- 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 2Xiangya School of Medicine, Central South University, Changsha, China
- 3Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
- 4Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, TX, United States
- 5National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: Rapid eye movement sleep behavior disorder (RBD) is thought to be a prodromal symptom of Parkinson's disease (PD). RBD is also thought to be involved in cognitive decline and dementia in PD. In PD, although the relationship between RBD and cognitive dysfunctions was confirmed by considerable studies, whether RBD was associated with distinct types of cognitive defects is worth of study.
Objectives: This systematic review summarizes the evidence relating to cognitive dysfunction in PD patients with RBD (PD-RBD) and those without and explores their specificity to cognitive domains.
Methods: A meta-analysis using a random-effects model was performed for 16 different cognitive domains, including global cognitive function, memory (long-term verbal recall, long-term verbal recognition, long-term visual recall, short-term spatial recall, and short-term verbal recall), executive function (general, fluid reasoning, generativity, shifting, inhibition, and updating), language, processing speed/complex attention/working memory, visuospatial/constructional ability, and psychomotor ability. The cognitive difference between the groups of patients was measured as a standardized mean difference (SMD, Cohen's d). PD-RBD patients were classified into Confirmed-RBD (definite diagnosis with polysomnography, PSG) and Probable-RBD (without PSG re-confirmation). In some domains, RBD patients could not be analyzed separately due to the exiguity of primary studies; this analysis refers to such RBD patients as “Mixed-RBD.”
Results: Thirty-nine studies with 6,695 PD subjects were finally included. Confirmed-RBD patients showed worse performance than those without in global cognitive function, long-term verbal recall, long-term verbal recognition, generativity, inhibition, shifting, language, and visuospatial/constructional ability; Probable-RBD, in global cognitive function and shifting; and Mixed-RBD, in long-term visual recall, short-term spatial recall, general executive function, and processing speed/complex attention/working memory.
Conclusion: This meta-analysis strongly suggests a relationship between RBD, Confirmed-RBD in particular, and cognitive dysfunctions in PD patients. Early and routine screening by sensitive and targeted cognitive tasks is necessary for all PD-RBD patients because it may offer the therapeutic time window before they evolve to irreversible dementia.
Introduction
Rapid eye movement sleep behavior disorder (RBD) is characterized by loss of the normal skeletal muscle atonia during rapid eye movement (REM) sleep, such that patients appear to act out the content of their dreams (1). Based on the third edition of the International Classification of Sleep Disorders (ICSD), the criteria for RBD were: (1) repeated episodes of behavior or vocalization that are either recorded by polysomnography (PSG) to arise from REM or are presumed to arise from REM based on reports of dream enactment and (2) evidence of REM sleep without atonia (RWA) on PSG (2). RBD has been linked to neurodegenerative pathology like Parkinson's disease (PD) (3). RBD was also identified as a key prodromal symptom of PD by the Movement Disorder Society (4). Approximately 75% of individuals who suffer from RBD progress to PD within 10 years (5, 6). Thus, the majority of patients manifesting RBD in sleep clinics are actually in the prodromal stages of PD.
Considerable studies suggested that RBD could be a key marker of a special subset of PD characterized by a non-tremor-dominant motor subtype or a kinetic-rigid motor phenotype (7–9) and symmetric disease (10, 11). RBD may also precede severe non-motor symptoms like increased autonomic dysfunction (12–15) and visual hallucinations (16–20). Of great interest, a concept— “RBD-PD phenotype” —was advanced. Poorer performance in memory, executive function (EF), and visuospatial abilities and a significantly greater risk of dementia were observed in PD patients who carry GBA gene mutations (21–23). GBA mutation carriers have a higher risk of developing probable RBD among PD patients (24). A study consisting 76 PD patients who were followed for an average of 4.5 years uncovered that the rate of deterioration was faster in the patients with RBD, mild cognitive impairment (MCI), and orthostatic hypotension at baseline (25). The similar conclusion that RBD is one of the most crucial prognosis determinants of PD was demonstrated by another follow-up study involving 421 PD patients for 32.8 ± 9.3 months (26). But two studies quarreled with this concept because neither significant gait disturbances and postural impairment nor specific worsening over time was observed in PD patients with RBD (27, 28).
Beyond these non-motor symptoms just mentioned, RBD usually predates, either by years or decades, the cognitive impairment or the diagnosis of MCI, which are the transitional states between normal aging and dementia in patients with PD (29, 30). Moreover, RBD increased the risk of cognitive decline and even dementia in PD patients (31–35). Considerable work reported an association between RBD and cognitive dysfunction and even dementia in PD [reviewed in (36)], but few suggested no significant declines in some targeted cognitive domains (37, 38) in PD patients with RBD.
The representative lineup includes RBD, “mild parkinsonian signs,” typical features of PD, PD with MCI, and a full dementia syndrome (PDD) (30), although not all of the patients follow this course of the disease. The endpoint of cognitive decline, PDD, is miserable and irreversible. These symptoms tend to appear in time intervals from months to years (39–41). The high conversion rate and the long latency of RBD to cognitive dysfunctions in PD patients make this study necessary and meaningful: cognitive dysfunction patients who suffer from both PD and RBD may be given opportunities for prevention and interventions before they progress to dementia.
Thus, this meta-analytic review was designed to shed light on the relationship between RBD and cognition in PD patients, as well as which cognitive domains are impaired. We also examined the influence of demographic and clinical confounders, like clinical stage, on cognitive performances in PD individuals.
Materials and Methods
Search Strategy
Consistent with PRISMA's suggestions (42), a systematic literature search was performed by two independent reviewers (JM and JY) up to 04 April 2020 using PsycInfo (PROQUEST), PubMed, Cochrane, and Embase. Searches were constructed using subtext headings and key words based on the following terms: RBD, cognitive impairment, and PD. For a detailed statement of the search, see Supplementary Table 1. The search was supplemented by hand searches of the reference lists cited in the original articles and review papers.
Study Eligibility Criteria
Inclusion Criteria
This systematic review included studies investigating the effects of RBD in patients with PD on cognitive functions, published in peer-reviewed journals in English. Participants needed to be adults diagnosed with idiopathic PD based on any established international clinical criteria (43–47). RBD should ideally be diagnosed with PSG, while validated questionnaires or targeted interviews were also acceptable. The comparison had to be performed between parkinsonians with and without RBD (PD-RBD and PD-NRBD, respectively). Moreover, only studies assessing cognitive domains through standardized tests were included and the results had to be reported as the mean and standard deviation (SD) or the corresponding original data to allow the calculation of these values. When more than one study was published by the same authors, we checked the independence of samples or used the study with the largest sample size.
Exclusion Criteria
Proceedings, commentaries, letters to the editor, theses, studies performed on animals, and single case studies were all unacceptable. Studies that recruited atypical PD or parkinsonian syndromes were excluded. Cognition measured by subjective report or ratings-based methods or did not report the performance data for each cognitive task were discarded. Studies that concentrated on cognitive functions without linking them directly to RBD in PD or reported a comparison between PD patients and healthy participants were also unacceptable.
Outcomes
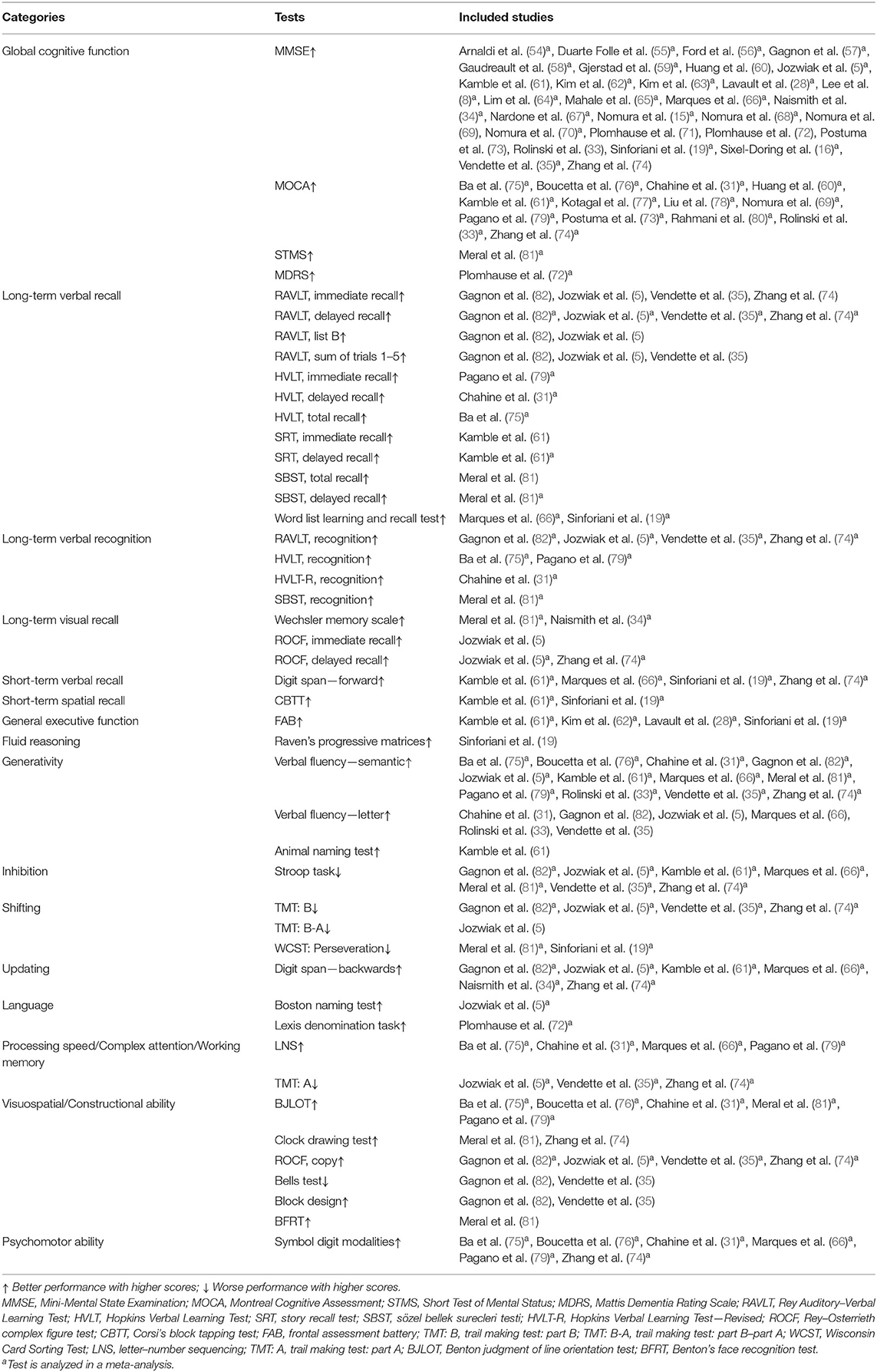
For each study, the primary outcomes were cognitive test scores. Our main objective was to meta-analyze these scores to determine whether RBD in PD was associated with distinct types of cognitive defects. We categorized the cognitive tests following the approach described by Litvan et al. (48), Wallace and Bucks (49), and Olaithe and Bucks (50) or the indication provided in the primary studies. Subsequently, seven major cognitive categories were analyzed to organize the findings of this meta-analysis: global cognitive function, memory, EF, language, processing speed/complex attention/working memory, visuospatial/constructional ability, and psychomotor ability. Memory was further divided into long-term verbal recall, long-term verbal recognition, long-term visual recall, short-term spatial recall, and short-term verbal recall. EF was subdivided into the following: general EF, fluid reasoning, generativity, inhibition, shifting, and updating. Therefore, in total, 16 cognitive domains were compartmentalized, and the following analyses were carried out, respectively, in these segmentations. With several previous meta-analyses available for consultation (51–53) or the instructions of the included tests, we categorized the cognitive tests and listed them in Table 1.
Cognitive domains assessed by only one study could not be included. When a cognitive function was explored by more than one test in a primary study, two different strategies were adopted by previous meta-analyses: some extracted data from the most sensitive and relevant instrument (52, 53), while some aggregated the results into a single effect size (ES) (83–85). These two strategies are both valid and have their own advantages; the first solution diminishes the risk of type II errors, while the second strategy decreases bias of a certain test. We decided to follow the first solution. As for the criteria for the “most sensitive and relevant instrument,” the sensitivity and relevance in the PD population of each test were checked on the basis of already-published research first, and preference was given to the highest sensitivity and/or relevant test if more than one test assessing the same cognitive domain were adopted in a primary study. If the sensitivity and/or relevance was not available, the most used test was analyzed in this domain. Thus, sensitivity, relevance, and popularity, in this order, are what we considered in choosing assessable tests. This criterion was consistently used across all domains in this meta-analysis.
Data Extraction and Coding
Data extracted and coded from the primary studies included: (1) characters of the publication (e.g., authors and year of publication); (2) diagnoses of PD and RBD; (3) characteristics of the sample [e.g., sample size, gender, age at evaluation, disease duration, education, severity of motor symptoms evaluated by the Unified Parkinson's Disease Rating Scale (UPDRS-III), stage of PD evaluated by Hoehn and Yahr (H&Y), and the levodopa equivalent daily dose (LEDD)]; and (4) cognitive tests.
Statistical Analysis
All statistical analyses were performed using RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and Stata/SE version 15 (StataCorp, College Station, TX, USA).
SMD was used as the outcome measure because, although the included studies all assessed the same cognitive function within one meta-analysis, different cognitive tests were employed. ESs were categorized using Cohen's d as 0.2, indicating a small effect, 0.5, a medium, and 0.8, large. When calculating ESs, the PD-NRBD scores were always subtracted from the PD-RBD scores. Cognitive tests broadly fit into two categories: one where higher scores indicate better performance, namely milder damage, and the other where higher scores conversely represent greater impairment. A negative ES in the former tests indicates that the PD-RBD participants were more impaired than the PD-NRBD participants, as opposed to the latter cases. Random-effects models were applied to all cognitive domains.
The methodological quality of the enrolled cohort and case–control studies was evaluated with the Newcastle–Ottawa Scale (86) and the cross-sectional studies with the modified Newcastle–Ottawa Scale (87). Reports that scored ≥6 points were considered to be of good quality. The quality assessment was performed independently by two authors (JM and JY) and disagreements were resolved by discussion.
Prior defined subgroup analyses were performed based on whether the diagnosis of RBD was confirmed by PSG. Hence, studies were placed in the “Confirmed-RBD” subgroup if the RBD patients met the ICSD criteria where PSG is mandatory. Conversely, studies were placed in the “Probable-RBD” subgroup if the diagnosis was made by questionnaires and/or interviews. In some domains, RBD patients could not be analyzed separately due to the exiguity of the primary studies in which they were enrolled, and they were referred to as “Mixed-RBD.” Concretely, in a certain domain, only one primary study used PSG to confirm RBD; therefore, patients from this study were Confirmed-RBD patients. Meanwhile, the patients included in the other studies were Probable-RBD patients not confirmed by PSG. Since one study could not be meta-analyzed, we combined and analyzed the results of all RBD patients, for both Confirmed-RBD and Probable-RBD, denoted as “Mixed-RBD.”
Another subgroup analysis was performed considering the possible differential effects of clonazepam, the major treatment for RBD, which may deteriorate cognitive dysfunctions (88–90). Therefore, studies were placed in either the “Mediated by Clonazepam” subgroup if medicated patients were recruited or “Unmediated by Clonazepam” subgroup when mediated patients were excluded or the dose they were taking was negligible.
Meta-regressions were performed to investigate whether the outcomes were affected by other characteristics, including demographic characteristics (age at evaluation, gender, and education), severity of PD (PD duration, UPDRS-III, H&Y stage, and LEDD), cognitive tests, and tools used to assess RBD. These covariates were meta-regressed individually in a random-effects meta-regression model. According to Borenstein et al. (91), a meta-regression could be generally conducted for outcomes where there are 10 samples at a minimum to one covariate. But given that the majority of domains included <10 reports, with reference to Taylor et al. (92), we liberalized the restriction to five.
The heterogeneity test was quantified using the I2 statistic. The I2 was set as low (25%), moderate (50%), or high (75%). Sensitivity analysis was conducted for meta-analysis where I2 ≥ 50% by omitting the enrolled studies, one at a time, to determine the effect of any individual study on the synthesized ES and between-study heterogeneity. Finally, publication bias analysis was performed with the funnel plot of which the asymmetry was further statistically confirmed by the Egger's regression method and the trim-and-fill procedure in the meta-analyses that included ≥10 studies.
All statistical tests were two-tailed, and P < 0.05 was considered significant.
Results
Study Selection and Risk of Bias
A total of 482 papers were produced according to our search strategy, and 15 additional records were identified from the references cited in the original articles and review papers. Following exclusion of duplicates and unrelated studies based on title and abstract screening, we retrieved 139 papers for full-text evaluation. The PRISMA flow diagram (Figure 1) summarizes the selection process. In total, 39 studies were enrolled after rigorous screening (5, 8, 15, 16, 19, 28, 31, 33–35, 54–82). A critical appraisal assessment found that all studies exhibited “good quality,” with the score ranging from 6 to 9, and no studies were excluded due to quality issues (Supplementary Tables 2, 3).
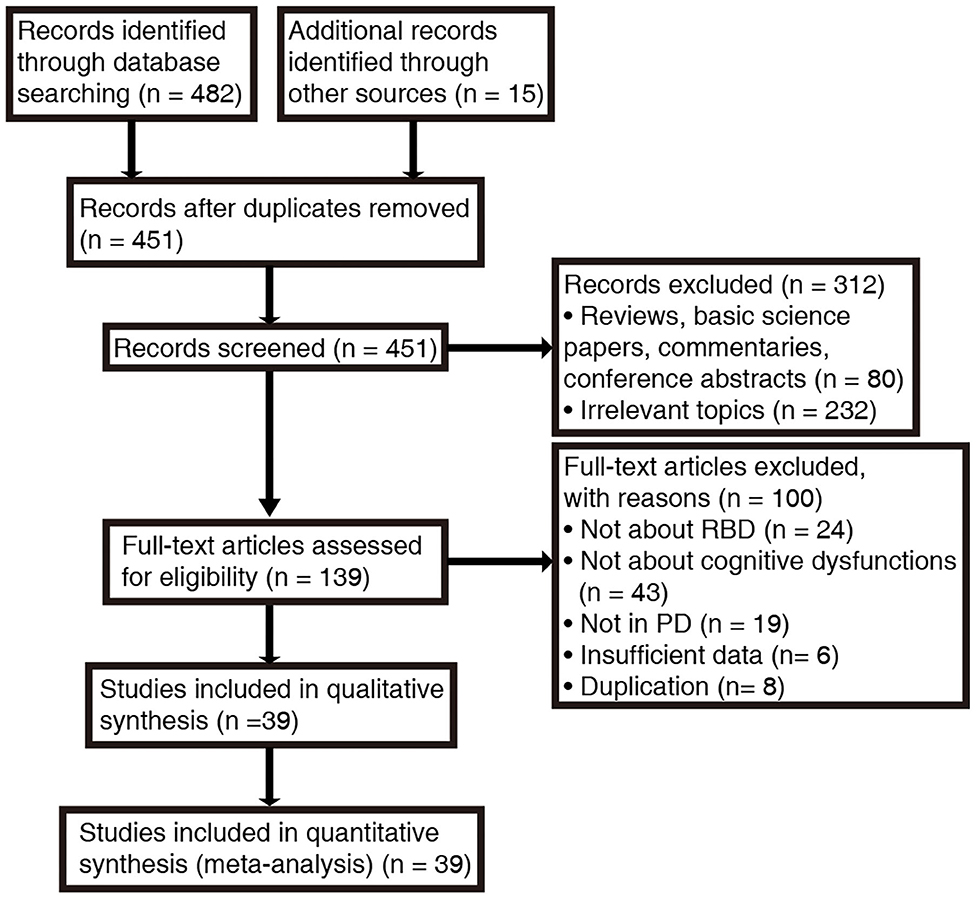
Figure 1. Search procedure and results according to PRISMA guidelines. RBD, rapid eye movement sleep behavior disorder; PD, Parkinson's disease.
Characteristics of the Included Studies
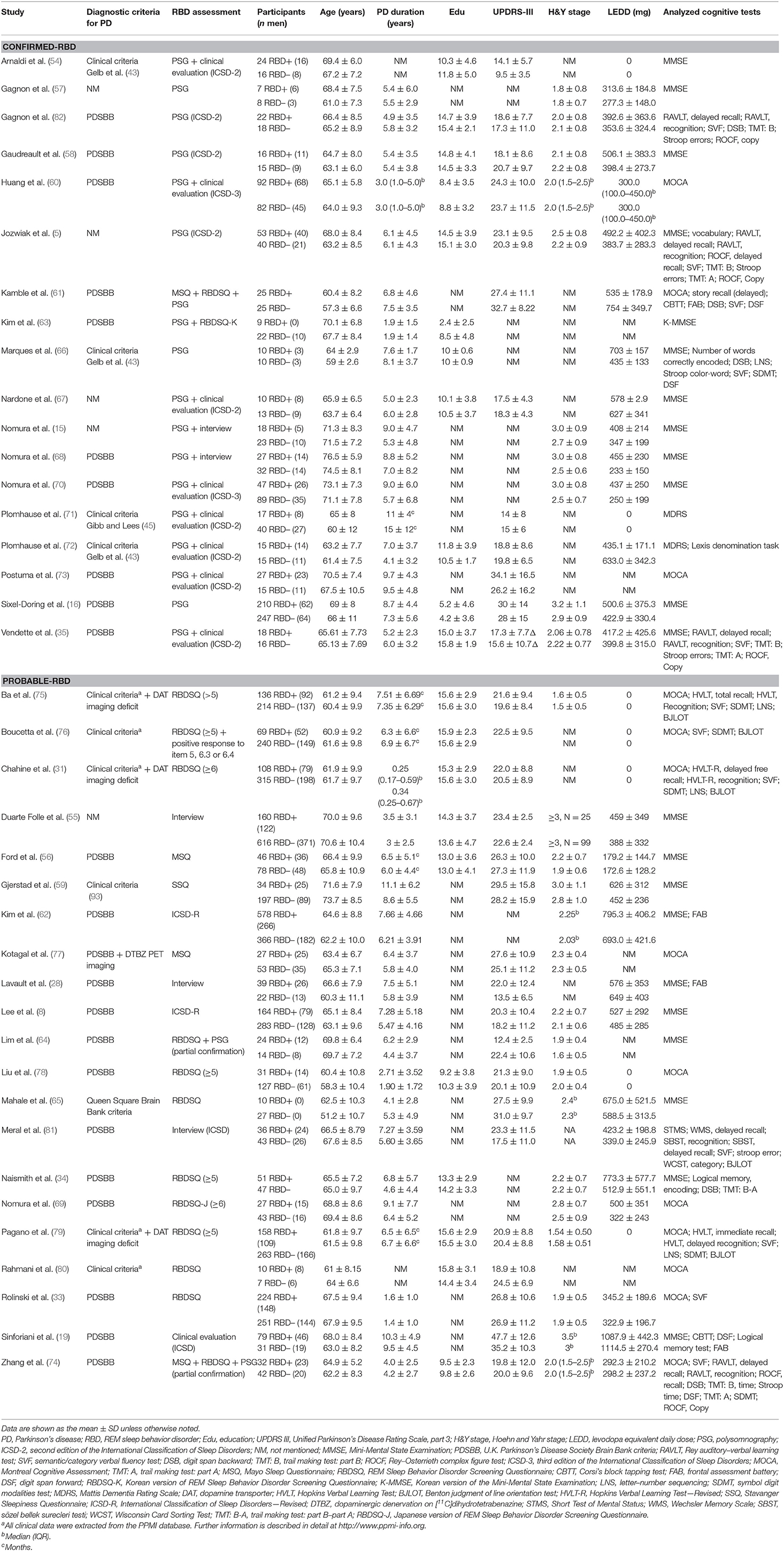
The characteristics of the included studies are summarized in Table 2. Across these 39 studies, 6,695 individuals with PD were investigated, with the mean age ranging from 57.3 to 76.5 years. The mean UPDRS-III scores were provided in 30 studies, while either the mean or median H&Y stage values were reported in 25. Two studies (63, 66) did not provide either measure of motor symptoms or disease stage for the entire sample. PSG was used in 18 studies alone or combined with the clinical interview (5, 15, 16, 35, 54, 57, 58, 60, 61, 63, 66–68, 70–73, 82). The remainder adopted sleep questionnaires and/or clinical interviews to identify Probable-RBD. Ten studies (16, 33, 57–59, 61, 69, 70, 78, 82) particularly pointed out the utilization of clonazepam.
Some reports (5, 35, 54, 58, 64, 66, 72, 74, 76, 82) also recruited idiopathic RBD patients and/or health controls in addition to our targeted individuals and did comparisons between any two, but we only extracted the statistics from PD-RBD and PD-NRBD patients. Moreover, PD-RBD patients were further classified into subgroups in four studies: clinical or subclinical PD-RBD (15, 68) and PD-RBD with and without visual hallucinations (VH) (19, 81). In the former two studies (15, 68), patients with both RWA on PSG and RBD symptoms were classified as the “RBD group”; patients who only manifested RWA without RBD symptoms were categorized as the “Subclinical RBD group.” Given that the rest of the reports did not recruit or subgroup the subclinical RBD patients, we only extracted data from the “RBD group” and the “NRBD group” and dropped the data from the “Subclinical RBD group.” The latter two studies (19, 81) examined VH besides RBD. Because VH was not the outcome of interest of this review, the groups were collapsed into RBD+ and RBD–.
Meta-Analytic Results
Global Cognitive Function
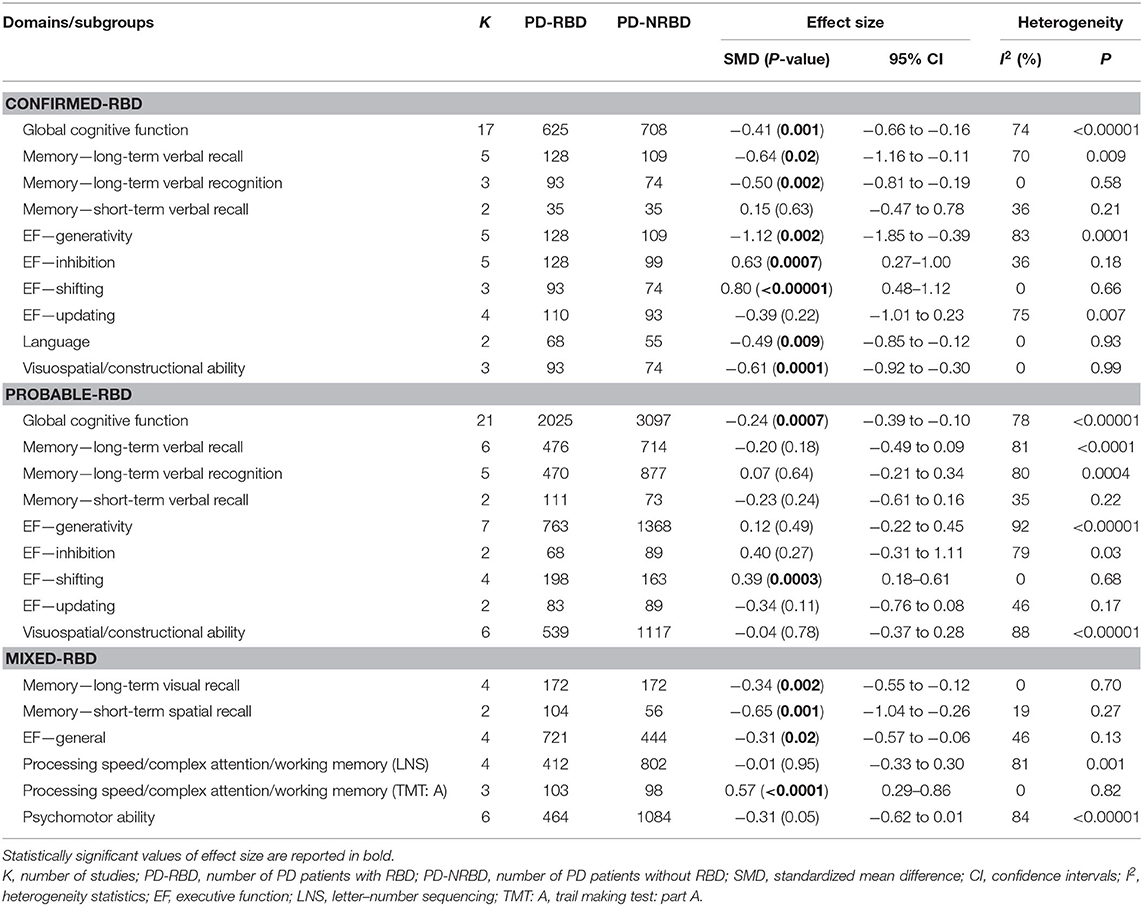
The meta-analysis included 17 “Confirmed-RBD” and 21 “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly lower scores than PD-NRBD patients, with a medium ES (SMD = −0.41, 95% CI = −0.66 to −0.16, P = 0.001); heterogeneity was moderate (I2 = 74%). For the “Probable-RBD” subgroup, PD-RBD patients also had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.24, 95% CI = −0.39 to −0.10, P = 0.0007); heterogeneity was high (I2 = 78%). No significant difference between these two subgroups was observed (Table 3, Figure 2).
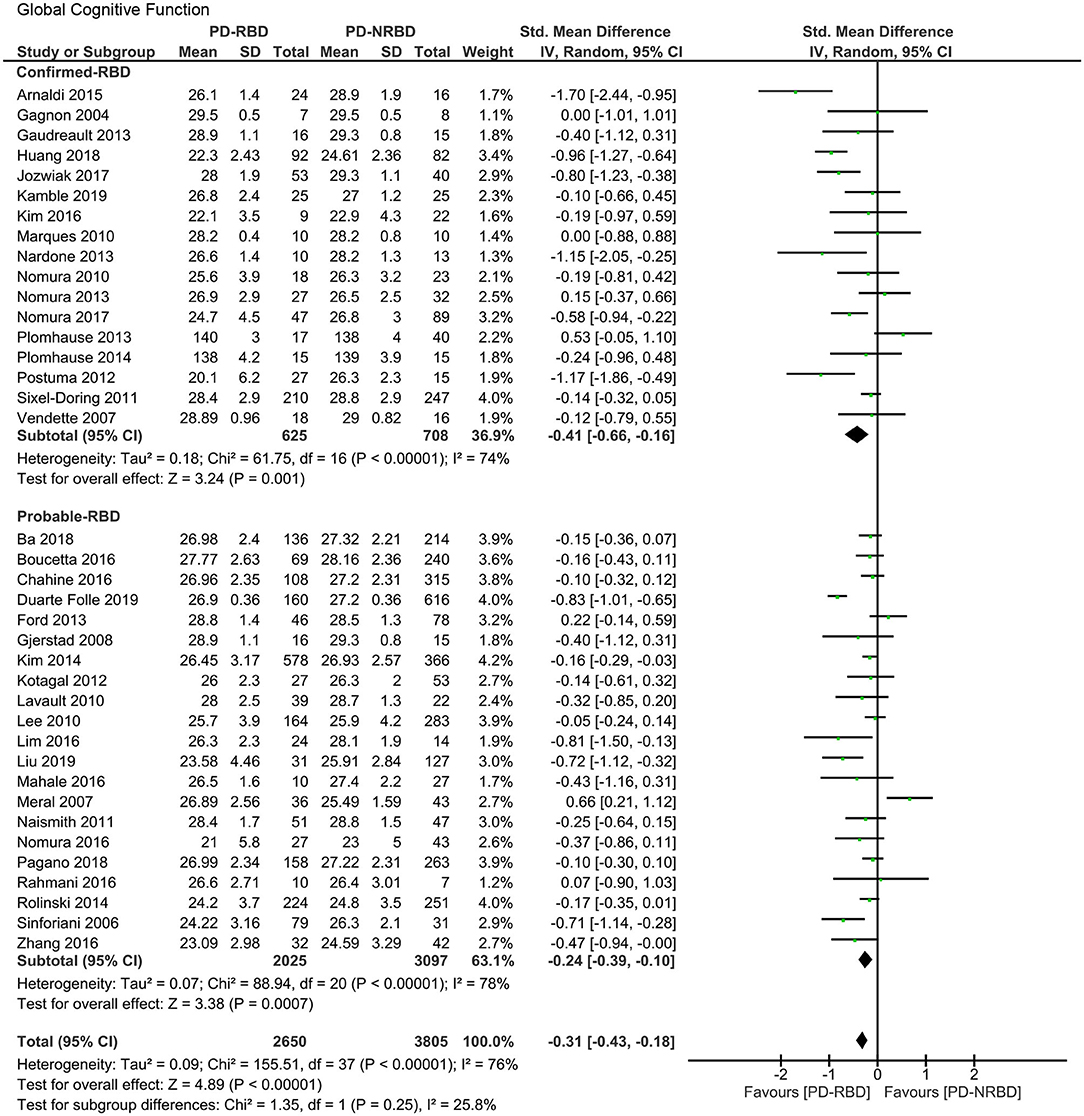
Figure 2. Forest plot for global cognitive function with subtotals by the diagnosis of rapid eye movement sleep behavior disorder (RBD) displaying the effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
Global cognitive function is the only domain where the second subgroup analysis could be performed. The meta-analysis included six “Mediated by Clonazepam” studies and three “Unmediated by Clonazepam” studies. For the “Mediated by Clonazepam” subgroup, PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.31, 95% CI = −0.51 to −0.12, P = 0.001); heterogeneity was moderate (I2 = 56%). For the “Unmediated by Clonazepam” group, the ES was not significant. No significant difference between these two subgroups was observed (Supplementary Figure 1).
Memory—Long-Term Verbal Recall
The meta-analysis included five “Confirmed-RBD” and six “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.64, 95% CI = −1.16 to −0.11, P = 0.02); heterogeneity was moderate (I2 = 70%). For the “Probable-RBD” subgroup, the ES was not significant. No significant difference between these two subgroups was observed (Table 3, Figure 3A).
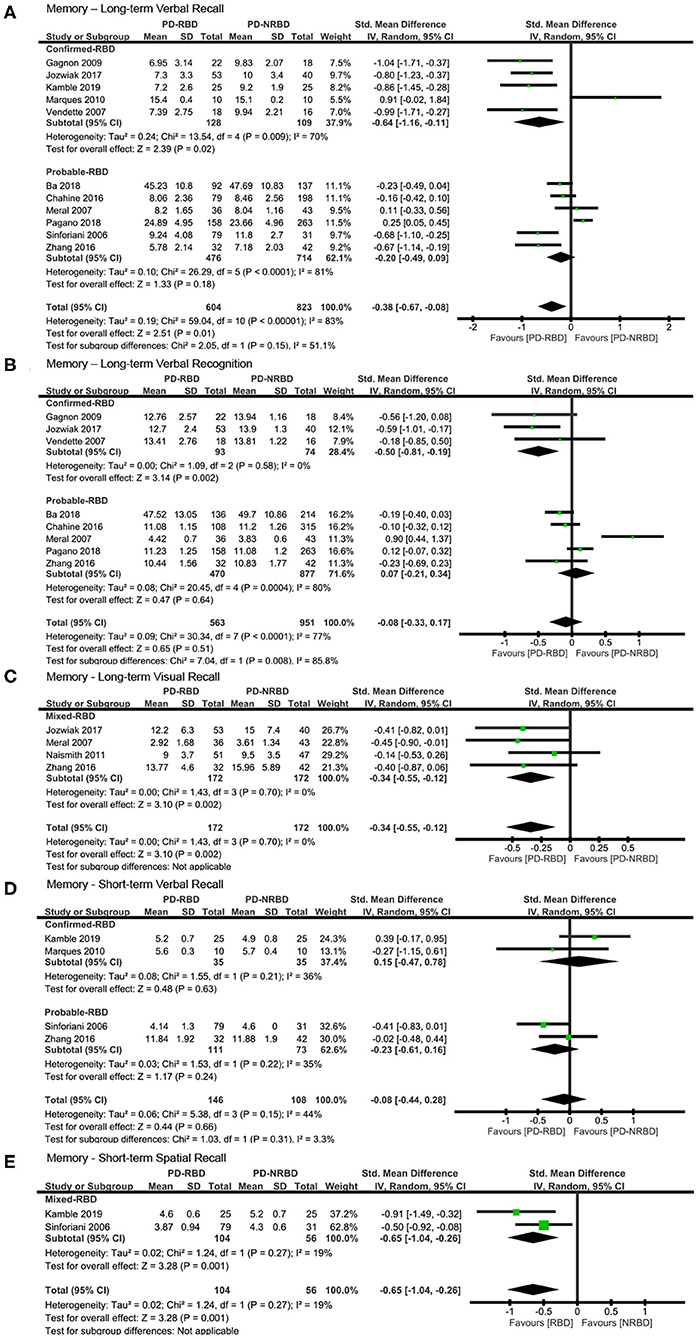
Figure 3. Forest plot for (A) long-term verbal recall with subtotals by the diagnosis of rapid eye movement sleep behavior disorder (RBD), (B) long-term verbal recognition with subtotals by the diagnosis of RBD, (C) long-term visual recall, (D) short-term verbal recall with subtotals by the diagnosis of RBD, and (E) short-term spatial recall displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
Memory—Long-Term Verbal Recognition
The meta-analysis included three “Confirmed-RBD” and five “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.50, 95% CI = −0.81 to −0.19, P = 0.002); heterogeneity was absent (I2 = 0%). For the “Probable-RBD” subgroup, the ES was not significant. The difference between these two subgroups was significant (P = 0.008) (Table 3, Figure 3B).
Memory—Long-Term Visual Recall
The meta-analysis included one “Confirmed-RBD” and three “Probable-RBD” primary studies. Given the exiguity of the primary studies in the “Confirmed-RBD” subgroup, we analyzed these two subgroups together. PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.34, 95% CI = −0.55 to −0.12, P = 0.002); heterogeneity was absent (I2 = 0%) (Table 3, Figure 3C).
Memory—Short-Term Verbal Recall
This meta-analysis included two “Confirmed-RBD” and two “Probable-RBD” studies. The ESs for both groups were insignificant (Table 3, Figure 3D).
Memory—Short-Term Spatial Recall
This meta-analysis included one “Confirmed-RBD” and one “Probable-RBD” study. Given the exiguity of the primary studies in both subgroups, we analyzed them together. PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.65, 95% CI = −1.04 to −0.26, P = 0.001); heterogeneity was absent (I2 = 19%) (Table 3, Figure 3E).
General EF
The meta-analysis included one “Confirmed-RBD” and three “Probable-RBD” studies. Given the exiguity of the primary studies in the “Confirmed-RBD” subgroup, we analyzed these two subgroups together. PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.31, 95% CI = −0.57 to −0.06, P = 0.02); heterogeneity was low (I2 = 46%) (Table 3, Figure 4A).
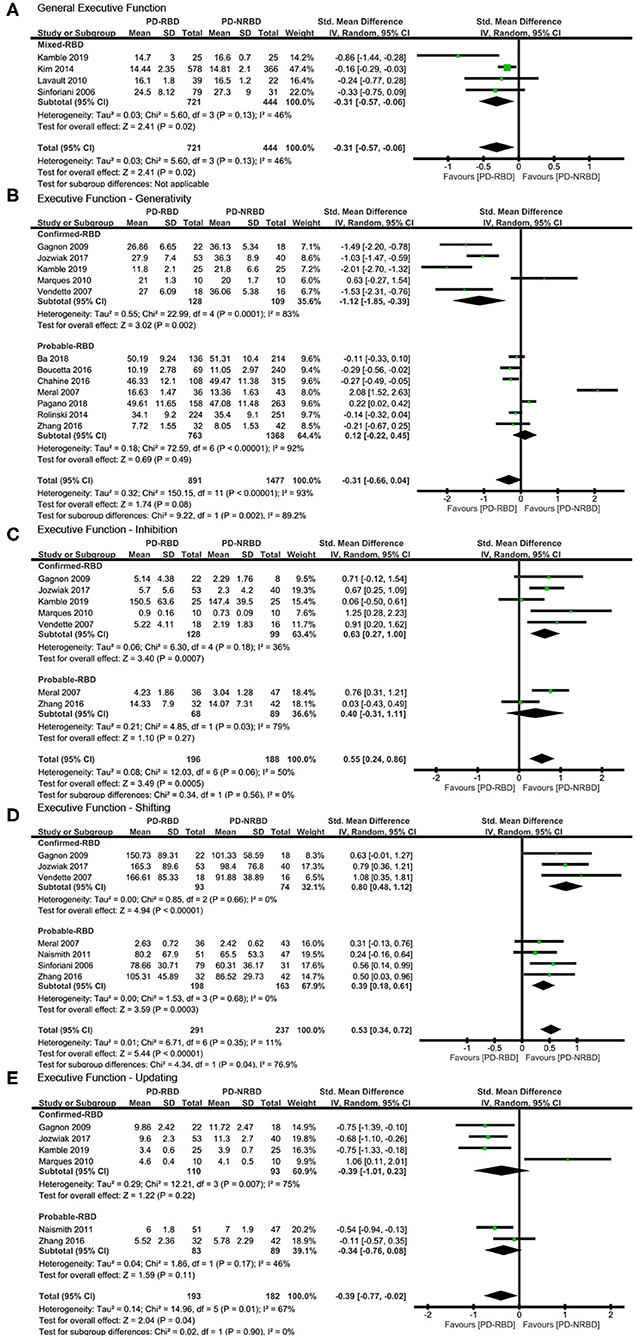
Figure 4. Forest plot for (A) general executive function (EF), (B) generativity with subtotals by the diagnosis of rapid eye movement sleep behavior disorder (RBD), (C) inhibition with subtotals by the diagnosis of RBD, (D) shifting with subtotals by the diagnosis of RBD, and (E) updating with subtotals by the diagnosis of RBD displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
EF—Fluid Reasoning
The meta-analytic study could not be performed due to the exiguity of the primary studies (n = 1). PD-RBD patients performed worse in this domain than did PD-RBD patients, according to the only primary study.
EF—Generativity
The meta-analysis included five “Confirmed-RBD” and seven “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a large ES (SMD = −1.12, 95% CI = −1.85 to −0.39, P = 0.002); heterogeneity was high (I2 = 83%). For the “Probable-RBD” subgroup, the ES was not significant. The difference between these two subgroups was significant (P = 0.002) (Table 3, Figure 4B).
EF—Inhibition
The meta-analysis included five “Confirmed-RBD” and two “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly higher scores than did PD-NRBD patients, with a medium ES (SMD = 0.63, 95% CI = 0.27–1.00, P = 0.0007); heterogeneity was low (I2 = 36%). For the “Probable-RBD” subgroup, the ES was not significant. No significant difference between these two subgroups was observed (Table 3, Figure 4C).
EF—Shifting
The meta-analysis included three “Confirmed-RBD” studies and four “Probable-RBD” primary studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly higher scores than did PD-NRBD patients, with a large ES (SMD = 0.80, 95% CI = 0.48–1.12, P < 0.00001); heterogeneity was absent (I2 = 0%). For the “Probable-RBD” subgroup, PD-RBD patients also had significantly higher scores than did PD-NRBD patients, with a medium ES (SMD = 0.39, 95% CI = 0.18–0.61, P = 0.0003); heterogeneity was absent (I2 = 0%). The difference between these two subgroups was significant (P = 0.04) (Table 3, Figure 4D).
EF—Updating
This meta-analysis included four “Confirmed-RBD” and two “Probable-RBD” studies. The ESs for both subgroups were insignificant (Table 3, Figure 4E).
Language
This meta-analysis only included two “Confirmed-RBD” studies. PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.49, 95% CI = −0.85 to −0.12, P = 0.009); heterogeneity was absent (I2 = 0%) (Table 3, Figure 5).
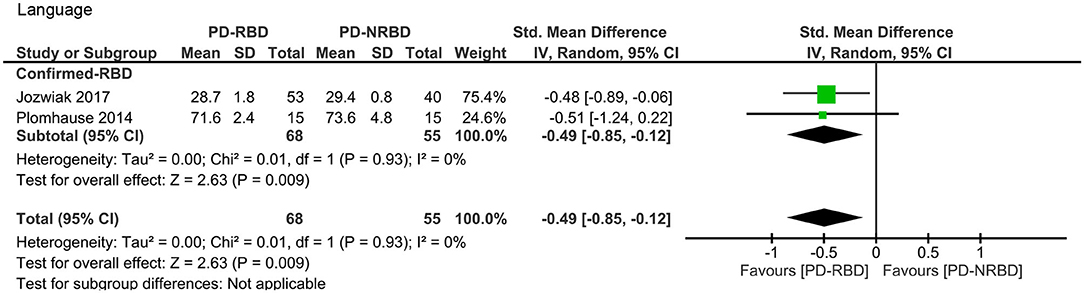
Figure 5. Forest plot for language with subtotals by the diagnosis of rapid eye movement sleep behavior disorder (RBD) displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
Processing Speed/Complex Attention/Working Memory
This domain was evaluated by seven studies in total, four of which used the letter–number sequence (LNS) and three adopted the trail making test, part A (TMT: A). Since these two tests could not be combined together, we analyzed them separately.
The meta-analysis focusing only on studies that used LNS scores included one “Confirmed-RBD” and three “Probable-RBD” primary studies. Given the exiguity of the primary studies in the “Confirmed-RBD” subgroup, we analyzed these two subgroups together. The ES was not significant (Table 3, Figure 6A).
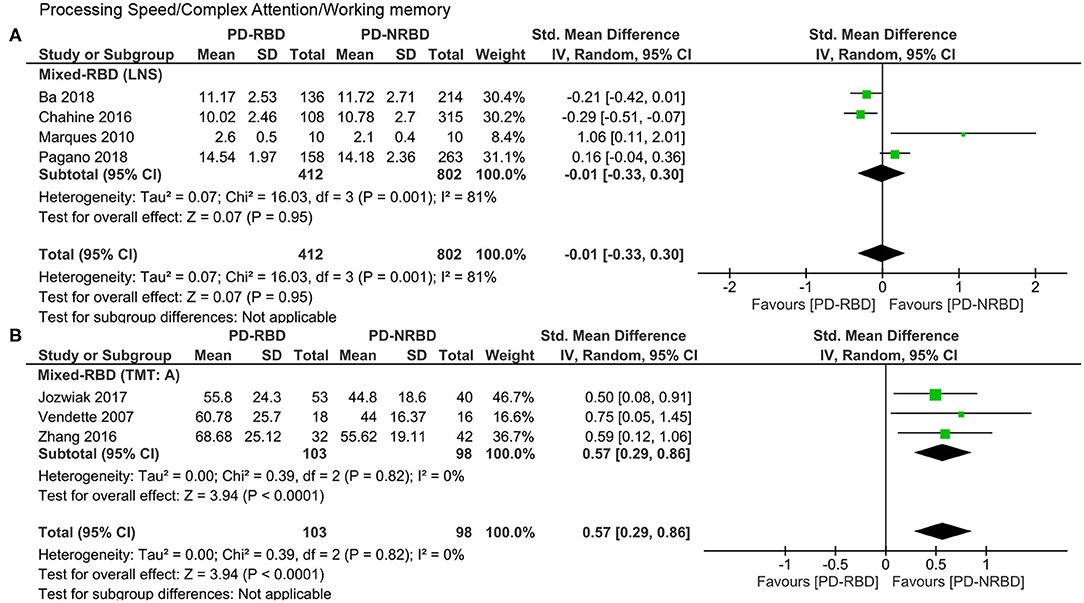
Figure 6. Forest plot for processing speed/complex attention/working memory evaluated by the (A) LNS and the (B) TMT: A displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval; LNS, letter–number sequence; TMT: A, trail making test, part A.
The meta-analysis focusing only on studies that used TMT: A scores included two “Confirmed-RBD” and one “Probable-RBD” primary studies. Given the exiguity of the primary studies in the “Probable-RBD” subgroup, we analyzed these two subgroups together. PD-RBD patients had significantly higher scores than did PD-NRBD patients, with a medium ES (SMD = 0.57, 95% CI = 0.29–0.86, P < 0.0001); heterogeneity was absent (I2 = 0%) (Table 3, Figure 6B).
Visuospatial and Constructional Ability
The meta-analysis included three “Confirmed-RBD” and six “Probable-RBD” studies. For the “Confirmed-RBD” subgroup, PD-RBD patients had significantly lower scores than did PD-NRBD patients, with a medium ES (SMD = −0.61, 95% CI = −0.92 to −0.30, P = 0.0001); heterogeneity was absent (I2 = 0%). For the “Probable-RBD” subgroup, the ES was not significant. The difference between these two subgroups was significant (P = 0.01) (Table 3, Figure 7).
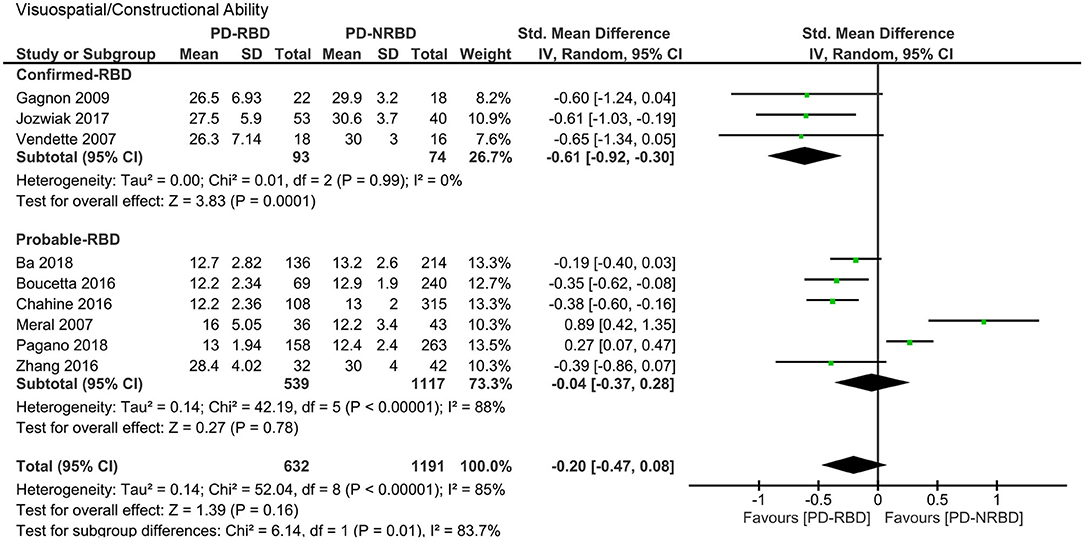
Figure 7. Forest plot for visuospatial/constructional ability with subtotals by the diagnosis of rapid eye movement sleep behavior disorder (RBD) displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
Psychomotor Ability
The meta-analysis included one “Confirmed-RBD” and five “Probable-RBD” primary studies. Given the exiguity of the primary studies in the “Confirmed-RBD” subgroup, we analyzed these two subgroups together. The ES was not significant (Table 3, Figure 8).
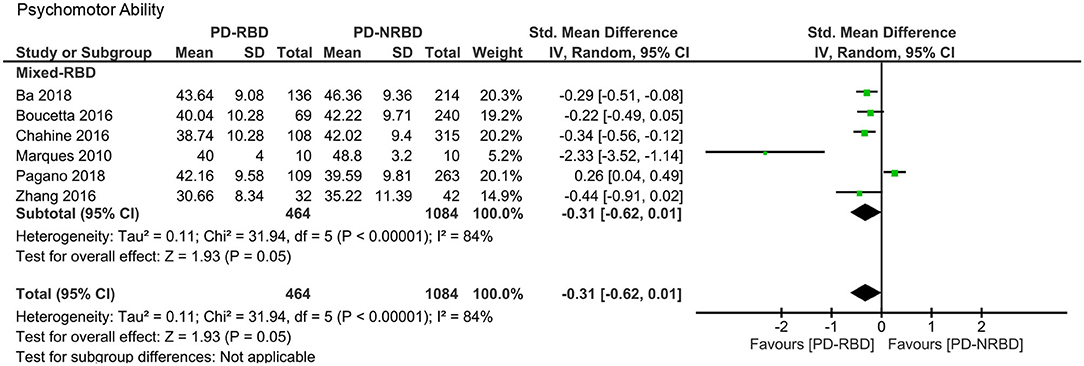
Figure 8. Forest plot for psychomotor ability displaying effect size calculated using a random-effects model. SD, standard deviation; Std. Mean Difference, standardized mean difference; CI, confidence interval.
Moderator Analysis
Meta-regression revealed that gender had a significant impact on the obtained ES for psychomotor ability (K = 6, β = 6.310, P = 0.001); PD duration for psychomotor ability (K = 6, β = −0.225, P = 0.005); H&Y for visuospatial/constructional ability (K = 6, β = −0.835, P = 0.033); LEDD for psychomotor ability (K = 6, β = −0.003, P = 0.005); cognitive test for global cognitive function (K = 38, β = 0.221, P = 0.041), long-term verbal recall (K = 11, β = 0.135, P = 0.039), long-term verbal recognition (K = 8, β = 0.336, P = 0.000), inhibition (K = 7, β = 0.558, P = 0.011), and visuospatial/constructional ability (K = 9, β = −0.576, P = 0.048); and RBD assessment for generativity (K = 12, β = 1.294, P = 0.013), shifting (K = 7, β = −0.417, P = 0.033), and psychomotor ability (K = 6, β = 2.239, P = 0.001). No other demographic and clinical factors manifested any significant effect on the ES for these outcomes. Finally, no other aspects had significant impact on the ES for the remaining outcomes (Supplementary Table 4).
Sensitivity Analysis
No obvious outliers were uncovered by the sensitivity analyses aiming at determining the effect of any individual study on the pooled ES, indicating the stability of the meta-analytic findings (Supplementary Figures 2–9).
Another sensitivity analysis identified that the study by Marques et al. (66) contributed dramatically to the heterogeneities in the “Confirmed-RBD” subgroups in the long-term verbal recall and updating domains, separately. After excluding this study, all heterogeneities plunged to 0%, and SMD decreased from −0.64 (95% CI = −1.16 to −0.11, P = 0.02) to −0.89 (95% CI = −1.17 to −0.61, P < 0.00001) in long-term verbal recall and from −0.39 (95% CI = −1.01 to 0.23, P = 0.22) to −0.71 (95% CI = −1.02 to −0.41, P < 0.00001) in updating. For the rest of the domains, excluding one single study did not change the heterogeneity dramatically.
Publication Bias
Global cognitive domain was the only domain where publication bias analysis could be performed, and the funnel plot for it suggested symmetry: Egger's test was insignificant and the trim-and-fill analysis did not remove any study for both the Confirmed and Probable-RBD subgroups.
Discussion
Summary of Findings
This study is the first systematic review and meta-analysis of the association between RBD and cognitive dysfunctions in patients with PD. This meta-analysis indicates that, relative to those without RBD, people with PD who were diagnosed with RBD, as confirmed or probable, demonstrate poorer cognitive performance that differs across cognitive domains. Specifically, Confirmed-RBD patients performed more poorly than those without RBD in global cognitive function, long-term verbal recall, long-term verbal recognition, generativity, inhibition, shifting, language, and visuospatial/constructional ability; Probable-RBD, in global cognitive function and shifting; and Mixed-RBD, in long-term visual recall, short-term spatial recall, general EF, and processing speed/complex attention/working memory that was evaluated by the TMT: A.
Our results put emphasis on PSG that provides objective statistics with which to compare subjective accounts in diagnosing RBD. Regarding the range and degree of cognitive damage, we found that Confirmed-RBD patients were more serious than did Probable-RBD patients when both were compared to PD-NRBD patients. Dream enactment behavior, the major diagnostic basis of Probable-RBD, is not specific for RBD (1, 94, 95), and presumably, Probable-RBD patients diagnosed accordingly are less generalizable to RBD patients. The results from our analyses clearly present the difference between patients with PSG-confirmed RBD and those with probable RBD based on subjective complaints. Although the gold standard for assessing RBD remains the laboratory PSG, there is heightened growing interest in home-based sleep monitoring by portable or wearable monitoring devices (96, 97). Identification of confirmed RBD cases will likely grow with advances in technology enabling home-based PSG assessment.
Clonazepam, the drug of choice in the treatment of RBD, was reported to deteriorate cognition, as noted above. Although PD-RBD patients treated with clonazepam performed significantly worse on global cognitive function compared to PD-NRBD patients while the unmediated group did not manifest any difference, the subgroup difference was statistically insignificant. This seeming contradiction needs to be further explored by large-sized investigations.
One sensitivity analysis revealed no outlier, and another identified that the results reported by Marques et al. (66) and Pagano et al. (79) contributed significantly to the heterogeneities found in the analysis of long-term verbal recall and updating. The investigations showed that the results of the tests were conflicting with the other studies in their respective domains and were statistically insignificant, which means that the PD-RBD patients performed insignificantly better than did PD-NRBD patients. These two primary studies did not mention the reason for these dissimilar results.
Some demographics and clinical phenomenology of RBD (30) and PD-RBD (25, 73) were identified, such as male gender, age at onset, and severity of PD. Therefore, these features could also affect the relationship between RBD and cognitive dysfunctions. Moderator analysis supported our hypothesis that the neuropsychological patterns of PD-RBD patients are dependent on some demographic and clinical aspects. Moreover, it also revealed the effects of evaluating cognition or RBD on some cognitive domains like global cognitive function. However, considering that the clinical data of patients were not reported consistently across studies, these results should be interpreted with more caution and examined further.
Many studies have revealed no relationship between sleep-related deficits and cognition when insensitive neuropsychological tests were used. For instance, the Mini-Mental State Examination (MMSE), designed to detect frank dementia (98) instead of cognitive dysfunction in PD, possesses a “strikingly low sensitivity” at only 50% when used to screen for dementia in people with PD (99). MMSE is thought to be less sensitive than the Montreal Cognitive Assessment (MOCA) in detecting MCI in PD patients (100). Although the MMSE was not recommended to evaluate cognition in PD by the 2010 Movement Disorders Task Force to detect cognitive impairment in PD (98), it remained the most commonly used global cognitive test. Impaired global cognitive functions detected mostly by the MMSE were related to “bad sleep” or “pure apathy” (51, 53) and were insignificantly associated with impulse control disorders (52) in PD patients in previous meta-analyses. In our meta-analysis, the MMSE was used in 22 of the 38 included studies assessing global cognition. Similarly, the Frontal Assessment Battery (FAB), designed to recognize frontal lobe dysfunction, has been validated in frontotemporal dementia, progressive supranuclear palsy (PSP), and PD. Regression analysis proved that 69.7% of individuals with PSP and frontotemporal dementia were classified correctly with FAB, which suggested that deficits associated with predominantly medial–prefrontal dysfunction could be captured successfully by FAB (101). However, FAB is insensitive to cognitive damage in PD because it detects frontal lobe dysfunction instead of disorders that primarily involve the dorsolateral and ventrolateral prefrontal cortices in PD (102). A study identified the sensitivity (66.3%) and specificity (72.3%) of FAB in detecting dementia in PD at a cutoff of 26 points (103). All the enrolled studies evaluating general EF used FAB in our meta-analysis. Thus, these two domains need to be confirmed by more sensitive tests. Moreover, due to the conflicting results of the processing speed/complex attention/working memory domain evaluated with LNS and TMT: A, this domain also need to be confirmed further.
The Process of Selecting Neurophysiological Tests
In diagnosing MCI in PD, Litvan et al. (48) suggested that two highly similar tests (e.g., two list learning tests or two story recall tests) or highly correlated scores from the same test (e.g., immediate and delayed recall of a word list) should not be used to meet the MCI criterion for two test-score abnormalities. As mentioned earlier, when a cognitive domain was determined with more than one test in a study, we extracted and analyzed data from the most sensitive, relevant, and frequently used tests and discussed in detail the process of assigning the tests in these domains.
In the global cognitive function domain, among the enrolled primary studies, eight (33, 60, 61, 69, 71–74) employed both the MMSE and MOCA. Because the sensitivity of the MOCA is higher than that of the MMSE in evaluating cognitive decline in PD (98–100), we analyzed the MOCA results of these eight studies.
In the long-term verbal recall domain, the tests used were several highly similar tests. We extracted the results of the delayed recall tasks from these tests, like Litvan et al. (48) who suggested in diagnosing MCI in PD or Jansen et al. (104) who selected evaluating cognition in individuals with MCI. This criterion was also applicable to the long-term visual recall domain.
Six studies (5, 31, 33, 35, 66, 82) used both verbal fluency, semantic and letter, to evaluate the generativity. It is controversial which aspect of verbal fluency was more affected in PD patients (105–107). In an attempt to resolve the inconsistency, a meta-analysis with 2,644 PD patients showed that, although PD patients manifested greater deficits in semantic than in letter fluency, the difference was small (108). Because several other studies only employed semantic verbal fluency, we extracted semantic results from these six studies and combined them with the results from other studies in order to minimize heterogeneity. Kamble et al. (61) used two highly similar tests in this domain, the semantic verbal fluency and animal naming tests. Similarly, we extracted the results from the semantic fluency test. This criterion was also applicable to the shifting domain where two tasks of the TMT were adopted in a study (5); we analyzed the task employed by other studies as well. Moreover, two tests, Stroop task and TMT, can be measure by both speed and accuracy; we extracted speed data when these are available.
Concerning the visuospatial and constructional ability, six different tests were adopted by the enrolled primary studies. The Benton judgment of line orientation test (BJLOT), one of the most extensively used visuospatial tasks, was sensitive to visuospatial deficits in PD (109). The copy of the Rey–Osterrieth complex figure test is another widely used test to assess visuo-constructional ability. However, due to its complexity, EF is also reflected in this test (110–112). Using the clock drawing test, PD patients manifested a low performance compared with healthy controls (113). However, the major reason for clock drawing difficulties in PD with early cognitive impairment is dysfunctional executive control of memory retrieval instead of visuospatial impairment (114). Similarly, the block design measures visual perception and organization and visual–motor coordination, and also non-verbal reasoning, analysis, and synthesis (115). The bells test is used to investigate visual perception, processing speed, and attention (116). However, it is considered a sensitive test to diagnose visual hemineglect (117, 118). The sensitivity of Benton's facial recognition in PD patients is rarely studied. A study pointed out that medicated people with PD did not show significant deficits in this test compared with those untreated (119). Therefore, in this domain, the BJLOT is our priority when it exists with other tests of this domain in one study, and the copy of the Rey–Osterrieth complex figure test is our second choice.
Possible Mechanisms of “RBD-PD Phenotype”
Although the relationship between RBD and cognitive dysfunction in PD was confirmed based on our results, the mechanisms behind this phenomenon are yet to be elucidated. The following dysregulations that affect both RBD and cognitive dysfunction in PD may be the targets.
Cholinergic dysfunction is strongly associated with RBD and cognitive decline in PD. RBD in the context of α-synucleinopathies was suggested to be a result of degeneration of the pontomedullary cholinergic pathways (67, 120, 121). A smaller volume of the pontomesencephalic tegmentum was found in PD patients with RBD than in those without (76). Moreover, dysfunctions of cholinergic systems and their projections were consistently associated with cognitive damage in PD (122–127). Cholinergic pedunculopontine nucleus neuronal loss in PD is believed to be attributable to cognitive damage (128–130). The decreased volume and the disrupted resting-state functional connectivity of the basal nucleus of Meynert (BNM), the main source of cholinergic innervation (131, 132), were found to be correlated with cognitive decline in PD (133–135). Rivastigmine, an inhibitor of acetylcholinesterase and butyrylcholinesterase, is effective in treating RBD and dementia associated with PD separately (136, 137).
Nigro-striatal dopaminergic impairment, limbic dysfunction, inflammation, and altered metabolism are also related to RBD and cognitive damage in PD. In PD-RBD patients, more severe nigro-striatal dopaminergic damage (138) and greater dopamine transporter loss (139) were discovered compared with PD-NRBD patients. A positive relationship between striatal dopamine transporter availability and fundamental cognitive capability was determined in PD patients (140). Compared to PD-NRBD patients, smaller volumes of the hypothalamus, thalamus, amygdala, anterior cingulate cortex, left posterior cingulate, and hippocampus were found in PD-RBD patients (64, 76). The volume loss of the thalamus and the accompanying damaged functional connectivity were also observed in PD patients with MCI (141). Elevations of peripheral inflammatory factors were found in the PD-RBD group compared with the PD-NRBD group (142). Cognitive damage in PD patients was associated with a higher level of circulating lymphocytes and—in drug-naive ones at least—with dysregulation of the T regulatory cells (143). In addition, an altered brain glucose metabolism was observed in PD patients with RBD and MCI (138, 144–147).
Therefore, it was previously suggested that PD-RBD represents a unique subtype of PD with severe non-motor symptoms. The positive relationship between RBD and cognitive decline in PD patients according to our results enriched and expanded this opinion. The above-mentioned dysfunctions in PD patients accompanied with either RBD or cognitive decline elucidated this relationship further, thus supplying possible therapeutic targets. Even though the results are encouraging, more cases and experiments are needed to confirm this phenotype.
Strengths and Limitations
This meta-analysis not only confirms the relationship between RBD and cognitive dysfunction in PD but also specifies which cognitive domains are involved. In addition, this meta-analysis scientifically distinguished probable RBD from true RBD and thus demonstrated the difference between objective and subjective evaluation of RBD.
However, two limitations warrant consideration when interpreting our results and designing further studies. The first is the pooling of other non-motor symptoms such as hallucinations and depression, which could also affect the cognitive status of patients with PD. Several longitudinal reports have revealed that hallucination can be a predictor of cognitive dysfunction in PD (148–151), specifically in the domain of EF (152). Moreover, a study confirmed cross-sectionally and longitudinally that hallucination was significantly related to the presence and development of dementia (153), and another separately confirmed the relationships between hallucination and depression with dementia in PD (154). In addition, the relationship between depression and cognitive dysfunction in individuals with PD was also confirmed in several studies, and the consensus was that PD patients with baseline depression manifested deteriorated cognition and motor ability (155–157). Given that nearly 60% of PD patients manifested more than one non-motor symptom and roughly 25% displayed more than two (158), the relationship between “pure RBD” and cognitive decline in PD patients is difficult to detect. The second limitation is that the symptoms of cognitive damage and RBD can both fluctuate (28, 59, 159, 160), so studies evaluating them at an arbitrary time point may not comprehensively and accurately reflect the condition. In addition to the fluctuation of symptoms, the effect of the appearance order of RBD and PD symptoms on cognition is controversial (70, 161).
Significance and Conclusion
This meta-analysis strongly suggests an association between RBD and cognitive dysfunctions in PD patients. Early and routine screening by cognitive tasks is simple and inexpensive and should be part of the standard assessment of all PD-RBD patients before they evolve to irreversible dementia in the study setting. Currently, there are no pharmacological therapeutics that could slow cognitive decline or dementia (162–164); however, there is some evidence suggesting that non-pharmacological interventions, like cognitive training, could enhance cognition in non-demented early-stage PD patients (165–167). This underscores the importance of a timely intervention of cognitive dysfunctions in PD. Our findings should be extended in larger prospective longitudinal studies to assess the progression of both cognitive decline and RBD in PD and to identify moderators that may help in a personalized care approach.
Author Contributions
JM and JG developed the concept for the study. JM and JY carried out search, quality assessment, and initial data interpretation. JM, XH, and LC carried out statistical analysis. JM prepared the manuscript draft, with input and revisions from LC, YH, BT, and JG. All authors approved the final version.
Funding
This research was supported by grants from the National Key Research and Development Program of China (grant nos. 2017YFC0909100 and 2016YFC1306000), the Central Public-Interest Scientific Institution Basal Research Fund of Chinese Academy of Medical Sciences (grant no. 2018-12M-HL-025), the National Natural Science Foundation of China (grant nos. 81571248, 81873785, and 81974202), the Natural Science Foundation of Hunan Province (grant no. 2017JJ1037), and Science and Technology Major Project of Hunan Provincial Science and Technology Department (grant no. 2018SK1030).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the authors of the included primary studies who provided the data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.577874/full#supplementary-material
References
1. Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. (2002) 25:120–38. doi: 10.1093/sleep/25.2.120
2. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
3. McKenna D, Peever J. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord. (2017) 32:636–44. doi: 10.1002/mds.27003
4. Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. (2015) 30:1600–11. doi: 10.1002/mds.26431
5. Jozwiak N, Postuma RB, Montplaisir J, Latreille V, Panisset M, Chouinard S, et al. REM sleep behavior disorder and cognitive impairment in Parkinson's disease. Sleep. (2017) 40:zsx101. doi: 10.1093/sleep/zsx101
6. Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE. (2014) 9:e89741. doi: 10.1371/journal.pone.0089741
7. Kumru H, Santamaria J, Tolosa E, Iranzo A. Relation between subtype of Parkinson's disease and REM sleep behavior disorder. Sleep Med. (2007) 8:779–83. doi: 10.1016/j.sleep.2007.02.005
8. Lee JE, Kim KS, Shin HW, Sohn YH. Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat Disord. (2010) 16:105–8. doi: 10.1016/j.parkreldis.2009.08.005
9. Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. (2008) 79:1117–21. doi: 10.1136/jnnp.2008.149195
10. Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Ann Neurol. (2010) 68:353–9. doi: 10.1002/ana.22076
11. Postuma RB, Gagnon JF. Symmetry of Parkinson's disease and REM sleep: one piece of the puzzle. Ann Neurol. (2011) 69:905, author reply 6. doi: 10.1002/ana.22259
12. Postuma RB, Montplaisir J, Lanfranchi P, Blais H, Rompre S, Colombo R, et al. Cardiac autonomic denervation in Parkinson's disease is linked to REM sleep behavior disorder. Mov Disord. (2011) 26:1529–33. doi: 10.1002/mds.23677
13. Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson's disease. Brain. (2009) 132:3298–307. doi: 10.1093/brain/awp244
14. Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. (2008) 23:1665–72. doi: 10.1002/mds.22099
15. Nomura T, Inoue Y, Högl B, Uemura Y, Kitayama M, Abe T, et al. Relationship between 123I-MIBG scintigrams and REM sleep behavior disorder in Parkinson's disease. Parkinsonism Related Disord. (2010) 16:683–5. doi: 10.1016/j.parkreldis.2010.08.011
16. Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. (2011) 77:1048–54. doi: 10.1212/WNL.0b013e31822e560e
17. Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson's disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol. (2009) 61:164–70. doi: 10.1159/000189269
18. Pacchetti C, Manni R, Zangaglia R, Mancini F, Marchioni E, Tassorelli C, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov Disord. (2005) 20:1439–48. doi: 10.1002/mds.20582
19. Sinforiani E, Zangaglia R, Manni R, Cristina S, Marchioni E, Nappi G, et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson's disease. Mov Disord. (2006) 21:462–6. doi: 10.1002/mds.20719
20. Onofrj M, Bonanni L, Albani G, Mauro A, Bulla D, Thomas A. Visual hallucinations in Parkinson's disease: clues to separate origins. J Neurol Sci. (2006) 248:143–50. doi: 10.1016/j.jns.2006.05.025
21. Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, et al. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord. (2016) 31:95–102. doi: 10.1002/mds.26359
22. Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. (2012) 78:1434–40. doi: 10.1212/WNL.0b013e318253d54b
23. Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. Brain. (2013) 136:392–9. doi: 10.1093/brain/aws318
24. Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with rapid eye movement sleep behavior disorder. Ann Clin Transl Neurol. (2015) 2:941–5. doi: 10.1002/acn3.228
25. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. (2015) 72:863–73. doi: 10.1001/jamaneurol.2015.0703
26. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain. (2017) 140:1959–76. doi: 10.1093/brain/awx118
27. Benninger DH, Michel J, Waldvogel D, Candia V, Poryazova R, van Hedel HJ, et al. REM sleep behavior disorder is not linked to postural instability and gait dysfunction in Parkinson. Mov Disord. (2010) 25:1597–604. doi: 10.1002/mds.23121
28. Lavault S, Leu-Semenescu S, Tezenas du Montcel S, Cochen de Cock V, Vidailhet M, Arnulf I. Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson's disease? J Neurol. (2010) 257:1154–9. doi: 10.1007/s00415-010-5482-y
29. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. (1999) 56:303–8. doi: 10.1001/archneur.56.3.303
30. Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. (2010) 1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x
31. Chahine LM, Xie SX, Simuni T, Tran B, Postuma R, Amara A, et al. Longitudinal changes in cognition in early Parkinson's disease patients with REM sleep behavior disorder. Parkinsonism Relat Disord. (2016) 27:102–6. doi: 10.1016/j.parkreldis.2016.03.006
32. Gong Y, Xiong KP, Mao CJ, Shen Y, Hu WD, Huang JY, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. (2014) 15:647–53. doi: 10.1016/j.sleep.2013.12.021
33. Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, Nithi K, Talbot K, Ben-Shlomo Y, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson's disease. J Neurol Neurosurg Psychiatry. (2014) 85:560–6. doi: 10.1136/jnnp-2013-306104
34. Naismith SL, Terpening Z, Shine JM, Lewis SJ. Neuropsychological functioning in Parkinson's disease: differential relationships with self-reported sleep-wake disturbances. Mov Disord. (2011) 26:1537–41. doi: 10.1002/mds.23640
35. Vendette M, Gagnon JF, Décary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. (2007) 69:1843–9. doi: 10.1212/01.wnl.0000278114.14096.74
36. Lin YQ, Chen SD. RBD: a red flag for cognitive impairment in Parkinson's disease? Sleep Med. (2018) 44:38–44. doi: 10.1016/j.sleep.2018.01.006
37. Erro R, Santangelo G, Picillo M, Vitale C, Amboni M, Longo K, et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol. (2012) 259:1808–13. doi: 10.1007/s00415-011-6407-0
38. Marion MH, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson's disease? J Neurol. (2008) 255:192–6. doi: 10.1007/s00415-008-0629-9
39. Louis ED, Bennett DA. Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord. (2007) 22:1681–8. doi: 10.1002/mds.21433
40. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. (2003) 60:387–92. doi: 10.1001/archneur.60.3.387
41. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. (2007) 22:1689–707; quiz 837. doi: 10.1002/mds.21507
42. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
43. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. (1999) 56:33–9. doi: 10.1001/archneur.56.1.33
44. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. (1992) 55:181–4. doi: 10.1136/jnnp.55.3.181
45. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. (1988) 51:745–52. doi: 10.1136/jnnp.51.6.745
46. National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: National Institute for Health and Clinical Excellence: Guidance. (2006).
47. Daniel SE, Lees AJ. Parkinson's disease society brain bank, London: overview and research. J Neural Transm Suppl. (1993) 39:165–72.
48. Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement disorder society task force guidelines. Mov Disord. (2012) 27:349–56. doi: 10.1002/mds.24893
49. Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. (2013) 36:203–20. doi: 10.5665/sleep.2374
50. Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. (2013) 36:1297–305. doi: 10.5665/sleep.2950
51. Pushpanathan ME, Loftus AM, Thomas MG, Gasson N, Bucks RS. The relationship between sleep and cognition in Parkinson's disease: a meta-analysis. Sleep Med Rev. (2016) 26:21–32. doi: 10.1016/j.smrv.2015.04.003
52. Santangelo G, Raimo S, Barone P. The relationship between impulse control disorders and cognitive dysfunctions in Parkinson's disease: a meta-analysis. Neurosci Biobehav Rev. (2017) 77:129–47. doi: 10.1016/j.neubiorev.2017.02.018
53. D'Iorio A, Maggi G, Vitale C, Trojano L, Santangelo G. “Pure apathy” and cognitive dysfunctions in Parkinson's disease: a meta-analytic study. Neurosci Biobehav Rev. (2018) 94:1–10. doi: 10.1016/j.neubiorev.2018.08.004
54. Arnaldi D, de Carli F, Picco A, Ferrara M, Accardo J, Bossert I, et al. Nigro-caudate dopaminergic deafferentation: a marker of REM sleep behavior disorder? Neurobiol Aging. (2015) 36:3300–5. doi: 10.1016/j.neurobiolaging.2015.08.025
55. Duarte Folle A, Paul KC, Bronstein JM, Keener AM, Ritz B. Clinical progression in Parkinson's disease with features of REM sleep behavior disorder: a population-based longitudinal study. Parkinsonism Relat Disord. (2019) 62:105–11. doi: 10.1016/j.parkreldis.2019.01.018
56. Ford AH, Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Burn DJ, et al. Rapid eye movement sleep behavior disorder in Parkinson's disease: magnetic resonance imaging study. Mov Disord. (2013) 28:832–6. doi: 10.1002/mds.25367
57. Gagnon JF, Fantini ML, Bédard MA, Petit D, Carrier J, Rompré S, et al. Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology. (2004) 62:401–6. doi: 10.1212/01.WNL.0000106460.34682.E9
58. Gaudreault PO, Gagnon JF, Montplaisir J, Vendette M, Postuma RB, Gagnon K, et al. Abnormal occipital event-related potentials in Parkinson's disease with concomitant REM sleep behavior disorder. Parkinsonism Relat Disord. (2013) 19:212–7. doi: 10.1016/j.parkreldis.2012.10.006
59. Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatr. (2008) 79:387–91. doi: 10.1136/jnnp.2007.116830
60. Huang JY, Zhang JR, Shen Y, Zhang HJ, Cao YL, Mao CJ, et al. Effect of rapid eye movement sleep behavior disorder on obstructive sleep apnea severity and cognition of Parkinson's disease patients. Chin Med J. (2018) 131:899–906. doi: 10.4103/0366-6999.229888
61. Kamble N, Yadav R, Lenka A, Kumar K, Nagaraju BC, Pal PK. Impaired sleep quality and cognition in patients of Parkinson's disease with REM sleep behavior disorder: a comparative study. Sleep Med. (2019) 62:1–5. doi: 10.1016/j.sleep.2019.04.001
62. Kim YE, Jeon BS, Yang HJ, Ehm G, Yun JY, Kim HJ, et al. REM sleep behavior disorder: association with motor complications and impulse control disorders in Parkinson's disease. Parkinsonism Relat Disord. (2014) 20:1081–4. doi: 10.1016/j.parkreldis.2014.03.022
63. Kim HJ, Im HK, Kim J, Han JY, De Leon M, Deshpande A, et al. Brain atrophy of secondary REM-sleep behavior disorder in neurodegenerative disease. J Alzheimer's Dis. (2016) 52:1101–9. doi: 10.3233/JAD-151197
64. Lim JS, Shin SA, Lee JY, Nam H, Lee JY, Kim YK. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson's disease. Parkinsonism Relat Disord. (2016) 23:31–6. doi: 10.1016/j.parkreldis.2015.11.027
65. Mahale RR, Yadav R, Pal PK. Rapid eye movement sleep behaviour disorder in women with Parkinson's disease is an underdiagnosed entity. J Clin Neurosci. (2016) 28:43–6. doi: 10.1016/j.jocn.2015.08.046
66. Marques A, Dujardin K, Boucart M, Pins D, Delliaux M, Defebvre L, et al. REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol. (2010) 257:383–91. doi: 10.1007/s00415-009-5328-7
67. Nardone R, Bergmann J, Brigo F, Christova M, Kunz A, Seidl M, et al. Functional evaluation of central cholinergic circuits in patients with Parkinson's disease and REM sleep behavior disorder: a TMS study. J Neural Trans. (2013) 120:413–22. doi: 10.1007/s00702-012-0888-6
68. Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med. (2013) 14:131–5. doi: 10.1016/j.sleep.2012.10.011
69. Nomura T, Tanaka K, Tajiri Y, Kishi M, Nakashima K. Screening tools for clinical characteristics of probable REM sleep behavior disorder in patients with Parkinson's disease. eNeurologicalSci. (2016) 4:22–4. doi: 10.1016/j.ensci.2016.04.004
70. Nomura T, Kishi M, Nakashima K. Differences in clinical characteristics when REM sleep behavior disorder precedes or comes after the onset of Parkinson's disease. J Neurol Sci. (2017) 382:58–60. doi: 10.1016/j.jns.2017.08.3247
71. Plomhause L, Dujardin K, Duhamel A, Delliaux M, Derambure P, Defebvre L, et al. Rapid eye movement sleep behavior disorder in treatment-naive Parkinson disease patients. Sleep Med. (2013) 14:1035–7. doi: 10.1016/j.sleep.2013.04.018
72. Plomhause L, Dujardin K, Boucart M, Herlin V, Defebvre L, Derambure P, et al. Impaired visual perception in rapid eye movement sleep behavior disorder. Neuropsychology. (2014) 28:388–93. doi: 10.1037/neu0000006
73. Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov Disord. (2012) 27:720–6. doi: 10.1002/mds.24939
74. Zhang JR, Chen J, Yang ZJ, Zhang HJ, Fu YT, Shen Y, et al. Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in parkinson's disease. Chin Med J. (2016) 129:379–85. doi: 10.4103/0366-6999.176077
75. Ba M, Yu G, Kong M, Liang H, Yu L. CSF Aβ1-42 level is associated with cognitive decline in early Parkinson's disease with rapid eye movement sleep behavior disorder. Transl Neurodegener. (2018) 7:22. doi: 10.1186/s40035-018-0129-5
76. Boucetta S, Salimi A, Dadar M, Jones BE, Collins DL, Dang-Vu TT. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson's disease. Sci Rep. (2016) 6:26782. doi: 10.1038/srep26782
77. Kotagal V, Albin RL, Müller MLTM, Koeppe RA, Chervin RD, Frey KA, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. (2012) 71:560–8. doi: 10.1002/ana.22691
78. Liu H, Ou R, Wei Q, Hou Y, Cao B, Zhao B, et al. Rapid eye movement behavior disorder in drug-naïve patients with Parkinson's disease. J Clin Neurosci. (2019) 59:254–8. doi: 10.1016/j.jocn.2018.07.007
79. Pagano G, De Micco R, Yousaf T, Wilson H, Chandra A, Politis M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology. (2018) 91:e894–905. doi: 10.1212/WNL.0000000000006134
80. Rahmani F, Ansari M, Pooyan A, Mirbagheri MM, Aarabi MH. Differences in white matter microstructure between Parkinson's disease patients with and without REM sleep behavior disorder. Annu Int Conf IEEE Eng Med Biol Soc. (2016) 2016:1124–6. doi: 10.1109/EMBC.2016.7590901
81. Meral H, Aydemir T, Ozer F, Ozturk O, Ozben S, Erol C, et al. Relationship between visual hallucinations and REM sleep behavior disorder in patients with Parkinson's disease. Clin Neurol Neurosurg. (2007) 109:862–7. doi: 10.1016/j.clineuro.2007.08.010
82. Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. (2009) 66:39–47. doi: 10.1002/ana.21680
83. Patel R, Silla F, Pierce S, Theule J, Girard TA. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): a quantitative meta-analysis in healthy adults. Neuropsychologia. (2020) 2020:107395. doi: 10.1016/j.neuropsychologia.2020.107395
84. Hu M, Wu X, Shu X, Hu H, Chen Q, Peng L, et al. Effects of computerised cognitive training on cognitive impairment: a meta-analysis. J Neurol. (2019). doi: 10.1007/s00415-019-09522-7. [Epub ahead of print].
85. Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. (2013) 17:341–7. doi: 10.1016/j.smrv.2012.09.002
86. Wells GA, Shea D, O'Connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. ohri.ca Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 25, 2019).
87. Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. (2013) 13:154. doi: 10.1186/1471-2458-13-154
88. Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. (2010) 6:85–95. doi: 10.5664/jcsm.27717
89. Salzman C. Do benzodiazepines cause Alzheimer's disease? Am J Psychiatry. (2020) 177:476–8. doi: 10.1176/appi.ajp.2020.20040375
90. Dokkedal-Silva V, Berro LF, Galduroz JCF, Tufik S, Andersen ML. Clonazepam: indications, side effects, and potential for nonmedical use. Harv Rev Psychiatry. (2019) 27:279–89. doi: 10.1097/HRP.0000000000000227
91. Michael B, Hedges LV, Higgins JPT, Rothstein HR. Meta-Regression. Introduction to Meta-Analysis. New York, NY: Wiley (2009). p. 187–203. doi: 10.1002/9780470743386
92. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. (2020) 158:1611–25.e12. doi: 10.1053/j.gastro.2020.01.043
93. Larsen JP, Dupont E, Tandberg E. Clinical diagnosis of Parkinson's disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol Scand. (1994) 89:242–51. doi: 10.1111/j.1600-0404.1994.tb01674.x
94. Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. (2005) 28:203–6. doi: 10.1093/sleep/28.2.203
95. Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Am J Psychiatry. (1989) 146:1166–73. doi: 10.1176/ajp.146.9.1166
96. Cooray N, Andreotti F, Lo C, Symmonds M, Hu MTM, De Vos M. Detection of REM sleep behaviour disorder by automated polysomnography analysis. Clin Neurophysiol. (2019) 130:505–14. doi: 10.1016/j.clinph.2019.01.011
97. Kelly JM, Strecker RE, Bianchi MT. Recent developments in home sleep-monitoring devices. ISRN Neurol. (2012) 2012:768794. doi: 10.5402/2012/768794
98. Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, et al. A recommended scale for cognitive screening in clinical trials of Parkinson's disease. Mov Disord. (2010) 25:2501–7. doi: 10.1002/mds.23362
99. Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease. results from the German study on epidemiology of Parkinson's disease with dementia (GEPAD). J Neurol. (2008) 255:255–64. doi: 10.1007/s00415-008-0720-2
100. Burdick DJ, Cholerton B, Watson GS, Siderowf A, Trojanowski JQ, Weintraub D, et al. People with Parkinson's disease and normal MMSE score have a broad range of cognitive performance. Mov Disord. (2014) 29:1258–64. doi: 10.1002/mds.25924
101. Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson's disease: tools for diagnosis and assessment. Mov Disord. (2009) 24:1103–10. doi: 10.1002/mds.22506
102. Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. (2003) 23:6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003
103. Kaszas B, Kovacs N, Balas I, Kallai J, Aschermann Z, Kerekes Z, et al. Sensitivity and specificity of addenbrooke's cognitive examination, mattis dementia rating scale, frontal assessment battery and mini mental state examination for diagnosing dementia in Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:553–6. doi: 10.1016/j.parkreldis.2012.02.010
104. Jansen WJ, Ossenkoppele R, Tijms BM, Fagan AM, Hansson O, Klunk WE, et al. Association of cerebral amyloid-beta aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. (2018) 75:84–95. doi: 10.1001/jamapsychiatry.2017.3391
105. Flowers KA, Robertson C, Sheridan MR. Some characteristics of word fluency in Parkinson's disease. J Neurolinguistics. (1995) 9:33–46. doi: 10.1016/0911-6044(95)00004-6
106. Azuma T, Bayles KA, Cruz RF, Tomoeda CK, Wood JA, McGeagh A, et al. Comparing the difficulty of letter, semantic, and name fluency tasks for normal elderly and patients with Parkinson's disease. Neuropsychology. (1997) 11:488–97. doi: 10.1037/0894-4105.11.4.488
107. Matison R, Mayeux R, Rosen J, Fahn S. “Tip-of-the-tongue” phenomenon in Parkinson disease. Neurology. (1982) 32:567–70. doi: 10.1212/WNL.32.5.567
108. Henry JD, Crawford JR. Verbal fluency deficits in Parkinson's disease: a meta-analysis. J Int Neuropsychol Soc. (2004) 10:608–22. doi: 10.1017/S1355617704104141
109. Montse A, Pere V, Carme J, Francesc V, Eduardo T. Visuospatial deficits in Parkinson's disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol. (2001) 23:592–8. doi: 10.1076/jcen.23.5.592.1248
110. Shorr JS, Delis DC, Massman PJ. Memory for the rey-osterrieth figure: perceptual clustering, encoding, and storage. Neuropsychology. (1992) 6:43–50. doi: 10.1037/0894-4105.6.1.43
111. Somerville J, Tremont G, Stern RA. The boston qualitative scoring system as a measure of executive functioning in rey-osterrieth complex figure performance. J Clin Exp Neuropsychol. (2000) 22:613–21. doi: 10.1076/1380-3395(200010)22:5;1-9;FT613
112. Beebe DW, Ris MD, Brown TM, Dietrich KN. Executive functioning and memory for the Rey-Osterreith complex figure task among community adolescents. Appl Neuropsychol. (2004) 11:91–8. doi: 10.1207/s15324826an1102_4
113. Scarpina F, Paschino C, Priano L, Mauro A. Performance at the clock drawing test of individuals affected by Parkinson's disease and healthy subjects: a retrospective study. Neurol Sci. (2020) 41:843–9. doi: 10.1007/s10072-019-04167-w
114. Saur R, Maier C, Milian M, Riedel E, Berg D, Liepelt-Scarfone I, et al. Clock test deficits related to the global cognitive state in Alzheimer's and Parkinson's disease. Dement Geriatr Cogn Disord. (2012) 33:59–72. doi: 10.1159/000336598
115. Coalson DL, Raiford SE, Saklofske DH, Weiss LG. CHAPTER 1 - WAIS-IV: advances in the assessment of intelligence. In: Weiss LG, Saklofske DH, Coalson DL, Raiford SE, editors. WAIS-IV Clinical Use and Interpretation. San Diego: Academic Press (2010) p. 3–23. doi: 10.1016/B978-0-12-375035-8.10001-1
116. Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int J Clini Neuropsychol. (1989) 11:49–54. doi: 10.1037/t28075-000
117. Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. (2002) 73:160–6. doi: 10.1136/jnnp.73.2.160
118. Cr O, Fc L. Use of bells test in the evaluation of the hemineglect post unilateral stroke. J Neurol Neurosci. (2016) 7:S3. doi: 10.21767/2171-6625.1000124
119. Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson's disease. Neuropsychologia. (2003) 41:1047–57. doi: 10.1016/S0028-3932(02)00295-6
120. Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. (2007) 130:2770–88. doi: 10.1093/brain/awm056
121. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
122. Muller ML, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. (2013) 13:377. doi: 10.1007/s11910-013-0377-9
123. Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson's disease. Mov Disord. (2011) 26:2496–503. doi: 10.1002/mds.23932
124. Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimer's disease. J Neurol Neurosurg Psychiatry. (1985) 48:413–21. doi: 10.1136/jnnp.48.5.413
125. Tiraboschi P, Hansen LA, Alford M, Sabbagh MN, Schoos B, Masliah E, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology. (2000) 54:407–11. doi: 10.1212/WNL.54.2.407
126. Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. (2003) 60:1745–8. doi: 10.1001/archneur.60.12.1745
127. Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain. (2014) 137:2493–508. doi: 10.1093/brain/awu193
128. Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA. (1987) 84:5976–80. doi: 10.1073/pnas.84.16.5976
129. Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson's disease. Ann Neurol. (1989) 26:41–6. doi: 10.1002/ana.410260106
130. Scarnati E, Florio T. The pedunculopontine nucleus and related structures. functional organization. Adv Neurol. (1997) 74:97–110.
131. Gratwicke J, Kahan J, Zrinzo L, Hariz M, Limousin P, Foltynie T, et al. The nucleus basalis of Meynert: a new target for deep brain stimulation in dementia? Neurosci Biobehav Rev. (2013) 37:2676–88. doi: 10.1016/j.neubiorev.2013.09.003
132. Lu YT, Chang WN, Chang CC, Lu CH, Chen NC, Huang CW, et al. Insula volume and salience network are associated with memory decline in Parkinson disease: complementary analyses of voxel-based morphometry versus volume of interest. Parkinsons Dis. (2016) 2016:2939528. doi: 10.1155/2016/2939528
133. Ray NJ, Bradburn S, Murgatroyd C, Toseeb U, Mir P, Kountouriotis GK, et al. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson's disease. Brain. (2018) 141:165–76. doi: 10.1093/brain/awx310
134. Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson's disease. Brain. (2018) 141:1501–16. doi: 10.1093/brain/awy072
135. Zhang C, Wu C, Zhang H, Dou W, Li W, Sami MU, et al. Disrupted resting-state functional connectivity of the nucleus basalis of meynert in Parkinson's disease with mild cognitive impairment. Neuroscience. (2020) 442:228–36. doi: 10.1016/j.neuroscience.2020.07.008
136. Poewe W, Wolters E, Emre M, Onofrj M, Hsu C, Tekin S, et al. Long-term benefits of rivastigmine in dementia associated with Parkinson's disease: an active treatment extension study. Mov Disord. (2006) 21:456–61. doi: 10.1002/mds.20700
137. Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson's disease. Mov Disord. (2012) 27:559–61. doi: 10.1002/mds.24909
138. Arnaldi D, Morbelli S, Brugnolo A, Girtler N, Picco A, Ferrara M, et al. Functional neuroimaging and clinical features of drug naive patients with de novo Parkinson's disease and probable RBD. Parkinsonism Relat Disord. (2016) 29:47–53. doi: 10.1016/j.parkreldis.2016.05.031
139. Cao R, Chen X, Xie C, Hu P, Wang K. Serial dopamine transporter imaging of nigrostriatal function in parkinson's disease with probable REM sleep behavior disorder. Front Neurosci. (2020) 14:349. doi: 10.3389/fnins.2020.00349
140. Vriend C, van Balkom TD, van Druningen C, Klein M, van der Werf YD, Berendse HW, et al. Processing speed is related to striatal dopamine transporter availability in Parkinson's disease. Neuroimage Clin. (2020) 26:102257. doi: 10.1016/j.nicl.2020.102257
141. Li MG, He JF, Liu XY, Wang ZF, Lou X, Ma L. Structural and functional thalamic changes in Parkinson's disease with mild cognitive impairment. J Magn Reson Imaging. (2020) 52:1207–15. doi: 10.1002/jmri.27195
142. Hu Y, Yu SY, Zuo LJ, Piao YS, Cao CJ, Wang F, et al. Investigation on abnormal iron metabolism and related inflammation in parkinson disease patients with probable RBD. PLoS ONE. (2015) 10:e0138997. doi: 10.1371/journal.pone.0138997
143. Magistrelli L, Storelli E, Rasini E, Contaldi E, Comi C, Cosentino M, et al. Relationship between circulating CD4+ T lymphocytes and cognitive impairment in patients with Parkinson's disease. Brain Behav Immun. (2020) 89:668–74. doi: 10.1016/j.bbi.2020.07.005
144. Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. (2008) 70:1470–7. doi: 10.1212/01.wnl.0000304050.05332.9c
145. Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Mov Disord. (2009) 24:854–62. doi: 10.1002/mds.22444
146. Lyoo CH, Jeong Y, Ryu YH, Rinne JO, Lee MS. Cerebral glucose metabolism of Parkinson's disease patients with mild cognitive impairment. Eur Neurol. (2010) 64:65–73. doi: 10.1159/000315036
147. Garcia-Garcia D, Clavero P, Gasca Salas C, Lamet I, Arbizu J, Gonzalez-Redondo R, et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson's disease. Eur J Nucl Med Mol Imaging. (2012) 39:1767–77. doi: 10.1007/s00259-012-2198-5
148. Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, et al. A neuropsychological longitudinal study in Parkinson's patients with and without hallucinations. Mov Disord. (2007) 22:2418–25. doi: 10.1002/mds.21746
149. Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Cognitive changes in Parkinson's disease patients with visual hallucinations. Dement Geriatr Cogn Disord. (2007) 23:281–8. doi: 10.1159/000100850
150. Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. (2014) 83:1253–60. doi: 10.1212/WNL.0000000000000842
151. Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, et al. The rate of cognitive decline in Parkinson disease. Arch Neurol. (2004) 61:1906–11. doi: 10.1001/archneur.61.12.1906
152. Creese B, Albertyn CP, Dworkin S, Thomas RS, Wan YM, Ballard C. Executive function but not episodic memory decline associated with visual hallucinations in Parkinson's disease. J Neuropsychol. (2020) 14:85–97. doi: 10.1111/jnp.12169
153. Gryc W, Roberts KA, Zabetian CP, Weintraub D, Trojanowski JQ, Quinn JF, et al. Hallucinations and development of dementia in Parkinson's disease. J Parkinsons Dis. (2020) doi: 10.3233/JPD-202116. [Epub ahead of print].
154. Zhu K, van Hilten JJ, Marinus J. Predictors of dementia in Parkinson's disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism Relat Disord. (2014) 20:980–5. doi: 10.1016/j.parkreldis.2014.06.006
155. Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. (1992) 55:377–82. doi: 10.1136/jnnp.55.5.377
156. Ng A, Chander RJ, Tan LC, Kandiah N. Influence of depression in mild Parkinson's disease on longitudinal motor and cognitive function. Parkinsonism Relat Disord. (2015) 21:1056–60. doi: 10.1016/j.parkreldis.2015.06.014
157. Park JH, Lee SH, Kim Y, Park SW, Byeon GH, Jang JW, et al. Depressive symptoms are associated with worse cognitive prognosis in patients with newly diagnosed idiopathic Parkinson disease. Psychogeriatrics. (2020). doi: 10.1111/psyg.12601. [Epub ahead of print].
158. Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O'Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. (2013) 80:276–81. doi: 10.1212/WNL.0b013e31827deb74
159. Bugalho P, da Silva JA, Neto B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson's disease. J Neurol. (2011) 258:50–5. doi: 10.1007/s00415-010-5679-0
160. Oudiette D, de Cock VC, Lavault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. (2009) 72:551–7. doi: 10.1212/01.wnl.0000341936.78678.3a
161. Ferri R, Cosentino FI, Pizza F, Arico D, Plazzi G. The timing between REM sleep behavior disorder and Parkinson's disease. Sleep Breath. (2014) 18:319–23. doi: 10.1007/s11325-013-0887-3
162. Emre M, Ford PJ, Bilgic B, Uc EY. Cognitive impairment and dementia in Parkinson's disease: practical issues and management. Mov Disord. (2014) 29:663–72. doi: 10.1002/mds.25870
163. Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. (2011) 26(Suppl. 3):S42–80. doi: 10.1002/mds.23884
164. Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. (2004) 351:2509–18. doi: 10.1056/NEJMoa041470
165. Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson's disease: a systematic review. Mov Disord. (2013) 28:1034–49. doi: 10.1002/mds.25377
166. Leung IH, Walton CC, Hallock H, Lewis SJ, Valenzuela M, Lampit A. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. (2015) 85:1843–51. doi: 10.1212/WNL.0000000000002145
Keywords: rapid eye movement sleep behavior disorder (RBD), parkinson's disease (PD), dementia, meta-analysis, cognitive dysfunction
Citation: Mao J, Huang X, Yu J, Chen L, Huang Y, Tang B and Guo J (2020) Association Between REM Sleep Behavior Disorder and Cognitive Dysfunctions in Parkinson's Disease: A Systematic Review and Meta-Analysis of Observational Studies. Front. Neurol. 11:577874. doi: 10.3389/fneur.2020.577874
Received: 30 June 2020; Accepted: 30 September 2020;
Published: 06 November 2020.
Edited by:
Anat Mirelman, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Peng Lei, Sichuan University, ChinaPedro Ribeiro, Federal University of Rio de Janeiro, Brazil
Copyright © 2020 Mao, Huang, Yu, Chen, Huang, Tang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jifeng Guo, Z3VvamlmZW5nMjAwM0AxNjMuY29t
 Jingrong Mao
Jingrong Mao Xiurong Huang1
Xiurong Huang1 Jiaming Yu
Jiaming Yu Lang Chen
Lang Chen Beisha Tang
Beisha Tang Jifeng Guo
Jifeng Guo