varying results of transcranial magnetic stimulation (TMS) treatment (36). A recent publication has demonstrated the feasibility of this concept for DBS (23). Here, the authors estimated the structural and functional connectivity profile of effective ventral intermediate (VIM) nucleus DBS by transforming an optimal literature-based DBS coordinate to standard stereotactic [Montreal Neurosciences Institute (MNI)] space and combining it with normative connectomes. A second study demonstrated that clinical DBS improvement can be predicted based on the connectivity profiles of electrodes alone (25) (Figure 3). Specifically, the structural and functional “connectivity fingerprints” of DBS electrodes in 95 PD patients operated on at two centers were highly predictive of their clinical motor improvement. In fact, the optimal connectivity profile of effective STN-DBS could be informed exclusively on data from the first DBS center and then used to accurately predict outcome in patients from the second center. The study demonstrated that brain connectivity may play a crucial role in the DBS mechanism of action and that it may be used to predict treatment outcome across cohorts and centers. Recently, the concept was transferred to essential tremor (37) and obsessive–compulsive disorder (OCD) (38). After further validation, resulting “effective treatment networks” of these and similar studies could in the future be used to guide both DBS programming and surgery. Moreover, networks could potentially be used to guide non-invasive brain stimulation since they define cortical areas that may play a role in disease-specific and therapeutic circuitries.
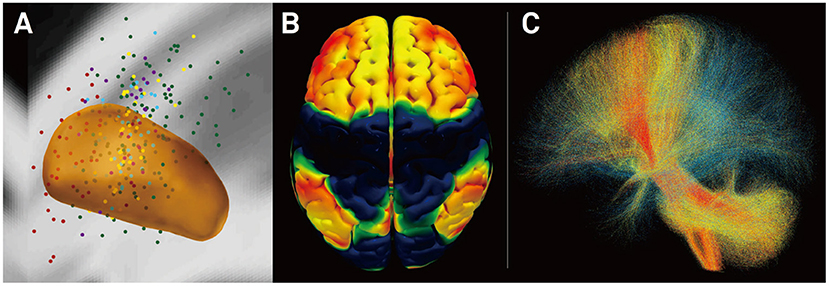
Figure 3. Connectivity predicts deep brain stimulation (DBS) outcome in Parkinson's disease (25). (A) Active stimulation coordinates from five cohorts out of two DBS centers mapped to subcortical anatomy [subthalamic nucleus (STN) shown in orange]. (B) Cortical connectivity map predictive of clinical outcome analyzed using normative-connectome based on resting-state functional MRI (rs-fMRI). Hot colors show areas that are associated with good clinical outcome if the electrode is strongly connected to them. In contrast, functional anticorrelation to areas in cold colors is associated with beneficial outcome. (C) Fiber tracts associated with good (red), intermediate (yellow), or poor (blue) clinical outcome. Connectivity profiles shown in (B,C) are able to predict motor improvement in out-of-sample data (across DBS cohorts and centers; R in the range of 0.5; ~20% variance explained).
Studying Effects of Deep Brain Stimulation in Individual Patients
DBS is a well-established functional neurosurgical technique that has recently observed rapid development as a potential treatment for neuropsychiatric disorders (27, 39). Not only does DBS mimic the effects of neuropharmacological treatment, but it currently offers key advantages with fewer side effects and greater adjustability. Although DBS has achieved great success in treating movement disorders such as PD and dystonia, a broader use of DBS for other neurological disorders is facing two major challenges. The first lies in accurate application in individual patients, as patients with neurological and psychiatric disorders are highly heterogeneous in terms of their symptom expression, disease progression, and more importantly their brain functional network organization. A personalized implantation/stimulation strategy is thus necessary to maximize the treatment benefits and improve patient care. The second challenge is the lack of in-depth understanding of how DBS impacts wider brain networks largely due to the lack of means to study the immediate and long-term stimulation effects on large-scale brain networks in vivo. A better appreciation of the mechanism of DBS is crucial in order to extend this important technique from treating movement disorders to a broader spectrum of brain diseases including Alzheimer's disease (AD), stroke, and neuropsychiatric disorders.
Understanding the neurophysiology, connectivity, and neuropathology at the level of individual patients is key to furthering the success of DBS treatment. To date, DBS applications are largely based on the presumption that current models of disease, which are predominantly derived from neuroimaging studies that identify brain abnormalities at a group level (40), can be directly applied to individual patients. However, it is becoming increasingly recognized that interindividual variability exists not only in macroscopic and microscopic brain anatomy (41–43) but also in the organization of functional systems, i.e., the topography and connectivity of functional regions may vary drastically across individuals (44, 45). Compared to unimodal sensory and motor functions, higher-order cognitive functions demonstrate substantial variability across individuals. Recent studies suggest that the high level of interindividual variability in higher-order functions may be a fundamental principle of brain organization and a critical outcome of human brain evolution (44–46). Disease models concerning motor circuits, which have a relatively low degree of interindividual variability, might be directly applied to individual patients to guide DBS treatment. For example, targeting the STN and globus pallidus internal segment (GPi) provides efficient treatment of akinesia, tremor, and rigidity in most PD patients (47). However, even with well-defined targets, not all patients seem to benefit from DBS to the same degree. The picture becomes more complicated with the application of DBS in psychiatric disorders. For example, a recent report of the application of DBS to the subcallosal cingulate for treatment-resistant depression yielded unsatisfactory results, with DBS failing to demonstrate a superior effect to sham stimulation (40). A subsequent trial has suggested that DBS targeting and parameters need to be optimized for individual patients in order to demonstrate treatment efficacy (48). Specifically, it was found that treatment responders shared a common pattern of white matter connectivity within the subcallosal cingulate region (49). These results suggest that it is necessary to develop patient-specific targets and cortical responsivity measures to identify precise DBS targets. On a systems level, it is crucial to develop non-invasive metrics of brain functional and structural connectivity, at an individual level, to make DBS treatment viable and to improve the cost–benefit ratio for patients. Recent technical advancement in functional connectivity MRI research has made it possible to localize functional networks at the single-subject level (50–52), which could thus be used to guide personalized DBS treatment. For example, the work by Wang et al. (52) has established a technology to parcellate cortical functional networks in individuals, which is highly sensitive to the characteristics of the individual and is able to capture intersubject variability. Functional networks localized using this parcellation technology were also validated by invasive cortical stimulation mapping in surgical patients. Such techniques may be essential for identifying DBS targets in individual patients in the near future.
Understanding the immediate and long-term effects of DBS on large-scale brain networks requires technologies that can read out brain signals in vivo. Until recently, due to technical constraints, the local and remote effects of DBS have only been measured with electroencephalography using external leads and formed the basis for the investigation of brain response to DBS (53). Recent technological developments allow for the concurrent recording of LFPs and high-field MRI during DBS (54). The implications of these developments are profound (47). DBS significantly suppressed beta activity (13~35 Hz), but the suppression effect appeared to gradually attenuate during a 6-month follow-up period after surgery (55). The concurrent recording of LFPs allows for the characterization of pathophysiological neuronal firing patterns, the investigation of the clinical response according to application parameters, and the development and testing of new disease models. This technological advancement has benefited the study of, for example, the abnormal oscillatory activity (13–35 Hz) in PD, the pivotal role of the STN in basal ganglia physiology and pathophysiology, and the use of β-band oscillations as biomarkers to devise closed-loop DBS systems to deliver a more neurophysiologically efficient therapy. Nevertheless, electrophysiological signals recorded from implanted electrodes only reflect neural responses at local structures rather than the effects on large-scale, distributed functional networks. Obtaining a comprehensive picture of DBS effects on the human brain is now possible, thanks to the recent development of DBS devices that are compatible with high-field MRI (54). Taking advantage of these novel devices, we are able to record functional activity across the entire brain during DBS using 3T MRI. The high-quality imaging data can capture the changes in the large-scale brain networks when DBS is turned on and turned off. The data presented by Liu et al. (52) demonstrate immediate, strong suppression of brain activity in the sensorimotor cortex after STN stimulation in PD patients (Figure 4). This DBS-fMRI technology allows for examining the validity of potential DBS targets for a variety of brain disorders, eventually leading to a broader use of DBS.
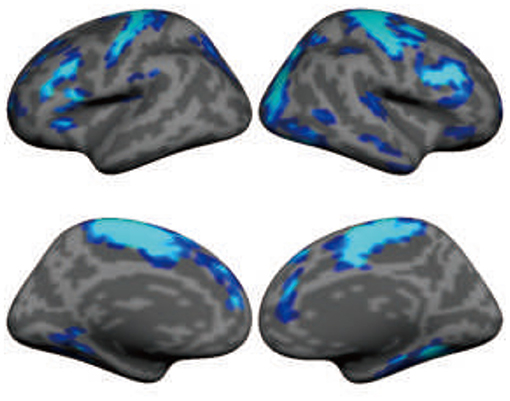
Figure 4. Subthalamic nucleus stimulation suppresses functional activity in large-scale brain networks, including sensorimotor and association regions in the frontal lobe. The map shows the functional MRI (fMRI) contrast between “DBS on” condition and “DBS off” condition in 11 patients with Parkinson's disease. Functional data were recorded using 3-Tesla MRI when deep brain stimulation (DBS) was turned on (36-s blocks) and off (24-s blocks) using a block design.
Taken together, DBS has the potential to revolutionize the treatment of neurological and psychiatric disorders and to improve our understanding of human brain function. However, before DBS can be implemented into standard practice for a broad range of disorders, a better understanding of how it affects large-scale brain networks and identification of precise targets in individual patients are necessary. The development of individualized functional imaging techniques and MRI-compatible DBS devices will greatly facilitate research in this important field.
Neurocircuitry Underlying Effective Deep Brain Stimulation for Mental Health Disorders
DBS is a promising therapeutic approach for patients with treatment-resistant mental health disease, including OCD and major depressive disorder (MDD) (56–58). MDD and OCD involve key elements of the cortico-cortical and cortico-basal ganglia networks. These networks include the ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), dorsal anterior cingulate cortex (dACC), and the basal ganglia structures, striatum, and STN (59). DBS primarily targets myelinated fibers that carry information from and to the above structures. As such, the most common DBS targets are (1) the subgenual cingulate gyrus white matter (SCGwm), the white matter adjacent to cortical areas 32 and 25; (2) the anterior limb of the internal capsule (ALIC) that carries descending and ascending cortical fibers; and (3) the connections of the STN, including the hyperdirect pathway that carries cortico- STN fibers (56–58).
The work by Haber et al. (59) has used a combination of nonhuman primate (NHP) tracing experiments and NHP and human dMRI to delineate the organization of PFC fiber pathways, which allows insight into which connections are likely to be involved at each DBS electrode site. The work has focused on how cortical fibers are organized within the SCGwm, ALIC, and STN and the fibers and terminal fields likely to be affected by DBS electrodes placed within those regions.
The SCGwm site is primarily used for treatment-resistant depression. The most effective SCGwm contacts (1 and 2) are at the border between the SCG and the inferior rostral gyrus (60). Fibers that pass through this region include multiple connection involving the entire ventral surface of the frontal cortex (Figure 5A). Contact 1 is within the inferior rostral gyrus white matter, contact 2 is within the SCG, and contracts 0 and 3 are ventral and dorsal, respectively. Contacts 0–2 will involve (1) all connections from vmPFC areas adjacent to the electrode contacts (both cortical and subcortical projections); (2) uncinate fasciculus fibers from non-adjacent vmPFC and medial OFC as they travel medially to other ventral PFC areas; (3) a subset of lateral OFC fibers traveling medially to innervate medial PFC areas; (4) axons traveling from the contralateral vmPFC and medial OFC; and (5) a subset of anterior vmPFC and medial OFC en route to the corpus callosum through the uncinate fasciculus. Contact 3 involves primarily fibers in the corpus callosum. In addition, this site captures a subset of fibers traveling from the medial OFC and posterior lateral OFC to the cingulum bundle and superior longitudinal fasciculus (61).
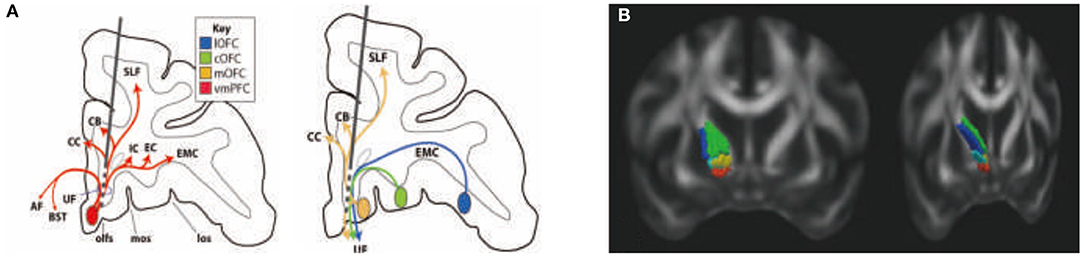
Figure 5. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness (61). Organization of fibers passing through the subgenual cingulate gyrus white matter (SCGwm) (A) and anterior limb of the internal capsule (ALIC) (B). (A) Schematic of an electrode passing through the SCGwm, indicating the cortical fibers involved at each contact. AF, amygdala fugal pathway; CB, cingulate bundle; CC, corpus callosum, EC, external capsule; EMC, extreme capsule; IC, internal capsule; los, lateral orbital sulcus; mos, medial orbital sulcus; olfs, olfactory sulcus; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus. (B) Organization of fibers in the human ALIC. Red, OFC and vmPFC fibers; yellow, ventrolateral PFC fibers; light blue, dACC fibers; green, dorsolateral PFC fibers; blue, dorsomedial PFC fibers.
The ALIC site is used for both treatment-resistant MDD and OCD. Fibers from different cortical regions follow predictable trajectories to, and locations within, the ALIC. The relative position of fibers from different cortical areas, within the ALIC, demonstrates the topology, and specifically the ALIC segmentation, based on PFC origin of fibers. Fibers arising from dorsal regions travel within the capsule dorsal to those from ventral cortical areas. Axons derived from medial areas travel within the capsule medial to those from lateral regions (Figure 5B). The organization shows how stimulation in different locations throughout the ALIC is likely to impact projections of different cortical areas, including the ventrolateral PFC (vlPFC), dorsolateral PFC (dlPFC), dorsomedial PFC, and dACC. Each contact placed within the ALIC activates a different subset of corticothalamic and brain stem fibers. In particular, an electrode contact targeting the ventralmost part of the ALIC will likely impact primarily fibers from the vmPFC and OFC. More dorsal contacts/lesions will impact lateral OFC, ventrolateral PFC, and dACC fibers. The most dorsal contacts/lesions will impact primarily the dorsomedial and dorsolateral PFC. In addition to consideration of the dorsoventral position of electrodes or lesions, the rostrocaudal position is also important (61, 62).
The effectiveness of DBS for depression at the SCGwm and ALIC sites has not been directly compared with respect to patient selection criteria. Nonetheless, both sites are effective in over 50% of otherwise treatment-resistant patients (63, 64). Stimulation at the SCGwm site captures all cortical and subcortical projections from the area surrounding each contact site. However, it also captures fibers from non-adjacent cortical areas passing through the target, including connections between different vPFC areas, and OFC fibers traveling to corpus callosum, medial forebrain bundle (MFB), cingulum bundle, and superior longitudinal fasciculus. In addition, this target captures the extensive brain stem connections from the SCG. In contrast, ALIC site does not directly involve corticocortical fibers. Rather, each contact in the ALIC site involves a different combination of thalamic and/or brain stem bundles. Of particular importance is that both DBS targets capture subsets of fibers that include both thalamic and brain stem fibers. Thus, an important part of the clinical effectiveness of DBS is likely to require a combination of thalamic and brain stem fibers.
The STN site, commonly used for PD is now experimentally used for OCD. In addition to the STN connections to both pallidal segments, there is an important direct cortico-STN connection that is referred to as the hyperdirect pathway. This pathway is also organized in a specific and general topographic manner. M1 projects to the dorsolateral STN, with area 6 projecting ventromedially to these terminals. Overall, PFC projections are concentrated and anterior to motor control projections. While dorsal PFC projections occupy the medial half of the STN, vmPFC and dACC dense terminal fields are located in the rostral and anterior, medial tip. This area also receives the limbic input from the pallidum, in particular, projections from the ventral pallidum (65). The delineation of limbic and cognitive hyperdirect pathways has been key for developing a DBS site for the treatment of OCD (58). It has also contributed to our understanding of non-motor effects of DBS in PD. Taken together, DBS for motor disorders such as PD targets the lateral STN regions, while the target for OCD targets the medial STN. Effectiveness of DBS for OCD at the ALIC and STN sites is now being compared at several clinical sites.
Deep Brain Stimulation for Major Depression and Addiction
MDD has been known to exist since the origins of humankind. Hippocrates (460–370 BC) referred to MDD as “melancholy.” Being a highly heterogeneous disorder, symptoms of MDD affect a range of behavioral domains including mood, sleep, sexual behavior, and motor functioning. MDD has a 1-year prevalence of 3–5% and lifetime prevalence of 15–20%. It has been reported that MDD is on the uprise (increase of 10% between 2005 and 2010), leading to an incremental economic burden for individuals with MDD. The cost increased by 21.5% (from $173.2 billion to $210.5 billion) (66).
DBS for MDD has demonstrated clinical benefit in three brain regions in open-label trials, including the ventral capsule/ventral striatum (VC/VS), the subgenual cingulate cortex (SCC), and the superolateral branch of the medial forebrain bundle (sl-MFB) (40, 67, 68). Two large, industry-sponsored sham-controlled randomized controlled trials (RCTs) of DBS for depression failed, one targeting BA25 and one targeting the ventral capsula (68). The BA25 trial (BROADEN) was halted when interim analyses showed a low likelihood of meeting primary endpoints. The outcomes of these trials have caused the field to question fundamental aspects of these therapies, including patient selection, trial design, network targeting, funding, and, of course, efficacy itself.
A number of lessons can be gleaned from these trials. (1) The underlying disorder, major depression, is not well-understood. We need better biomarkers to further delineate and stratify various forms of depression. (2) There is a need for identification of better outcome measures analogous to those used in DBS for movement disorders. For example, specific motor variables such as bradykinesia, rigidity, and tremor are utilized to determine the outcome of DBS for PD while psychiatric scales tend to focus on more holistic and subjective disease outcomes. Furthermore, these variables could also be used to identify patients most likely to respond to DBS. Thus, the best PD candidate is not simply the patient with the worst overall disease but one with specific levodopa-responsive symptoms. (3) There is shift, now, from the single-target approach to a network-based model of neuropsychiatry in which target selection is patient and disease specific. (4) We need to leverage structural and functional neuroimaging to better identify these network nodes. (5) We need to change our attitudes with regard to trial designs to support more flexible designs and to aggregate data across trials. (6) Finally, ongoing advancements in DBS hardware and software (such as directional electrodes and closed-loop devices) will result in increasing the therapeutic index and efficacy of DBS for psychiatric disorders. Thus, the field of psychiatric neurosurgery finds itself at a crossroads. Despite the setbacks of these so-called “failed” trials, the clinical and financial burden of psychiatric diseases continues to grow, as does the theoretical rationale for DBS therapy, with increased commitment from various national and international agencies.
In addition to major depression, substance addiction is one of the most prevalent and costly health problems globally. Standard medical therapy is often not curative, and relapse is common. Research over the past several decades on the neural underpinnings of addiction has implicated a network of structures within the brain shown to be altered in patients with substance abuse. While invasive neuromodulation such as DBS and VNS have proven to be effective in treating depression, OCD, and epilepsy, there is increasing interest and data with regard to their potential application in the treatment of severe, intractable substance abuse and addiction. Several neuromodulatory techniques and brain targets are currently under investigation in patients with various substance abuse disorders (69).
The current work by Bari et al. (70, 71) is aimed to apply the lessons learned from DBS for depression toward the application of DBS and other forms of invasive neuromodulation toward addiction. Thus, using probabilistic tractography, Bari et al. have identified specific limbic structures associated with nicotine addiction and impulsivity (currently under review with human brain mapping). In addition, brain mapping data from patients undergoing DBS for post-traumatic stress disorder (PTSD) combined with a normative connectomic approach has supported the role of the amygdala in regulating reward-related emotions. These efforts provide the background on which to design more informed trials of invasive neurmodulation for nicotine and other forms of addiction.
Toward Deep Brain Stimulation Targets and Stimulation Designs for Depression
The neural correlates of MDD have only been partially unraveled and involve both activity and connectivity changes. Based on neuroimaging, a neural circuit taxonomy for depression and anxiety has been developed (72). This suggests that rumination is the consequence of hyperconnectivity within the default mode network, inattention due to hypoconnectivity within the frontoparietal attentional networks, anhedonia and context insensitivity to a dysfunctional positive affect network, and anxious avoidance to hypoconnectivity within the salience network and hyperconnectivity between salience and default mode network (72).
Before medications were discovered that could treat MDD, psychosurgical techniques were developed to address this complex pathology. Whereas, the initial approach was to perform a large frontal lobotomy, the development of stereotactic approaches in 1947 permitted smaller and better targeted lesions resulting in four kinds of psychosurgery: (1) cingulotomy, (2) anterior capsulotomy, (3) subcaudate tractotomy, and (4) limbic leucotomy (combination of 1+3). Even though psychosurgery came under public attack and was nearly forbidden, a dilemma arises with recent disinterest and disengagement of the big pharma in developing novel medications for brain disorders.
Based on modern structural imaging with tractography, it is now clear that these four targeted regions functionally converge at the pgACC, extending into the OFC, and are connected via the forceps minor and the anterior thalamic radiations to subgenual cingulate regions. Anatomically, this convergence may derive from the superolateral branch of the MFB, a structure that connects these frontal areas to the origin of the mesolimbic dopaminergic “reward” system in the midbrain ventral tegmental area, which is a possible final common pathway.
From the initial work by Mayberg et al. (49) the subgenual anterior cingulate has been selected as a target for depression, yet others have targeted the MFB. However, there seems to be a problem. Open-label studies for MDD are all positive including a meta-review of meta-analyses. Yet two controlled trials for MDD were both negative, one targeting BA25 and one targeting ventral capsule/striatum. This is in keeping with a larger problem that today, no target—whatever the disease—can meet the criteria for clinical efficacy as recently defined by an international committee for neurosurgery for psychiatric disorders. How can we overcome this problem? Should the neuromodulation community look for new targets, use novel stimulation designs, or a combination of the two?
Based on historical data from destructive psychosurgery, as well as modern functional and structural imaging, new targets for neuromodulation can be proposed: the dACC has been a target for the treatment of MDD with lesioning, TMS, and implants. Similarly, functional imaging suggests that also the left amygdala, right parahippocampal area, pgACC, caudate nucleus, insula, as well as the DLPFC and VLPFC could be potential targets for neuromodulation for MDD.
Another critical question is what is the ideal pattern of stimulation? DBS for movement disorders applies fairly standardized stimulation parameters, consisting of a frequency of 130 Hz, pulse widths <300 μs and variable amplitudes. Recently, burst stimulation has been developed, and this stimulation design applies the natural frequency of the targeted area, e.g., 6 Hz at the ACC, 20 Hz at the DLPFC, 4–8 Hz at the somatosensory and auditory cortex. Thus, effective outcomes can only be expected if both the target and stimulation design match. Interestingly, novel stimulation designs such as noise stimulation can be developed to prevent the brain of habituating to the stimulation. Noise can come in various forms, also called colors, from white to pink to brown and black, with an increasing steeper slope, following a 1/fβ with β = 0, 1, 2, 3 for white, pink, brown, and black, respectively. Considering that connectivity is both hypo- and hyper-, a combination of burst and noise may be essential to normalize dysconnectivity in MDD, with burst stimulation potentially increasing connectivity and noise stimulation desynchronizing activity, i.e., decreasing connectivity.
Yet still another form of neurostimulation can be developed, called reconditioning stimulation. The concept is that electrical stimulation is paired to external stimuli, as first proposed in a seminal paper in tinnitus. This concept can be adjusted to treating depression and has as advantage that the paired stimulation exerts a learning effect on the brain, instead of only suppressing hyperactivity or blocking hyperconnectivity, which is the mainstay of current neurostimulation approaches. The adaptation would be to pair external hedonic stimuli to rewarding stimuli delivered at different parts of the reward circuitry.
In summary, novel targets combined with novel stimulation designs pave the way for improved treatments for MDD and entirely novel approaches, such as reconditioning stimulation, might be yet another approach to treat this most debilitating of brain disorders.
Pathway-Specific Targeting for Subcallosal Cingulate Deep Brain Stimulation
DBS of the subcallosal cingulate white matter (SCC DBS) is an emerging strategy for treatment-resistant depression (56). Clinical trials show response rates at 6 months across studies range from 41 to 66% with sustained and increased response over time (40, 73). A challenge to effectively disseminate this nascent treatment remains in the fact that there is an absence of biomarkers to guide lead placement or to titrate stimulation parameters during follow-up care. Unlike PD, where intraoperative electrophysiology is routinely employed to define the anatomical–functional placement of the lead and titrate stimulus parameters to moderate symptoms in real time, such mechanistically guided biomarkers for depression are lacking. Furthermore, the SCC target is in the white matter, without demarcated anatomical boundaries. As such, individualized mapping of the target and its precise cortical connections is a critical first step to standardize the procedure.
Targeting the SCC white matter was based on converging imaging data demonstrating changes in SCC activity with antidepressant response to a variety of standard treatments (74, 75). Selection of this target was further supported by an extensive literature demonstrating monosynaptic connections between the subcallosal cingulate and specific frontal, limbic, subcortical, and brain stem sites involved in mood regulation, depression, and the antidepressant response (76, 77). Specific placement of the DBS electrodes was therefore determined by local anatomy. Approximate coordinates were derived from PET imaging studies localizing the subcallosal cingulate region (Brodmann area 25) and adjacent white matter and were then combined with anatomical landmarks identified in standard neurosurgical atlases. Tractography-guided connectomic approach to SCC electrode implantation has been found to improve the precision of surgical targeting following an initial feasibility study (49, 78).
In the latest developments by Choi et al. (79) refinement of surgical targeting has been aided by using tractography guidance. White matter pathways have now been mapped in responders and non-responders in the first study cohort to define the necessary and sufficient pathways that must be stimulated to achieve a full antidepressant effect (78). These maps utilize individualized models of the volume of tissue activated (VTA) derived from each patient's diffusion tractography scan (80). Successful prospective targeting in the most recent cases of 11 patients has resulted in successful mapping of responders group. These latest results confirm that prospective targeting of four key white matter bundles (cingulum, uncinate fasciculus, forceps minor, and frontal-striatal; Figure 6) can be performed reliably in individual patients, and use of this method improves long-term outcomes.
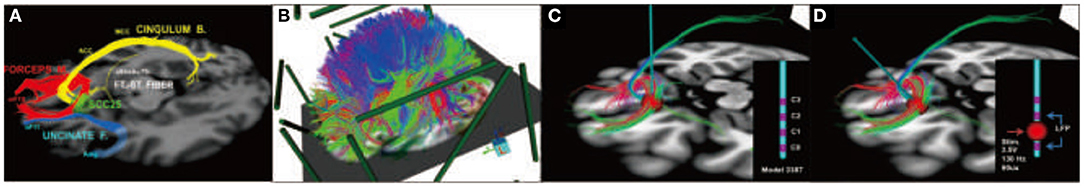
Figure 6. Prospective targeting of these four white matter bundles can be performed reliably in individual patients, and use of this method improves long-term outcomes. (A) Four-bundle white matter “blueprint”: cingulum (yellow), uncinate fasciculus (blue), forceps minor (red), frontal-striatal (white). (B) Whole-brain tractography loaded in patient-specific stereotactic frame space using StimVision. (C) Visualizing tracts passing through the volume of tissue activated (VTA) to define optimal target location that best visually matched the “blueprint.” (D) Finalization of lead trajectory with the neurosurgeon to avoid cerebral vasculature and choosing the point of entry.
Perspectives of Deep Brain Stimulation for Memory Disorders
Memory deficits are a characteristic feature of numerous neuropsychiatric disorders, including various forms of dementias. To date, no effective treatment exists for memory deficits in dementia. Commonly used medications are acetylcholinesterase inhibitors, but the overall response is not very satisfactory (81). Neuromodulation strategies, including DBS, have been recently proposed for the treatment of these conditions.
The use of electrical stimulation for the study of memory problems is not new. In animal models, stimulation of various brain regions has been conducted in attempts to investigate physiological aspects in several learning and memory paradigms (82, 83). Early reports administering high current intensities to limbic structures in rodents undergoing memory tests reported stimulation-induced memory deterioration (82, 84). In contrast, stimulation regimens suited to induce plasticity (85, 86) were found to improve memory.
In humans, memory improvement has been reported in patients with epilepsy receiving entorhinal cortex (EC) (87) or anterior nucleus of thalamus (ANT) DBS (88). However, impairment has also been described particularly when stimulation was delivered acutely at relatively high currents (89). A patient with morbid obesity treated with DBS in the hypothalamic/forniceal region presented dejà vu sensations (90). In this same patient, stimulation was found to modulate the activity of the mesial temporal lobe and improve hippocampal memory function, as measured with neuropsychological testing (90).
A few years ago, a phase 1 clinical trial was conducted to test the safety of fornix DBS in six patients with AD (91). In addition to promising clinical findings, DBS was shown to modulate the activity of mesial temporal lobe structures and increase brain metabolism in temporal and parietal regions, as revealed by positron emission tomography (PET) scan (91). Following that study, a phase II trial of fornix DBS in mild AD (ADVANCE) was conducted (92). Forty-two patients were recruited in centers across the United States and Canada. It consisted of a 12-month double-blinded randomized controlled study comparing active and sham stimulation. When all patients were considered, no significant differences were observed in the Alzheimer's Disease Assessment Scale–Cognitive (ADASCog) 13 scores between groups (92). Interestingly, however, patients older than 65 receiving DBS seemed to have had a slower disease progression (though no significant differences were detected compared to sham-treated individuals of the same age group) (92). Another DBS target proposed for the treatment of AD is the nucleus basalis of Meynert, with preliminary studies showing promising results in different clinical types (93).
To explain potential mechanisms of stimulation, preclinical work has been conducted. In one of these studies, Gratwicke et al. (94, 95) stimulated the EC of AD transgenic animals. The authors found significant improvements in animals receiving DBS compared to sham treatment in the Morris water maze and novel object recognition tests. Remarkably, DBS reduced the number of Aβ plaques, as well as tau, phosphorylated tau, and amyloid precursor protein (APP) in the hippocampus of transgenic animals (94).
Taken together, the effects of stimulation on memory seem to vary as a function of the current intensity, target, and duration of treatment. Promising results have been reported in degenerative disorders, including AD. In animal models, DBS has been shown not only to improve memory function but also to have neuroprotective effects. Recent clinical trials in AD patients have shown promising enough results to warrant large-scale studies.
Latest Approaches in Deep Brain Stimulation for Movement Disorders
From Primate to Man: Pedunculopontine Nucleus Stimulation as a Therapy for Patients With Parkinsonian Disorders
FOG and falls are two of the most disabling symptoms of PD affecting upward of 10% of such patients (96). The introduction of L-DOPA in 1975 was so miraculous in reversing the cardinal signs of PD that it led to an initial discontinuation of functional neurosurgery, as it was felt that a single drug could be found to have similar beneficial effects with less potential side effects in movement disorders (97). However, it became apparent that with time, after 5 years, upward of 60% of patients would develop crippling medication-induced side effects such as dyskinesias. To move forward, a better understanding of the disease was needed. The problem was that, at that time, there was no animal model to better understand the disease pathology.
Serendipity came into play with the report in 1983 by Langston and Ballard (98) and Langston et al. (99) of a young man admitted in a presumed catatonic state unresponsive to psychiatric therapies. However, when given L-DOPA, his symptoms were reversed. Subsequently, a series of these patients were reported to have been rendered parkinsonian by self-administration of a pethidine analog, methyl-phenyl-tetrahydropyridine (MPTP). After one patient overdosed on cocaine and died, the autopsy conducted showed the changes in the brain similar to those seen in PD, particularly loss of nigral dopaminergic neurons. In 1983, Burns et al. (100) administered MPTP to NHPs and produced an experimental model of PD, mimicking bradykinesia, forward flexed posture, and rigidity. These animals were also highly responsive to L-DOPA therapy.
Subsequent studies using the MPTP-lesioned NHP model of PD with electrophysiology and 2-deoxyglucose (2-DG) studies (101) led to a key pathophyisiological understanding of PD, in which loss of nigral dopamine leads to disinhibition of the STN, causing an excessive inhibitory drive from the medial pallidum to ascending and descending pathways to the thalamus and upper brain stem. Two pioneering studies confirmed that lesioning the STN, using either neurotoxin (102) or surgical radiofrequency electrodes (103), reversed experimental parkinsonism. Lesioning the STN was not considered an option for fear of inducing hemiballism; therefore, it was the finding that high-frequency stimulation of the STN in the NHP model that made it clinically applicable. Very soon after, STN HFS became to be the most accepted treatment for advanced PD.
With time, it became apparent that even with medication and STN-DBS, PD patients were not resistant to FOG and falls. Certain lines of evidence suggest that the upper brain stem might be relevant to understanding this. For example, in decerebrate rats, cats, and dogs, electrical or chemical stimulation in the mesencephalic motor region induces walking. The particular brain region, the pedunculopontine nucleus (PPN), was shown to degenerate in PD and other akinetic disorders such as multiple system atrophy (MSA) and progressive supranuclear palsy (PSP). In addition, 2-DG studies have indicated that MPTP-lesioned NHPs have excessive inhibition of the PPN.
A lesion or high-frequency stimulation of the PPN in healthy NHPs induces akinesia (104). Once rendered parkinsonian with MPTP administration, microinjections of bicuculline [gamma aminobutyric acid (GABA) antagonist] directly into the PPN reverses akinesia and imbalance, as does low-frequency PPN stimulation (105). The finding that PPN stimulation alleviated movement abnormalities in the MPTP-lesioned NHP was translated rapidly to treat PD patients by clinical groups in the UK (Bristol) and Italy (Rome), with low-frequency stimulation (around 20–30 Hz) being employed (106, 107). These early clinical studies noted an effect of PPN stimulation that mimicked those found in the NHPs, which was an improvement in akinesia, in addition to gait and posture. This was later followed by a clinical study of six PD patients with dual STN and PPN stimulation (108). Results suggested modest improvements in akinesia and more marked beneficial effects in FOG and postural instability, with the suggestion of STN and PPN DBS being complementary. However, a major issue arose regarding the target that appeared to lie in the neighboring peri-peduncular nucleus. This has since prompted a debate on the exact location of the PPN, and this remains a controversial issue (109–112).
Two further clinical series have reported PPN stimulation in two differing scenarios (1) dual bilateral STN and PPN stimulation and (2) single-target unilateral PPN stimulation (113, 114). These studies have reported very modest therapeutic effects of PPN stimulation, largely limited to FOG and postural instability. One study found very limited effects even on FOG and questioned the clinical utility of this treatment (113). However, several aspects in the clinical application of PPN stimulation in these studies could have affected therapeutic efficacy. Indeed, some patients were selected for PPN stimulation with severe motor fluctuations requiring STN stimulation and variable degrees of gait disturbance (108, 113). For example, PD patients were selected for PPN stimulation to treat FOG that developed during STN stimulation. Other patients had been selected for PPN stimulation who had not experienced FOG that persisted “on medication” or having recurrent falls (113, 114). It remains possible that co-stimulation of the STN could influence the efficacy of PPN stimulation due to the substantial reciprocal connections between the two targets (115). In this regard, it should be noted that high-frequency stimulation required for STN stimulation (i.e., 130 Hz) appears to worsen gait when delivered to the PPN. It has been found that the PPN was targeted above the pontomesencephalic junction with choline-acetyltransferase 5 (ChAT5) staining studies in humans, suggesting that lead placement could have missed the caudal extent of the nucleus, which is most degenerate in PD (116–118). Moreover, a clinical study by Thevathasan et al. (119) demonstrated that bilateral PPN stimulation provides a greater therapeutic effect over unilateral stimulation.
It is apparent that there are some questions remaining regarding the effect of PPN stimulation in parkinsonian FOG and falls, i.e., the exact target and the best patient candidates are still debated. It is noteworthy that the outcomes measured with UPDRS may lack sensitivity for gait and posture. Indeed, the precise effects of PPN stimulation on motor function in PD including gait are not established (120). However, in recent years, it has been reported in a meta-analysis of several well-documented PPN stimulation studies that patients ON or OFF medication are better with PPN stimulation (121).
Novel Neuromodulation Applications for Gait Disorders in Parkinson's Disease
Postural instability and gait disorders (PIGDs) are debilitating phenomena that frequently impair locomotion and can significantly affect quality of life in PD patients (122). Prevalence of PIGD tends to follow the severity of disease; it is the most common cause of falls, which are associated with an increase in morbidity and mortality in PD (122). Besides the effects of STN and GPi DBS on PIGD responsive to levodopa, PPN DBS was the first neuromodulatory technique directly applied for the treatment of PIGD, with success recorded in many reports (123). In addition, there have been some reports on dual stimulation STN/SNr and VFS of STN as possible neuromodulatory methods for PIGD (124, 125).
Since the first experimental report from Fuentes et al. (126) showing that spinal cord stimulation (SCS) could enhance locomotion in murine PD models, and more recently in NHPs (127), SCS has been considered as a possible treatment for FOG in PD. Increasing evidence suggests that SCS improves treatment-resistant PIGD in PD patients (128). Recently, Pinto de Souza et al. (129) have reported positive effects of high-frequency SCS (300 Hz) on gait, improving the performance in various gait tests, which were reproduced during double-blinded assessments. It was also seen that continuous SCS chronically reduced FOG episodes, improved UPDRS-III motor scale scores, and self-reported quality of life (129). These results are in line with previous clinical observations and findings recorded in parkinsonian animal models (126, 127). More recently, Samotus et al. (130) also showed positive effects of SCS for gait dysfunction in PD patients. In comparison to early reports, the selection of PD patients with locomotor problems were more precise (130), in which motor symptoms, gait performance, and FOG were closely followed with adequate evaluations.
Despite that positive results have been reported, the mechanisms by which SCS may improve FOG are still elusive. Considering that the exact mechanism of FOG itself is also not completely understood, the study of SCS might converge with the study of FOG. Normal gait requires an exact coordination of postural adjustment in advance of each step forward, namely, anticipatory postural adjustment (APA). During imminent FOG episodes, the intention to walk is uncoupled from the triggering of APA, with consequent failure of the forward movement. This often results in knee trembling and failure to initiate gait. In PD, FOG episodes are associated with deficient APA. Physiological evidence, functional imaging, and clinicopathologic studies suggest that FOG is mainly associated with disorders of frontal cortical regions [e.g., supplementary motor area (SMA)] that comprise a known brain circuitry dedicated to APA control (131). In NHPs, SCS increases neuronal firing of the primary motor cortex and decreases pathological cortico-striatal synchronous low-frequency waves showing that SCS does influence the oscillatory activity in multiple structures of brain motor circuits (127). In fact, SCS may disrupt the aberrant inhibition from the GPi to the thalamus and SMA. As part of the circuit that controls APA, the SMA has corticofugal projections to the PPN, a region particularly involved in gait initiation (as described above). Since the activity of SMA, globus pallidus, and PPN is abnormal in PD patients with FOG, SCS could potentially modulate this circuit and improve APA and gait initiation.
Recent work by de Lima-Pardini et al. (132) has recently reported that SCS at 300 Hz effectively reduces the time of FOG with simultaneous correction of altered APA, which is reported in PD patients with FOG (Figure 7). These results corroborated with initial clinical data demonstrating significant progress toward revealing mechanisms by which SCS may improve FOG. It is possible that by stimulating ascending spinal pathways, SCS may correct pathological oscillatory activity in the circuits that mediate FOG, subsequently inhibiting episodes of FOG in PD patients. Conversely, SCS has failed to improve reactive posture control. It is possible that SCS may have different effects on the two mechanisms of postural control that are known to be reactive and anticipatory. While APA mechanisms are thought to be dependent on thalamo-cortical-striatal loops highly influenced by attentional and environmental changes, reactive posture control to external and unpredictable triggers relies on neuronal circuitries involving the brain stem and spinal cord with less influence from the cortex.
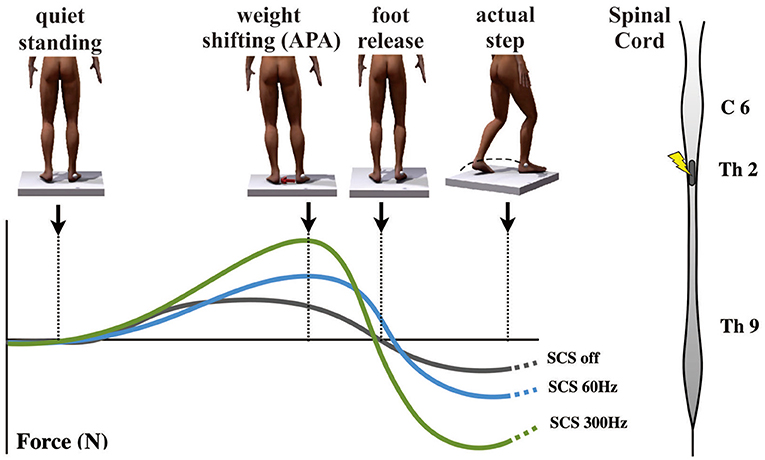
Figure 7. Effects of spinal cord stimulation on postural control in Parkinson's disease patients with freezing of gait (132). On the top left, the figures show the representation of the step initiation task from quiet standing to the actual step, showing the marker on the right malleolus to detect the moment that the foot clears the floor. The graph below shows body weight shifting toward the supporting leg [anticipatory postural adjustment (APA)] during the three different conditions [gray curve, spinal cord stimulation (SCS) OFF; blue curve, 60 Hz-CS; green curve, 300 Hz-SCS]. On the right is a representation of the spinal cord showing the site of stimulation (T2) used in the same report.
Despite various reports on the positive effects of SCS on FOG, there is still skepticism regarding this treatment since some PD symptoms can improve remarkably with placebo or during startle responses upon threats. In order to differentiate the effect of SCS from placebo, Pinto de Souza et al. (129) performed a double-blind study comparing the effects of stimulation at 300 and 60 Hz, once both frequencies elicited indistinguishable paresthesia. It was found that only the stimulation at 300 Hz improved gait performance and reduced episodes of FOG, while the effects of the lower frequency were similar to no stimulation.
In summary, there is increasing evidence for SCS-induced improvements in gait disturbances for PD, especially FOG. However, evidence from comparative studies with larger patient populations and data from prospective placebo-controlled trials is still lacking. The exact mechanisms and circuits mediating the expression of FOG are still uncertain. In addition, the optimal level for spinal stimulation, specific structures (segmental short circuits or ascending tracts) for effects of SCS, the most effective electrode geometry and specific parameters of stimulation remain undefined (133). Besides its potential therapeutic use, the development of SCS for the treatment of PD symptoms may also contribute to a better understanding of locomotor behaviors and complex pathophysiology of neurological disturbances, specifically FOG (134).
Deep Brain Stimulation to Enhance Chronic Post-stroke Rehabilitation
Ischemic stroke is a major cause of long-term disability in the industrialized world, with chronic debilitating motor impairments significantly impacting quality of life for more than one third of stroke survivors. Current standard-of-care treatment for those left with motor sequelae is largely limited to subacute physical therapy. However, long-term disabling deficits for most patients persist despite best efforts. As a result, there is substantial interest in identifying new ways to enhance post-stroke recovery and rehabilitation, including invasive and non-invasive neurostimulation-based approaches. Progress has been limited to date, however, with most clinical studies yielding limited or variable efficacy in improving motor function using current approaches.
Machado and Baker (135) have previously proposed that chronic stimulation of cerebellar dentate nucleus (DN; Figure 8A) should, through activation of the net excitatory glutamatergic dentatothalamocortical pathway, upregulate thalamocortical activity and cerebral cortical excitability across prefrontal, frontal, and parietal cortical regions (Figure 8B), establishing a basal environment more compatible with functional neuroplastic reorganization. The work over the past decade, using preclinical models of middle cerebral artery ischemia, that stimulation of the lateral cerebellar nucleus (the rodent homolog of the human DN) does indeed facilitate motor recovery when paired with rehabilitation, with the magnitude of the effect sensitive to stimulation frequency (136, 137). Moreover, the electrophysiological and histological data implicate frequency-specific changes in cortical excitability and enhanced functional reorganization of surviving perilesional cortex as potential therapeutic mechanisms, with improvements in motor function accompanied by increased expression of markers of synaptic plasticity, synaptogenesis, and neurogenesis in the perilesional cortex (138, 139).
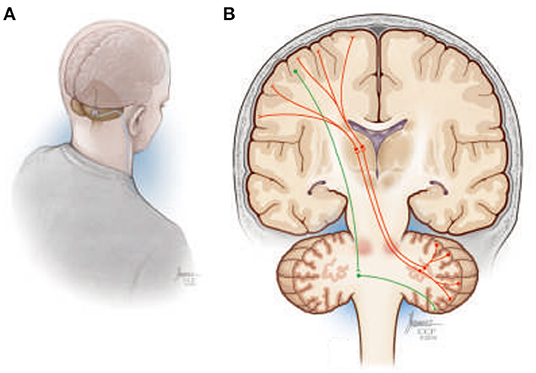
Figure 8. (A) Illustration depicting a suboccipital approach to delivering deep brain stimulation therapy to the cerebellar dentate nucleus. (B) Simplified overview of the human dentatothalamocortical (DTC) and corticopontocerebellar (CPC) pathways. The DTC (red) projects through the ipsilateral superior cerebellar peduncle, decussating at the level of the pons, to terminate in contralateral thalamus, where its activity influences widespread thalamocortical interactions. The CPC is shown in green descending from the cortex, decussating in the ipsilateral pons, and terminating in the contralateral cerebellar hemisphere.
Based upon the preclinical work, a first-in-human trial (NCT02835443) to translate DN-DBS as a treatment for upper extremity hemiparesis in chronic post-stroke patients commenced in 2016. With additional support from the National Institutes of Health Brain Initiative, the trial will extend beyond establishing the safety, feasibility, and efficacy of the approach to directly examine the acute and chronic effects of DN-DBS on cerebral cortical excitability and motor representation using TMS-based techniques as well as the topography of motor-related LFP activity in the DN region. All these data are further being incorporated into MRI-based patient-specific anatomical models of the deep cerebellar region to facilitate next-generation lead design and targeting techniques, while using the LFP data to develop physiological classifiers to inform future treatment paradigms, including possible closed-loop approaches. Finally, ongoing preclinical studies are focusing on therapy refinement and optimization, including a potential role for a more physiologic-based, closed-loop stimulation system that more directly stimulates delivery with motor activity.
Current Clinical Deep Brain Stimulation Approaches for Dystonia
DBS of the GPi is an effective treatment for medical refractory dystonia and reduces not only motor impairment but also other disabilities (140–144). Within the last two decades, DBS has progressively evolved into a widely available therapeutic strategy for generalized and segmental dystonia. More recently, it was also successfully applied in patients with cervical dystonia or cases that were resistant to botulinum toxin treatment (145). Long-term effects and side effects of this potentially lifelong therapy are of special interest. More recent retrospective reports of DBS for dystonia includes follow-up periods of 5–7 years (146–149) with sometimes even longer observation periods in individual cases, i.e., 6–10 years (148) and overall good clinical outcome of 50–80% mean improvement of dystonia. However, individual factors that remain reliable for predicting DBS outcome in dystonia are still difficult to define. Several factors such as gene mutation status, age at surgery, disease duration, presence of musculoskeletal deformities, predominance of phasic vs. tonic movements, the size of the globus pallidus, and optimal stimulation parameters are widely discussed to have a possible influence on stimulation effects (148–152). Brüggemann et al. (150) now report that patients genetically confirmed with DYT1 and DYT6 dystonia have significant and enduring effects of pallidal stimulation. Furthermore, Isaias et al. (149) and Lumsden et al. (151) report cases with disease duration being an important factor, e.g., with respect to developing fixed musculoskeletal deformities, also highlighting the importance to differentiate between isolated dystonia and patients with combined/complex dystonia because the latter shows a significantly reduced benefit of pallidal DBS.
While the clinical benefit of DBS in cervical and other focal dystonias is well-documented, the underlying therapeutic mechanism remains to be elucidated. Converging evidence points to a modulation of aberrant neural population activity in the basal ganglia through high-frequency stimulation (9, 153, 154). In recent years, DBS has enabled the unique opportunity to record oscillatory activity as LFPs directly from the basal ganglia during surgery and in a postoperative interval, with the DBS electrodes externalized. Here, oscillatory patterns of pallidal LFPs were found to differ in a disease-specific manner (155, 156). The best characterized pathological oscillatory phenomenon has been described in patients with PD, where STN β oscillatory activity (13–30 Hz) at rest is suppressed by dopaminergic medication and is directly correlated with patient symptom severity (9). In dystonia, low-frequency activity in the θ-α range (4–12 Hz; subsequently referred to as θ, as most peaks in dystonia are in the 4–10-Hz range) is predominant in the GPi and correlates with symptom severity (157). Indeed, θ activity in dystonia patients with phasic movements has been shown to be suppressed by high-frequency DBS (154). Thus, pallidal θ activity has been proposed as a potential pathophysiological hallmark of dystonia. It can be envisioned that adaptive closed-loop DBS using pallidal θ activity as a biomarker could be efficiently used for controlling dystonic motor symptoms in patients.
Automatic Classification of Pallidal Borders During Awake and Asleep Deep Brain Stimulation Procedures for Dystonia
DBS of the GPi in patients with dystonia can reduce motor symptoms and improve their quality of life (158–160). With the current limits of today's brain imaging techniques in resolution, distortion, and possible brain shift (161), together with the broad distribution of DBS centers (>1,000 worldwide with many non-academic centers), the outcome of many DBS procedures might be less optimal because of mis-localization of the DBS leads (13, 162). To enable better localization of the DBS target, pallidal borders can be visibly and audibly detected by electrophysiological microelectrode recordings (MERs) during DBS procedures. Even given ideal conditions, the detection of the striato-pallidal borders is never an easy task even for an expert electrophysiologist.
Previously, it has reported a real-time automated procedure (163, 164) for the detection of the borders and subdomains of the STN using hidden Markov models (HMMs) in PD patients (165). Bergman et al. also reported an algorithm for detection of the striato-pallidal borders, with a dataset including 116 GPi trajectories from 42 patients consisting of 11,774 MERs in five classes of disease (awake PD patients, awake and lightly anesthetized genetic and non-genetic dystonia patients (166), with the current work now under review for publication; Journal of Neuro-engineering). Using the L1-distance measure in root mean square (RMS) and power spectral densities of the MER, Bergman et al. has found that awake and light anesthesia (with sevoflurane and N2O, minimum alveolar concentration (MAC) = 0.3–0.6) dystonia classes with and without anesthesia can be merged. Therefore, depth (MAC) of anesthesia was reduced 10–15 min before the beginning of the MER and restored deep surgical anesthesia after the end of the MER exploration in each hemisphere. It was found that significant differences exist between the RMS and spectral features of the striato-pallidal trajectory. Bergman et al. reported training on the HMM on trajectories with striato-pallidal labels as inputs in three different disease classes (PD, genetic and non-genetic dystonia) using the decision of an expert electrophysiologist as gold standard labels. Then, the performance of the HMM algorithm was tested with a leave-one-out cross-validation. The HMM was found to achieve performance on par with an expert electrophysiologist across the striatum-GPe, GPe-GPi, and GPi-exit transitions in the three disease classes (167).
In conclusion, as for STN DBS, GPi automated navigation systems can potentially shorten the length of electrophysiological mapping to <15 min per hemisphere, while implanting the DBS lead within the optimal location. A reduced procedure time and improved targeting would be expected to lead to better clinical outcomes in GPi DBS therapy for dystonia.
Long-Term Awake Multi-Electrode Monitoring in Children With Acquired Combined Dystonia
Acquired combined dystonia remains a difficult disorder to treat partly because of the variability of causes and symptoms. Dystonia has multiple potential anatomic origins, including basal ganglia, cerebellum, thalamus, and prefrontal cortex. Despite these origins, the specific postures generated are often remarkably similar, and a review of 3 years of clinic videos shows that almost all children have at least one of seven stereotypical postures, no matter what their underlying etiology (168). This has led to the conjecture that these postures are due to similar somatotopy within the motor cortex, so that any brain region capable of stimulating contiguous regions of motor cortex will cause a similar posture. This finding is reminiscent of the very limited sets of postures seen with long-train electrical stimulation of the motor cortex (169). If unfocused stimulation of motor cortex is the final common pathway, then dystonia is a very non-specific symptom, and successful treatment using DBS will require uncovering the anatomic origin of the disorder in each child.
In order to do this, Sanger et al. (170) have developed a new procedure that includes test stimulation and recording from multiple externalized electrodes while children are admitted to a neuromodulation monitoring unit (NMU). Up to 10 electrodes (Figure 9), each with 16 contacts, are implanted in multiple regions of the basal ganglia and thalamus, targeting the most likely pathways for uncontrolled cortical stimulation. Test stimulation can be used to determine efficacy and any side effects of stimulation of each region. This is particularly helpful in the thalamus, for which the effects of stimulation are usually immediate. In the pallidum, effects of stimulation may require weeks or months to determine; therefore, test stimulation is primarily used to find regions that are free of adverse effects that might limit flexibility of stimulation.
Figure 9. Pediatric Deep Brain Stimulation Using Awake Recording and Stimulation for Target Selection in an Inpatient Neuromodulation Monitoring Unit (170). Axial (A,B) and coronal (C,D) MRI showing the position of temporary leads within the basal ganglia and thalamus. Stereotactic planning trajectories are shown in (B,D).
Recording yields single-unit activity that can be correlated against surface electromyography (EMG) to determine regions that are more or less likely to be carrying dystonic signals. In particular, a region that does not change activity during dystonic contractions is unlikely to be a mediator of dystonia and unlikely to be a candidate target for DBS. Sanger et al. (170) have recorded so far from 20 children with acquired combined dystonia. Contrary to data from adults, in NHPs and children with primary dystonia, the group have found that baseline activity in the pallidum is low and increases with dystonic muscle contractions. In fact, all regions activate with dystonic spasms, including pallidum and multiple thalamic subnuclei (VIM, VPL, Vo, and VA). Although the pallidum inhibits the thalamus, activity in the pallidum is positively correlated with activity in the thalamus in all children. This suggests a loss of the normal inhibition and loss of specificity of activity within these regions. Overflow to contralateral muscles is evident within cerebellar projection pathways, and dystonic activity is always much higher and less focused than voluntary activity. Taken together, the results reported here suggest a generalized lack of specificity and both reversal of normal activity and hyperexcitability throughout the basal ganglia/thalamus circuit. Since the output of this circuit projects to motor cortex, these findings are consistent with the hypothesis of non-specific cortical drive as the mediator of dystonic postures.
Use of the NMU procedure by Sanger et al. (170) has allowed for finding more precise patient-specific targets for children. The group always implants four leads: usually two within the GPi and two within the optimal region of the thalamus for each child. Subsequent programming suggests that GPi DBS is the most effective for hypertonic components of dystonia, whereas thalamic DBS is the most effective for the hyperkinetic components of dystonia. Preliminary analysis of outcomes data shows significantly improved outcomes using the new procedure and four-lead DBS. This is a promising new procedure that will yield both improved outcomes for children with acquired combined dystonia, as well as detailed knowledge on the physiological mechanisms underlying this disabling condition.
Conclusions
This report summarizes the information presented in the first DBS initiative meeting held at the NELN of Tsinghua University. The collective group addressed foreseeable challenges in DBS therapy and recent clinical approaches with technological advancements. In-depth discussions were held on the connectome approach in DBS, novel developments in 3T MRI-compatible DBS devices and neural recording technologies for understanding disease pathophysiology, and pursuing new clinical approaches and indications using such advancements. This meeting marks a unique milestone in developing global DBS research using state-of-the-art technologies for rapid clinical translation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
2-DG, 2-deoxyglucose; 3T MRI, 3-Tesla magnetic resonance imaging; AD, Alzheimer's disease; AI, artificial intelligence; ALIC, anterior limb of the internal capsule; ANT, anterior nucleus of thalamus; APA, anticipatory postural adjustment; APP, amyloid precursor protein; ChAT5, choline-acetyltransferase 5; dACC, dorsal anterior cingulate cortex; DBS, deep brain stimulation; dMRI, diffusion magnetic resonance imaging; DN, dentate nucleus; EC, entorhinal cortex; FOG, freezing of gait; GPi, globus pallidus internal segment; HMMs, hidden Markov models; IEC, International Electrotechnical Commission; IPG, implantable pulse generator; LFPs, local field potentials; MDD, major depressive disorder; MERs, microelectrode recordings; MFB, medial forebrain bundle; MPTP, methyl-phenyl-tetrahydropyridine; MSA, multiple system atrophy; NELN, National Engineering Laboratory for Neuromodulation; NHP, nonhuman primate; NMU, neuromodulation monitoring unit; OCD, obsessive–compulsive disorder; OFC, orbitofrontal cortex; PD, Parkinson's disease; PET, positron emission tomography; PIGDs, postural instability and gait disorders; PPN, pedunculopontine nucleus; PSP, progressive supranuclear palsy; RF, radiofrequency; RMS, root mean square; SAR, specific absorption rate; SCC, subgenual cingulate cortex; SCGwm, subgenual cingulate gyrus white matter; SCS, spinal cord stimulation; sl-MFB, superolateral branch of the medial forebrain bundle; STN, subthalamic nucleus; TMS, transcranial magnetic stimulation; TUG, Timed Up and Go; VC/VS, ventral capsule/ventral striatum; VIM, ventral intermediate; vmPFC, ventromedial prefrontal cortex; VFS, variable frequency stimulation; VTA, volume of tissue activated.
References
1. Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. (2005) 28:326–8 doi: 10.1111/j.1540-8159.2005.50024.x
2. Falowski S, Safriel Y, Ryan MP, Hargens L. The rate of magnetic resonance imaging in patients with deep brain stimulation. Stereotact Funct Neurosurg. (2016) 94:147–53. doi: 10.1159/000444760
3. Henderson JM, Tkach J, Phillips M, Baker K, Shellock FG, Rezai AR. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson's disease: case report. Neurosurgery. (2005) 57:E1063. doi: 10.1227/01.NEU.0000180810.16964.3E
4. Horn A, Wenzel G, Irmen F, Huebl J, Li N, Neumann WJ, et al. Deep brain stimulation induced normalization of the human functional connectome in Parkinson's disease. Brain J Neurol. (2019) 18:130–15. doi: 10.1093/brain/awz239
5. IEC 60601-2-33:2010+AMD1:2013+AMD2:2015 CSV: Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonanceequipment for medical diagnosis.
6. Shen L, Jiang C, Hubbard CS, Ren J, He C, Wang D, et al. Subthalamic nucleus deep brain stimulation modulates 2 distinct neurocircuits. Ann Neurol. (2020) 88:1178–93. doi: 10.1002/ana.25906
7. Brown P. Oscillatory nature of human basal ganglia activity: relationship to thepathophysiology of Parkinson's disease. Mov Disord. (2003) 18:357–63. doi: 10.1002/mds.10358
8. Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation medi-ate some of the therapeutic effects of DBS in patients with Parkinson's disease? Front Integr Neurosci. (2012) 6:47. doi: 10.3389/fnint.2012.00047
9. Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. (2006) 23:1956–60. doi: 10.1111/j.1460-9568.2006.04717.x
10. Neumann WJ, Jha A, Bock A, Huebl J, Horn A, Schneider GH, et al. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain. (2015) 138:1894–906. doi: 10.1093/brain/awv109
11. Chen Y, Gong C, Hao H, Guo Y, Xu S, Zhang Y, et al. Automatic sleep stage classification based on subthalamic local field potentials. IEEE Trans Neural Syst Rehabil Eng. (2019) 27:118–28. doi: 10.1109/TNSRE.2018.2890272
12. Farris S, Giroux M. Retrospective review of factors leading to dissatisfaction with subthalamic nucleus deep brain stimulation during long-term management. Surg Neurol Int. (2013) 4:69. doi: 10.4103/2152-7806.112612
13. Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. (2005) 62:1250–5. doi: 10.1001/archneur.62.8.noc40425
14. Chen Y, Hao H, Chen H, Tian Y, Li L. The study on a real-time remote monitoring system for Parkinson's disease patients with deep brain stimulators. Annu Int Conf IEEE Eng Med Biol Soc. (2014) 2014:1358–61. doi: 10.1109/EMBC.2014.6943851
15. Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. (2015) 11:98–110. doi: 10.1038/nrneurol.2014.252
16. Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain. (2010) 133:2664–76. doi: 10.1093/brain/awq221
17. Fagundes VC, Rieder CR, da Cruz AN, Beber BC, Portuguez MW. Deep brain stimulation frequency of the subthalamic nucleus affects phonemic and action fluency in Parkinson's disease. Parkinsons Dis. (2016) 2016:6760243. doi: 10.1155/2016/6760243
18. Jia F, Hu W, Zhang J, Wagle Shukla A, Almeida L, Meng FG, et al. Variable frequency stimulation of subthalamic nucleus in Parkinson's disease: rationale and hypothesis. Parkinsonism Relat Disord. (2017) 39:27–30. doi: 10.1016/j.parkreldis.2017.03.015
19. Sui Y, Yue Y, Burdick JW. Correlational dueling bandits with application to clinical treatment in large decision spaces. In: Sierra C, editor. Proceedings of the Twenty-Sixth International Joint Conference on Artificial Intelligence. AAAI Press (2017). doi: 10.24963/ijcai.2017/389
20. Sui Y, Zhuang V, Burdick JW, Yue Y. Stagewise safe Bayesian optimization with Gaussian processes. In: Thirtyb-fifth International Conference on Machine Learning. (2018).
21. Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. (2018) 563:65–71. doi: 10.1038/s41586-018-0649-2
22. Accolla EA, Herrojo Ruiz M, Horn A, Schneider G.-H., Schmitz-Hubsch T, Draganski B, Kühn AA. Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain. (2016) 139:2503–15. doi: 10.1093/brain/aww182
23. Horn A, Kühn AA, Merkl A, Shih L, Alterman R, Fox M. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. NeuroImage. (2017) 150:395–404. doi: 10.1016/j.neuroimage.2017.02.004
24. Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. (2019) 184:293–316. doi: 10.1016/j.neuroimage.2018.08.068
25. Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. (2017) 82:67–78. doi: 10.1002/ana.24974
26. Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain. (2018) 141:2445–56. doi: 10.1093/brain/awy161
27. Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. (2013) 77:406–24. doi: 10.1016/j.neuron.2013.01.020
28. Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comp Biol. (2005) 1:e42. doi: 10.1371/journal.pcbi.0010042
29. Fernandes HM, van Hartevelt TJ, Boccard SGJ, Owen SLF, Cabral J, Deco G, et al. Novel fingerprinting method characterises the necessary sufficient structural connectivity from deep brain stimulation electrodes for a successful outcome. New J Phys. (2015) 17:015001–15. doi: 10.1088/1367-2630/17/1/015001
30. van Hartevelt TJ, Cabral J, Deco G, Møller A, Green AL, Aziz TZ, Kringelbach ML. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson's disease. PLoS ONE. (2014) 9:e86496. doi: 10.1371/journal.pone.0086496
31. Vanegas Arroyave N, Lauro PM, Huang L, Hallett M, Horovitz SG, Zaghloul KA, Lungu C. Tractography patterns of subthalamic nucleus deep brain stimulation. Brain. (2016) 139:1200–10. doi: 10.1093/brain/aww020
32. Ewert S, Plettig P, Li N, Chakravarty MM, Collins DL, Herrington TM, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. NeuroImage. (2018) 170:271–82. doi: 10.1016/j.neuroimage.2017.05.015
33. Horn A, Blankenburg F. Toward a standardized structural-functional group connectome in MNI space. NeuroImage. (2016) 124:310–22. doi: 10.1016/j.neuroimage.2015.08.048
34. Horn A, Ostwald D, Reisert M, Blankenburg F. The structural-functional connectome and the default mode network of the human brain. NeuroImage. (2014) 102:142–51. doi: 10.1016/j.neuroimage.2013.09.069
35. Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA. (2017) 56:201706587–6. doi: 10.1073/pnas.1706587115
36. Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. (2018) 84:28–37. doi: 10.1016/j.biopsych.2017.10.028
37. Al-Fatly B, Ewert S, Kübler D, Kroneberg D, Horn A, Kühn AA. Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain J Neurol. (2019) 18:130. doi: 10.1093/brain/awz236
38. Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, et al. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol Psychiatry. (2019) 85:735–43. doi: 10.1016/j.biopsych.2018.12.019
39. Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. (2017) 13:548–54 doi: 10.1038/nrneurol.2017.105
40. Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. (2017) 4:839–49. doi: 10.1016/S2215-0366(17)30371-1
41. Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). (2005) 210:343–52. doi: 10.1007/s00429-005-0025-5
42. Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comparative Neurol. (1999) 412:319–41. doi: 10.1002/(SICI)1096-9861(19990920)412:2<319::AID-CNE10>3.0.CO;2-7
43. Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. (2010) 30:2268–76. doi: 10.1523/JNEUROSCI.4682-09.2010
44. Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, et al. Individual variability in functional connectivity architecture of the human brain. Neuron. (2013) 77:586–95. doi: 10.1016/j.neuron.2012.12.028
45. Wang D, Liu H. Functional connectivity architecture of the human brain: not all the same. Neuroscientist. (2014) 20:432–8. doi: 10.1177/1073858414543290
46. Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends Cognitive Sci. (2013) 17:648–65. doi: 10.1016/j.tics.2013.09.017
47. Neumann WJ, Staub-Bartelt F, Horn A, Schanda J, Schneider GH, Brown P, et al. Long term correlation of subthalamic beta band activity with motor impairment in patients with Parkinson's disease. Clin Neurophysiol. (2017) 128:2286–91. doi: 10.1016/j.clinph.2017.08.028
48. Waters AC, Veerakumar A, Choi KS, Howell B, Tiruvadi V, Bijanki KR, et al. Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp. (2018) 39:4844–56. doi: 10.1002/hbm.24327
49. Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. (2018) 23:843–9. doi: 10.1038/mp.2017.59
50. Fox MD, Qian T, Madsen JR, Wang D, Li M, Ge M, et al. Combining task-evoked and spontaneous activity to improve pre-operative brain mapping with fMRI. Neuroimage. (2016) 124:714–23. doi: 10.1016/j.neuroimage.2015.09.030
51. Langs G, Wang D, Golland P, Mueller S, Pan R, Sabuncu MR, et al. Identifying shared brain networks in individuals by decoupling functional and anatomical variability. Cereb Cortex. (2016) 26:4004–14. doi: 10.1093/cercor/bhv189
52. Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, et al. Parcellating cortical functional networks in individuals. Nat Neurosci. (2015) 18:1853–60. doi: 10.1038/nn.4164
53. Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. (2008) 213:108–13. doi: 10.1016/j.expneurol.2008.05.008
54. Qian X, Chen Y, Feng Y, Ma B, Hao H, Li, et al. A platform for long-term monitoring the deep brain rhythms. Biomed Phys Eng Express. (2017) 3:015009. doi: 10.1088/2057-1976/aa50d6
55. Chen Y, Gong C, Tian Y, Orlov N, Zhang J, Guo Y, et al. Neuromodulation effects of deep brain stimulation on beta rhythm: a longitudinal local field potential study. Brain Stimul. (2020) 13:1784–92. doi: 10.1016/j.brs.2020.09.027
56. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005) 45:651–60. doi: 10.1016/j.neuron.2005.02.014
57. Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation deep brain stimulation for OCD. Neuropsychopharmacology. (2010) 35:317–36. doi: 10.1038/npp.2009.128
58. Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. (2008) 359:2121–34. doi: 10.1056/NEJMoa0708514
59. Haber SN, Behrens TE. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. (2014) 83:1019–39. doi: 10.1016/j.neuron.2014.08.031
60. Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. (2009) 111:1209–15. doi: 10.3171/2008.10.JNS08763
61. Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. (2011) 31:10392–402. doi: 10.1523/JNEUROSCI.0595-11.2011
62. Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NCR, et al. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci. (2018) 38:2106–17. doi: 10.1523/JNEUROSCI.2335-17.2017
63. Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. (2011) 168:502–10. doi: 10.1176/appi.ajp.2010.10081187
64. Greenberg BD, Gabriels LA, Malone DA Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. (2010) 15:64–79. doi: 10.1038/mp.2008.55
65. Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. (2013) 33:4804–14. doi: 10.1523/JNEUROSCI.4674-12.2013
66. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. (2015) 76:155–62. doi: 10.4088/JCP.14m09298
67. Tsolaki E, Espinoza R, Pouratian N. Using probabilistic tractography to target the subcallosal cingulate cortex in patients with treatment resistant depression. Psychiatry Res. (2017) 261:72–4. doi: 10.1016/j.pscychresns.2017.01.006
68. Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O'Reardon JP, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. (2015) 78:240–8. doi: 10.1016/j.biopsych.2014.11.023
69. Wang TR, Moosa S, Dallapiazza RF, Elias JW, Lynch WJ. Deep brain stimulation for the treatment of drug addiction. Neurosurg Focus. (2018) 45:E11. doi: 10.3171/2018.5.FOCUS18163
70. Bari A, DiCesare J, Babayan D, Runcie M, Sparks H, Wilson B. Neuromodulation for substance addiction in human subjects: a review. Neurosci Biobehav Rev. (2018) 95:33–43. doi: 10.1016/j.neubiorev.2018.09.013
71. Bari AA, Mikell CB, Abosch A, Ben-Haim S, Buchanan RJ, Burton AW, et al. Charting the road forward in psychiatric neurosurgery: proceedings of the 2016 American Society for Stereotactic and Functional Neurosurgery workshop on neuromodulation for psychiatric disorders. J Neurol Neurosurg Psychiatry. (2018) 89:886–96. doi: 10.1136/jnnp-2017-317082
72. Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. (2016) 3:472–80. doi: 10.1016/S2215-0366(15)00579-9
73. Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. (2012) 69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456
74. Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Investigation. (2009) 119:717–25. doi: 10.1172/JCI38454
75. Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. (2000) 48:830–43. doi: 10.1016/S0006-3223(00)01036-2
76. Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. (1997) 9:471–81. doi: 10.1176/jnp.9.3.471
77. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. (1999) 156:675–82. doi: 10.1176/ajp.156.5.675
78. Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. (2014) 76:963–9. doi: 10.1016/j.biopsych.2014.03.029
79. Choi KS, Noecker AM, Riva-Posse P, Rajendra JK, Gross RE, Mayberg HS, et al. Impact of brain shift on subcallosal cingulate deep brain stimulation. Brain Stimul. (2018) 11:445–53. doi: 10.1016/j.brs.2017.12.001
80. Chaturvedi A, Lujan J, McIntyre C. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng. (2013) 10:1–17. doi: 10.1088/1741-2560/10/5/056023
81. Lockhart IA, Mitchell SA, Kelly S. Safety and tolerability of donepezil, rivastigmine and galantamine for patients with Alzheimer's disease: systematic review of the 'real-world' evidence. Dement Geriatr Cogn Disord. (2009) 28:389–403. doi: 10.1159/000255578
82. Kesner RP. Brain stimulation: effects on memory. Behav Neural Biol. (1982) 36:315–67. doi: 10.1016/S0163-1047(82)90762-2
83. Hamani C, Dubiela FP, Soares JC, Shin D, Bittencourt S, Covolan L, et al. Anterior thalamus deep brain stimulation at high current impairs memory in rats. Exp Neurol. (2010) 225:154–62. doi: 10.1016/j.expneurol.2010.06.007
84. Wilburn MW, Kesner RP. Differential amnestic effects produced by electrical stimulation of the caudate nucleus and nonspecific thalamic system. Exp Neurol. (1972) 34:45–50. doi: 10.1016/0014-4886(72)90186-0
85. Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. (2012) 4:142rv148. doi: 10.1126/scitranslmed.3003722
86. Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. (2011) 31:13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011
87. Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. (2012) 366:502–10. doi: 10.1056/NEJMoa1107212
88. Oh YS, Kim HJ, Lee KJ, Kim YI, Lim SC, Shon YM. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. (2012) 21:183–7. doi: 10.1016/j.seizure.2011.12.003
89. Brandling-Bennett EM, Bookheimer SY, Horsfall JL, Moftakhar P, Sedrak M, Barkulis CT, et al. A paradigm for awake intraoperative memory mapping during forniceal stimulation. Neurocase. (2012) 18:26–38. doi: 10.1080/13554794.2010.547509
90. Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. (2008) 63:119–23. doi: 10.1002/ana.21295
91. Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. (2010) 68:521–34. doi: 10.1002/ana.22089
92. Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos JM, Munro C, Oh E, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis. (2016) 54:777–87. doi: 10.3233/JAD-160017
93. Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia. Mol Psychiatry. (2015) 20:353–60. doi: 10.1038/mp.2014.32
94. Gratwicke J, Zrinzo L, Kahan J, Peters A, Brechany U, McNichol A, et al. Bilateral nucleus basalis of Meynert deep brain stimulation for dementia with Lewy bodies: a randomised clinical trial. Brain Stimul. (2020) 13:1031–9. doi: 10.1016/j.brs.2020.04.010
95. Mann A, Gondard E, Tampellini D, Milsted JAT, Marillac D, Hamani C, et al. Chronic deep brain stimulation in an Alzheimer's disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimul. (2018) 11:435–44. doi: 10.1016/j.brs.2017.11.012
96. Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. (2001) 56:1712–21. doi: 10.1212/WNL.56.12.1712
97. Cotzias GC. L-Dopa for Parkinsonism. N Engl J Med. (1968) 278:630. doi: 10.1056/NEJM196803142781127
98. Langston JW, Ballard PA Jr. Parkinson's disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med. (1983) 309:310. doi: 10.1056/NEJM198308043090511
99. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. (1983) 219:979–80. doi: 10.1126/science.6823561
100. Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. (1983) 80:4546–50. doi: 10.1073/pnas.80.14.4546
101. Crossman AR, Mitchell IJ, Sambrook MA. Regional brain uptake of 2-deoxyglucose in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the macaque monkey. Neuropharmacology. (1985) 24:587–91 doi: 10.1016/0028-3908(85)90070-X
102. Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. (1990) 249:1436–8 doi: 10.1126/science.2402638
103. Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. (1991) 6:288–92. doi: 10.1002/mds.870060404
104. Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci. (2002) 9:170–4 doi: 10.1054/jocn.2001.0943
105. Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. (2002) 125:2418–30. doi: 10.1093/brain/awf259
106. Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. (2005) 16:1877–81. doi: 10.1097/01.wnr.0000187629.38010.12
107. Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. (2005) 16:1883–7. doi: 10.1097/01.wnr.0000187637.20771.a0
108. Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. (2007) 130:1596–607. doi: 10.1093/brain/awl346
109. Yelnik J. PPN or PPD, what is the target for deep brain stimulation in Parkinson's disease? Brain. (2007) 130:e79. doi: 10.1093/brain/awm138
110. Zrinzo L, Hariz M. The peripeduncular nucleus: a novel target for deep brain stimulation? Neuroreport. (2007) 18:1631–2. doi: 10.1097/WNR.0b013e3282638603
111. Zrinzo L, Zrinzo LV, Hariz M. The pedunculopontine and peripeduncular nuclei: a tale of two structures. Brain. (2007) 130:e73. doi: 10.1093/brain/awm079
112. Zrinzo L, Zrinzo LV, Tisch S, Limousin PD, Yousry TA, Afshar F, et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. (2008) 131:1588–98. doi: 10.1093/brain/awn075
113. Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. (2010) 133:205–14. doi: 10.1093/brain/awp229
114. Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. (2010) 133:215–24. doi: 10.1093/brain/awp261
115. Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, et al. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. (2009) 24:319–28. doi: 10.1002/mds.22189
116. Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. (1989) 283:611–33. doi: 10.1002/cne.902830414
117. Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, et al. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. (1999) 89:759–70. doi: 10.1016/S0306-4522(98)00380-7
118. Rinne JO, Ma SY, Lee MS, Collan Y, Röyttä M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson's disease is related to disability of the patients. Parkinsonism Relat Disord. (2008) 14:553–7. doi: 10.1016/j.parkreldis.2008.01.006
119. Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery. (2011) 69:1248–53 doi: 10.1227/NEU.0b013e31822b6f71
120. Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, et al. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson's disease. Gait Posture. (2010) 32:512–8. doi: 10.1016/j.gaitpost.2010.07.012
121. Wang H, Gao H, Jiao T, Luo Z. A meta-analysis of the pedunculopontine nucleus deep-brain stimulation effects on Parkinson's disease. Neuroreport. (2016) 27:1336–44. doi: 10.1097/WNR.0000000000000697
122. Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. (2014) 71:884–90. doi: 10.1001/jamaneurol.2014.753
123. Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain J-S, et al. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. (2012) 135:1446–54. doi: 10.1093/brain/aws039
124. Jia F, Guo Y, Wan S, Chen H, Hao H, Zhang J, et al. Variable frequency stimulation of subthalamic nucleus for freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. (2015) 21:1471–2. doi: 10.1016/j.parkreldis.2015.10.002
125. Weiss SA, Banks GP, McKhann GM Jr, Goodman RR, Emerson RG, Trevelyan AJ, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. (2013) 136:3796–808. doi: 10.1093/brain/awt276
126. Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MAL. Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science. (2009) 323:1578–82. doi: 10.1126/science.1164901
127. Santana MB, Halje P, Simplício H, Richter U, Freire MAM, Petersson P, et al. Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron. (2014) 84:716–22. doi: 10.1016/j.neuron.2014.08.061
128. de Andrade EM, Ghilardi MG, Cury RG, Barbosa ER, Fuentes R, Teixeira MJ, et al. Spinal cord stimulation for Parkinson's disease: a systematic review. Neurosurg Rev. (2016) 39:27–35. doi: 10.1007/s10143-015-0651-1
129. Pinto de Souza C, Hamani C, Oliveira Souza C, Lopez Contreras WO, Dos Santos Ghilardi MG, Cury RG, et al. Spinal cord stimulation improves gait in patients with Parkinson's disease previously treated with deep brain stimulation. Mov Disord. (2017) 32:278–82. doi: 10.1002/mds.26850
130. Samotus O, Parrent A, Jog M. Spinal cord stimulation therapy for gait dysfunction in advanced Parkinson's disease patients. Mov Disord. (2018) 33:783–92. doi: 10.1002/mds.27299
131. de Lima-Pardini AC, de Azevedo Neto RM, Coelho DB, Boffino CC, Shergill SS, de Oliveira Souza C, et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci Rep. (2017) 7:43088. doi: 10.1038/srep43088
132. de Lima-Pardini AC, Coelho DB, Souza CP, Souza CO, Ghilardi MGDS, Garcia T, et al. Effects of spinal cord stimulation on postural control in Parkinson's disease patients with freezing of gait. Elife. (2018) 7:e37727. doi: 10.7554/eLife.37727
133. de Souza CP, Dos Santos MGG, Hamani C, Fonoff ET. Spinal cord stimulation for gait dysfunction in Parkinson's disease: essential questions to discuss. Mov Disord. (2018) 33:1828–9. doi: 10.1002/mds.27508
134. Fonoff ET, Lima-Pardini AC, Coelho DB, Mônaco BA, Machado BM, Pinto de Souza C, et al. Spinal cord stimulation for freezing of gait: from the bench to the bedside. Front Neurol. (2019) 10:905. doi: 10.3389/fneur.2019.00905
135. Machado A, Baker KB. Upside down crossed cerebellar diaschisis: proposing chronic stimulation of the dentatothalamocortical pathway for post-stroke motor recovery. Front Integr Neurosci. (2012) 6:20. doi: 10.3389/fnint.2012.00020
136. Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. (2009) 1280:107–16. doi: 10.1016/j.brainres.2009.05.007
137. Machado AG, Cooperrider J, Furmaga HT, Baker KB, Park HJ, Chen Z, et al. Chronic 30-Hz deep cerebellar stimulation coupled with training enhances post-ischemia motor recovery and peri-infarct synaptophysin expression in rodents. Neurosurgery. (2013) 73:344–53. doi: 10.1227/01.neu.0000430766.80102.ac
138. Baker KB, Schuster D, Cooperrider J, Machado AG. Deep brain stimulation of the lateral cerebellar nucleus produces frequency-specific alterations in motor evoked potentials in the rat in vivo. Exp Neurol. (2010) 226:259–64. doi: 10.1016/j.expneurol.2010.08.019
139. Cooperrider J, Furmaga H, Plow E, Park HJ, Chen Z, Kidd G, et al. Chronic deep cerebellar stimulation promotes long-term potentiation, microstructural plasticity, and reorganization of perilesional cortical representation in a rodent model. J Neurosci. (2014) 34:9040–50. doi: 10.1523/JNEUROSCI.0953-14.2014
140. Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, et al. Deep-Brain Stimulation for Dystonia Study Group. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. (2006) 355:1978–90. doi: 10.1056/NEJMoa063618
141. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. French Stimulation du Pallidum Interne dans la Dystonie (SPIDY) Study Group. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. (2005) 352:459–67. doi: 10.1056/NEJMoa042187
142. Vidailhet M, Pollak P. Deep brain stimulation for dystonia: make the lame walk. Ann Neurol. (2005) 57:613–4. doi: 10.1002/ana.20491
143. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. French SPIDY Study Group. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. (2007). 6:223–9. doi: 10.1016/S1474-4422(07)70035-2
144. Volkmann J, Wolters A, Kupsch A, Müller J, Kühn AA, Schneider GH, et al. DBS study group for dystonia. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. (2012) 11:1029–38. doi: 10.1016/S1474-4422(12)70257-0
145. Volkmann J, Mueller J, Deuschl G, Kühn AA, Krauss JK, Poewe W, et al. DBS study group for dystonia. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. (2014) 13:875–84. doi: 10.1016/S1474-4422(14)70143-7
146. Markun LC, Starr PA, Air EL, Marks WJ Jr, Volz MM, Ostrem JL. Shorter disease duration correlates with improved long-term deep brain stimulation outcomes in young-onset DYT1 dystonia. Neurosurgery. (2012) 71:325–30. doi: 10.1227/NEU.0b013e318258e21b
147. Walsh RA, Sidiropoulos C, Lozano AM, Hodaie M, Poon YY, Fallis M, et al. Bilateral pallidal stimulation in cervical dystonia: blinded evidence of benefit beyond 5 years. Brain. (2013) 136:761–9. doi: 10.1093/brain/awt009
148. Cif L, Vasques X, Gonzalez V, Ravel P, Biolsi B, Collod-Beroud G, et al. Long-term follow-up of DYT1 dystonia patients treated by deep brain stimulation: an open-label study. Mov Disord. (2010) 25:289–99. doi: 10.1002/mds.22802
149. Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain. (2008) 131:1895902. doi: 10.1093/brain/awn120
150. Brüggemann N, Kühn A, Schneider SA, Kamm C, Wolters A, Krause P, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. (2015) 84:895–903. doi: 10.1212/WNL.0000000000001312
151. Lumsden DE, Kaminska M, Gimeno H, Tustin K, Baker L, Perides S, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol. (2013) 55:567–74. doi: 10.1111/dmcn.12117
152. Krause P, Brüggemann N, Völzmann S, Horn A, Kupsch A, Schneider GH, et al. Long-term effect on dystonia after pallidal deep brain stimulation (DBS) in three members of a family with a THAP1 mutation. J Neurol. (2015) 262:2739–44. doi: 10.1007/s00415-015-7908-z
153. Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends in neurosciences. (2007) Jul;30:357-64. doi: 10.1016/j.tins.2007.05.004
154. Barow E, Neumann WJ, Brücke C, Huebl J, Horn A, Brown P, et al. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain. (2014) Nov;137(Pt 11):3012-3024. doi: 10.1093/brain/awu258
155. Silberstein P, Kühn AA, Kupsch A, Trottenberg T, Krauss JK, Wöhrle JC, et al. Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia. Brain. (2003) Dec;126(Pt 12):2597-608. doi: 10.1093/brain/awg267
156. Wang DD, de Hemptinne C, Miocinovic S, Qasim SE, Miller AM, Ostrem JL, et al. Subthalamic local field potentials in Parkinson's disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol Dis. (2016) 89:213–22. doi: 10.1016/j.nbd.2016.02.015
157. Neumann WJ, Horn A, Ewert S, Huebl J, Brücke C, Slentz C, et al. A localized pallidal physiomarker in cervical dystonia. Ann Neurol. (2017) 82:912–24. doi: 10.1002/ana.25095
158. Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. (2008) 5:320–30. doi: 10.1016/j.nurt.2008.01.002
159. Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. (2004) 101:189–94. doi: 10.3171/jns.2004.101.2.0189
160. Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V. Pallidal stimulation for dystonia. Neurosurgery. (2004) 55:1361–8; discussion 1368–70. doi: 10.1227/01.NEU.0000143331.86101.5E
161. Khan MF, Mewes K, Gross RE, Škrinjar O. Assessment of Brain Shift Related to Deep Brain Stimulation Surgery. Stereotact Funct Neurosurg. (2008) 86:44–53. doi: 10.1159/000108588
162. Rolston JD, Englot DJ, Starr PA, Larson PS. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: analysis of multiple databases. Parkinsonism Relat Disord. (2016) 33:72–7. doi: 10.1016/j.parkreldis.2016.09.014
163. Zaidel A, Spivak A, Shpigelman L, Bergman H, Israel Z. Delimiting subterritories of the human subthalamic nucleus by means of microelectrode recordings and a hidden Markov model. Mov Disord. (2009) 24:1785–93. doi: 10.1002/mds.22674
164. Valsky D, Marmor-Levin O, Deffains M, Marmor-Levin O, Deffains M, Eitan R, et al. Stop! border ahead: automatic detection of subthalamic exit during deep brain stimulation surgery. Mov Disord. (2017) 32:70–9. doi: 10.1002/mds.26806
165. Rabiner LR. A tutorial on hidden Markov models and selected applications in speech recognition. Proc IEEE. (1989) 77:257–86. doi: 10.1109/5.18626
166. Venkatraghavan L, Rakhman E, Krishna V, Sammartino F, Manninen P, Hutchison W. The effect of general anesthesia on the microelectrode recordings from pallidal neurons in patients with dystonia. J Neurosurg Anesthesiol. (2016) 28:256–61. doi: 10.1097/ANA.0000000000000200
167. Valsky D, Blackwell KT, Tamir I, Eitan R, Bergman H, Israel Z. Real-time machine learning classification of pallidal borders during deep brain stimulation surgery. J Neural Eng. (2020) 17:016021. doi: 10.1088/1741-2552/ab53ac
168. Sanger TD, Ferman D. Similarity of involuntary postures between different children with dystonia. Mov Disord Clin Pract. (2017) 4:870–4. doi: 10.1002/mdc3.12533
169. Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. (2002) 34:841–51. doi: 10.1016/S0896-6273(02)00698-0
Keywords: neuromoxdulation, depression, deep brain stimulation, MRI compatibility, gait disability
Citation: Sui Y, Tian Y, Ko WKD, Wang Z, Jia F, Horn A, De Ridder D, Choi KS, Bari AA, Wang S, Hamani C, Baker KB, Machado AG, Aziz TZ, Fonoff ET, Kühn AA, Bergman H, Sanger T, Liu H, Haber SN and Li L (2021) Deep Brain Stimulation Initiative: Toward Innovative Technology, New Disease Indications, and Approaches to Current and Future Clinical Challenges in Neuromodulation Therapy. Front. Neurol. 11:597451. doi: 10.3389/fneur.2020.597451
Received: 21 August 2020; Accepted: 23 November 2020;
Published: 28 January 2021.
Edited by:
Vitor Engracia Valenti, São Paulo State University, BrazilReviewed by:
Stephen Tisch, St Vincent's Hospital Sydney, AustraliaRon Alterman, Beth Israel Deaconess Medical Center and Harvard Medical School, United States
Laura Cif, University Hospital Montpellier, Montpellier, France
Luigi M. Romito, Fondazione IRCCS Istituto Neurologio Carlo Besta, Italy
Copyright © 2021 Sui, Tian, Ko, Wang, Jia, Horn, De Ridder, Choi, Bari, Wang, Hamani, Baker, Machado, Aziz, Fonoff, Kühn, Bergman, Sanger, Liu, Haber and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hesheng Liu, bGl1aGVAbXVzYy5lZHU=; Suzanne N. Haber, U3V6YW5uZV9IYWJlckB1cm1jLnJvY2hlc3Rlci5lZHU=; Luming Li, bGlsbUB0c2luZ2h1YS5lZHUuY24=
†These authors have contributed equally to this work
 Yanan Sui
Yanan Sui Ye Tian
Ye Tian Wai Kin Daniel Ko
Wai Kin Daniel Ko Zhiyan Wang1
Zhiyan Wang1 Andreas Horn
Andreas Horn Dirk De Ridder
Dirk De Ridder Ki Sueng Choi
Ki Sueng Choi Clement Hamani
Clement Hamani Kenneth B. Baker
Kenneth B. Baker Andre G. Machado
Andre G. Machado Tipu Z. Aziz
Tipu Z. Aziz Erich Talamoni Fonoff
Erich Talamoni Fonoff Andrea A. Kühn
Andrea A. Kühn Hagai Bergman
Hagai Bergman Terence Sanger
Terence Sanger Suzanne N. Haber
Suzanne N. Haber Luming Li
Luming Li