- 1Department of Neurology, First Hospital, Peking University, Beijing, China
- 2Department of Neurology, Temple University, Philadelphia, PA, United States
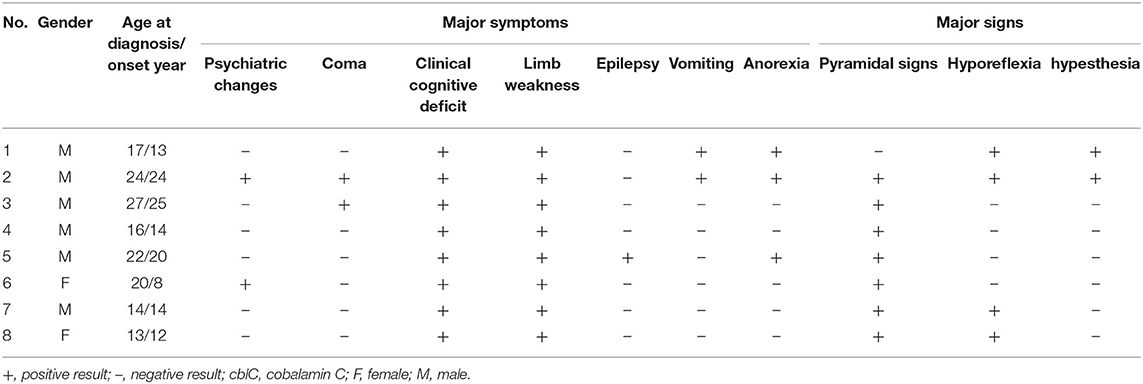
Background: Cobalamin C (cblC) has a fundamental role in both central and peripheral nervous system function at any age. Neurologic manifestations may be the earliest and often the only manifestation of hereditary or acquired cblC defect. Peripheral neuropathy remains a classical but underdiagnosed complication of cblC defect, especially in late-onset cblC disease caused by mutations in the methylmalonic aciduria type C and homocysteinemia (MMACHC) gene. So the clinical, electrophysiological, and pathological characteristics of late-onset cblC disease are not well-known.
Methods: A retrospective study of patients with late-onset cblC disease was conducted at our hospital on a 3-year period. The neuropathy was confirmed by the nerve conduction study. Sural biopsies were performed in 2 patients.
Results: Eight patients were identified, with a mean onset age of 16.25 ± 6.07 years. All patients had methylmalonic aciduria, homocysteinemia, compound heterozygous MMACHC gene mutations were detected in all patients, and 7/8 patients with c.482G>A mutation. One patient concomitant with homozygote c.665C>T mutation in 5,10-methylenetetrahydrofolate reductase (MTHFR) gene. All patients showed limb weakness and cognitive impairment. Five patients had possible sensorimotor axonal polyneuropathy predominantly in the distal lower limbs. Sural biopsies showed loss of myelinated and unmyelinated fibers. Electro microscopy revealed crystalline-like inclusions bodies in Schwann cells and axonal degeneration.
Conclusion: Late-onset cblC disease had possible heterogeneous group of distal axonal neuropathy. c.482G>A mutation is a hot spot mutation in late-onset cblC disease.
Introduction
The main causes of cobalamin deficiency are inadequate dietary intake (veganism, etc.), malabsorption (atrophic gastritis, parasitic Infection, chronic pancreatitis, pernicious anemia, etc.), and metabolic disturbance (transcobalamin gene polymorphisms, etc.) (1). Cobalamin C (cblC) disease is the most common inborn error of intracellular vitamin B12 metabolism caused by mutations in the methylmalonic aciduria type C and homocysteinemia (MMACHC) gene. It impairs conversion of dietary vitamin B12 and causes deficient synthesis of methylcobalamin and adenosylcobalamin. Diagnostic laboratory findings of cblC disease include elevated levels of methylmalonic acid in the urine, homocysteine in the blood (2–6), and serum propionylcarnitine (C3). Moreover, the ratios of C3-to-acetylcarnitine (C2) are remarkably elevated (6). Depending on the onset time of disease, the cblC disease can be divided into two types, early-onset type in the infancy period (2) and late-onset type in adolescents or adults (3–5).
The late-onset cblC disease is characterized by involvement of both the central nervous system and peripheral nerve system. The patients showed commonly cognitive and psychiatric disturbances (2, 3, 5–8), epilepsy (3–9), spastic paraplegia (3, 7, 8, 10, 11), with optic atrophy (3, 12). Some patients showed typical subacute combined degeneration (7, 13). Neuroimaging findings of the central nervous system were dominated by cerebral and cerebellar atrophy, with white matter hyperintensities (14) and spinal cord hyperintensities (13, 15).
Peripheral neuropathy remains a classical but underdiagnosed complication of cblC defect. Some reports indicated length dependent peripheral neuropathy (7, 8, 10). Nerve conduction study revealed reductions of conduction velocity or amplitude in the motor and sensory nerves (6–8, 14). Sural biopsy showed mixed neuropathy with demyelination and axonal degeneration in patients with central nerve involvement (7, 10). The objective of the present study was to verify and summarize the clinical, electrophysiological, and pathological features of peripheral nerve involvement in late-onset cblC disease.
Materials and Methods
Clinical data from 8 unrelated patients with late-onset cblC disease who underwent peripheral nerve electrophysiological test in Peking University First Hospital from January 2016 to October 2019 were retrospectively analyzed (Table 1). The present study was approved by the institutional review board and ethics committee at Peking University First Hospital.
Nerve Conduction Study
All patients were performed peripheral nerve conduction study (Alpine bioMed Aps corporation in Denmark), compound muscle action potentials (CMAPs) were recorded for the median nerve, the ulnar nerve, the tibial nerve, and the peroneal nerve with surface electrodes. Sensory nerve action potentials were recorded with surface electrodes from the median nerve, the ulnar nerve, and the sural nerve.
Pathological Examination
Patient 1 and 2 were performed sural biopsies, part of sample was fixed in 4% formaldehyde, then paraffin-embedded, and sections were stained with Luxol fast blue, Congo red, hematoxylin, and eosin. The rest of each section was fixed in 2.5% glutaraldehyde then 1% buffered osmium tetroxide, followed by dehydrated and embedded in Epon. Semi-thin sections were stained with toluidine blue for light microscopy. Ultrathin sections were with uranyl acetate and lead citrate double-staining, then examined under an electron microscope.
Results
Clinical Data
All patients included 6 men and 2 women with body mass index ranging from 15.24 to 27.04 kg/m2. Of all 8 patients, the average age was 16.25 ± 6.07 years (13–27 years). The duration from onset to diagnostic time was 13.75 ± 12.71 months (2–35 months). Limb weakness (8/8) and cognitive impairment (8/8) were the most frequent clinical manifestations, followed by anorexia (3/8), psychiatric changes (2/8), vomiting (2/8), coma (2/8), numbness (1/8), and epilepsy (1/8). Additionally, 7 of 8 patients showed positive Babinski sign bilaterally in their lower limbs, hyporeflexia appeared in 4 of 8 patients, decreased vibration and pinprick sensations in lower limbs in 2 of 8 patients. Some patients presented with relapsing and remitting course. After 2–8 weeks treatments with intramuscular adenosylcobalamin (1 mg/d), oral L-carnitine (3 g/d), folic acid (5 mg/d), betaine (9 g/d) and compound vitamin B, all patients showed alleviation of clinical manifestations, the homocysteine and methylmalonic acid were in normal range.
Laboratory and Imaging Findings
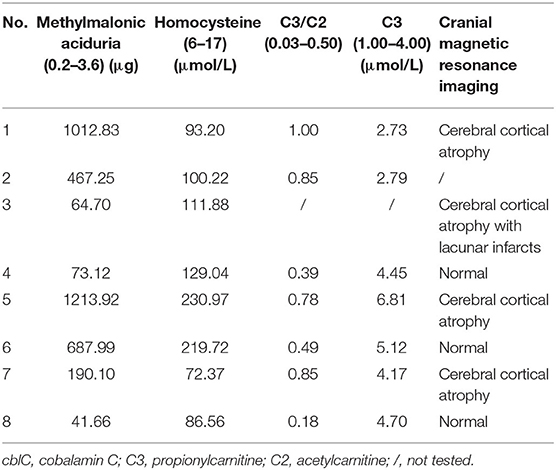
Laboratory investigations were remarkably elevated levels of urine methylmalonic acid and plasma homocysteine in 8 patients. Serum C3 levels were elevated in 3 patients, and the ratios of C3/C2 were elevated in 4 patients. Proteinuria was detected in 4 patients and hyperuricemia in 2. Cranial Magnetic resonance imaging revealed cerebral and cerebellar atrophy in 4 patients, ischemic infarcts in cerebral or basal ganglion in 2 patients (Table 2).
Mutation Analysis
Next generation sequencing revealed all patients with compound heterozygous mutations in the MMACHC gene. A missense mutation of c. 482G > A (VCV000001425.8) was detected in patient 1–5, 7, and 8. A deletion mutation of c.658_660delAAG (VCV000095707.7) was detected in patient 1, 6. A nonsense mutation of c.217C>T (VCV000203825.5) in patient 2, a frameshift mutation of c.440_441del (VCV000203834.4) in patient 3, a duplication mutation of c.271dupA (VCV000001421.17) in patient 8. A nonsense mutation of c.609G>A (VCV000030800.6) was detected in patient 4, 5, and 7. A missense mutation of c.347T>C (VCV000001422.3) was detected in patient 6. The parents of patient 1, 3, 4, 7, and 8 were confirmed to be heterozygous carriers of the MMACHC gene mutations, while patient 2, 5, and 6 did not perform pedigree verification. Finally, Patient 1 further showed homozygous missense mutations of c.665C>T (VCV000003520.13) in 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, whose parents were heterozygous carriers of the MTHFR gene mutations.
Nerve Conduction Study
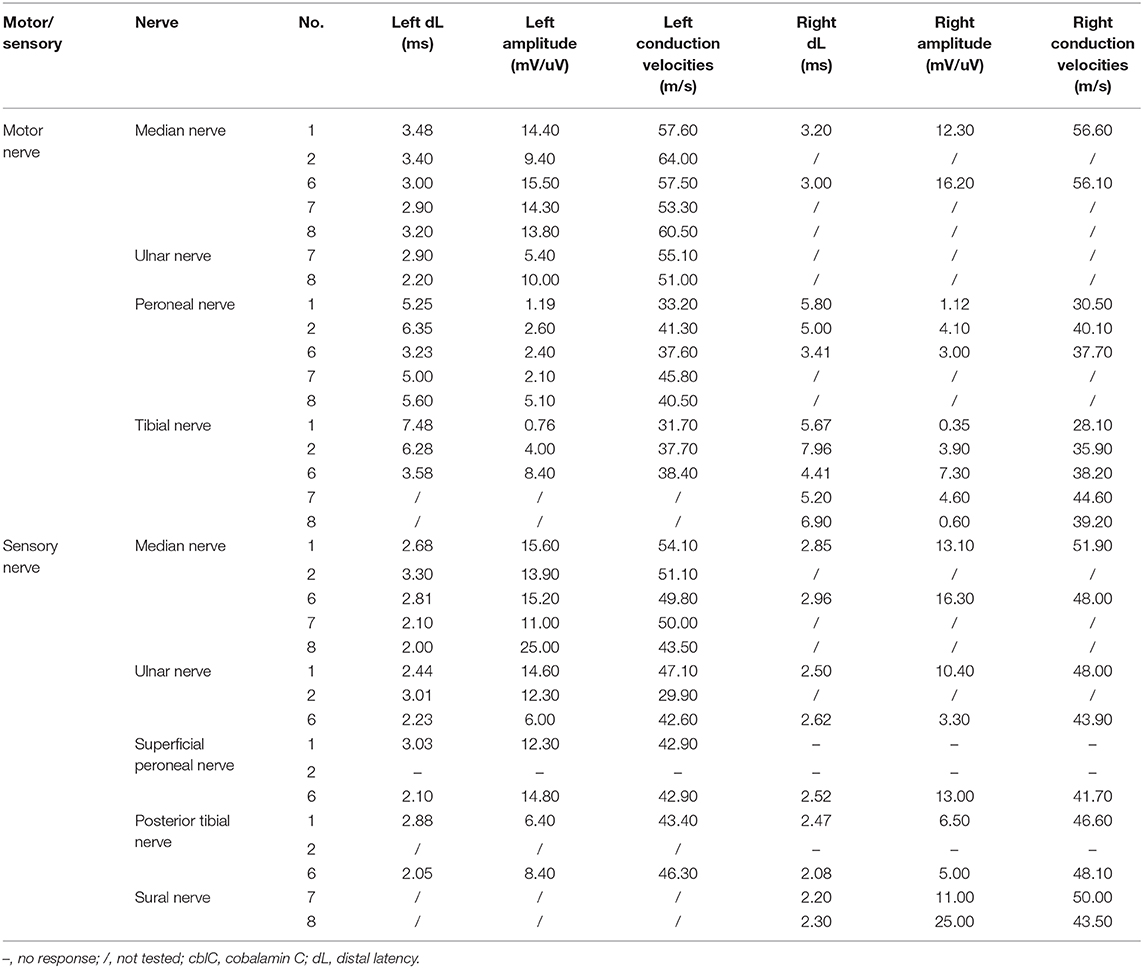
Nerve conduction study (Table 3) revealed decreased motor nerve conduction velocities with reduced amplitude of CMAPs of common peroneal nerve and tibial nerve in patient 1 and 6. Reduction of motor nerve conduction velocities with reduced amplitude of CMAPs and prolonged distal motor latencies in bilateral tibial nerve and prolonged distal motor latencies of bilateral common peroneal nerve, absence of the sensory nerve action potentials amplitude in the bilateral posterior tibial nerve, superficial peroneal nerve, were recorded in patient 2. Reduction of sensory nerve conduction velocities of the right sural nerve, the prolonged distal motor latencies and decreased amplitude of the CMAPs and reduction of motor nerve conduction velocities of the right tibial nerve and the left common peroneal nerve were recorded in patient 8. Decreased amplitude of Sensory nerve action potentials of right sural nerve and decreased amplitude of CMAPs of left common peroneal nerve were recorded in patient 7. No obvious abnormality was found in the nerve conduction study of patient 3 and 5. The somatosensory evoked potentials P30 and P38, and the bilateral visual evoked potentials P100 were not recorded in patient 3 and 5. The T10-L4 evoked potentials of spinal cord conduction velocity in patient 3 were not recorded. The somatosensory evoked potential N9 was not recorded in patient 4. No sympathetic skin response in the right lower limb and prolonged latency of the left lower limb were recorded in patient 6. The sympathetic skin response was prolonged in both upper limbs and lower limbs in patient 4.
Nerve Pathology
A pathological examination was conducted on two patients who showed moderate loss of myelinated fibers (Figure 1A). The density of myelinated fiber was 2,787/mm2 (9,663/mm2 in the control), with predominantly loss of large myelinated fibers (Figure 1B). Immunohistochemical staining with an anti-neurofilament antibody showed severe loss of both myelinated fibers and unmyelinated fibers (Figure 1C). The rest unmyelinated fibers loosely distributed, without a normal cluster profile (Figure 1D). Additionally, clusters of regenerating myelinated fibers, degeneration of myelinated fibers and atypical onion bulb formations were observed. Ultrastructural assessment further confirmed axonal degeneration with regenerated clusters (Figure 2A). The axonal degeneration characterized by myelin remnants with irregular laminated structure within Schwann cells. Frequently, some myelin remnants with irregular circular laminated structure nearby an abnormal axon surrounded by Schwann cell cytoplasm (Figure 2B). The axons are filled with fine fibrillar material. Some Schwann cells contain crystalline-like inclusions bodies (Figure 2C). Occasionally, Schwann cell processes were arranged myelinated fibers in circular configurations (Figure 2D). The collagen pocket was observed generally.
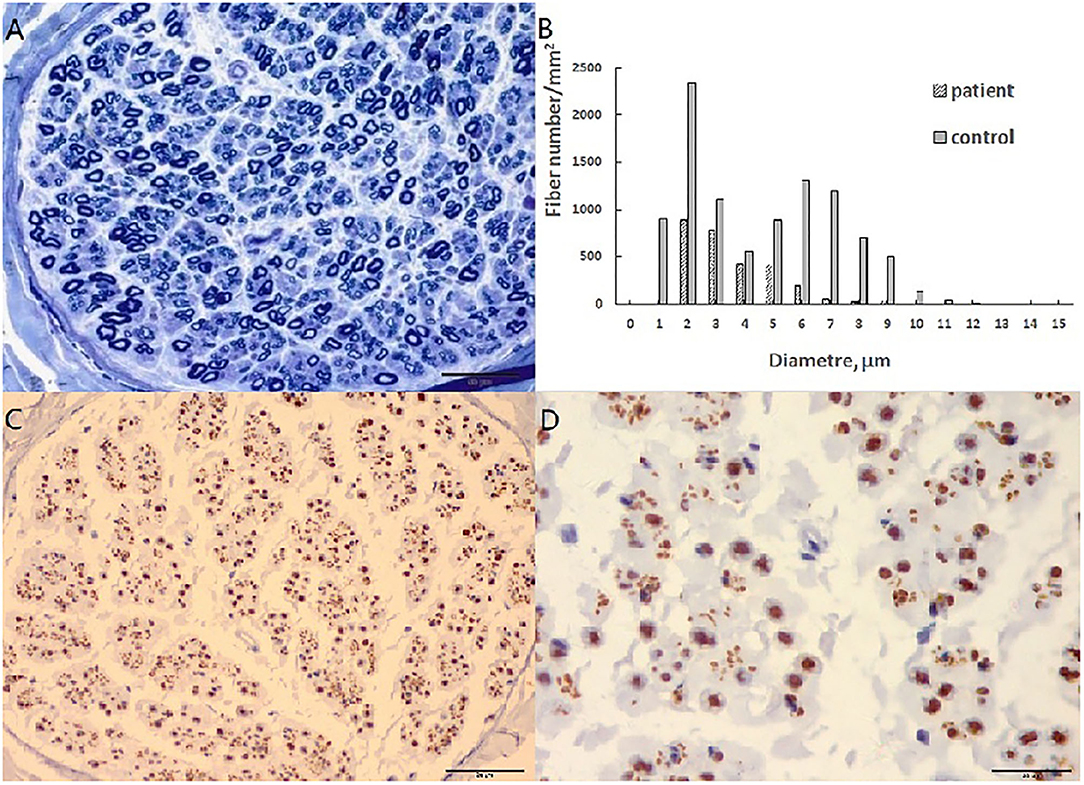
Figure 1. Sural biopsy in cblC disease. (A) Diffuse loss of myelinated fibers, predominant the large myelinated fibers. (B) Histograms of myelinated fibers, the entity is the control, and the slash is the patient with loss of large myelinated fibers. (C) Moderate loss of axons with different diameter. (D) High magnificent showed unmyelinated fibers loosely distributed without a normal cluster profile. Scale bar = 50 μm in (A,C). Scale bar = 20 μm in (D).
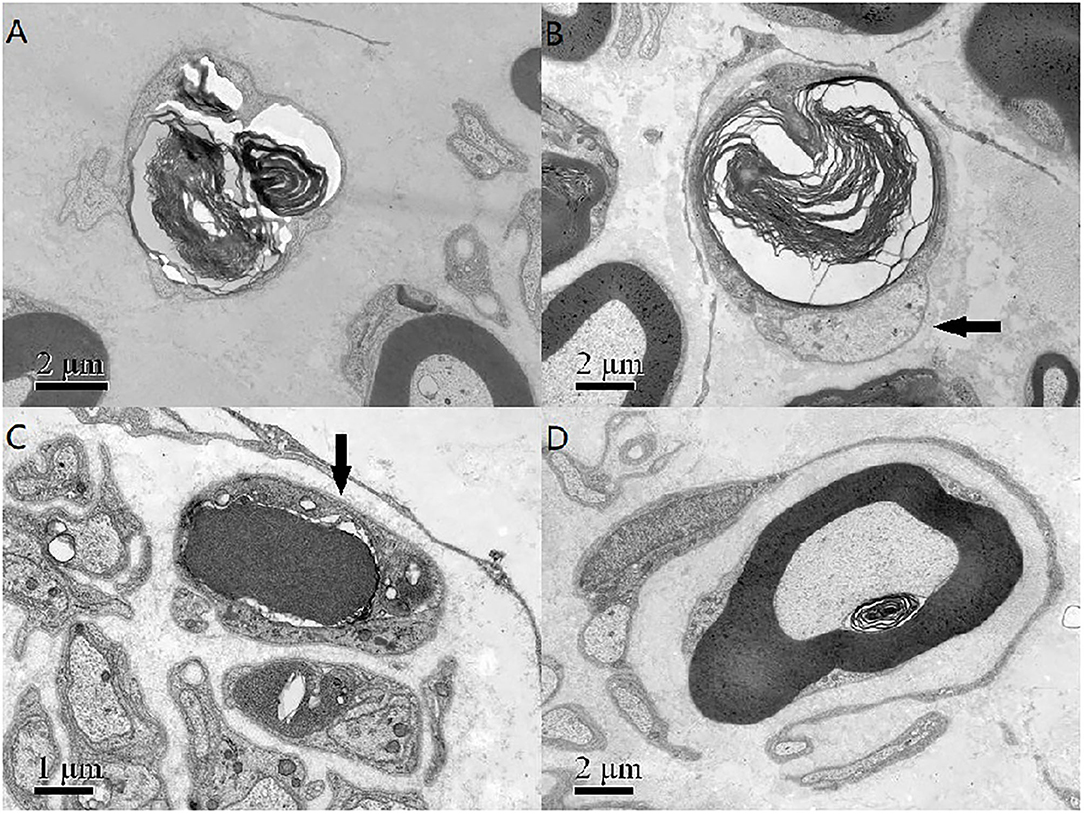
Figure 2. (A) The axonal degeneration characterized by myelin remnants with irregular laminated structure within Schwann cells. (B) Myelin remnants with circular arrangement of myelin debris with an axon (arrow) surrounded by Schwann cell cytoplasm. (C) There was a non-membranous bounded osmiophilic dense crystalline-like inclusion bodies in a Schwann cell (arrow), and an osmiophilic inclusion with a fissure and organelle in another Schwann cells. (D) The Schwann cell processes and unmyelinated nerve fibers around a myelinated fibers formed an atypical onion bulb structure. Scale bar = 2 μm in (A,B,D). Scale bar = 1 μm in (C).
Discussion
All patients in present series showed adolescence onset, similar to reported patients in China (3, 6). The main symptoms were involvement of central nervous system, similar to other reports (3, 6, 8, 16). The gastrointestinal disorders appeared also frequently in our cohort, which were rarely reported among late-onset patients (3). Cranial magnetic resonance imaging revealed cerebral atrophy with lacunar infarcts in our patients and also other reports (3, 14). All patients had high levels of urine methylmalonic acid and the plasma homocysteine. Compound heterozygous mutations in the MMACHC gene were identified and confirmed the diagnosis of late-onset cblC disease in them.
Sensorimotor peripheral demyelinating neuropathy appeared in early-onset methylmalonic aciduria and homocystinuria, cblC type (17). We found that peripheral nerve involved in late-onset cblC disease, the peripheral nerve involvement was a predominant manifestation in one patient without pyramidal signs, the most symptoms other patients showed were mild sensory or autonomic symptoms, combined with pyramidal signs (3, 14, 18). Like our patient with multivitamin therapy, relapsing and remitting myelopathy and neuropathy as main symptoms were also described in late-onset cblC disease (10). The vitamin B12 responsive neuropathy should systematically be ruled out in the clinical setting of sensory neuropathy or idiopathic neuronopathy because of potential reversibility (1).
The neurophysiological studies in present series indicated that 75% patients presented with peripheral nerve involvement, similar to other report with 83% patients presented with mild to moderate peripheral nerve damage (6). We also found that the neurophysiological findings varied largely due to the severity variation in different patients. Some patients showed mixed axonal and myelinated neuropathies in both sensory and motor nerves, usually intermediate conduction velocity with reduction of amplitudes (7, 14). We found that some patients showed possible axonal neuropathy with a decrease in the amplitudes of sensory and motor nerves, similar to a recent case report (19). Decreased conduction velocity was less commonly detected also in other reported late-onset cblC disease (6, 14). Similar results appeared in other vitamin B12 responsive neuropathy (1, 20).
A sural biopsy specimen displayed general axonal degeneration with demyelination in the peripheral nerves in late-onset cblC disease. Both demyelination and axonal degeneration in peripheral nerves were described in other report (7). In addition of regeneration cluster, we found axonal degeneration characterized by myelin remnants with irregular laminated structure within Schwann cells. Some myelin remnants with circular arrangement of myelin debris with abnormal axon surrounded by Schwann cell cytoplasm (Figure 2B) which might be a specific form of axonal degeneration rather than a demyelination process. Some Schwann cells had crystalline-like inclusions bodies, which were also described as Fardeau-Engel bodies in other neuropathy (21). Appearance of atypical onion ball structure indicated a mininal demyelination which was described in axonal CMT (22). Moreover, we found the loss of unmyelinated fibers in late-onset cblC disease, which was described also in vitamin B12 deficiency (23).
We confirmed that c.482G>A mutation in MMACHC gene was the hot spot mutation (3, 6, 24). Patients with c.482G>A mutation had late-onset symptoms and easier metabolic control (25). A reported late-onset patient with mutations (c.217C>T and c.482G>A) same as our patient 2 showed more severer peripheral nerve impairment than patient 2 (19). Peripheral nerves were more frequently identified in late-onset type with point mutation (3). We found that peripheral nerve impairment was most severe in the patient concomitant with a homozygous c.665C>T mutation in MTHFR gene. Elevated homocysteine could have contributed to increased peripheral nerve damage (26). However, given our limited data, it is difficult to determine the linear relationship between homocysteine and peripheral nerve damage.
In fact, only one patient presented with typical peripheral neuropathy, and 5 patients showed subclinical peripheral nervous system involvement. It is difficult to identify the levels of the lesions such as limb weakness concerning the central nervous system as spinal cord or the peripheral nervous system or both, our electrophysiological data and pathology findings were not very specific and significant of peripheral nerve lesions, the examinations provided the possible evidences of the peripheral nervous system involvement. We need to follow patients in a long time to re-evaluate levels of the peripheral nerve lesions.
In summary, our study highlights peripheral nervous system involvement in late-onset cblC disease. Pathological findings in our patients reveal new insights that possible distal axonal neuropathy in cblC patients cannot be ignored.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XC and YY contributed to the conception and design of the study. XC collected and organized the database. XC wrote the first draft of the manuscript. LM and WZ wrote sections of the manuscript. ZW and JL participated in the design. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patients and their families for their cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.594905/full#supplementary-material
References
1. Franques J, Chiche L, De Paula André M, Grapperon A, Attarian S, Pouget J, et al. Characteristics of patients with vitamin B12-responsive neuropathy: a case series with systematic repeated electrophysiological assessment. Neurol Res. (2019) 41:569–76. doi: 10.1080/01616412.2019.1588490
2. Hu S, Mei S, Liu N, Kong X. Molecular genetic characterization of cblC defects in 126 pedigrees and prenatal genetic diagnosis of pedigrees with combined methylmalonic aciduria and homocystinuria. BMC Med Genet. (2018) 19:154. doi: 10.1186/s12881-018-0666-x
3. Wang X, Yang Y, Li X, Li C, Wang C. Distinct clinical, neuroimaging and genetic profiles of late-onset cobalamin C defects (cb1C): a report of 16 Chinese cases. Orphanet J Rare Dis. (2019) 14:109. doi: 10.1186/s13023-019-1058-9
4. Thauvin-Robinet C, Roze E, Couvreur G, Horellou M-H, Sedel F, Grabli D, et al. The adolescent and adult form of cobalamin c disease: clinical and molecular spectrum. J Neurol Neurosurg Psychiatry. (2008) 79:725–8. doi: 10.1136/jnnp.2007.133025
5. Gilson RC, Wallis L, Yeh J, Gilson RT. Dementia, diarrhea, desquamating shellac-like dermatitis revealing late-onset cobalamin C deficiency. JAAD Case Rep. (2018) 4:91–4. doi: 10.1016/j.jdcr.2017.09.016
6. Wang S, Yan C, Wen B, Zhao Y. Clinical feature and outcome of late-onset cobalamin C disease patients with neuropsychiatric presentations: a Chinese case series. Neuropsychiatr Dis Treat. (2019) 15:549–55. doi: 10.2147/NDT.S196924
7. Roze E, Gervais D, Demeret S, Ogier de B, Zittoun J, Benoist J-F, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin c disease. Arch Neurol. (2003) 60:1457–62. doi: 10.1001/archneur.60.10.1457
8. Liu Y, Ji Y, Wang Y, Zhang B, Fang G, Wang J, et al. Clinical analysis of late-onset methylmalonic acidaemia and homocystinuria, cblc type with a neuropsychiatric presentation. J Neurol Neurosurg Psychiatry. (2015) 86:472–5. doi: 10.1136/jnnp-2014-308203
9. Rahmandar MH, Bawcom A, Romano ME, Hamid R. Cobalamin C deficiency in an adolescent with altered mental status and anorexia. Pediatrics. (2014) 134:e1709–14. doi: 10.1542/peds.2013-2711
10. Gold R, Bogdahn U, Kappos L, Toyka KV, Baumgartner ER, Fowler B, et al. Hereditary defect of cobalamin metabolism (homocystinuria and methylmalonic aciduria) of juvenile onset. J Neurol Neurosurg Psychiatry. (1996) 60:107–8. doi: 10.1136/jnnp.60.1.107
11. Wei Y, Zhou Y, Yuan J, Ni J, Peng B. Treatable cause of hereditary spastic paraplegia: eight cases of combined homocysteinaemia with methylmalonic aciduria. J Neurol. (2019) 266:2434–9. doi: 10.1007/s00415-019-09432-8
12. Ku CA, Ng JK, Karr DJ, Reznick L, Harding CO, Weleber RG, et al. Spectrum of ocular manifestations in cobalamin c and cobalamin a types of methylmalonic acidemia. Ophthalmic Genet. (2016) 37:404–14. doi: 10.3109/13816810.2015.1121500
13. Cui J, Wang Y, Zhang H, Cui X, Wang L, Zheng H. Isolated subacute combined degeneration in late-onset cobalamin C deficiency in children: Two case reports and literature review. Medicine. (2019) 98:e17334. doi: 10.1097/MD.0000000000017334
14. Wang X, Sun W, Yang Y, Jia J, Li C. A clinical and gene analysis of late-onset combined methylmalonic aciduria and homocystinuria, cblC type, in China. J Neurol Sci. (2012) 318:155–9. doi: 10.1016/j.jns.2012.04.012
15. Higashimoto T, Kim AY, Ogawa JT, Sloan JL, Almuqbil MA, Carlson JM, et al. High-dose hydroxocobalamin achieves biochemical correction and improvement of neuropsychiatric deficits in adults with late onset cobalamin C deficiency. JIMD Rep. (2020) 51:17–24. doi: 10.1002/jmd2.12087
16. Powers JM, Rosenblatt DS, Schmidt RE, Cross AH, Morgan D. Neurological and neuropathologic heterogeneity in two brothers with cobalamin C deficiency. Ann Neurol. (2001) 49:396–400. doi: 10.1002/ana.78
17. Frattini D, Fusco C, Ucchino V, Tavazzi B, Della GE. Early onset methylmalonic aciduria and homocystinuria cblC type with demyelinating neuropathy. Pediatr Neurol. (2010) 43:135–8. doi: 10.1016/j.pediatrneurol.2010.04.007
18. Ji T, Zhang Y, Li F, Qin J, Yang Y. Peripheral nervous impairment in a patient with methylmalonic aciduria combined with hyperhomocysteinemia. J Peking Univ Health Sci. (2013) 45:303–6.
19. Wei Y, Guan Y, Hao H. Late-onset cobalamin C disease presenting with acute progressive polyneuropathy. Muscle Nerve. (2020) 61:37–40. doi: 10.1002/mus.26865
20. Puri V, Chaudhry N, Goel S, Gulati P, Nehru R, Chowdhury D. Vitamin B12 deficiency: a clinical and electrophysiological profile. Electromyogr Clin Neurophysiol. (2005) 45:273–84.
21. Vital C, Bouillot S, Canron M-H, Vital A. Schwannian crystalline-like inclusions bodies (Fardeau-Engel bodies) revisited in peripheral neuropathies. Ultrastruct Pathol. (2002) 26:9–13. doi: 10.1080/01913120252934279
22. Lv H, Wang L, Zhang W, Wang Z, Zuo Y, Liu J, et al. A cohort study of Han Chinese MFN2-related Charcot-Marie-Tooth 2A. J Neurol Sci. (2015) 358:153–7. doi: 10.1016/j.jns.2015.08.1528
23. Güneş HN, Bekircan-Kurt CE, Tan E, Erdem-Özdamar S. The histopathological evaluation of small fiber neuropathy in patients with vitamin B12 deficiency. Acta Neurol Belg. (2018) 118:405–10. doi: 10.1007/s13760-017-0847-y
24. Morel CF, Lerner-Ellis JP, Rosenblatt DS. Combined methylmalonic aciduria and homocystinuria (cblC): phenotype-genotype correlations and ethnic-specific observations. Mol Genet Metab. (2006) 88:315–21. doi: 10.1016/j.ymgme.2006.04.001
25. Almannai M, Marom R, Divin K, Scaglia F, Sutton VR, Craigen WJ, et al. Milder clinical and biochemical phenotypes associated with the c.482G>A (p.Arg161Gln) pathogenic variant in cobalamin C disease: implications for management and screening. Mol Genet Metab. (2017) 122:60–6. doi: 10.1016/j.ymgme.2017.06.011
Keywords: cobalamin C disease, methylmalonic acid, homocysteine, peripheral neuropathy, MMACHC gene, MTHFR gene
Citation: Chu X, Meng L, Zhang W, Luo J, Wang Z and Yuan Y (2020) Peripheral Nervous System Involvement in Late-Onset Cobalamin C Disease? Front. Neurol. 11:594905. doi: 10.3389/fneur.2020.594905
Received: 14 August 2020; Accepted: 28 October 2020;
Published: 26 November 2020.
Edited by:
Angelo Schenone, University of Genoa, ItalyReviewed by:
Jean-Michel Vallat, Centre Hospitalier Universitaire de Limoges, FranceChristian Lunetta, Centro Clinico NEMO, Italy
Copyright © 2020 Chu, Meng, Zhang, Luo, Wang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Yuan, eXVhbnl1bjIwMDJAMTI2LmNvbQ==
 Xujun Chu
Xujun Chu Lingchao Meng1
Lingchao Meng1 Wei Zhang
Wei Zhang Jinjun Luo
Jinjun Luo Zhaoxia Wang
Zhaoxia Wang Yun Yuan
Yun Yuan