- 1Division of Movement Disorders, Department of Neurology, Yale School of Medicine, Yale University, New Haven, CT, United States
- 2Department of Rehabilitation and Regenerative Medicine (Program in Physical Therapy), Gertrude H. Sergievsky Center, College of Physicians and Surgeons, Columbia University, New York, NY, United States
- 3Taub Institute for Research on Alzheimer's Disease and the Aging Brain, College of Physicians and Surgeons, Columbia University, New York, NY, United States
- 4Department of Neurology, College of Physicians and Surgeons, Columbia University, New York, NY, United States
- 5Department of Neurology, University of Texas Southwestern, Dallas, TX, United States
Background: Essential tremor (ET) encompasses a variety of features, including tremor, cognitive dysfunction, and gait and balance impairments. Gait and balance impairments in ET are often mild, but they can be severe and are, in some cases, associated with functional sequelae in terms of increased fall risk and reduced balance confidence. Previous research on gait and balance in ET has been limited to cross-sectional comparisons. There have been no longitudinal studies or prospective studies. As such, our understanding of natural history and possible predictors of declines in ET-related gait and balance impairments is incomplete.
Objectives: We (1) present natural history data on the change in gait and balance measures over time, (2) provide estimates of annual rate of change in each gait and balance metric, and (3) examine the relationship between baseline clinical predictors and changes in gait and balance over time.
Methods: 149 ET participants (mean age 78.7 years), enrolled in a prospective, longitudinal, clinical-pathological study, underwent an extensive evaluation of cognition, tremor, and gait and balance at three distinct intervals performed every 18 months. Gait and balance measures included a combination of performance-based tests (e.g., tandem gait, tandem stance) and self-reported assessments (e.g., number of falls, use of a walking aid).
Results: Between the baseline and final assessments, numerous balance and gait measures showed evidence of decline and annual rates of change were quantified for each. We examined the predictive utility of clinical variables at baseline for five gait and balance outcomes, with global cognition and executive function standing out as the most consistent predictors.
Conclusions: We present a much-needed look into the course of disease for elderly patients with ET, focusing on changes observed in gait and balance and the predictors of these changes. These results also add another dimension to the relevance of cognitive impairment observed in ET; such impairment can now be viewed as predictive of poorer gait and balance over time in ET. These findings are a useful tool for clinicians, patients, and their families to better understand and plan for changing disease-features over time.
Introduction
Essential tremor (ET), the most prevalent tremor disorder, is a progressive neurological disease that affects up to 6.3% of the population aged 65 years and older (1). Historically, ET has been considered a benign, mono-symptomatic disorder with the single characterizing feature of tremor (2). However, it is now understood that ET patients can have other motor and non-motor symptoms and signs (3–5). Impairments in gait and balance have been documented across numerous studies (6–8), as have problems with cognition (9, 10), sleep (11, 12), and mood (4, 13). Gait and balance impairments in ET are often mild, but in some patients they can be severe; furthermore, in some cases they are associated with functional sequelae in terms of increased risk of falls and reduced balance confidence (8, 14–16).
Impaired gait and balance have been studied to a limited extent in patients with ET, and these impairments associated with cranial tremor and poorer cognition in ET (17–19), yet all previous research on gait and balance in ET has been limited to cross-sectional designs. There have been no longitudinal studies and no prospective studies assessing (1) natural history, (2) rate of change in gait and balance over time, or (3) the predictive utility of baseline clinical features for future gait and balance outcomes. The natural history and predictors of ET-related gait and balance impairments need to be systematically examined. We sought to bridge this knowledge gap.
Participants with ET were enrolled in a prospective, longitudinal study. In these analyses, which used data from three discrete time intervals, we (1) present natural history of change in gait and balance measures over time, (2) provide estimates of annual rate of change in each gait and balance metric, and (3) examine the predictive utility of baseline clinical predictors for subsequent changes in gait and balance over time. Broadly, the baseline predictors of interest were tremor severity, cognition, daytime sleepiness, capacity to perform activities of daily living, mood, and alcohol use. More specifically, based on existing data in ET, in the elderly, and in individuals with other neurological diseases of late life, a priori we hypothesized that baseline features such as higher total tremor score (20) and cranial tremor score (17); lower global cognition, (18) higher Clinical Dementia Rating (CDR) (18, 21); worse aggregate scores in the cognitive domains of executive function (22, 23), attention (22, 24) and visuospatial skills (25); greater daytime sleepiness (26); lower ability for daily activities (27); and finally, higher levels of depression, (28) and anxiety (29) and alcohol use (30) could function as predictors of long-term gait and balance impairment or greater decline in gait and balance in ET participants.
Methods
Recruited Participants
Two hundred and thirty participants from across the United States were enrolled in the Clinical-Pathological Study of Cognition in Essential Tremor (NINDS R01NS086736), a prospective, longitudinal study of cognitive function in ET. The study, which began in July 2014, recruited participants through the International Essential Tremor Foundation (IETF) as well as other study websites. As described previously (31, 32), participants who met the following eligibility criteria were recruited: (1) diagnosis of ET, (2) minimum age of 55 years, (3) no brain surgery for the treatment of ET, (4) willingness to participate in testing and enroll as a brain donor. All portions of the study were approved by the Yale University and Columbia University Internal Review Boards and all participants signed informed consent upon enrollment.
General Evaluation
The evaluation of all participants was performed by thoroughly trained research associates. Initial assessments were completed at Time 1 (T1), then follow-up assessments at Time 2 (T2), and final assessments at Time 3 (T3), at intervals of ~18 months. At each assessment, clinical and demographic information, including a detailed tremor history, was collected using structured questionnaires. Details regarding the comprehensive videotaped neurological examination and neuropsychological evaluation, performed at each assessment, are also presented further below. In addition, several other assessments were performed. Participants were asked to report their general level of daytime sleepiness using the Epworth Sleepiness Scale (ESS) (33). The ESS asks participants to rate on a scale from 0 to 3 how likely they would be to doze off or fall asleep in eight situations (0 = would never doze, 3 = high chance of dozing), with total ESS scores ranging from 0 to 24 (34). The participant's self-reported competence in activities of daily living such as using a telephone, doing laundry, and handling finances, were measured using the Instrumental Activities of Daily Living Scale (IADL) (35). Participants' responses were scored on a 0−1 scale, with zero reflecting limited function for that activity. Total scores for the IADL range from 0 to 8. Depression and anxiety were measured with the Geriatric Depression Scale Thirty-Item (GDS-30) (36) and the General Anxiety Disorder Seven-Item (GAD-7) (37) test, respectively. The GDS-30 uses 30 questions in which each is rated 0 (no; symptom absent) or 1 (yes; symptom present). Total scores for the GDS-30 range from 0 to 30, with a cutoff score of 10 indicating the presence of significant depression. The GAD-7 consists of seven questions with each answer scored on a scale from 0 to 3, with increasing scores indicating greater symptom severity. Total GAD-7 scores range from 0 to 21, and a score of 8 or more indicates the likely presence of an anxiety disorder. Alcohol use was self-reported based on how many drinks the participant reported to consume in an average week during their adult life. Participants were identified as drinkers if they consumed greater or equal to one drink per week on average during their adult life. Current medications were also documented, including those with the potential to impair balance and gait (e.g., benzodiazepines, hypnotics, opioids, central analgesics, and anticonvulsants). For each participant, the number of such types of medications was counted and reported. Because of constraints in the personal environment, health concerns, and time restrictions of the home visit, certain tests were omitted for certain participants.
As in our previous studies (14), each participant underwent a comprehensive videotaped neurological examination at each assessment interval, which included a detailed evaluation of various types of tremor. A senior movement disorders neurologist (E.D.L.) reviewed all videotaped examinations and confirmed each participant's ET diagnosis using reliable and valid diagnostic criteria for ET (moderate or greater amplitude kinetic tremor on ≥ 3 tests, or head tremor in the absence of Parkinson's disease [PD] or dystonia or other causes) (38). Severity of postural and kinetic tremors was rated (0−3), resulting in a total tremor score (0−36), which is a measure of the severity of action tremor (39). A cranial tremor score (0−3) was calculated for each participant based on the number of locations (neck, jaw, voice) in which cranial tremor was present on examination (17). Intention tremor was identified during 10 repetitions of the finger-nose-finger maneuver. As described previously, intention tremor was considered present if the participant either had a score of 0.5 (probable intention tremor) in both arms or a score of 1 (definite intention tremor) in at least one arm (40).
Neuropsychological Evaluation
The neuropsychological evaluation at each assessment comprised a comprehensive in-person cognitive test battery in each participant's home, a telephone interview with an informant (a close family member or friend of the participant), and subjective written summaries from the trained research associates summarizing qualitative observations regarding cognitive and functional abilities for each participant. The cognitive test battery excluded tests whose scores relied on the accuracy of motor responses so as not to disadvantage participants with more severe tremor (31, 32). The battery included two global cognitive screening measures [Mini-Mental State Examination (MMSE) (41) and Montreal Cognitive Assessment (MoCA) (42)] as well as multiple tests per cognitive domain as follows: attention [Wechsler Adult Intelligence Scale (WAIS) Digit Span subtest (43) and Symbol-Digit Modalities test (44)], executive function [Delis-Kaplan Executive Function System (D-KEFS) (45) Verbal Fluency, Color-Word Interference, Sorting, and Twenty Questions], language [Boston Naming test (46) and the Multilingual Aphasia Examination Token subtest (47)], memory [California Verbal Learning Test – II (48), Wechsler Memory Scale (WMS) Verbal Paired Associates (49), and WMS Logical Memory subtest A (50)], and visuospatial function [Judgement of Line Orientation (51), Benton Facial Recognition (52), and WAIS Visual Puzzles subtest (50)]. Aggregate scores for each cognitive domain were calculated as an average of the z-scores for each test. Z-scores of ≤ −1.5 were considered impaired.
During case conferences with collaborating expert neuropsychologists and neuropsychiatrists (E.D.H., S.C.), the neuropsychological evaluations were used to classify participants into cognitive groups (31, 32, 53). Classifications were assigned based on results from the clinical questionnaire, neuropsychological test battery, informant interview, and research associate impressions. Using these measures, each participant was assigned a rating from the CDR scale, ranging from zero to three (0 = no dementia, 0.5 = questionable dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia) (54, 55). Participants were also assigned one of three primary cognitive diagnoses: normal cognition, mild cognitive impairment (MCI), or dementia. The criteria for assigning these three cognitive diagnoses has been described previously (31). Normal cognition included: No impairment (CDR 0, no impairment on any test); Impairment of unlikely clinical significance (CDR 0, impairment on 1 test); Impairment of possible clinical significance (CDR 0 or 0.5, impairment on ≥ 1 test but not meeting operational criteria for MCI); Questionable or isolated functional impairment (CDR 0.5, no impairment on any test). MCI was defined as a CDR of 0.5 and impairment on 2 MCI-designated tests (31). Dementia was defined as a CDR ≥ 1 and impairment in multiple cognitive domains.
Gait and Balance Evaluation
As a part of the videotaped neurological examination, participants were asked to complete two gait and balance tasks: tandem gait and tandem stance. In tandem gait, patients were asked to walk 10 steps on a straight path with the heel of the leading foot touching the toe of the following foot. The number of missteps, defined as the number of steps off of the straight line, was the reported outcome (6, 56). During tandem stance, the participant was instructed to stand with the heel of one foot touching the toe of their other foot and hold the position for 10 s. The reported outcome was the number of seconds the participant could hold the position. Additionally, we administered the Transformed Six-Item Activities of Balance Confidence Scale (ABC-6), an abbreviated version of the full Activities of Balance Confidence Scale (57). For each item, participants were asked to rate their confidence in performing six activities (e.g., walk on icy sidewalks, step onto or off of an escalator without using the handrails) without losing their balance or becoming unsteady on a scale from 0 (not at all confident) to 100 (completely confident). The total score ranged from 0 to 600. Participants reported how many falls and near falls, in which they felt like they were going to fall but did not actually fall, they had in the past year and past month. We also inquired whether each participant used a walking aid, including a cane, walker, or wheelchair, inside or outside of the home.
Final Sample
Forty-seven out of 230 enrolled participants did not receive all three assessments. Of these 47 participants, 34 had died and 13 had withdrawn from the study before all assessments could be completed. This left 183 of 230 participants. Next, only participants with a confirmed ET diagnosis at every assessment interval, based on the videotaped neurological examinations assessed by E.D.L., were included. We therefore excluded 34 participants who received an alternative diagnosis or whose ET diagnosis was not confirmed at any of the three intervals (6 ET-PD, 4 dystonia, 5 who did not meet the criteria for an ET diagnosis, 19 with no videotaped neurological examination). As a result of these exclusions (total = 81), 149 of 230 enrolled participants were eligible for analyses.
Statistical Analyses
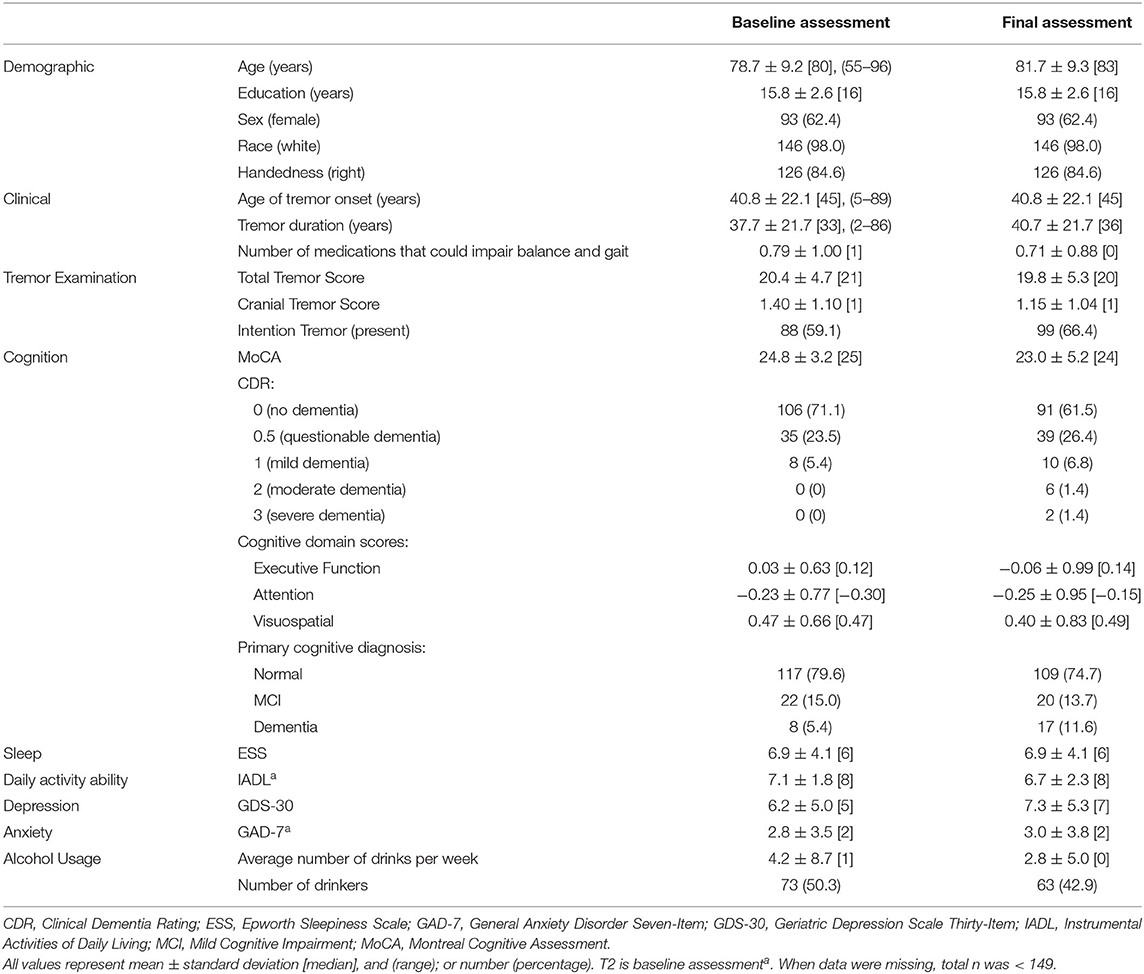
Statistical analyses were performed using SPSS (Version 26; Chicago, IL, USA). We report demographic and clinical characteristics of participants, both at baseline and at the final assessment (Table 1). Second, we report gait and balance variables at both time intervals, the change in these variables over time, the annual rate of change in these variables, and a statistical comparison between baseline and final values of these variables (Table 2). Most baseline measures used information from the T1 assessment, however, a small number of measures were added at T2 (see Tables) and for these, T2 was used as the baseline assessment. T3 data were always used for the final assessment measurements.
All gait and balance measures at baseline and final assessments were tested for normality using the Kolmogorov-Smirnov test, which determined that each of the gait and balance parameters of interest were non-normally distributed; thus, non-parametric tests were used (Table 2) and all models were logistic rather than linear regressions (Tables 3 and 4).
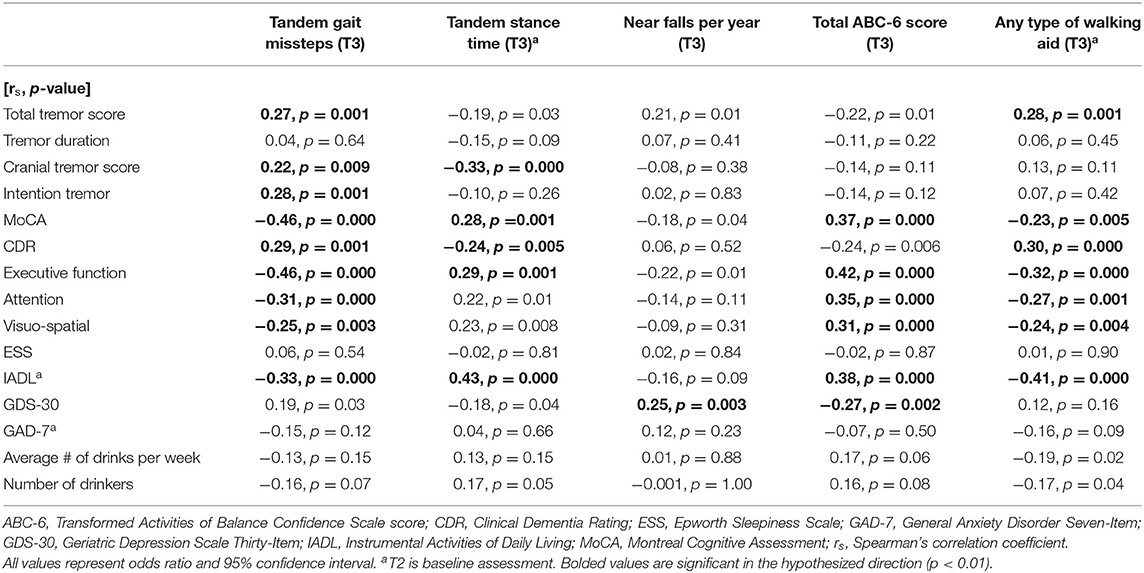
In unadjusted logistic regression models, we examined associations between baseline clinical predictors of interest and five gait and balance outcomes at the final (i.e., T3) assessment; the outcome variables were coded as “more impaired” = 1 (e.g., more tandem missteps, less tandem stance time) vs. “less impaired” = 0 based on a median split (i.e., < median vs. > median) (Table 3A). In a second set of unadjusted logistic regression models, we examined the predictive utility of baseline clinical predictors of interest for change in five gait and balance parameters over time, which was defined as the T3 value subtracted by the baseline value; the outcome variables were coded as “greater decline in function” = 1 (e.g., increase in tandem gait missteps, decrease in tandem stance time) vs. “less decline in function” = 0 based on a median split (i.e., < median vs. > median) (Table 3B). In a third set of unadjusted logistic regression models, we examined associations between baseline clinical predictors of interest and five gait and balance outcomes at the final assessment (i.e., similar to those presented in Table 3A); however, in these analyses, the outcome variables were coded as “more impaired” = 1 (e.g., more tandem missteps, less tandem stance time) vs. “less impaired” = 0 based on current benchmarks available in the ET literature rather than a median split (i.e., based on available empiric data rather than a mathematical split, Table 3C). The cut-offs for impairment were as follows: ≥2 tandem gait missteps (8), ≥26 near falls per year (15), and ≤ 300 total ABC-6 score (15). All of the logistic regression analyses resulted in odds ratios (OR) with 95% confidence intervals (CI) and p-values. To adjust for multiple comparisons, significance for Table 3 was set at p < 0.01 in Tables 3A,B (Bonferroni correction because there were five outcome measures of interest) and p < 0.017 in Table 3C (because there were three outcome measures of interest). In Table 3D, we performed correlational analyses between baseline clinical predictors of interest and five gait and balance outcomes at the final (i.e., T3) assessment; these analyses used Spearman's correlation coefficients.
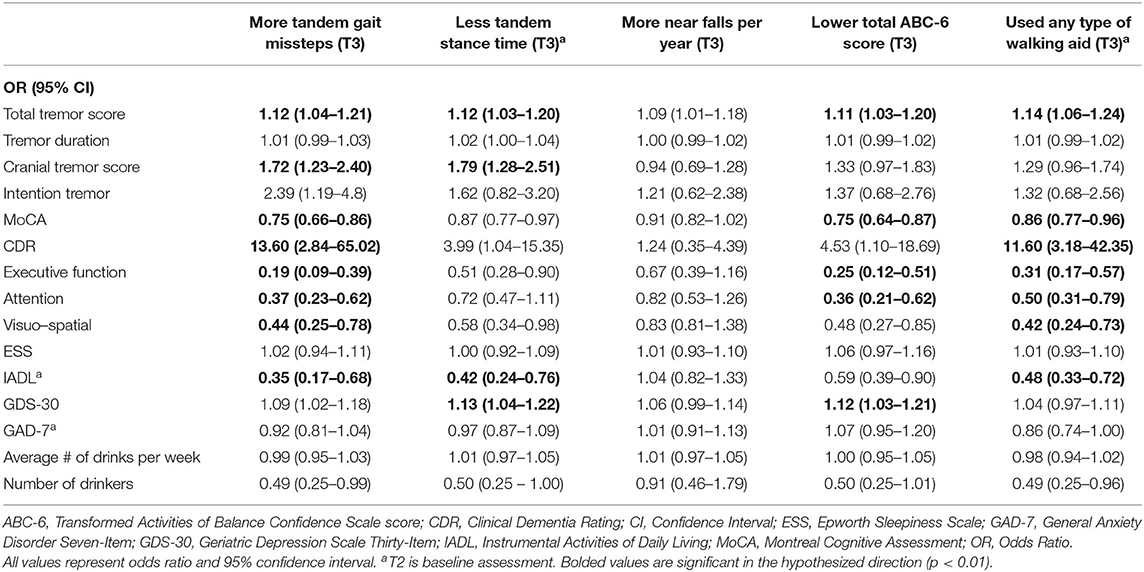
Table 3A. Unadjusted logistic regression models: Baseline characteristics as predictors of T3 gait and balance outcomes.
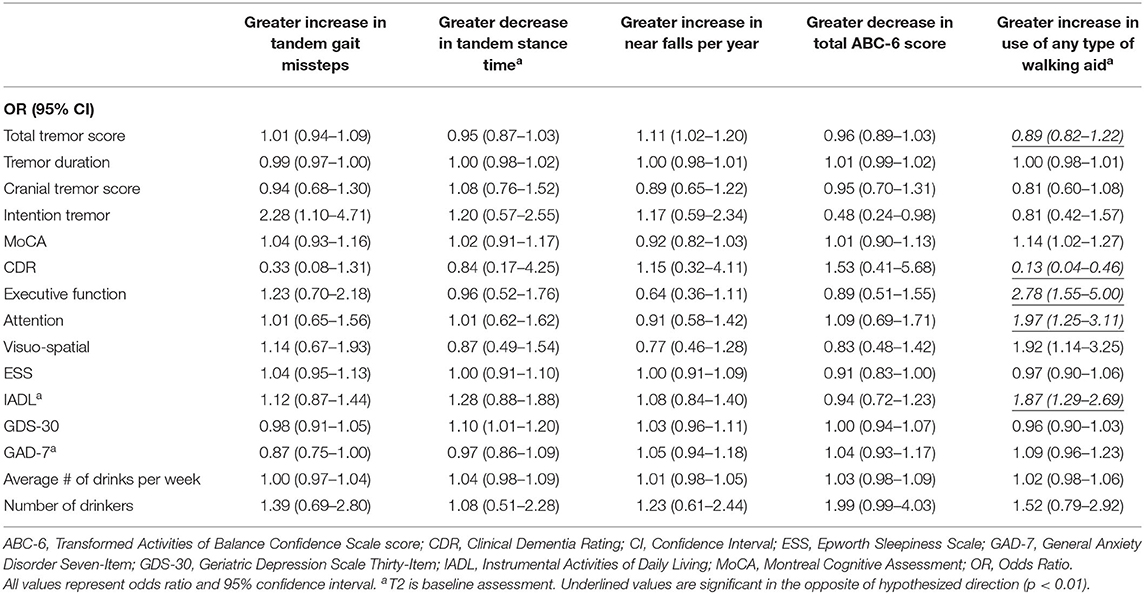
Table 3B. Unadjusted logistic regression models: Baseline characteristics as predictors of change in gait and balance outcomes.
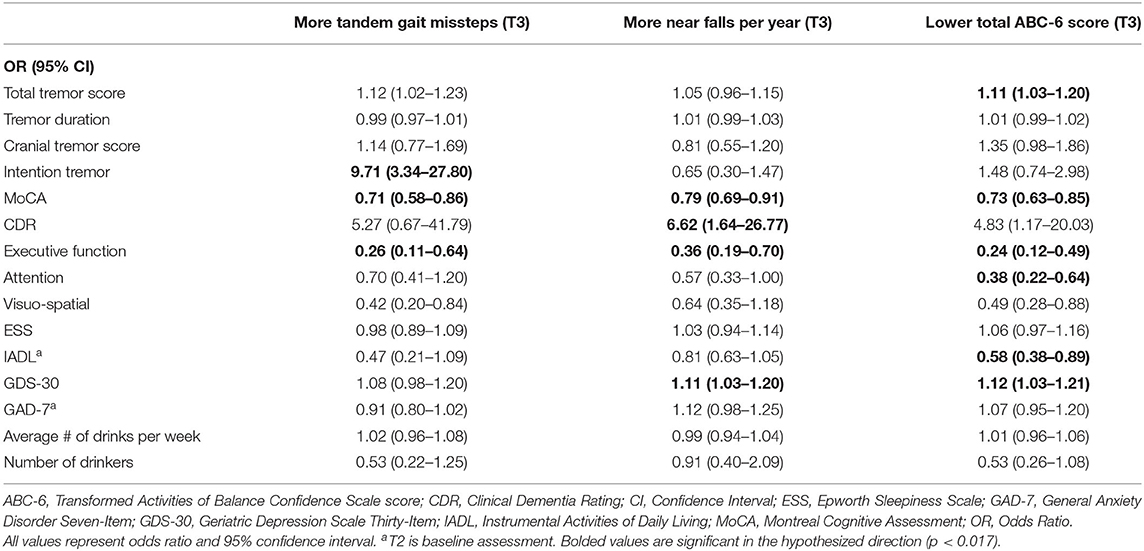
Table 3C. Unadjusted logistic regression models: Baseline characteristics as predictors of T3 gait and balance outcomes (based on available literature).
We then considered the effects of potential confounding variables, specifically, age, medication that can impair gait and balance, education, and gender (Tables 4A–C). In adjusted model 1, we used a less restrictive approach to confounding—confounding variables were included in the model if they were associated with either the independent variable or the dependent variable. In adjusted model 2, we used a more restrictive model, and confounding variables were only included if they were associated with both the independent and dependent variables. Significance in Tables 4A–C were corrected for multiple comparisons, as they were in Tables 3A–C.
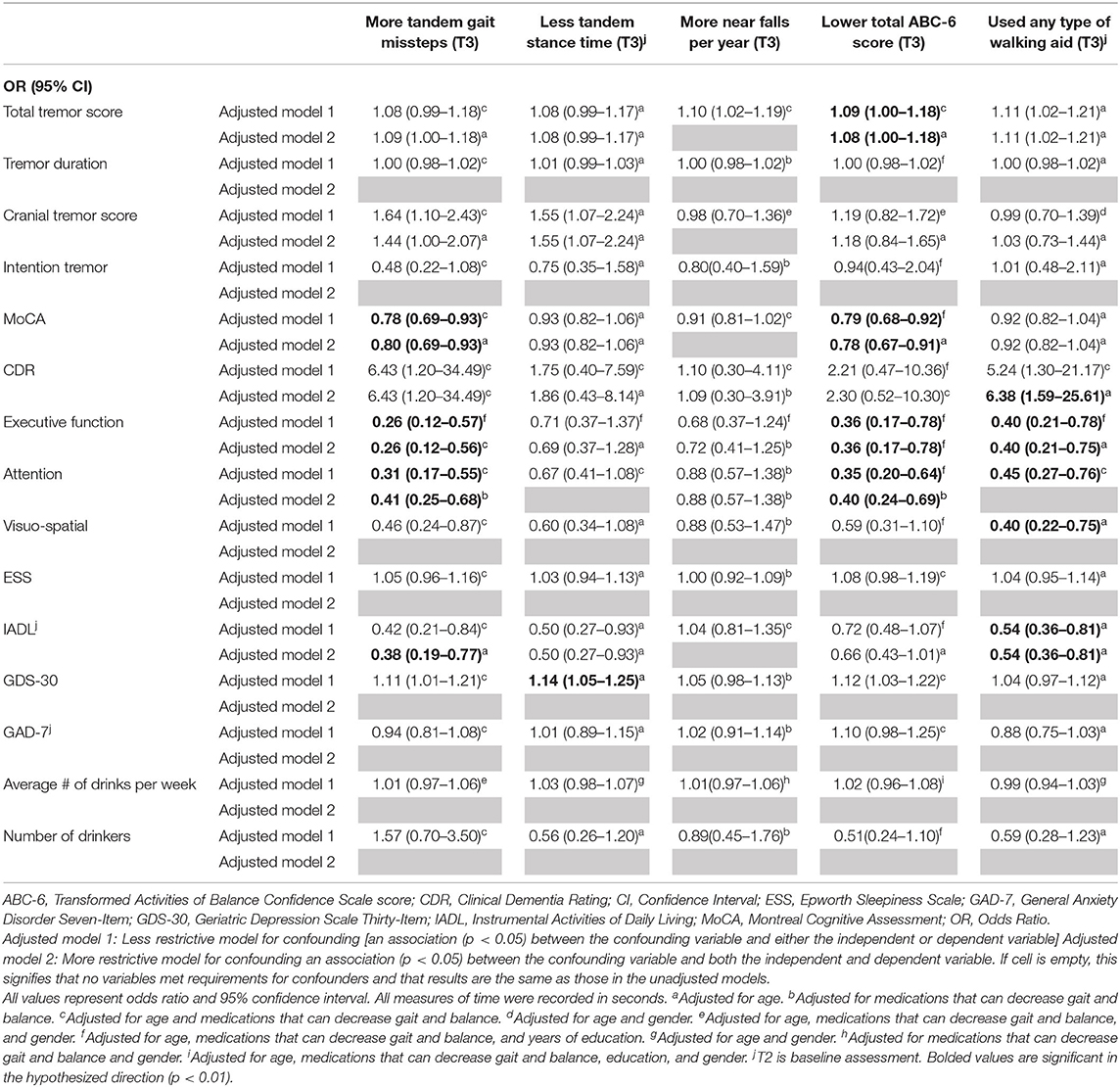
Table 4A. Adjusted logistic regression models: Baseline characteristics as predictors of T3 gait and balance outcomes.
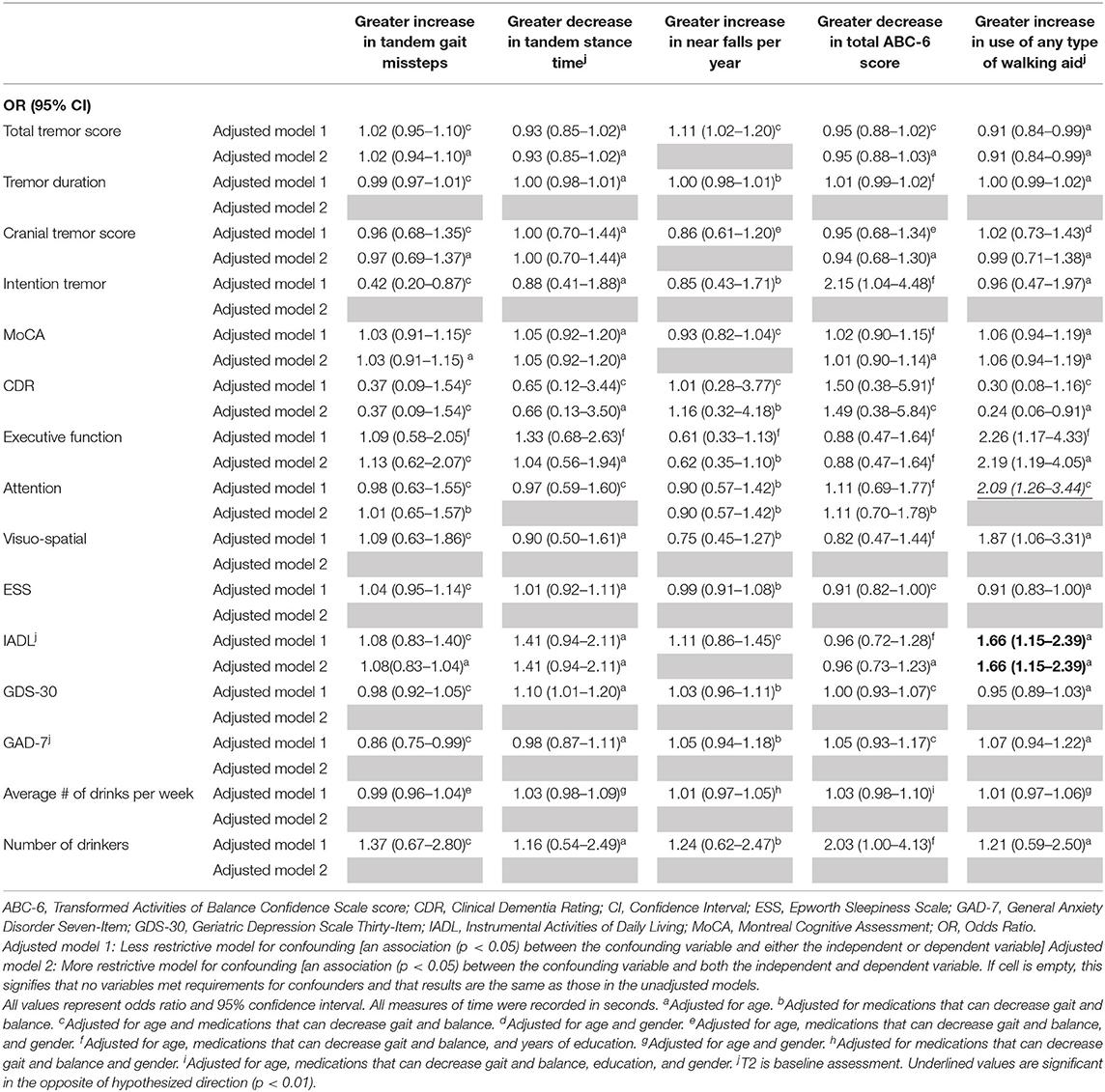
Table 4B. Adjusted logistic regression models: Baseline characteristics as predictors of change in gait and balance outcomes.
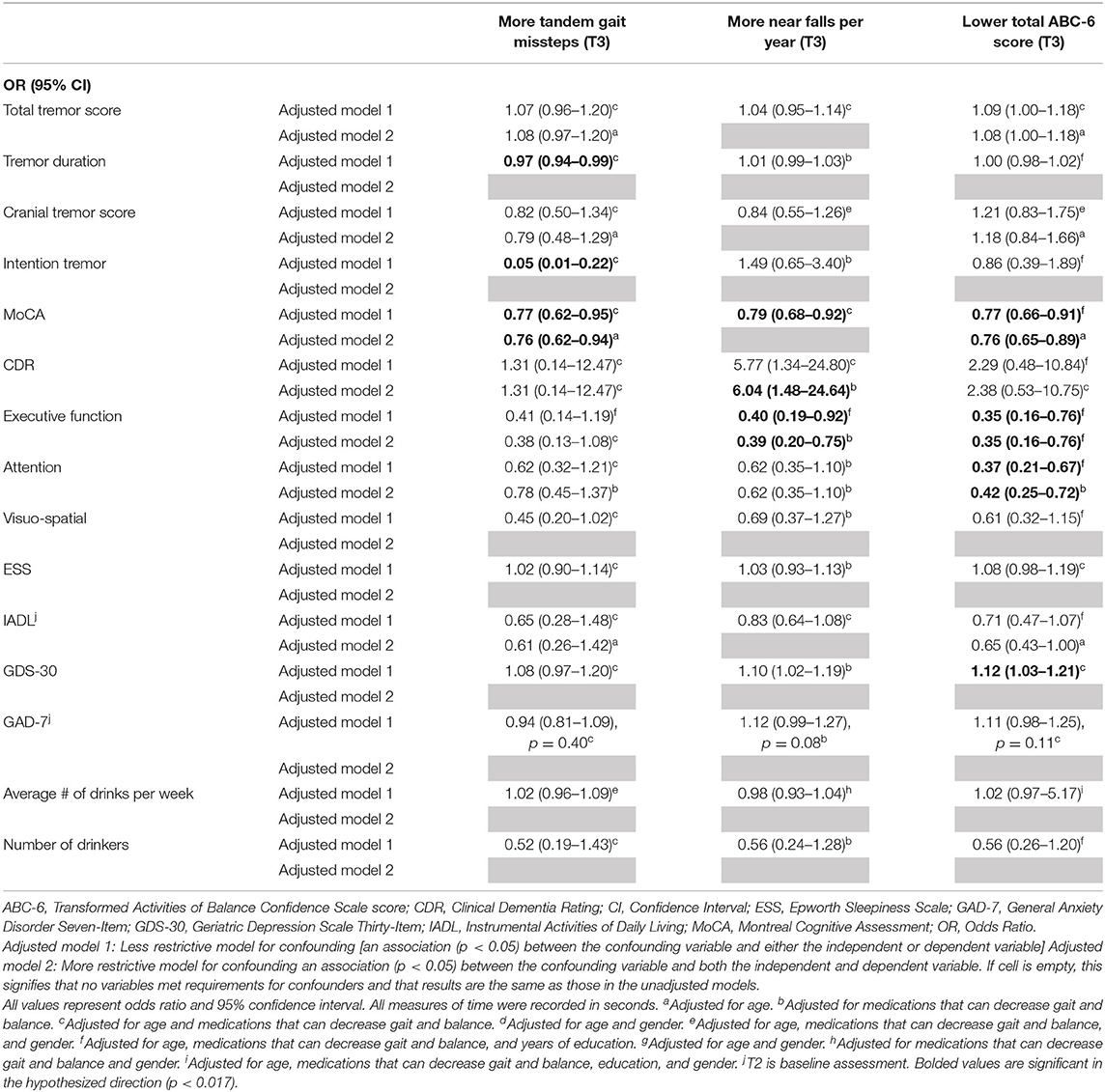
Table 4C. Adjusted logistic regression models: Baseline characteristics as predictors of T3 gait and balance outcomes (based on available literature).
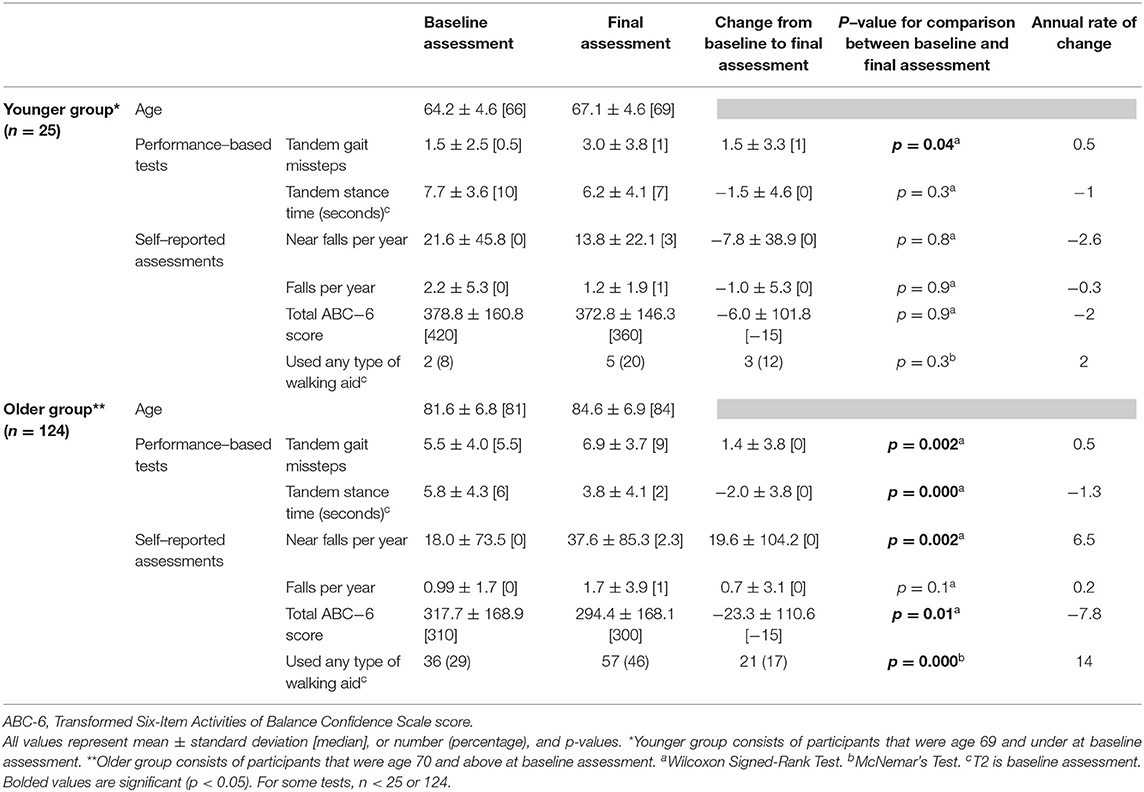
We also performed an additional analysis in which we stratified our sample into relatively younger (<70 years) vs. older ET cases, and presented data from the baseline and final assessments on numerous balance and gait measures (Table 5).
Results
There were 149 ET participants available for longitudinal analysis. At baseline, the mean age was 78.7 ± 9.2 [median = 80, range = 55–96] years (Table 1). Of these 149, 107 (71.8%) had one or more of the following features, qualifying them for a diagnosis of “ET-plus:” dystonia, rest tremor, intention tremor, MCI or dementia (Supplementary Table 1).
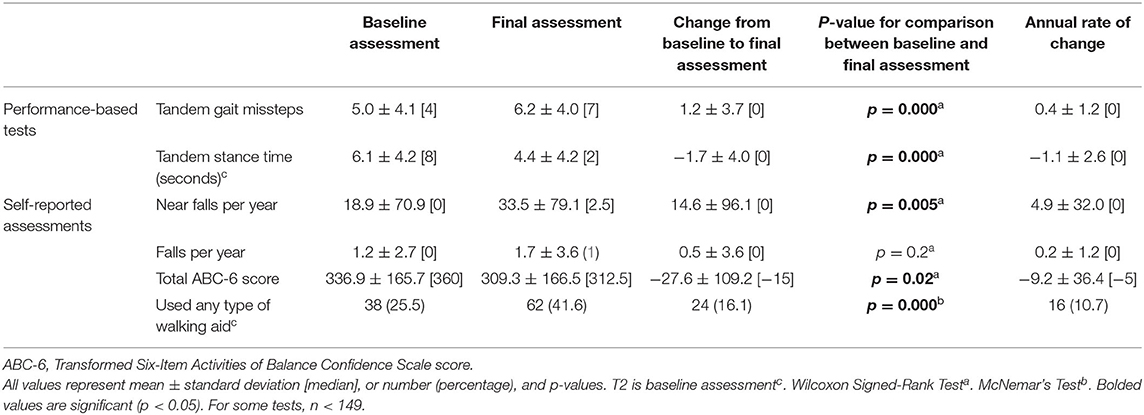
The mean ± standard deviation time elapsed between assessments T1 and T2 was 1.51 ± 0.19 [median = 1.5] years, and between T2 and T3, it was 1.49 ± 0.24 [median = 1.5] years. Between the baseline and final assessments, numerous balance and gait measures showed evidence of decline: more tandem missteps, fewer seconds in maintaining tandem stance, more near falls per year, lower total ABC-6 score (i.e., decline in balance confidence), and an increased proportion of participants using walking aids (Table 2). Annual rates of change in each gait and balance measure are also shown (Table 2).
In the first set of unadjusted logistic regression models, we examined associations between baseline clinical predictors of interest and five gait and balance outcomes at T3 (Table 3A). Baseline clinical predictors of interest were tremor (total tremor score and cranial tremor score), cognition (MoCA, CDR, executive function, attention, and visuo-spatial function), daytime sleepiness (ESS), activities of daily living (IADL), depression (GDS-30), anxiety (GAD-7), and average number of alcoholic drinks per week (Table 3A). Numerous baseline clinical predictors of interest were associated with greater number of tandem missteps on gait performance (e.g., higher total tremor score, higher cranial tremor score, lower MoCA, higher CDR, lower executive function, lower attention, lower visuospatial function), less tandem stance time (higher total tremor score, higher cranial tremor score, lower IADL, more depression), lower balance confidence (higher total tremor score, lower MoCA, lower executive function, lower attention, lower IADL, and more depression) and with use of a walking aid (e.g., higher total tremor score, lower MoCA, higher CDR, lower executive function, lower attention, lower visuospatial function) at T3 (Table 3A). We also performed correlational analyses between baseline clinical predictors of interest and five gait and balance outcomes at the final (i.e., T3) assessment (Table 3D), and the vast bulk of associations (21 of 25) in Table 3A remained significant. In adjusted models, many of these associations persisted between higher baseline tremor severity, more impaired baseline cognitive function, lower baseline IADL, more baseline depression and several of our outcomes of interest—greater tandem mis-steps, greater use of walking aid and lower balance confidence (Table 4A).
In the second set of unadjusted logistic regression models, we examined associations between baseline clinical predictors of interest and change in five gait and balance parameters. We only observed a paradoxical association between lower baseline tremor severity and better baseline cognitive function on the one hand and increase in use of any type of walking aid on the other hand (Table 3B). In adjusted models, there was an association between higher attention and higher baseline IADL and greater use of any type of walking aid (Table 4B).
In the third set of unadjusted logistic regression models, we examined baseline clinical predictors of gait and balance outcomes at T3, using cut-offs that had been established in the literature (i.e., tandem missteps, near falls per year, total ABC-6 score). In these analyses, lower MoCA and more executive dysfunction were associated with more tandem mis-steps; lower MoCA, higher CDR, and more executive dysfunction were associated with more near falls per year; higher total tremor score, lower MoCA, more executive dysfunction, more inattention and more depression were associated with lower balance confidence (Table 3C). In the adjusted models, many of these results were replicated (Table 4C).
We also performed an additional analysis in which we stratified our sample into relatively younger vs. older ET cases (Table 5), with respective mean ages of 64.2 and 81.6 years old at baseline. The younger group was small (n = 25); hence, it was statistically underpowered. Even with this, we saw a significant increase in tandem missteps over time. Declines in balance confidence and increased use of walking aids over time in this younger group did not reach statistical significance (Table 5). Interestingly, annual rate of change in tandem gait missteps and tandem stance time were strikingly similar across the two age groups (Table 5).
Discussion
In this study, 149 elderly ET participants were evaluated prospectively and longitudinally to quantitatively assess change in gait and balance measures over time (i.e., natural history) and examine the relationship between baseline clinical predictors and changes in these gait and balance measures. To our knowledge, it is the only such study of its kind. Between the baseline and final assessments, numerous balance and gait measures showed evidence of decline and annual rates of changes were carefully quantified for each. We examined associations between baseline clinical predictors of interest and five gait and balance outcomes, with baseline lower MoCA and lower executive function standing out as the most consistently significant predictors of greater gait and balance impairment at follow up. Analyses that assessed changes in gait and balance parameters over time were less successful in terms of identifying meaningful baseline predictors of such change.
Previously published work has primarily been successful in establishing abnormalities in gait and balance in ET in cross sectional studies, sampling ET cases at one point in time. The comparison of 104 ET patients and 40 age-matched controls using gait analysis showed that ET patients demonstrated reduced gait speed, dynamic imbalance, and gait asymmetry during standard walk and clear impairment in tandem gait (58). Even at an advanced age, ET patients performed more poorly than controls. In another study that compared ET patients to age-matched PD patients, dystonia patients, and controls, patients with movement disorders had lower balance confidence, increased falls, and greater need for walking aids compared to controls (59). Further comparison between movement disorder groups revealed a stepwise severity trend for all measures with PD patients experiencing the most imbalance, then ET patients, followed by dystonia patients. The longitudinal data we now present are in agreement with our cross-sectional data, which showed that ET patients with lower cognitive scores had more gait impairments (60) and greater number of falls (14).
Our study is the first to use longitudinal data to quantitatively assess the natural evolution of gait and balance deficits over the course of ET. Our findings also indicate that there are baseline clinical features of ET, especially level of cognitive performance, that can predict the level of gait and balance function later in life. The implications of these results are that physicians may be able to identify which ET patients are at higher risk for dangerous falls. If this risk could be identified early, preventative interventions could be suggested, such as balance-focused physical therapy, in hopes of mitigating the consequence of ET-related gait and balance disorder later in life. These findings also add another dimension to the cognitive changes observed in ET; these changes can now be viewed as predictive of poorer gait and balance performance over time in ET.
The mechanistic basis for the gait and balance impairment in ET is not entirely clear. While aging and aging-related factors play a role, the gait impairment in ET has been shown to be in excess of that seen in age-matched controls, indicating that other factors are involved. In clinical studies as well as quantitative gait studies, ET results in significant impairments in gait speed, asymmetry, dynamic balance, and variability, which lead to functional consequences of increased falls risk. The impairments seen in ET are qualitatively similar to what has been reported in classic cerebellar ataxia, although present to a milder degree (8). This is not surprising given the links, both in neuroimaging and postmortem studies, between ET and changes in the cerebellum.
We should comment on the fact that in our models, we were able to identify more predictors of T3 gait and balance outcomes (Tables 3A,B, 4A,C) than of change in these gait and balance outcomes (Tables 3B, 4B). This could be related to the fact that the duration of follow up was <5 years and hence, change was modest in absolute terms. Additionally, because our outcome variables were not normally distributed, our models were logistic regressions rather than linear regressions; logistic regression forces all observations into one of two categories and hence may not be optimal for detecting more nuanced change over time.
We observed an association between baseline cognitive function in ET and poorer balance performance at follow-up. This raises the broader question, what is the prior literature linking baseline cognitive deficits with declines in gait and balance over time in the elderly? There is a robust literature that highlights the roles of executive function and attention in gait, and that deficits in these domains are associated with deterioration in gait in the elderly (22–24). There are similar data in PD (25). More specifically with respect to ET, in cross-sectional studies of ET, individuals with ET and lower cognitive test scores had impaired gait, lower balance confidence and a higher number of falls than their counterparts with ET and higher cognitive test scores (14). In another cross-sectional study of ET cases, the authors reported that more cognitive difficulty was associated with more tandem gait difficulty. The authors noted that walking requires the concurrent use of cognitive and motor neural systems and that it is possible that the cognitive and gait problems in ET reflect an underlying pervasive disorder affecting both cognitive and motor circuits (19).
We noted an interesting association between poorer baseline cognitive features and reduction in use of any type of walking aid over time (Table 3B). This may be due to the fact that use of such assist devices require the participant to understand and cognitively manage the device.
Several issues merit special comment and consideration. First, our study did not include age-matched control participants. However, the goal of these analyses was to collect and present natural history data rather than to test the hypothesis that decline in gait and balance measures in ET cases occurs at a rate that is greater than that seen in age-matched controls. The data that we presented on ET were therefore valid and they fill a gap in knowledge, even without a direct comparison group. The observation, from numerous cross-sectional studies of ET patients of various ages, that gait and balance are compromised in ET, however, is heavily suggestive that the rate of change is likely to be in excess of that seen in age-matched controls; however, our study design does not allow us to make this conclusion. It has been documented that problems with balance increase over time in the normal aging population. Two previous studies have found that 13% of patients at age 65 years, 35% at age 75 years, and 46% of patients at 85 years reported balance difficulties (61, 62). According to one study that investigated objective measures of gait and balance over a wide continuum of age ranges in healthy adults, there are significant age-related changes in measures such as tandem gait, steady state gait, and dual task gait, although age-stratified data were not provided in that study, making direct comparisons with our data impossible (63). Second, we recognize that our findings do not apply to all ET, as ours was an older cohort. This is an issue of generalizability rather than validity. Nonetheless, epidemiological studies indicate that the majority of prevalent ET cases are in older age groups, such as the one we studied, and hence, older individuals are the group of greatest interest. Nevertheless, it is valuable to assess gait and balance in a younger ET cohort. As such, we were able to perform an additional analysis in which we stratified our sample into relatively younger vs. older ET cases (Table 5), with respective mean ages of 64.2 and 81.6 years, and reported data on annual rates of change in these groups. The younger group was small; hence, it was statistically underpowered. Even so, we saw a significant increase in tandem missteps over time. The observable declines in balance confidence and increased use of walking aids over time in this younger group, however, did not reach statistical significance (Table 5). Future studies may wish to sample a larger cohort of younger ET cases so as to sample the other end of the age spectrum in ET.
Our findings are admittedly not without their limitations. Subjective measures of balance confidence are open to self-report bias, especially as cognition may decline between baseline and final assessments. As a related point, our assessment of gait and balance intentionally used clinically-grounded and functional measures which were possible in the field (i.e., in patient's homes), and which are more likely to be of clinical significance; we did not perform quantitative gait testing and such studies would have aided in the precision of our estimates of rates of change. Also, we did not perform imaging on our study subjects and this would have been of value in terms of studying the underlying structural correlates of some of our findings.
Yet, despite these limitations, our study also has several strengths, the most important being our novel longitudinal data. The comprehensive evaluation provided a wealth of clinical data across three time intervals, allowing us to assess a broad array of predictors as well as potential confounding factors.
We present a much-needed look into the course of disease for elderly patients with ET, focusing on the changes observed in gait and balance and the predictors of these changes. These findings present a useful tool for clinicians, patients, and their families to better understand and plan for changing disease-features over time.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Yale University and Columbia University Internal Review Boards.
Author Contributions
HD: acquisition of data, analysis and interpretation of data, drafting/editing manuscript, and final approval of work. MZ, KR, and TC: acquisition of data, drafting/editing manuscript, and final approval of work. AR, EH, and SC: drafting/editing manuscript and final approval of work. EL: conception and design of study, analysis and interpretation of data, drafting/editing manuscript, and final approval of work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Neurological Diseases and Stroke (NINDS R01NS086736).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.581703/full#supplementary-material
References
1. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. (2010) 25:534–41. doi: 10.1002/mds.22838
2. Benito-Leon J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. (2008) 31:191–2. doi: 10.1159/000154933
3. Louis ED. Essential tremor as a neuropsychiatric disorder. J Neurol Sci. (2010) 289:144–8. doi: 10.1016/j.jns.2009.08.029
4. Jhunjhunwala K, Pal PK. The non-motor features of essential tremor: a primary disease feature or just a secondary phenomenon? Tremor Other Hyperkinet Mov. (2014) 4:255. doi: 10.5334/tohm.230
5. Bermejo-Pareja F, Puertas-Martin V. Cognitive features of essential tremor: a review of the clinical aspects and possible mechanistic underpinnings. Tremor Other Hyperkinetic Mov. (2012) 2:106. doi: 10.5334/tohm.106
6. Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. (1994) 9:193–6. doi: 10.1002/mds.870090212
7. Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain. (2001) 124:2278–86. doi: 10.1093/brain/124.11.2278
8. Rao AK, Louis ED. Ataxic gait in essential tremor: a disease-associated feature? Tremor Other Hyperkinetic Mov. (2019) 9:507. doi: 10.5334/tohm.507
9. Benito-Leon J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES). J Alzheimer Dis. (2011) 23:727–35. doi: 10.3233/JAD-2011-101572
10. Benito-Leon J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. (2006) 66:1500–5. doi: 10.1212/01.wnl.0000216134.88617.de
11. Sengul Y, Sengul HS, Yucekaya SK, Yucel S, Bakim B, Pazarci NK, et al. Cognitive functions, fatigue, depression, anxiety, and sleep disturbances: assessment of nonmotor features in young patients with essential tremor. Acta Neurol Belgica. (2015) 115:281–7. doi: 10.1007/s13760-014-0396-6
12. Gerbin M, Viner AS, Louis ED. Sleep in essential tremor: a comparison with normal controls and Parkinson's disease patients. Parkinsonism Relat Disord. (2012) 18:279–84. doi: 10.1016/j.parkreldis.2011.11.004
13. Monin JK, Gutierrez J, Kellner S, Morgan S, Collins K, Rohl B, et al. Psychological suffering in essential tremor: a study of patients and those who are close to them. Tremor Other Hyperkinetic Mov. (2017) 7:526. doi: 10.5334/tohm.338
14. Rao AK, Gilman A, Louis ED. Balance confidence and falls in nondemented essential tremor patients: the role of cognition. Archiv Phys Med Rehabil. (2014) 95:1832–7. doi: 10.1016/j.apmr.2014.04.001
15. Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait Posture. (2012) 35:43–7. doi: 10.1016/j.gaitpost.2011.08.002
16. Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinetic Mov. (2013) 3:138. doi: 10.5334/tohm.138
17. Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord. (2010) 25:1633–8. doi: 10.1002/mds.23144
18. Louis ED, Kellner S, Morgan S, Collins K, Rohl B, Huey ED, et al. Cognitive dysfunction is associated with greater imbalance and falls in essential tremor. Front Neurol. (2017) 8:154. doi: 10.3389/fneur.2017.00154
19. Louis ED, Rao AK. Tandem gait performance in essential tremor patients correlates with cognitive function. Cerebellum Ataxias. (2014) 1:19. doi: 10.1186/s40673-014-0019-2
20. Cinar N, Sahin S, Okluoglu Onay T, Karsidag S. Balance in essential tremor during tandem gait: is the first mis-step an important finding? J Clin Neurosci. (2013) 20:1433–7. doi: 10.1016/j.jocn.2013.01.013
21. Sverdrup K, Bergh S, Selbæk G, Røen I, Kirkevold Ø, Tangen GG. Mobility and cognition at admission to the nursing home—a cross-sectional study. BMC Geriatrics. (2018) 18:30. doi: 10.1186/s12877-018-0724-4
22. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. (2008) 23:329–42. doi: 10.1002/mds.21720
23. Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol Ser A Biol Sci Med Sci. (2013) 68:726–32. doi: 10.1093/gerona/gls224
24. Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. (2002) 16:1–14. doi: 10.1016/S0966-6362(01)00156-4
25. Chastan N, Bair WN, Resnick SM, Studenski SA, Decker LM. Prediagnostic markers of idiopathic Parkinson's disease: gait, visuospatial ability and executive function. Gait Posture. (2019) 68:500–5. doi: 10.1016/j.gaitpost.2018.12.039
26. Tyagi S, Perera S, Brach JS. Balance and mobility in community-dwelling older adults: effect of daytime sleepiness. J Am Geriatr Soc. (2017) 65:1019–25. doi: 10.1111/jgs.14735
27. Weiss A, Mirelman A, Buchman AS, Bennett DA, Hausdorff JM. Using a body-fixed sensor to identify subclinical gait difficulties in older adults with IADL disability: maximizing the output of the timed up and go. PLoS ONE. (2013) 8:e68885. doi: 10.1371/journal.pone.0068885
28. Kincses P, Kovács N, Karádi K, Feldmann A, Dorn K, Aschermann Z, et al. Association of gait characteristics and depression in patients with parkinson's disease assessed in goal-directed locomotion task. Parkinson's Dis. (2017) 2017:6434689. doi: 10.1155/2017/6434689
29. Avanzino L, Lagravinese G, Abbruzzese G, Pelosin E. Relationships between gait and emotion in Parkinson's disease: a narrative review. Gait Posture. (2018) 65:57–64. doi: 10.1016/j.gaitpost.2018.06.171
30. Klebe S, Stolze H, Grensing K, Volkmann J, Wenzelburger R, Deuschl G. Influence of alcohol on gait in patients with essential tremor. Neurology. (2005) 65:96–101. doi: 10.1212/01.wnl.0000167550.97413.1f
31. Cersonsky TEK, Kellner S, Chapman S, Huey ED, Louis ED, Cosentino S. Profiles of normal cognition in essential tremor. J Int Neuropsychol Soc. (2020) 26:197–209. doi: 10.1017/S1355617719001140
32. Cersonsky TEK, Morgan S, Kellner S, Robakis D, Liu X, Huey ED, et al. Evaluating mild cognitive impairment in essential tremor: how many and which neuropsychological tests? J Int Neuropsychol Soc. (2018) 24:1084–98. doi: 10.1017/S1355617718000747
33. Johns MW. Epworth Sleepiness Scale. (2020). Available online at: http://epworthsleepinessscale.com (accessed April 17, 2020).
34. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
35. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
36. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatric Res. (1982) 17:37–49. doi: 10.1016/0022-3956(82)90033-4
37. Spitzer RL KK, Williams BW, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
38. Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. (1997) 16:124–33. doi: 10.1159/000109681
39. Louis ED, Wendt KJ, Albert SM, Pullman SL, Yu Q, Andrews H. Validity of a performance-based test of function in essential tremor. Arch Neurol. (1999) 56:841–6. doi: 10.1001/archneur.56.7.841
40. Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. (2009) 24:626–7. doi: 10.1002/mds.22370
41. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
42. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatrics Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
43. Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R). San Antonio: The Psychological Corporation. (1981).
44. Smith A. Symbol Digit Modalities Test. Los Angekes: Western Psychological Services (1973). doi: 10.1037/t27513-000
45. Delis DC, Kaplan E, Kramer JH, Delis D, Kramer J. Delis-Kaplan executive function system (D-KEFS) Examiner's manual. Washington, DC: American Psychological Association (2001). doi: 10.1037/t15082-000
47. Benton AL, deS K, Sivan AB. Multilingual aphasia examination. AJA Associates. Iowa City, IA (1994).
48. Delis DC. California Verbal Learning Test, Adult Version. San Antonio, TX: Psychological Corporation (2000).
49. Wechsler D. Wechsler Memory Scale. Washington, DC: American Psychological Association (1945). doi: 10.1037/t27207-000
50. Wechsler D. Wechsler memory scale-revised. Psychological Corporation. Washington, DC: American Psychological Association (1987).
51. Benton AL, Abigail B, Sivan AB, Hamsher KD, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press (1994).
52. Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Trans Am Neurol Assoc. (1968) 93:38–42.
53. Rohl B, Collins K, Morgan S, Cosentino S, Huey ED, Louis ED. Daytime sleepiness and nighttime sleep quality across the full spectrum of cognitive presentations in essential tremor. J Neurol Sci. (2016) 371:24–31. doi: 10.1016/j.jns.2016.10.006
54. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
55. Woolf C, Slavin MJ, Draper B, Thomassen F, Kochan NK, Reppermund S, et al. Can the clinical dementia rating scale identify mild cognitive impairment and predict cognitive and functional decline? Dementia Geriatric Cogn Disord. (2016) 41:292–302. doi: 10.1159/000447057
56. Hubble JP, Busenbark KL, Pahwa R, Lyons K, Koller WC. Clinical expression of essential tremor: effects of gender and age. Mov Disord. (1997) 12:969–72. doi: 10.1002/mds.870120620
57. Schepens S, Goldberg A, Wallace M. The short version of the Activities-specific Balance Confidence (ABC) scale: its validity, reliability, and relationship to balance impairment and falls in older adults. Arch Gerontol Geriatr. (2010) 51:9–12. doi: 10.1016/j.archger.2009.06.003
58. Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture. (2011) 34:65–70. doi: 10.1016/j.gaitpost.2011.03.013
59. Louis ED, Rao AK. Functional aspects of gait in essential tremor: a comparison with age-matched Parkinson's disease cases, dystonia cases, and controls. Tremor Other Hyperkinetic Mov. (2015) 5:242. doi: 10.5334/tohm.242
60. Rao AK, Uddin J, Gillman A, Louis ED. Cognitive motor interference during dual-task gait in essential tremor. Gait Posture. (2013) 38:403–9. doi: 10.1016/j.gaitpost.2013.01.006
61. Sixt E, Landahl S. Postural disturbances in a 75-years-old population: i. prevalence and functional consequences. Age Ageing. (1987) 16:393–8. doi: 10.1093/ageing/16.6.393
62. Gerson LW, Jarjoura D, McCORD G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. (1989) 18:31–4. doi: 10.1093/ageing/18.1.31
Keywords: Essential Tremor, longitudinal, gait, balance, clinical
Citation: Dowd H, Zdrodowska MA, Radler KH, Cersonsky TEK, Rao AK, Huey ED, Cosentino S and Louis ED (2020) Prospective Longitudinal Study of Gait and Balance in a Cohort of Elderly Essential Tremor Patients. Front. Neurol. 11:581703. doi: 10.3389/fneur.2020.581703
Received: 09 July 2020; Accepted: 08 October 2020;
Published: 13 November 2020.
Edited by:
Sanjay Pandey, University of Delhi, IndiaReviewed by:
Shweta Prasad, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaSoumya Sharma, London Health Sciences Center, Canada
Copyright © 2020 Dowd, Zdrodowska, Radler, Cersonsky, Rao, Huey, Cosentino and Louis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elan D. Louis, ZWxhbi5sb3Vpc0BVVFNvdXRod2VzdGVybi5lZHU=
 Hollie Dowd1
Hollie Dowd1 Ashwini K. Rao
Ashwini K. Rao Elan D. Louis
Elan D. Louis