- Department of Neurology, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, Chengdu, China
Purpose: To evaluate the incidence and risk of tremor in patients treated with valproic aid (VPA) monotherapy.
Methods: We searched the PubMed, Embase, and Cochrane Library databases to gather relevant data on tremor in patients taking VPA and other drugs and performed a meta-analysis using Stata15.1 software.
Results: Twenty-nine randomized controlled trials (RCTs) met the inclusion criteria and were included in the meta-analysis. The overall incidence of tremor in patients receiving VPA therapy was 14% [OR = 0.14, 95% CI (0.10–0.17)]. The pooled estimate risk of tremor showed a significant difference between patients treated with VPA and all other drugs [OR = 5.40, 95% CI (3.22–9.08)], other antiepileptic drugs (AEDs) [OR = 5.78, 95% CI (3.18–10.50)], and other non-AEDs [OR = 4.77, 95% CI (1.55–14.72)]. Both a dose of <1,500 mg/d of VPA [included 500 mg/d: OR = 3.57, 95% CI (1.24–10.26), 500–999 mg/d: OR = 3.99, 95% CI (1.95–8.20), 1,000–1,499 mg/d: OR = 8.82, 95% CI (3.25–23.94)] and a VPA treatment duration of <12 m [included ≤ 3 months: OR = 3.06, 95% CI (1.16–8.09), 3–6 months: OR = 16.98, 95% CI (9.14–31.57), and 6–12 months: OR = 4.15, 95% CI (2.74–6.29)] led to a higher risk of tremor than did other drugs, as did higher doses and longer treatment times.
Conclusion: Compared with other drugs, VPA led to a higher risk of tremor, and the level of risk was associated with the dose and duration of treatment.
Introduction
VPA (valproic acid), a clear, colorless, eight-carbon branched-chain fatty acid, was first produced in 1882 as an organic solvent (1). Then, in 1963, the therapeutic potential of VPA was fortuitously discovered by Carraz et al. (2), who recognized that VPA itself has anticonvulsant properties. VPA has been approved by the Food and Drug Administration (FDA) and is the first-generation broad-spectrum antiepileptic drug (AED) most commonly administered to treat generalized and focal epilepsies in children and adults, and it is also used to treat bipolar disorder, posttraumatic stress disorder (PTSD), schizophrenia, neuropathic pain, and migraine headaches (3–7). It may be useful in novel applications that are currently being researched, such as cancer therapy and prevention (8). Despite its utility, VPA is associated with several common side effects, including tremors, weight gain, alopecia, liver dysfunction, gastrointestinal disturbances, increased triglyceride levels, thrombocytopenia, etc. (9–12).
Tremor, one of the most common neurological symptoms, is defined as an involuntary, rhythmical, oscillatory movement of a body part produced by either synchronous or alternating contractions of antagonist muscles (13). Tremor is the most common side effect involving the central nervous system, occurring in as many as one quarter of chronically treated patients (14, 15). VPA-related tremors are usually action or postural tremors, but sometimes they are rest tremors (16). Tremors do not usually abate with continued treatment (10), so we investigated and diagnosed with the utmost caution, but in some cases, tremors may respond to smaller dosages or changes in the dosing regimen. Additionally, tremors also occur relatively commonly in patients taking antipsychotics, antidepressants, sympathomimetics, antiarrhythmics, AEDs, and other drugs (14, 17), such as lithium, phenytoin (PHT), carbamazepine (CBZ), topiramate (TPM), vigabatrin (VGT), lamotrigine (LTG), and gabapentin (TGB).
However, the relationship between VPA dosage and tremor is currently unclear. In a study by Karas et al., tremors usually appeared with dosages >750 mg per day (16). Patients exhibit tremors with doses exceeding 1,000 mg/d, according to Hyman's reports (18). Additionally, trials have shown that patients with doses of <500 mg also experience tremor (19, 20). In addition, it is not clear whether the dose of VPA compared to other drugs increases the risk of tremors. At present, there is relatively little information about VPA-associated tremors in the existing medical literature, and most of the existing studies are limited to studies with small sample sizes or case reports. To provide more evidence about the incidence of VPA-associated tremor as well as compare the risk of tremor between VPA and other drugs, we systematically reviewed related articles and analyzed them.
Methods
Our study did not require ethical approval because patients were not involved. According to the PRISMA (21) principles and MOOSE (22) guidelines, the search strategy, selection criteria, data extraction process, quality assessment, and statistical analysis were predesigned based on the Cochrane Review Methods. The protocol was not registered on any website.
Search Strategy
Two researchers searched online databases, including the PubMed (1977 to February 2, 2020), Embase (1982 to February 2, 2020), and Cochrane Library databases (2001 to February 2, 2020), for potentially relevant studies; there were no language restrictions, but the search was restricted to human studies. The search was conducted with a combination of medical subject headings and term words, including “Valproic acid”[Mesh], “Propylisopropylacetic Acid,” “2-propylpentanoic acid,” “Divalproex,” “Depakene,” “Divalproex Sodium,” “Valproate,” “Valproate Sodium,” “Valproate Calcium,” “VPA,” “Depakine,” “Depakote,” “tremor” [Mesh], “Tremor,” “Tremors,” and “drug-induced tremor.” Additionally, the references of all the included studies or related reviews were screened to avoid accidental omissions.
Selection Criteria
Clinical trials that met the following criteria were included: (1) the design of the trial was an RCT (randomized controlled trial), (2) VPA was the only therapy provided to the control group or test group, and (3) the study provided the original data for VPA-associated tremor and comparisons with the control group. The exclusion criteria were as follows: (1) observational studies (including cross-sectional studies, case–control studies, cohort analyses, and so on), (2) studies in which VPA was combined with one or more other drugs and compared with a control group, (3) comparative studies with two different valproate preparations, (4) studies in which VPA-associated tremor was not mentioned or the data provided were incomplete, and (5) studies based on the same study population. According to the inclusion and exclusion criteria, we identified a total of 29 randomized controlled trials (six studies were placebo-controlled studies).
Data Extraction
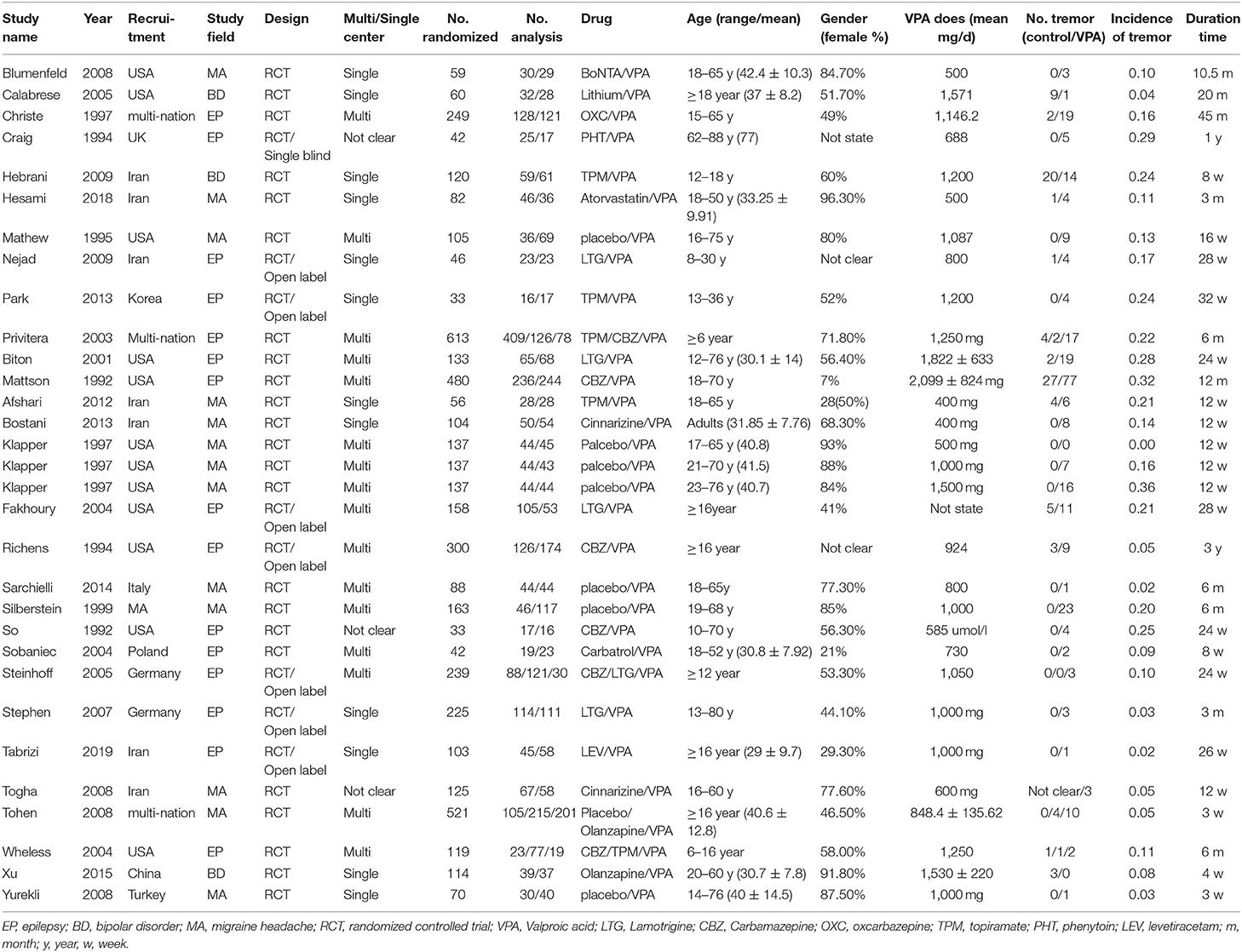
Two reviewers independently extracted relevant information from each eligible study, and any discrepancies between the two reviewers were resolved by consulting with the senior author. We established a data extraction form (Table 1), which included the first author's name, publication year, study recruitment method, study field, design, whether the trial include one or multiple centers, sample sizes of the test group and control group, median age of the participants, percentage of females, dose of VPA, duration of treatment, and number of adverse outcomes related to tremor.
Quality Assessment
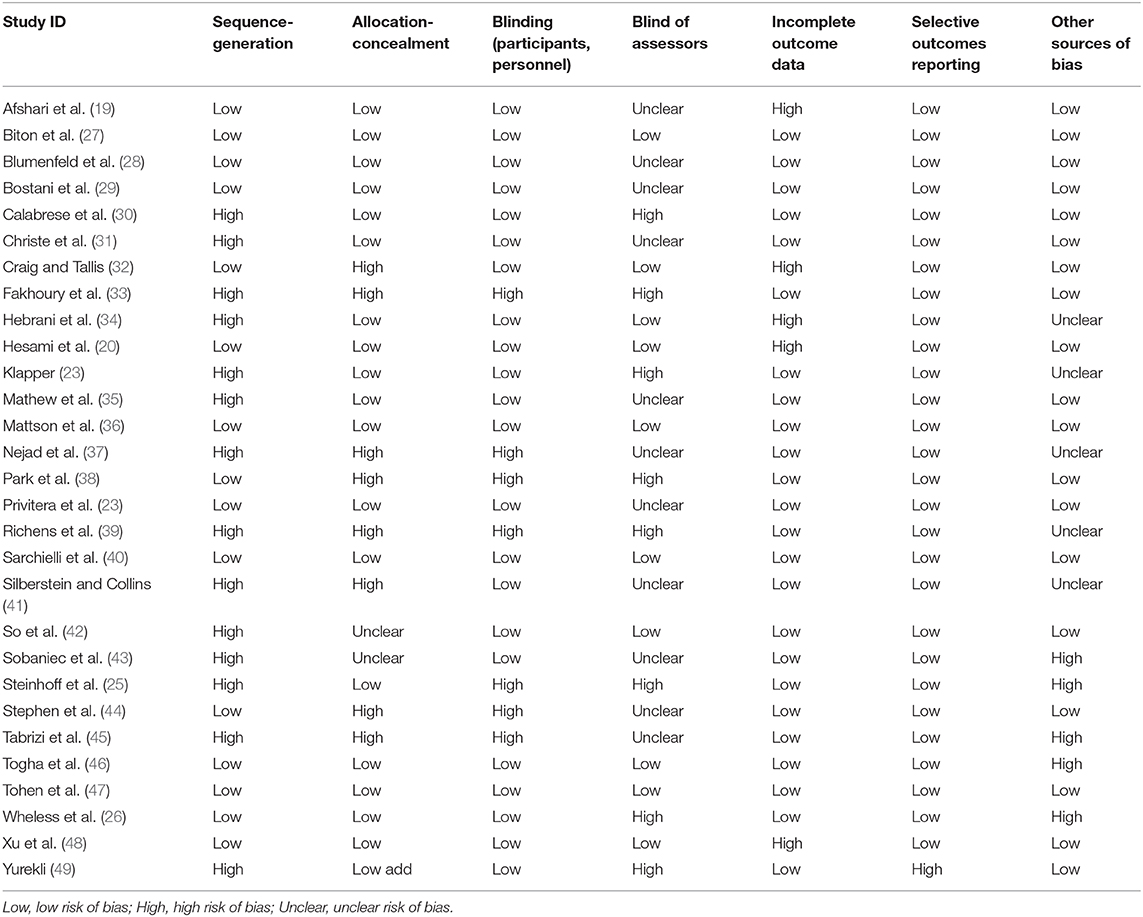
The quality of the RCTs was assessed by two authors, and any disagreements were resolved via discussion with a third reviewer, after which the trial was reevaluated. The guidelines for assessing risk of bias provided in the Cochrane handbooks http://community.cochrane.org/handbook were used to assess the quality of the included studies, and the domains assessed included random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the assessors, incomplete outcome data, and selective outcome reporting.
Statistical Analysis
We performed statistical analyses using Stata 15.1 software. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) to demonstrate the pooled effects of tremor with VPA compared to other drugs. Forest plots were used to visually display the meta-analysis results. We used fixed-effect models to weight the studies by the amount of available information, whereas a random-effect model was used for heterogeneity between studies, and Cochran's Q statistic and I2 metric statistics were used to assess the level of heterogeneity. For the Cochran Q test, heterogeneity was considered statistically significant when P < 0.05. For I2, if I2 > 50%, the level of heterogeneity was considered unacceptable, and the data were analyzed with a random-effect model. A fixed-effect model was applied when I2 < 50%. P < 0.05 indicated statistical significance, and all tests were two-sided. In our study, we performed analyses of different control groups [AEDs (excluding VPA) and other non-AEDs]. Obvious heterogeneity was assessed by subgroups based on the sample size, VPA dose, and treatment duration.
Results
Included Studies
A total of 1,169 records were retrieved from the Embase, PubMed, and Cochrane library databases. As shown in Figure 1, after duplicates were removed, 1,113 articles remained. During our initial screening process, the titles and abstracts were read, and we eliminated reviews, meta-analyses, single case studies, case series, conference abstracts, commentaries, letters, and editorials. Fifty-three human clinical studies remained. After the full-text review, 24 studies were excluded. Finally, based on the inclusion criteria, 29 RCT studies (a total of 1,986 participants) that presented information on VPA-associated tremor were included in the evaluation of tremor incidence (Table 1).
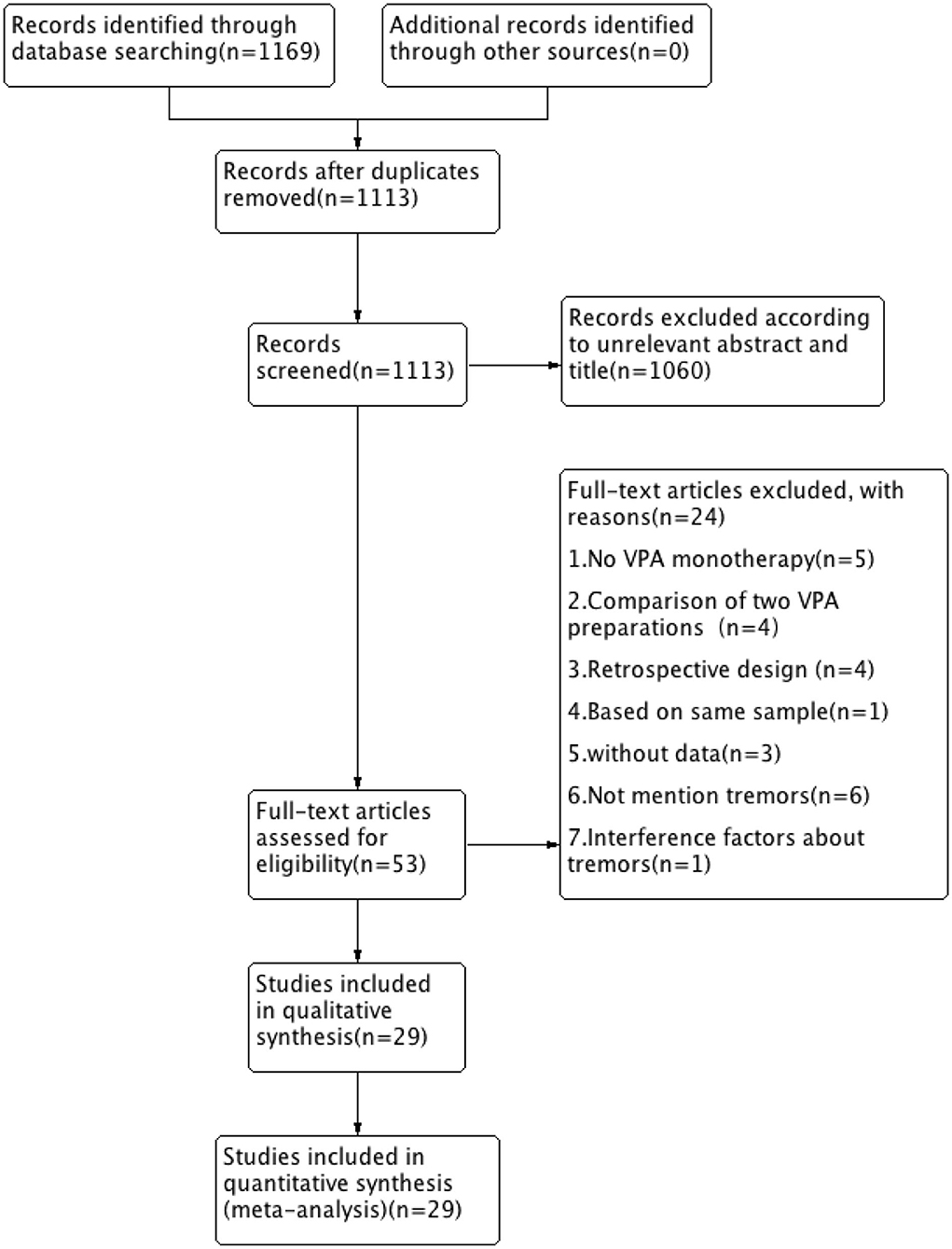
Figure 1. Flow chart of selection of studies about tremor in patients taking VPA therapy. VPA, valproic acid.
In one study (23), three different doses of VPA were used and compared with a placebo, so we counted this study as three trials. In three studies (24–26), two different AEDs were compared to VPA, so we considered these three studies as six trials. Thus, the total number of comparisons was 34. Most trials were multicenter trials and recruited patients from the USA and Europe, seven trials were conducted in Iran, and other studies were conducted in various countries, such as Korea, Germany, Turkey, and China. These trials included different diseases, all of which were treated by VPA, including epilepsy (15), migraine headache (11), and bipolar disorder (3).
The sample size of the included trials ranged from 33 to 613, and the number of participants treated with VPA ranged from 16 to 244. The age range of the study patients was 6–88 years. The mean VPA dosage ranged from 400 to 2,099 mg/day. The trial performed by Mattson, was the only one in which a 2,099-mg/day dose was tested. The treatment period ranged from 0.75 to 45 months.
Quality Assessment of Included Studies
The results of quality assessments of the included 29 studies are presented in Table 2.
Incidence of Tremor
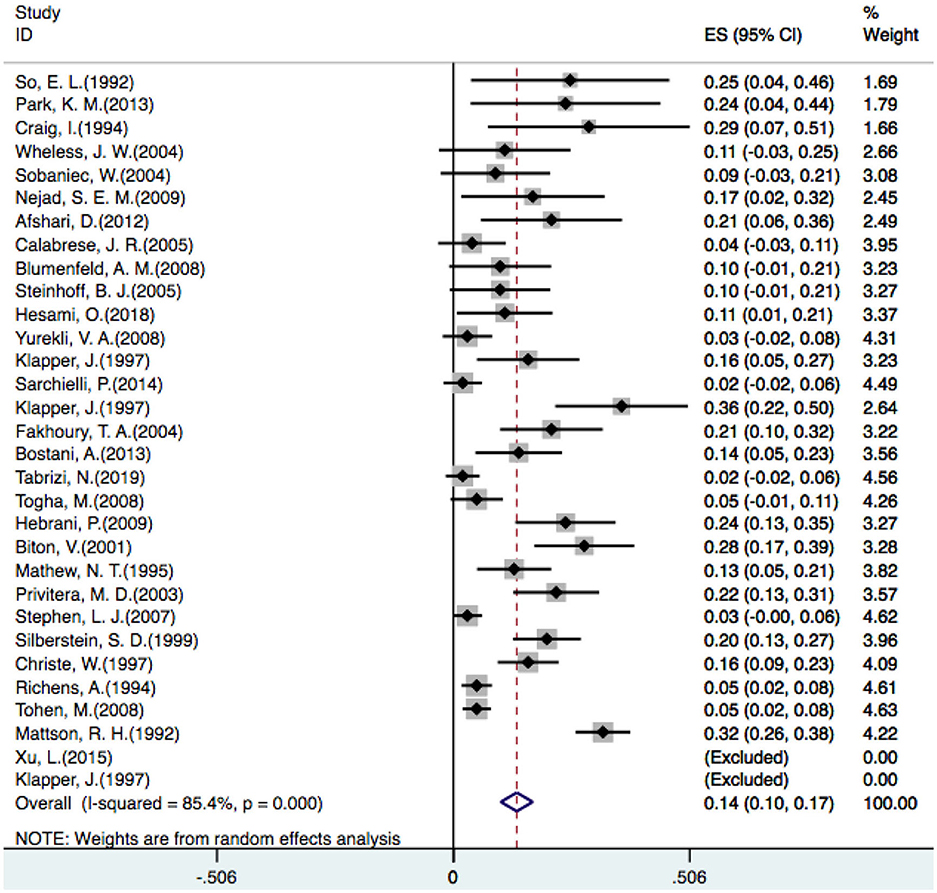
In one placebo-controlled trial (23), three different doses of VPA were compared with a placebo, so we counted it as three trials. Additionally, two studies did not identify patients who developed tremors (23, 48), so 29 studies were included in our analysis. A random-effect model revealed that the overall incidence of tremor with VPA treatment was 14% [OR = 0.14, 95% CI (0.10–0.17)] (Figure 2).
Comparison Between Various Drugs
Twenty-eight articles were included because one prospective study did not clearly determine the incidence of tremor in the control group (46). We independently evaluated the pooled ORs of VPA-induced tremor compared with tremor induced by all other drugs (other AEDs, other non-AEDs) to investigate the specific effect of VPA on tremors. According to our analysis, the use of VPA was significantly associated with an increased risk of tremor compared to that of the control group (Figure 3), including patients taking other drugs [28 articles, OR = 5.40, 95% CI (3.22–9.08)], other AEDs [17 articles, OR = 5.78, 95% CI (3.18–10.50)], and other non-AEDs [11 articles, OR = 4.77, 95% CI (1.55–14.72)]. In addition, we separately compared VPA to other AEDs and found a significant difference in the tremor risk between patients treated with VPA and other AEDs (Figure 4) [LTG OR = 7.46, 95% CI (3.43–16.20); CBZ OR = 3.53, 95% CI (1.91–6.50)]. However, there was no significant difference in the tremor risk between patients treated with VPA and TPM [OR = 4.35, 95% CI (0.681–27.76), P = 0.120]. There was only one article on OXC, LEV, and PHT. These comparisons exhibited heterogeneity, so random-effect models were used.
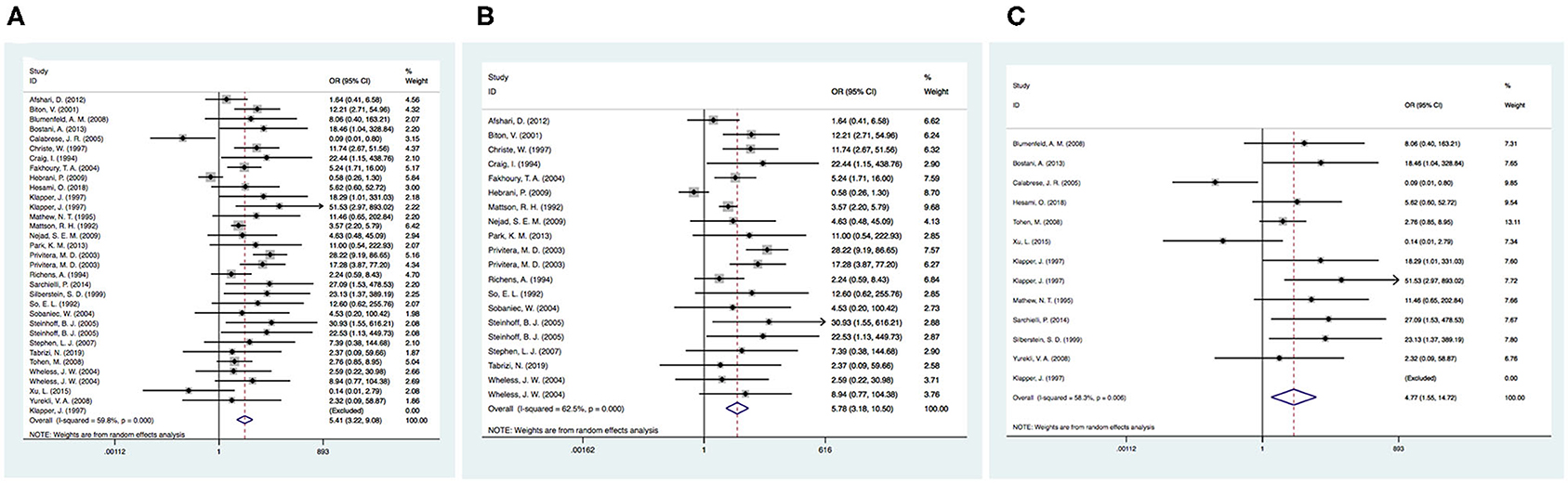
Figure 3. (A) The pooled OR of VPA-associated tremor compared with all other drugs. (B) The pooled OR of VPA-associated tremor compared with all other AEDs (except VPA), (C) The pooled OR of VPA-associated tremor compared with all other non-AEDs. AEDs, antiepileptic drugs; VPA, valproic acid.
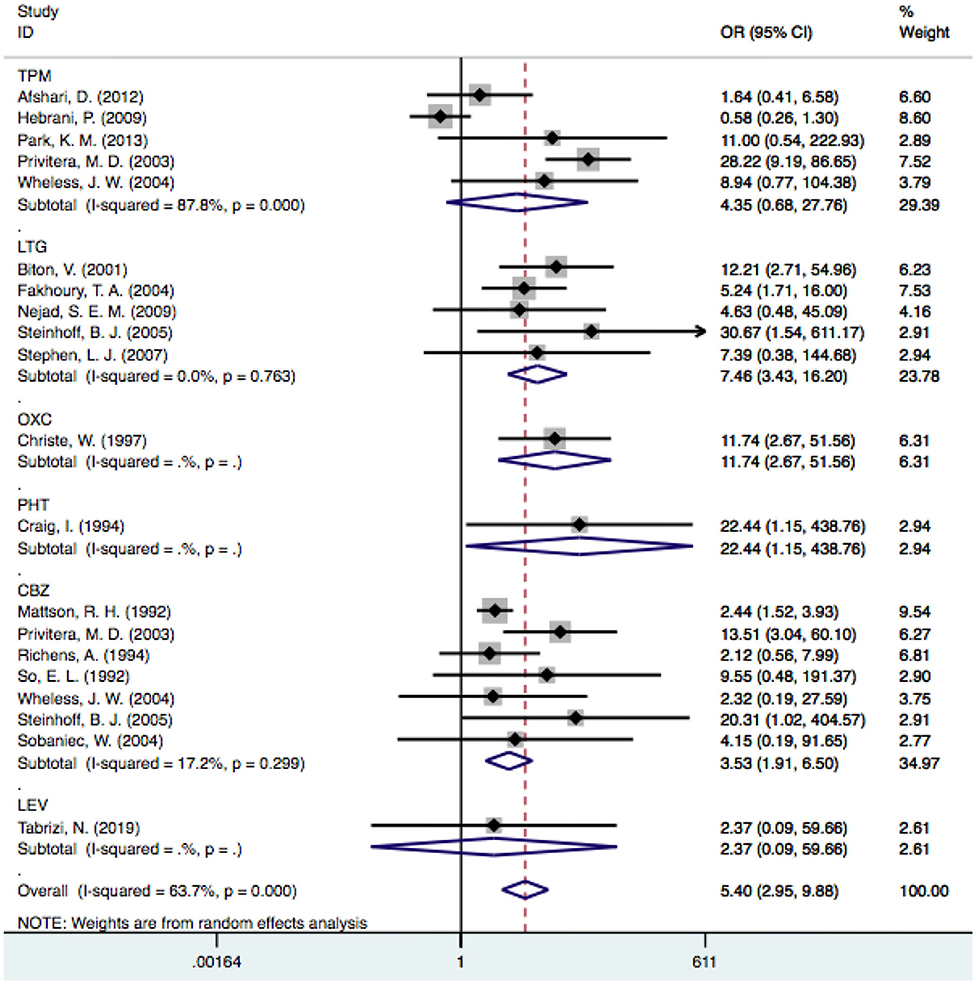
Figure 4. The pooled OR of VPA-associated tremor with other AEDs respectively. VPA, valproic acid; AEDs, antiepileptic drugs; TPM, topiramate; LTG, Lamotrigine; OXC, oxcarbazepine; PHT, phenytoin; CBZ, carbamazepine; LEV, levetiracetam.
Subgroup Analysis
The former comparisons of VPA-induced tremor compared with all drugs exhibited heterogeneity, and we considered that the sample size, VPA dosage, and follow-up time influenced the outcomes. Therefore, subgroup analysis was performed according to the following three factors.
Sample Size
In the subgroup analysis performed by sample size, we used a threshold of 100 patients. It was noted that the risk of VPA-associated tremors in the studies with more than 100 patients differed significantly from the risk of tremors associated with other drugs [OR = 6.23, 95% CI (3.35–11.59)], and this significant difference persisted in the groups with fewer than 100 patients [OR = 3.85, 95% CI (1.41–10.50)] (Figure 5). Among the studies with larger sample sizes, the risk of VPA-related tremors was larger than that in the studies with a small sample size, which reflected the stability of our meta-analysis. Neither group exhibited a significant difference in heterogeneity (I2 = 42.2% and I2 = 66.4%, respectively).
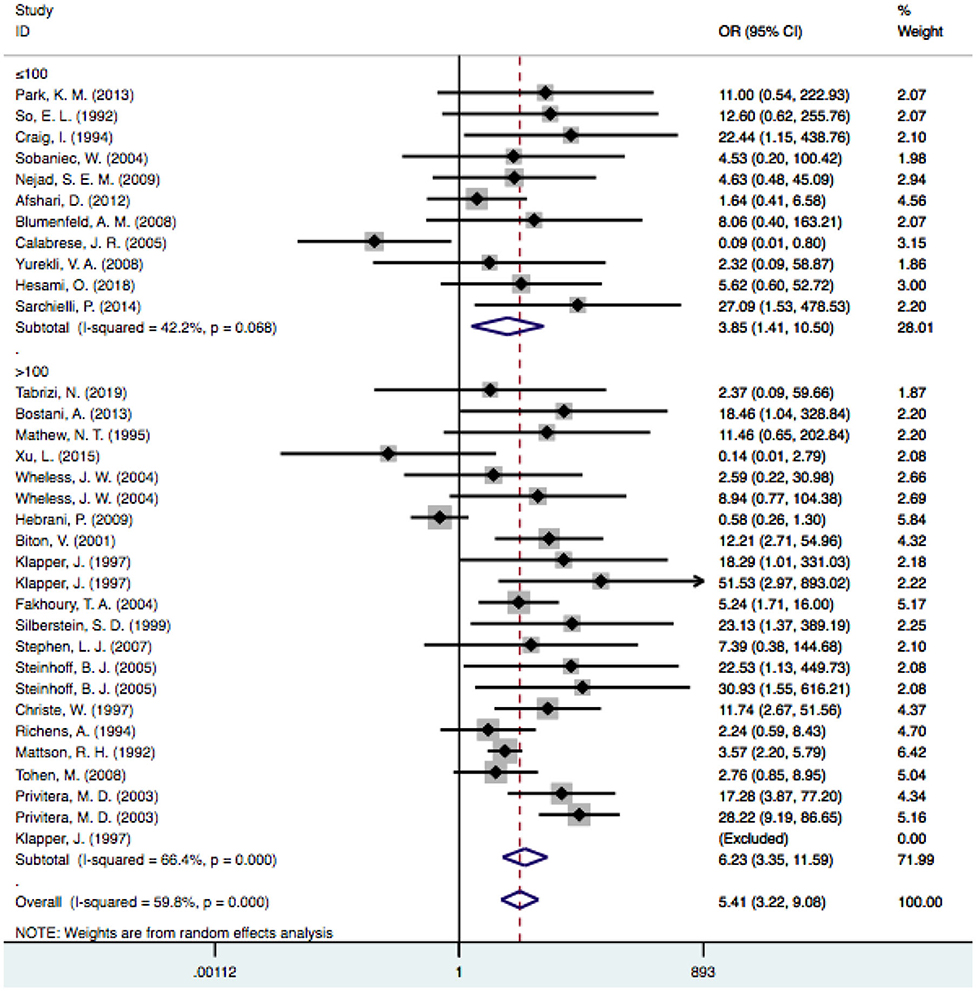
Figure 5. Subgroup analysis of VPA-associated tremor compared with other drugs according to the sample size. VPA, valproic acid.
VPA Doses Used
In the subgroup analysis of the doses, we excluded one study because it did not clearly provide the mean VPA dosage (33). According to the dosage used, we divided the patients into four subgroups: the ≤ 500-mg/d, 500–999-mg/d, 1,000–1,499-mg/d, and ≥1,500-mg/d subgroups. When the dose reached ≥1,500 mg/d, the level of heterogeneity was considered significant (I2 = 81.4% vs. I2 = 59.8%). Considering that dose-related factors may influence the results, we excluded articles that reported doses >1,500 mg/d (23, 27, 30, 36, 48), but the level of heterogeneity did not decrease significantly (I2: 54.6 vs. 59.8%). Therefore, we did not consider studies with a mean dose >1,500 mg/d as a source of heterogeneity. The pooled estimate for VPA-related tremors was significantly different from that of tremors related to other drugs at doses of 500 mg/d [OR = 3.57, 95% CI (1.24–10.26)], 500–999 mg/d [OR = 3.99, 95% CI (1.95–8.20)] and 1,000–1,499 mg/d [OR = 8.82, 95% CI (3.25–23.94)], and there were no statistically significant differences regarding other drugs at doses ≥1,500 mg/d [OR = 2.17, 95% CI (0.38–12.44); P = 0.381] (Figure 6).
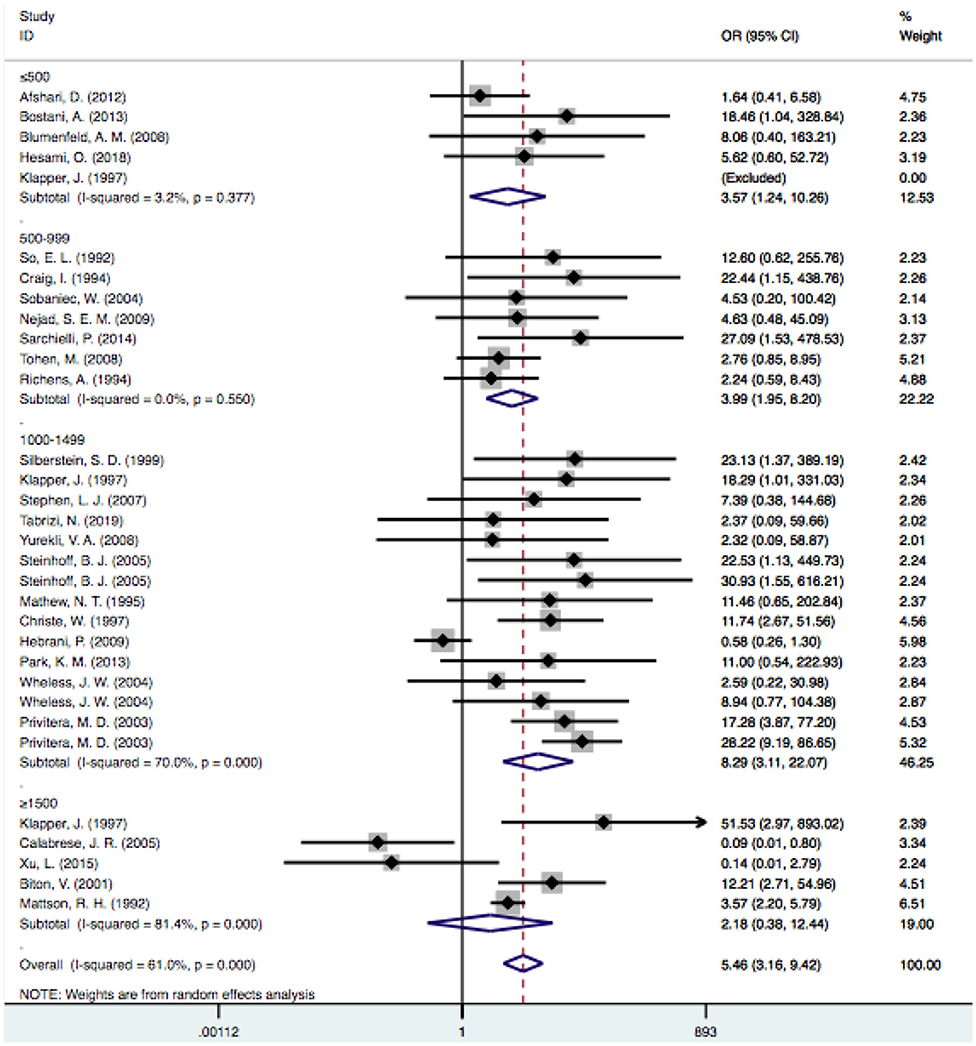
Figure 6. Subgroup analysis of VPA-associated tremor compared with other drugs according to the different drug dose. VPA, valproic acid.
Duration Times
Based on the follow-up time, the pooled estimate of VPA-associated tremor was statistically significantly higher than that of tremors associated with other drugs at duration times of ≤ 3 months [OR = 3.06, 95% CI (1.16–8.09)], 3–6 months [OR = 16.98, 95% CI (9.14–31.57)] and 6–12 months [OR = 4.15, 95% CI (2.74–6.29)]. VPA-induced tremor had a higher incidence during treatment durations of 6–12 months than during durations of ≤ 3 and 3–6 months. However, the risk of VPA-related tremor was not significantly different for durations >12 months [OR = 1.53, 95% CI (0.14–16.79); P=0.730] (Figure 7).
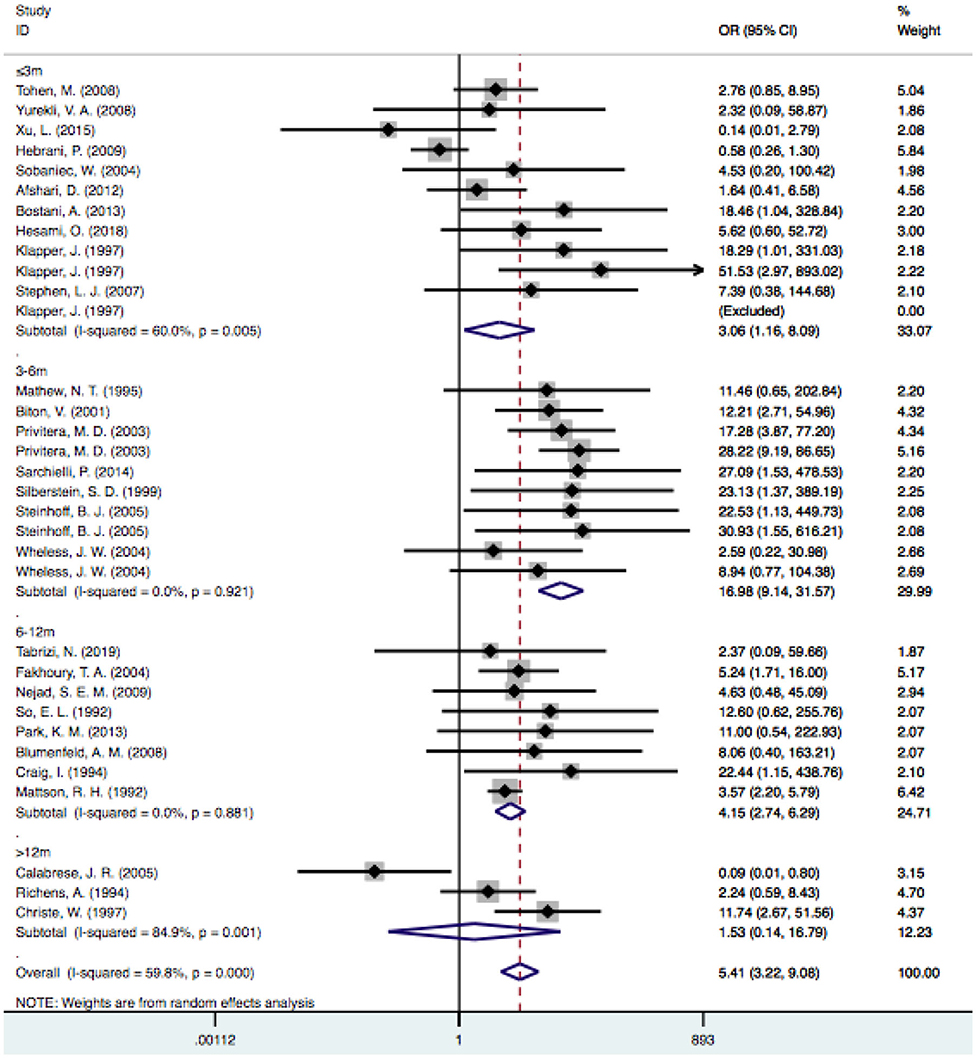
Figure 7. Subgroup analysis of VPA-associated tremor compared with other drugs according to the duration time. VPA, valproic acid.
Sensitivity Analysis
The stability of the pooled estimate was assessed by excluding ineligible studies one by one and reconducting the analysis. The outcomes of VPA-associated tremors were not significantly affected, indicating that the results of our analysis were stable (Figure 8).
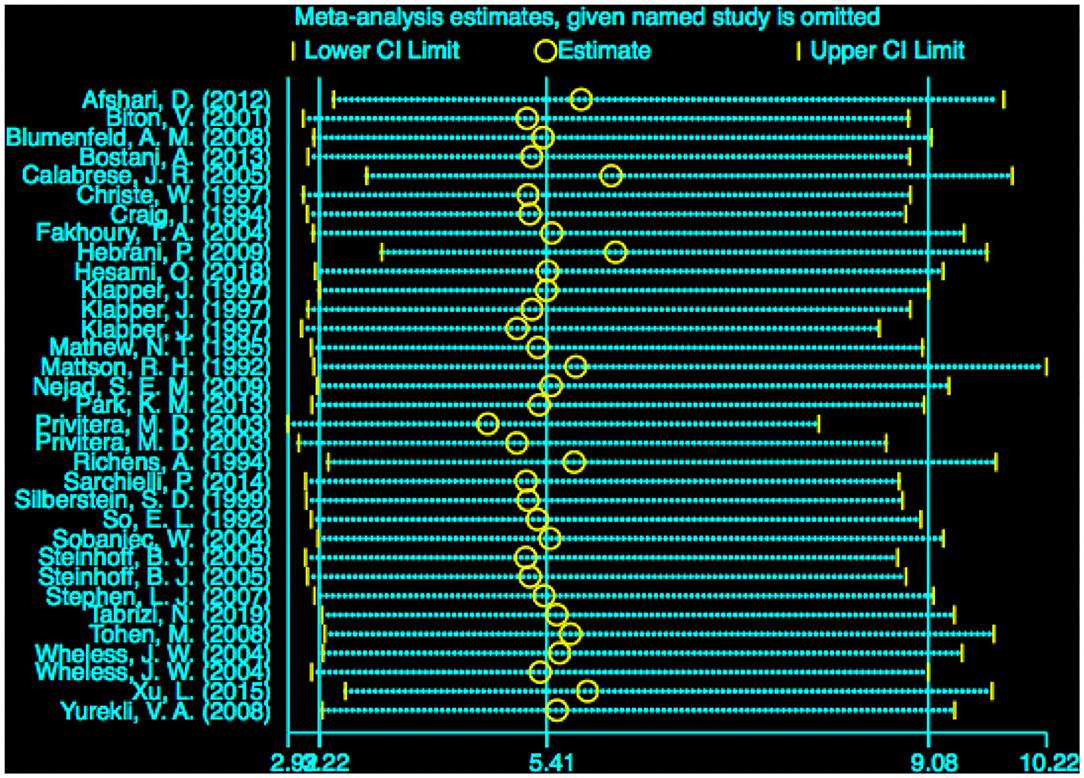
Figure 8. Sensitivity analysis of VPA-associated tremor compared with other drugs. VPA, valproic acid.
Publication Bias
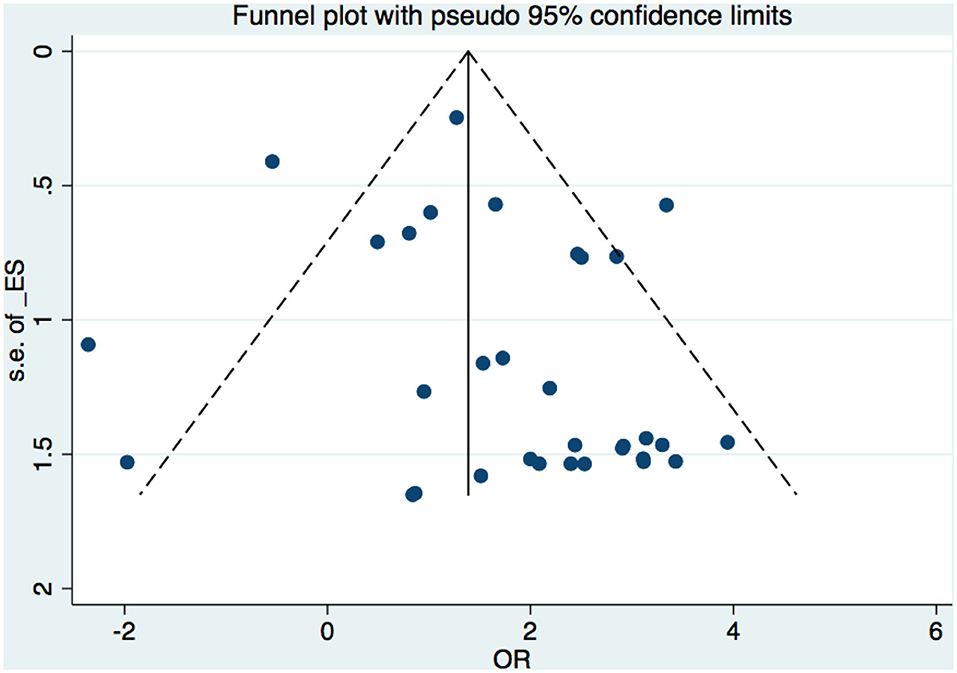
A funnel plot was used to detect bias in these studies, and the plot seemed to be asymmetrically distributed (Figure 9). Begg's test and Egger's test were conducted, and both tests indicated a lack of publication bias for VPA-associated tremors compared to tremors associated with other drugs (P = 0.922, P = 0.094, Figures 10, 11).
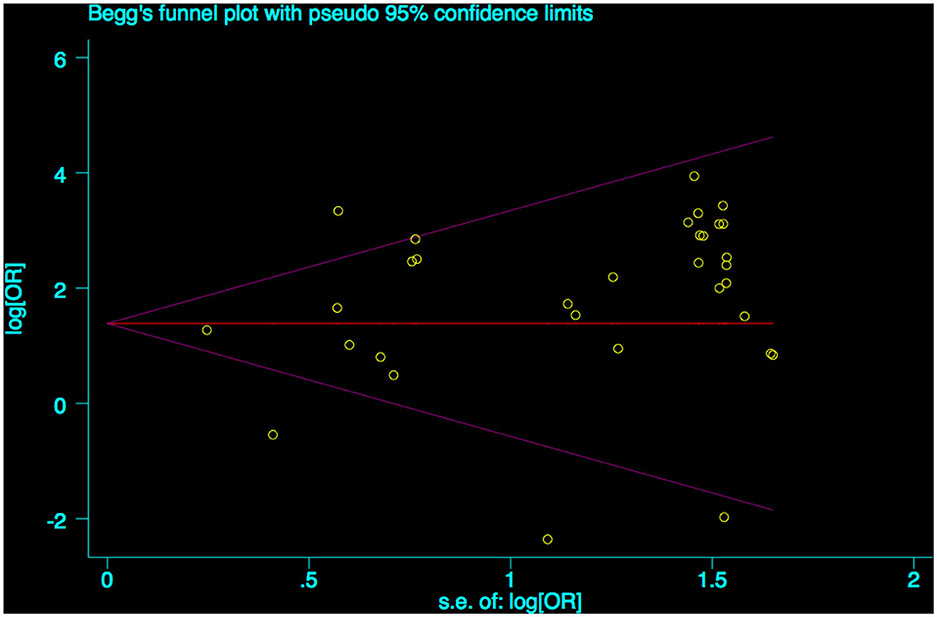
Figure 10. Begg's funnel plot test of publication bias for VPA-associated tremor. VPA, valproic acid.
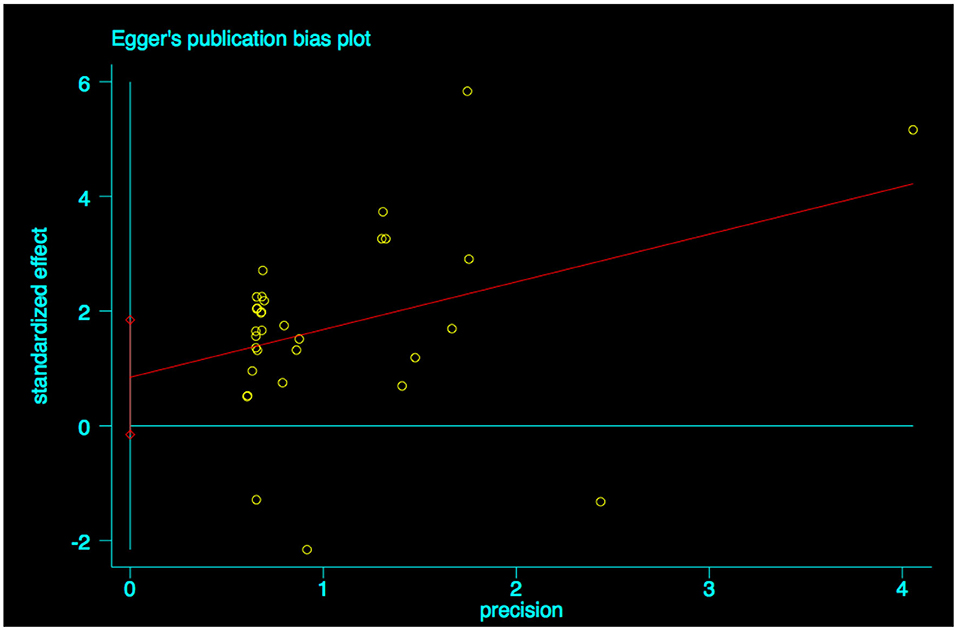
Figure 11. Egger's linear regression test of publication bias for VPA-associated tremor. VPA, valproic acid.
Discussion
According to previous studies, the evidence for VPA-induced tremor has been well documented. In epilepsy patients, VPA-related tremor is presumed to be one of the most common side effects, with an incidence of 6–64% (36, 39, 50). The mechanism underlying VPA-induced tremors is not completely understood; it has been suggested that the occurrence of tremors with VPA could be explained by the marked changes in the GABA synthesis rate in the substantia nigra and corpus striatum (51), and disturbances of the GABAergic pathways in the basal ganglia system may result in DA inhibition and subsequent changes in catecholamine (NE and E) concentrations (52–54). There is considerable evidence indicating that VPA increases the concentration of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), and this mechanism is thought to be a major cause of tremor (55, 56). The increase in transmitters occurs mainly through the following two pathways: (1) inhibition of the activation of glutamate transaminase and succinic acid dehydrogenase to reduce its metabolism and (2) activation and increase glutamate decarboxylase synthesis. At the same time, VPA increased GABA receptor-mediated hyperylation and inhibited the activation of the N-methyl-aspartic acid receptor. The factors of tremor severity may include the patient's sex and age and the dosage and formulations of VPA (55, 56). VPA often exaggerates familial tremor, and this sort of tremor becomes more prevalent and more noticeable as people age. However, whether the relation of VPA-associated tremor with aging is partly a consequence of previously unrecognized essential tremor cases worsening is unknown.
This meta-analysis provides a valuable and relatively complete description of the incidence and risk of VPA-associated tremor compared to tremor associated with other drugs. Our study included 29 RCT trials, and the overall incidence of tremor in patients treated with VPA was estimated to be 14%. According to Farkas et al. (57), the true incidence of VPA-induced tremor may be underestimated, as quantitative methods suggest that motor disturbances may predate symptoms of tremor. Tremor is probably not the first appearing motor adverse effect of valproate treatment. Quantitative methods might reveal a higher incidence of valproate-related motor disturbances than is currently considered (57). Furthermore, we observed a significant difference among patients treated with VPA and all other drugs (other AEDs, non-AEDs). Patients taking VPA had an approximately 4.5-fold higher (17.5 vs. 3.9%) risk of developing tremor than did patients taking other AEDs and an approximately 5-fold higher (11.7 vs. 2.5%) risk than did patients taking other non-AEDs. Compared to a single AED, the risk of VPA-induced tremor was greater than that of LTG- (7.47 times) and CBZ-induced tremor (3.53 times). Since only a few included studies reported the incidence of tremor associated with other AEDs, we were unable to calculate the pooled estimates. This meta-analysis could provide insights into alternative antiepileptic drugs to VPA.
Subgroup analyses were conducted to evaluate the impact of different drug dosages. VPA-induced tremor was found to be dose-related, but the means of the serum levels of VPA were within the normal therapeutic ranges (54). Patients with high blood VPA concentrations tend to develop adverse effects (58). VPA-induced tremor was reported to be reversible upon reduction or withdrawal of the drug and worsened as the dose increased (18, 57). Based on our meta-analysis, we divided the included trials into four groups (≤500 mg/d, 500–999 mg/d, 1,000–1,499 mg/d, ≥1,500 mg/d) according to the mean daily drug dose, and we drew a similar conclusion regarding VPA dose-related. A dose-dependent effect of VPA-related tremor was observed when the four dose groups were compared. Patients taking doses of 1,000–1,500 mg/d had an increased risk of developing tremor compared to patients who were administered both doses ≤ 500 mg/d and doses ranging from 500 to 999 mg/d. It is worth mentioning that VPA is often supplied as a sodium salt, and the molecular weight differed across the included studies. Therefore, future research should provide a more detailed description of the VPA dose.
Medication retention includes all possible reasons for effectiveness and intolerability (59), especially due to the side-effect profile (60). If patients experience VPA-related tremors, clinicians should consider whether the VPA dose is within the therapeutic dosage and whether it is possible to reduce the VPA dose. It may be a wise approach to choose another drug instead of VPA if the effect cannot be maintained after reducing the dosage.
According to the reports of Karas et al. (16), tremors occur as early as within 1 month of therapy. Tremors are present and exacerbated by intentional positions, and as the treatment continues, tremors gradually appear or become more severe. Our meta-analysis confirmed this result. We also divided the studies into four groups according to the follow-up time (≤ 3 m, 3–6 m, 6–12 m, >12 m); when we pooled the results of studies that followed up patients for 6–12 months, the risk of tremor was higher than that in studies that followed up patients for <3 months or from 3 to 6 months. This finding reminds us that the risk of VPA-related tremor may be time-related. At present, no studies have clearly examined the relation between the duration of treatment and risk of tremor. Hence, these data may lay the foundation for future studies, which may be profoundly meaningful for clinical therapy.
Due to the comprehensive search strategy used, high-quality RCTs were included, and the statistical heterogeneity of the outcomes in the pooled estimate analyses and stable sensitivity analysis results suggested our results have good reliability. However, our study also had several limitations. First, in the 29 trials, only 1,986 participants were treated with VPA, and among them, only a small number experienced tremor due to VPA therapy. Second, all the included studies were RCTs, but not all of the studies employed a double-blind design, so the quality of some studies was not relatively high. Third, VPA-related tremor is more common in elderly people and women, but relevant data were unavailable for us to perform a pooled estimation and confirm this conclusion. In addition, the number of the included studies and the AEDs included in comparisons were relatively small; therefore, we need to be cautious when drawing general conclusions.
Conclusion
VPA-associated tremor is more common than many clinicians have realized, with an overall incidence of 14%. VPA poses a higher risk of tremor than do other AEDs. VPA-induced tremor does depend on a dose <1,500 mg/d, and reducing the drug dose can reduce the risk. If the effect cannot be maintained, we recommend replacing VPA with other drugs (such as LTG or CBZ). Additionally, VPA-induced tremor can occur in every period after VPA treatment and gradually worsen over time. Patients taking VPA have a higher risk of experiencing tremor than do patients taking other drugs within 12 months, and patients should be advised about the risk. In summary, these results support more large population-based studies, and longer duration clinical trials are urgently needed to confirm whether VPA increases the risk of developing tremor compared to other drugs and whether the risk is related to dose and duration treatment times.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CqZ and HbS performed the experiments. BmH and HbS supervised the data collection. MlH and HbS wrote the initial draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
VPA, valproic aid; RCTs, randomized controlled trials; AEDs, antiepileptic drugs; FDA, Food and Drug Administration; PTSD, posttraumatic stress disorder; CBZ, carbamazepine; TPM, topiramate; VGT, vigabatrin; LTG, lamotrigine; TGB, gabapentin; ORs, odds ratios; CIs, confidence intervals.
References
1. Burton BS. On the propyl derivatives and decomposition products of ethylacetoacetate. Am Chem J. (1882) 3:385–95.
2. Meunier H, Carraz G, Meunier Y, Eymard P, Aimard M. Proprietes pharmacodynamiques de l'acide n-diproplyacetique. Therapie. (1963) 18:435–8.
3. Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al. A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder. Health Technol Assess. (2007) 11:iii–iv, ix−206. doi: 10.3310/hta11390
4. Adamou M, Puchalska S, Plummer W, Hale AS. Valproate in the treatment of PTSD: systematic review and meta analysis. Curr Med Res Opin. (2007) 23:1285–91. doi: 10.1185/030079907X188116
5. Wang Y, Xia J, Helfer B, Li C, Leucht S. Valproate for schizophrenia. Cochrane Database Syst Rev. (2016) 1:CD004028. doi: 10.1002/14651858.CD004028.pub4
6. Ross EL. The evolving role of antiepileptic drugs in treating neuropathic pain. Neurology. (2000) 55 (5 Suppl. 1):S41–6. Discussion: S54–48.
7. Bialer M, Yagen B. Valproic acid: second generation. Neurotherapeutics. (2007) 4:130–7. doi: 10.1016/j.nurt.2006.11.007
8. Brodie SA, Brandes JC. Could valproic acid be an effective anticancer agent? The evidence so far. Expert Rev Anticancer Ther. (2014) 14:1097–100. doi: 10.1586/14737140.2014.940329
9. Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. (2016) 15:210–8. doi: 10.1016/S1474-4422(15)00314-2
11. Hassamal S, Waller S, Reese K, Testa C. Reversible valproic acid-induced parkinsonism and cognitive impairment in an elderly patient with bipolar disorder I. Turk Psikiyatri Dergisi. (2016) 27:213. doi: 10.5080/u12251
12. Nasreddine W, Beydoun A. Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia. (2008) 49:438–45. doi: 10.1111/j.1528-1167.2007.01429.x
13. Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. (2018) 33:75–87. doi: 10.1002/mds.27121
14. Zadikoff C, Munhoz RP, Asante AN, Politzer N, Wennberg R, Carlen P, et al. Movement disorders in patients taking anticonvulsants. J Neurol Neurosurg Psychiatry. (2007) 78:147–51. doi: 10.1136/jnnp.2006.100222
15. Mattson RH, Cramer JA. Tremor due to sodium valproate. Neurology. (1981) 31:114. doi: 10.1212/WNL.31.1.113-b
16. Karas BJ, Wilder BJ, Hammond EJ, Bauman AW. Valproate tremors. Neurology. (1982) 32:428–32. doi: 10.1212/WNL.32.4.428
17. Block F, Dafotakis M. Drug-induced tremor. Fortschr Neurol Psychiatr. (2011) 79:570–5. doi: 10.1055/s-0031-1281687
18. Hyman NM, Dennis PD, Sinclair KG. Tremor due to sodium valproate. Neurology. (1979) 29:1177–80. doi: 10.1212/WNL.29.8.1177
19. Afshari D, Rafizadeh S, Rezaei M. A comparative study of the effects of low-dose topiramate versus sodium valproate in migraine prophylaxis. Int J Neurosci. (2012) 122:60–8. doi: 10.3109/00207454.2011.626908
20. Hesami O, Sistanizad M, Asadollahzade E, Johari MS, Beladi-Moghadam N, Mazhabdar-Ghashghai H. Comparing the effects of atorvastatin with sodium valproate (divalproex) on frequency and intensity of frequent migraine headaches: a double-blind randomized controlled study. Clin Neuropharmacol. (2018) 41:94–7. doi: 10.1097/WNF.0000000000000280
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
23. Klapper J. Divalproex sodium in migraine prophylaxis: a dose-controlled study. Cephalalgia. (1997) 17:103–8. doi: 10.1046/j.1468-2982.1997.1702103.x
24. Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. (2003) 107:165–75. doi: 10.1034/j.1600-0404.2003.00093.x
25. Steinhoff BJ, Ueberall MA, Siemes H, Kurlemann G, Schmitz B, Bergmann L, et al. The LAM-SAFE Study: lamotrigine versus carbamazepine or valproic acid in newly diagnosed focal and generalised epilepsies in adolescents and adults. Seizure. (2005) 14:597–605. doi: 10.1016/j.seizure.2005.09.011
26. Wheless JW, Neto W, Wang S. Topiramate, carbamazepine, and valproate monotherapy: double-blind comparison in children with newly diagnosed epilepsy. J Child Neurol. (2004) 19:135–41. doi: 10.1177/08830738040190020901
27. Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. (2001) 56:172–7. doi: 10.1212/WNL.56.2.172
28. Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. (2008) 48:210–20. doi: 10.1111/j.1526-4610.2007.00949.x
29. Bostani A, Rajabi A, Moradian N, Razazian N, Rezaei M. The effects of cinnarizine versus sodium valproate in migraine prophylaxis. Int J Neurosci. (2013) 123:487–93. doi: 10.3109/00207454.2013.765419
30. Calabrese JR, Shelton MD, Rapport DJ, Youngstrom EA, Jackson K, Bilali S, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. (2005) 162:2152–61. doi: 10.1176/appi.ajp.162.11.2152
31. Christe W, Krämer G, Vigonius U, Pohlmann H, Steinhoff BJ, Brodie MJ, et al. A double-blind controlled clinical trial: oxcarbazepine versus sodium valproate in adults with newly diagnosed epilepsy. Epilepsy Res. (1997) 26:451–60. doi: 10.1016/S0920-1211(96)01013-3
32. Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single? Blind randomized comparative study. Epilepsia. (1994) 35:381–90. doi: 10.1111/j.1528-1157.1994.tb02448.x
33. Fakhoury TA, Hammer AE, Vuong A, Messenheimer JA. Efficacy and tolerability of conversion to monotherapy with lamotrigine compared with valproate and carbamazepine in patients with epilepsy. Epilepsy Behav. (2004) 5:532–8. doi: 10.1016/j.yebeh.2004.04.006
34. Hebrani P, Behdani F, Manteghi AA. Double-blind, randomized, clinical trial of topiramate versus sodium valproate for the treatment of bipolar disorder in adolescents. Pak J Med Sci. (2009) 25:247–52.
35. Mathew NT, Saper JR, Silberstein SD. Migraine prophylaxis with divalproex. Arch Neurol. (1995) 52:281–6. doi: 10.1001/archneur.1995.00540270077022
36. Mattson RH, Cramer JA, Collins JF, Mccutcheon CB, Fish SL, Mamdani MB, et al. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. N Engl J Med. (1992) 327:765–71. doi: 10.1056/NEJM199209103271104
37. Nejad SEM, Nikpour MRA, Rahim F, Nahibi SN, Bahrammi MA. A randomized open-label comparison of lamotrigine and valproate in patients with juvenile myoclonic epilepsy. Int J Pharmacol. (2009) 5:313–8. doi: 10.3923/ijp.2009.313.318
38. Park KM, Kim SH, Nho SK, Shin KJ, Park J, Ha SY, et al. A randomized open-label observational study to compare the efficacy and tolerability between topiramate and valproate in juvenile myoclonic epilepsy. J Clin Neurosci. (2013) 20:1079–82. doi: 10.1016/j.jocn.2012.10.020
39. Richens A, Davidson D, Cartlidge N, Easter D. A multicentre comparative trial of sodium valproate and carbamazepine in adult onset epilepsy. Adult EPITEG Collaborative Group. J Neurol Neurosurg Psychiatry. (1994) 57:682–7. doi: 10.1136/jnnp.57.6.682
40. Sarchielli P, Messina P, Cupini LM, Tedeschi G, Di Piero V, Livrea P, et al. Sodium valproate in migraine without aura and medication overuse headache: a randomized controlled trial. Euro Neuropsychopharmacol. (2014) 24:1289–97. doi: 10.1016/j.euroneuro.2014.03.010
41. Silberstein SD, Collins SD. Long-term safety of depakote in headache prophylaxis study group. Safety of divalproex sodium in migraine prophylaxis: an open-label, long-term study. Headache. (1999). 39:633–43. doi: 10.1046/j.1526-4610.1999.3909633.x
42. So EL, Lai CW, Pellock J, Mashman JA. Safety and efficacy of valproate and carbamazepine in the treatment of complex partial seizures. J Epilepsy. (1992) 5:149–52. doi: 10.1016/S0896-6974(05)80133-1
43. Sobaniec W, Kułak W, Smigielska-Kuzia J, Boćkowski L, Majkowski J, Jedrzejczak J. A multicenter, placebo-controlled, double-blind study of efficacy of a new form of carbamazepine (Carbatrol∧R) in refractory epileptic patients. Polish J Pharmacol. (2004) 56:195–202.
44. Stephen LJ, Sills GJ, Leach JP, Butler E, Parker P, Hitiris N, et al. Sodium valproate versus lamotrigine: a randomised comparison of efficacy, tolerability and effects on circulating androgenic hormones in newly diagnosed epileps. Epilepsy Res. (2007) 75:122–9. doi: 10.1016/j.eplepsyres.2007.04.009
45. Tabrizi N, Zarvani A, Rezaei P, Cheraghmakani H, Alizadeh-Navaei R. Levetiracetam in genetic generalized epilepsy: a prospective unblinded active-controlled trial. Epilepsy Res. (2019) 157:106214. doi: 10.1016/j.eplepsyres.2019.106214
46. Togha M, Rahmat Jirde M, Nilavari K, Ashrafian H, Razeghi S, Kohan L. Cinnarizine in refractory migraine prophylaxis: efficacy and tolerability. A comparison with sodium valproate. J Headache Pain. (2008) 9:77–82. doi: 10.1007/s10194-008-0013-2
47. Tohen M, Vieta E, Goodwin M, Sun B, Amsterdam JD, Banov M, et al. Olanzapine versus divalproex versus placebo in the treatment of mild to moderate mania: a randomized, 12-week, double-blind study. J Clin Psychiatry. (2008) 69:1776–89. doi: 10.4088/JCP.v69n1113
48. Xu L, Lu Y, Yang Y, Zheng Y, Chen F, Lin Z. Olanzapine–valproate combination versus olanzapine or valproate monotherapy in the treatment of bipolar imania: a randomized controlled study in a chinese population group. Neuropsychiatr Dis Treat. (2015) 11:1265–71. doi: 10.2147/NDT.S81146
49. Yurekli VA, Akhan G, Kutluhan S, Uzar E, Koyuncuoglu HR, Gultekin F. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. (2008) 9:37–41. doi: 10.1007/s10194-008-0002-5
50. Beydoun A, Sackellares J, Shu VJN. Safety and efficacy of divalproex sodium monotherapy in partial epilepsy: a double-blind, concentration-response design clinical trial. Neurology. (1997) 48:182–8. doi: 10.1212/WNL.48.1.182
51. Kanner AM, Balabanov A. Valproate: a practical review of its uses in neurological and psychiatric disorders. Expert Rev Neurother. (2002) 2:151–65. doi: 10.1586/14737175.2.2.151
52. Isaias IU, Marzegan A, Pezzoli G, Marotta G, Canesi M, Biella GE, et al. A role for locus coeruleus in Parkinson tremor. Front Hum Neurosci. (2011) 5:179. doi: 10.3389/fnhum.2011.00179
53. Hamed SA, Elserogy YM, Abd-Elhafeez HA. Psychopathological and peripheral levels of neurobiological correlates of obsessive-compulsive symptoms in patients with epilepsy: a hospital-based study. Epilepsy Behav. (2013) 27:409–15. doi: 10.1016/j.yebeh.2013.01.022
54. Hamed SA, Abdellah MM. The relationship between valproate induced tremors and circulating neurotransmitters: a preliminary study. Int J Neurosci. (2017) 127:236–42. doi: 10.1080/00207454.2016.1181631
55. Gutiérrez-Gutiérrez G, Poza-Aldea JJ, Tellería-Elmezábal J, et al. A retrospective study comparing two formulations of valproic acid. Rev Neurol. (2006) 42:129–32. doi: 10.33588/rn.4203.2005351
56. Xiao Y, Xiong W, Lu L, Chen J, Zhang Y, Jiang X, et al. The clinical characteristics and related factors of tremor in patients with epilepsy. Seizure. (2019) 66:70–5. doi: 10.1016/j.seizure.2019.02.002
57. Farkas Z, Gulyás S, Molnár R, Szirmai I, Kamondi AJS. Quantitative analysis of motor performance in epilepsy patients treated with valproate. Seizure. (2010) 19:173–7. doi: 10.1016/j.seizure.2010.01.013
58. Klotz U, Schweizer CJ. Valproic acid in childhood epilepsy: anticonvulsive efficacy in relation to its plasma levels. Int J Clin Pharmacol Ther Toxicol. (1980) 18:461–5.
59. Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. (2007) 16:296–304. doi: 10.1016/j.seizure.2007.01.004
Keywords: valproic aid, tremor, random control trials, meta-analysis, systematic reveiw
Citation: Zhang Cq, He Bm, Hu Ml and Sun Hb (2020) Risk of Valproic Acid-Related Tremor: A Systematic Review and Meta-Analysis. Front. Neurol. 11:576579. doi: 10.3389/fneur.2020.576579
Received: 26 June 2020; Accepted: 09 October 2020;
Published: 15 December 2020.
Edited by:
Rosanna Cardani, IRCCS Policlinico San Donato, ItalyReviewed by:
Mervyn Eadie, The University of Queensland, AustraliaCarlo Trompetto, University of Genoa, Italy
Copyright © 2020 Zhang, He, Hu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong bin Sun, c2hiMTM2OUBhbGl5dW4uY29t
 Chen qi Zhang
Chen qi Zhang Hong bin Sun
Hong bin Sun