
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 10 November 2020
Sec. Neuro-Otology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.585747
This article is part of the Research Topic Third Window Syndrome View all 21 articles
 Han Matsuda1
Han Matsuda1 Yasuhiko Tanzawa1
Yasuhiko Tanzawa1 Tatsuro Sekine1
Tatsuro Sekine1 Tomohiro Matsumura2
Tomohiro Matsumura2 Shiho Saito1
Shiho Saito1 Susumu Shindo1
Susumu Shindo1 Shin-ichi Usami3
Shin-ichi Usami3 Yasuhiro Kase1
Yasuhiro Kase1 Akinori Itoh1
Akinori Itoh1 Tetsuo Ikezono1*
Tetsuo Ikezono1*Introduction: Recent third window syndrome studies have revealed that the intact bony labyrinth and differences in the stiffness of the oval and round windows are essential for proper cochlear and vestibular function. Herein we report a patient with a congenital dehiscence of the right stapes footplate. This dehiscence caused long-standing episodic pressure-induced vertigo (Hennebert sign). At the time of presentation, her increased thoracic pressure changes induced the rupture of the membranous stapes footplate. Perilymph leakage was confirmed by imaging and a biochemical test [perilymph-specific protein Cochlin-tomoprotein (CTP) detection test].
Case Report: A 32-year-old woman presented with a sudden onset of right-sided hearing loss and severe true rotational vertigo, which occurred immediately after nose-blowing. CT scan showed a vestibule pneumolabyrinth. Perilymphatic fistula (PLF) repair surgery was performed. During the operation, a bony defect of 0.5 mm at the center of the right stapes footplate, which was covered by a membranous tissue, and a tear was found in this anomalous membrane. A perilymph-specific protein CTP detection test was positive. The fistula in the footplate was sealed. Postoperatively, the vestibular symptoms resolved, and her hearing improved. A more detailed history revealed that, for 15 years, she experienced true rotational vertigo when she would blow her nose. After she stopped blowing her nose, she would again feel normal.
Discussion: There is a spectrum of anomalies that can occur in the middle ear, including the ossicles. The present case had a dehiscence of the stapes, with a small membranous layer of tissue covering a bony defect in the center of the footplate. Before her acute presentation to the hospital, this abnormal footplate with dehiscence induced pathological pressure-evoked fluid-mechanical waves in the inner ear, which resulted in Hennebert sign. When patients have susceptibility (e.g., weak structure) to rupture, such as that identified in this case, PLF can be caused by seemingly insignificant events such as nose-blowing, coughing, or straining.
Conclusion: This case demonstrates that PLF is a real clinical entity. Appropriate recognition and treatment of PLF can improve a patient's condition and, hence, the quality of life.
Third window syndrome (TWS) was first identified in patients with superior semicircular canal dehiscence (SCD) (1); now it includes cochlea-internal carotid artery dehiscence and posterior semicircular canal-jugular bulb dehiscence, cochlea-facial nerve dehiscence, and others (2). Wackym et al. classified these conditions as CT+ TWS or CT+ OCDS (i.e., third window syndrome with positive findings on CT imaging or otic capsule dehiscence syndrome with positive findings on CT imaging). The patient reported herein had no visible CT evidence of a bony dehiscence creating a third mobile window. The diagnostic findings and symptoms were similar to those of patients with CT+ TWS (3, 4).
We report a patient identified as a CT- TWS who had a bony defect of 0.5 mm at the center of the right stapes footplate which was covered by a membranous structure. This dehiscence caused a long-standing, pressure-induced vertigo. At the time of presentation, her increased thoracic pressure induced the rupture of the membranous stapes footplate, resulting in severe true rotational vertigo and hearing loss. Perilymph leakage was confirmed by imaging and a biochemical test utilizing a perilymph-specific protein Cochlin-tomoprotein (CTP) detection test.
A 32-year-old woman presented to another hospital with a sudden onset of right-sided hearing loss and severe true rotational vertigo, which occurred immediately after nose-blowing. She was treated with corticosteroids and bedrest for 1 week, and her vestibular symptom initially resolved. However, on the 7th day, her severe rotational vertigo recurred, and her hearing loss persisted, and she was referred to our hospital.
An otoscopic examination showed bilateral intact tympanic membranes. Pure tone audiometry showed a severe right-sided mixed hearing loss (Figure 1). Left-beating horizontal and rotatory nystagmus was mainly observed in the supine position with Frenzel glasses. A high-resolution temporal bone CT scan on the 10th day showed pneumolabyrinth in the right vestibule (Figure 2). She was again treated with bedrest and corticosteroids. After the conservative treatment, however, her vertigo and severe hearing loss did not resolve. Therefore, we decided to perform perilymphatic fistula (PLF) repair surgery on the 17th day. The operation was done under general anesthesia; a transcanal approach with tympanomeatal flap elevation enabled the observation of a dehiscence in the center of the stapes footplate with a bony defect 0.5 mm in diameter, which was covered by a membranous tissue, and a tear was found in this membrane (Figure 3). A small amount of perilymph leakage was observed from this tear, and middle ear lavage with 0.3 ml of saline for a CTP detection test was collected during the operation. The fistula in the footplate was sealed with connective tissue, and the round window was reinforced with connective tissue and cartilage to stabilize the labyrinth further. The vestibular symptoms and nystagmus disappeared immediately after the operation, and at her 1-month follow-up assessment, her hearing improved (Figure 4). Postoperatively, a CTP detection test revealed a concentration of 0.84 ng/ml, which is positive, with the cutoff criteria being CTP ≧ 0.8 positive, 0.8 > CTP > 0.4 intermediate, and 0.4 > CTP negative (ng/ml) (5).
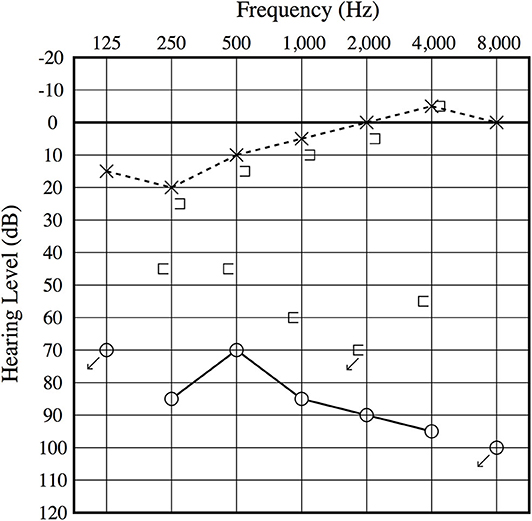
Figure 1. Preoperative audiogram. A severe mixed sensory and conductive hearing loss is observed on the right ear, and an air–bone gap is present at low frequencies.
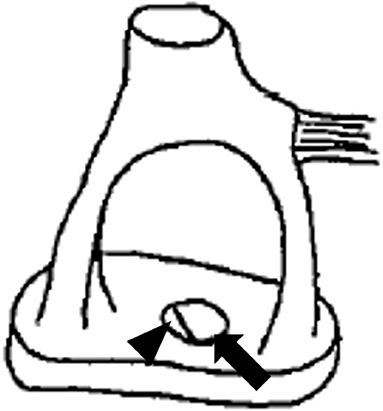
Figure 3. Illustration depicting the anomalous stapes footplate. The arrow illustrates the bony defect, while the arrowhead illustrates a tear in the membranous stapes footplate.
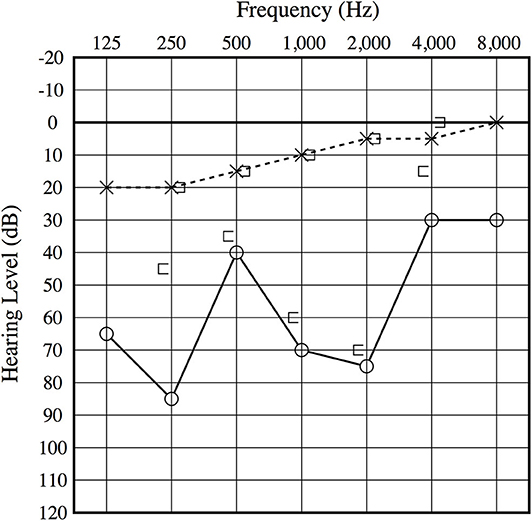
Figure 4. Postoperative audiogram. The 1-month postoperative audiogram had thresholds somewhat improved compared to the preoperative audiogram.
A more detailed history revealed that, for 15 years, she felt vertigo as if her brain was shaken upward when she would blow her nose. True rotational vertigo would continue for 3 to 4 s. After she stopped blowing her nose, she would again feel normal. She did not hear any internal sounds such as echoing, resonant voice, pulse/heartbeat, or hearing her eyes move or blink. She did not have any history of traumatic events to her head or ears. Based upon her history, intraoperative findings, and postoperative resolution of her vestibular symptoms, the dehiscence of the stapes footplate caused pressure-induced vertigo symptoms. At 1 year after the surgery, she had no recurrence of the vestibular symptoms, and her cochlear function remained unchanged.
There is a spectrum of anomalies that can occur in the middle ear, including the ossicles. In mild cases, they can be the cause of conductive hearing loss, and in severe cases, it can cause cerebrospinal fluid (CSF) leakage. Dysplasia of the inner ear is often associated with an abnormal otic capsule, resulting in congenital weakness or fistula formation in the stapes footplate or annular ligament, which is one of the most common causes of cerebrospinal fluid otorrhea and meningitis (6). The present case had normal hearing prior to this episode, and the imaging showed an intact inner/middle ear structure. Intraoperatively, a minor anomaly of the stapes was identified, with a small membranous layer of tissue covering a bony defect in the center of the footplate. This type of congenital anomalous footplate has not been described. Recent developmental studies show that the possibility of this type of anomaly can still exist since the otic capsule may not be involved in the formation of the base of the stapes (7, 8). Because of her negative history of past traumatic events to her head or ears, this abnormal finding of the footplate is most probably due to developmental malformation.
Third window syndrome studies revealed that the intact bony labyrinth and differences in the stiffness of the oval and the round windows are essential for proper cochlear and vestibular function (9). Before her acute presentation to the hospital, this dehiscence in the footplate induced pathological pressure-evoked fluid-mechanical waves in the inner ear and caused pressure-induced vertigo, which is one of the TWS symptoms. Although she had vertigo induced by nose-blowing since she was 17 years old, the etiology remained undiagnosed. The rapid change in the middle ear/intracranial pressure by her nose-blowing resulted in membrane rupture, perilymph leakage, and pneumolabyrinth. When patients have susceptibility (e.g., weak structure) to rupture, such as that identified in this case, PLF can be caused by seemingly insignificant events such as nose-blowing.
The diagnosis has been established in CT+ TWS, which has typical symptoms and CT findings. Ward et al. synthesized the diagnostic criteria for SCD (10). On the other hand, the clinical entity of PLF with leakage has remained a topic of controversy for more than 50 years due to the lack of specific biomarkers. The manifestations of PLF with leakage include a broad spectrum of neuro-otological symptoms such as hearing loss, vertigo/dizziness, disequilibrium, aural fullness, tinnitus, and cognitive dysfunction. The hearing loss may range from high frequency to low frequency and can mimic Menière disease or cochlear endolymphatic hydrops. Therefore, the difficulty of making a definitive diagnosis of PLF has caused a long-standing debate regarding its prevalence, natural history, management, and even its very existence (11).
We can overcome this controversy if we could make the definite diagnosis of PLF with leakage using an appropriate biomarker. Based on proteomic analysis, we have identified an isoform of Cochlin CTP (12) as a perilymph-specific protein that is not expressed in the blood, CSF, or saliva (13). The leaked perilymph can be recovered by middle ear lavage (MEL) with 0.3 ml of saline. We have developed an ELISA for human CTP and defined the cutoff criteria as CTP ≧ 0.8 positive, 0.8 > CTP ≧ 0.4 intermediate, and 0.4 > CTP negative (ng/ml). The sensitivity and the specificity of the test to detect perilymph leakage was 86.4 and 100%, respectively (5). The detection of CTP in the middle ear indicates the presence of a fistula and perilymph leakage. The CTP test is the most extensively studied biomarker so far. In terms of perilymph-specific expression and diagnostic accuracy, a large-scale study has been reported (14). Using this novel test, the Japanese diagnostic criteria were established (Table 1). This test is continuously available as an investigator-initiated trial throughout Japan by Ikezono et al. (5) and funded by Saitama Medical University. In June 2020, the Japanese Ministry of Health Labor Standards approved the CTP ELISA Test, which has qualities for medical diagnosis.

Table 1. Diagnostic criteria for perilymph fistula (PLF) (based on the criteria of the Intractable Hearing Loss Research Committee of the Ministry of Health and Welfare, Japan revised in 2016).
Perilymph leakage may be located in the round or the oval window (15), which may be associated with an anomalous stapes footplate, such as in this case. We have also reported a case with patent fistula ante-fentestram, showing that this microfissure can be the route for perilymph leakage (16). Most pneumolabyrinth patients reported to date were due to temporal bone trauma or otologic surgery (17–19). Pneumolabyrinth was shown by CT imaging in this case, which has a strong diagnostic value for perilymph leakage induced by the barotraumatic event. Imaging by high-resolution temporal bone CT did not show a CT+ TWS or any other inner ear abnormalities, suggesting that the pressure-induced vertigo that she experienced before her acute presentation was due to bony dehiscence of the center of the stapes footplate.
This case demonstrates that PLF is a real clinical entity. It is noteworthy that, unlike other causes of sensorineural hearing loss and dizziness, PLF with leakage is surgically correctable by sealing the fistula. By sealing the fistula, PLF is a surgically curable disease. Also, appropriate recognition and treatment of PLF can improve a patient's condition and, hence, quality of life.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Saitama Medical University Hospital (IRB No.13086). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
TI and HM: data curation and conceptualization. TM: formal analysis. TI, S-iU, and YK: funding acquisition. HM, YT, TS, TM, SSa, SSh, YK, AI, and TI: investigation. TI, TM, SSa, and HM: methodology. TI: project administration, resources, supervision, validation, and visualization. HM: writing–original draft. All authors contributed to the article and approved the submitted version.
This work was supported by the Ministry of Health and Welfare, Japan (H29-Nanchitou(Nan)-Ippan-031) (http://www.mhlw.go.jp/english/).
TI has a patent on this CTP detection test.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. (1998) 124:249–58. doi: 10.1001/archotol.124.3.249
2. Ho ML, Moonis G, Halpin CF, Curtin HD. Spectrum of third window abnormalities: semicircular canal dehiscence and beyond. AJNR Am J Neuroradiol. (2017) 38:2–9. doi: 10.3174/ajnr.A4922
3. Wackym PA, Wood SJ, Siker DA, Carter DM. Otic capsule dehiscence syndrome: superior semicircular canal dehiscence syndrome with no radiographically visible dehiscence. Ear Nose Throat J. (2015) 94:E8–24. doi: 10.1177/014556131509400802
4. Wackym PA, Balaban CD, Zhang P, Siker DA, Hundal JS. Third window syndrome: surgical management of cochlea-facial nerve dehiscence. Front Neurol. (2019) 10:1281. doi: 10.3389/fneur.2019.01281
5. Ikezono T, Matsumura T, Matsuda H, Shikaze S, Saitoh S, Shindo S, et al. The diagnostic performance of a novel ELISA for human CTP (Cochlin-tomoprotein) to detect perilymph leakage. PLoS ONE. (2018) 13:e0191498. doi: 10.1371/journal.pone.0191498
6. Wang B, Dai WJ, Cheng XT, Liuyang WY, Yuan YS, Dai CF, et al. Cerebrospinal fluid otorrhea secondary to congenital inner ear dysplasia: diagnosis and management of 18 cases. J Zhejiang University Sci. (2019) 20:156–63. doi: 10.1631/jzus.B1800224
7. Rodríguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anatomy. (2005) 207:165–73. doi: 10.1111/j.1469-7580.2005.00441.x
8. Ozeki-Satoh M, Ishikawa A, Yamada S, Uwabe C, Takakuwa T. Morphogenesis of the middle ear ossicles and spatial relationships with the external and inner ears during the embryonic period. Anat Rec. (2016) 299:1325–37. doi: 10.1002/ar.23457
9. Merchant SN, Nakajima HH, Halpin C, Nadol JB Jr, Lee DJ, Innis WP, et al. Clinical investigation and mechanism of air-bone gaps in large vestibular aqueduct syndrome. Ann Otol Rhinol Laryngology. (2007) 116:532–41. doi: 10.1177/000348940711600709
10. Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. (2017) 8:177. doi: 10.3389/fneur.2017.00177
11. Hornibrook J. Perilymph fistula: fifty years of controversy. ISRN Otolaryngol. (2012) 2012:1–9. doi: 10.5402/2012/281248
12. Ikezono T, Shindo S, Sekiguchi S, Hanprasertpong C, Li L, Pawankar R, et al. Cochlin-tomoprotein: a novel perilymph-specific protein and a potential marker for the diagnosis of perilymphatic fistula. Audiol Neurotol. (2009) 14:338–44. doi: 10.1159/000212113
13. Ikezono T, Shindo S, Sekiguchi S, Morizane T, Pawankar R, Watanabe A, et al. The performance of Cochlin-tomoprotein detection test in the diagnosis of perilymphatic fistula. Audiol Neurotol. (2010) 15:168–74. doi: 10.1159/000241097
14. Matsuda H, Sakamoto K, Matsumura T, Saito S, Shindo S, Fukushima K, et al. A nationwide multicenter study of the Cochlin tomo-protein detection test: clinical characteristics of perilymphatic fistula cases. Acta Oto-Laryngol (Stockh). (2017) 137:S53–9. doi: 10.1080/00016489.2017.1300940
15. Kohut RI, Hinojosa R, Howard G, Ryu JH. The accuracy of the clinical diagnosis (predictability) of patencies of the labyrinth capsule (perilymphatic fistulas): a clinical histopathologic study with statistical evaluations. Acta Oto-Laryngol Suppl. (1995) 520:235–7. doi: 10.3109/00016489509125236
16. Fujita T, Kobayashi T, Saito K, Seo T, Ikezono T, Doi K. Vestibule-middle ear dehiscence tested with perilymph-specific protein Cochlin-Tomoprotein (CTP) detection test. Front Neurol. (2019) 10:47. doi: 10.3389/fneur.2019.00047
17. Achache M, Sanjuan Puchol M, Santini L, Lafont B, Cihanek M, Lavieille JP, et al. Delayed pneumolabyrinth after undiagnosed perilymphatic fistula. A illustrative clinical case justifying a standardised management in an emergency setting. Eur Ann Otorhinolaryngol Head Neck Dis. (2013) 130:283–7. doi: 10.1016/j.anorl.2012.04.012
18. Hidaka H, Miyazaki M, Kawase T, Kobayashi T. Traumatic pneumolabyrinth: air location and hearing outcome. Otol Neurotol. (2012) 33:123–31. doi: 10.1097/MAO.0b013e318241bc91
Keywords: cochlin-tomoprotein, CTP, pneumolabyrinth, perilymph fistula, PLF, stapes, superior canal dehiscence, third window syndrome
Citation: Matsuda H, Tanzawa Y, Sekine T, Matsumura T, Saito S, Shindo S, Usami S-i, Kase Y, Itoh A and Ikezono T (2020) Congenital Membranous Stapes Footplate Producing Episodic Pressure-Induced Perilymphatic Fistula Symptoms. Front. Neurol. 11:585747. doi: 10.3389/fneur.2020.585747
Received: 21 July 2020; Accepted: 19 October 2020;
Published: 10 November 2020.
Edited by:
Stefan K. Plontke, Martin Luther University of Halle-Wittenberg, GermanyReviewed by:
Ja-Won Koo, Seoul National University, South KoreaCopyright © 2020 Matsuda, Tanzawa, Sekine, Matsumura, Saito, Shindo, Usami, Kase, Itoh and Ikezono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsuo Ikezono, aWtlem9uby50ZXRzdW9AMTk3Mi5zYWl0YW1hLW1lZC5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.