
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 15 February 2021
Sec. Multiple Sclerosis and Neuroimmunology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.574748
Background: Multiple sclerosis (MS), a disabling demyelinating disease of the central nervous system, is associated with cognitive impairment, spasticity, and fatigue. There are still no established guidelines on the management of MS-related sequela. Memantine has the potential to reduce glutamate toxicity, thereby reducing consequent cognitive impairment, spasticity, and fatigue.
Objectives: This study aims to determine the efficacy and safety of memantine in preventing cognitive impairment, reducing spasticity and fatigue, and controlling disability in MS patients through a review of relevant randomized trials.
Methods: MEDLINE, CENTRAL, Scopus, Embase, LILACS, ClinicalTrials.gov, and HERDIN were searched from inception to May 2020 for relevant trials.
Results: The search yielded 203 articles; four studies were included in the analysis. Pooled evidence shows that memantine compared with placebo does not significantly improve PASAT, ASS, MFIS, and EDSS scores of patients with MS. Memantine is associated with mild adverse drug events such as dizziness, fatigue, and anxiety.
Conclusion: There is not enough evidence to support the efficacy of memantine in preventing cognitive decline, controlling spasticity, reducing fatigue, and preventing disability. Future researches should consider the different MS subtypes, effect of co-administration of disease-modifying therapies, longer duration of administration, and more sensitive outcome measures to evaluate the potential benefit of memantine in MS.
Multiple sclerosis (MS) is a disabling inflammatory demyelinating disease affecting the central nervous system (CNS) (1). With an increasing prevalence of 50–300 per 100,000 individuals, it is the most common non-traumatic neurologic cause of permanent disability among young adults (2–4).
Cognitive impairment affects 45–65% of patients with MS causing considerable burden and disability (5). Previous studies suggest that although cognitive impairment is prevalent among patients with relapsing-remitting phenotype, more severe forms of impairment are observed among patients with primary progressive and secondary progressive diseases (6). It should be noted, however, that the widely used scale to assess disability in MS, the Expanded Disability Status Scale (EDSS), poorly describes cognitive impairment in MS (7). Early studies suggest that cognitive impairment in MS is a form of white matter subcortical dementia presenting with disruption in memory, processing speed, and executive function (8). A recent case–control study concluded that patients with MS have more pronounced deep gray matter atrophy compared with controls, supporting the claim that cognitive impairment in MS may also be due to cortical and deep gray matter lesions (9). Another proposed mechanism is glutamate excitotoxicity. This is supported by in vivo models which showed that a milieu of elevated glutamate activity causes neuronal excitotoxicity and subsequent cognitive decline (10).
There are still no established clinical guidelines on the management of cognitive impairment in MS. Recent studies suggest that disease-modifying therapies (DMT) significantly reduce the effect of neurodegeneration and subsequent cognitive impairment (11, 12). Studies investigating the role of drugs commonly used for dementia such as rivastigmine and donepezil for cognitive impairment in MS failed to demonstrate a significant benefit (13, 14).
Damage to the descending motor tracts brought about by autoimmune processes and subsequent neuroplasticity seen in MS is thought to cause disinhibition of motor reflexes leading to spasticity (15). Early studies involving rodent models of MS also suggest the role of glutamate toxicity in spasticity (16). Although cannabinoids, gamma amino butyric acid receptor agonists, imidazoline and alpha 2 receptor agonists, certain anti-epileptic drugs, and ryanodine receptor antagonists have been used for the symptomatic management of spasticity, there are still no specific guidelines on the treatment of MS-related spasticity (15).
Fatigue, or paucity of physical and mental drive, affects 83.1% of patients with MS (17). MS-related fatigue is understood to be related to axonal injury secondary to glutamate toxicity as manifested by the reduction in N-acetylaspartate levels seen in multivoxel spectroscopic studies (18, 19). Several non-pharmacologic modalities (cognitive behavioral therapy, exercise, and energy conservation) and pharmacologic agents (modafinil, amantadine, methylphenidate, and aspirin) have shown to decrease fatigue symptoms using several scales; however, the findings of these trials have been inconsistent (18).
Memantine, a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, has been theorized to have a fundamental role in reducing glutamate toxicity (20). This agent has been the focus of recent clinical trials investigating its potential benefit in cognitive impairment (21, 22), spasticity (23), and fatigue (24) among patients with MS.
This study aims to determine the efficacy and safety of memantine among patients with multiple sclerosis in terms of improvement in cognitive function and reduction of spasticity, fatigue, and disability using a review of relevant trials.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) consensus guidelines were followed in this review (25).
We considered clinical trials that employed randomized, double-blind, parallel group, placebo- and/or active-controlled designs in this review. Studies using other designs such as quasi-experimental, cluster-randomized, cross-over, prospective or retrospective cohort, case–control, and cross-sectional designs were excluded to prevent selection bias and the possibility of carryover effects of the intervention. We included trials involving patients who were diagnosed with multiple sclerosis satisfying the 2017 McDonald criteria (26, 27) regardless of the MS subtype (relapsing-remitting, secondary progressive, and primary progressive multiple sclerosis) including previous versions of this criteria. No restrictions in terms of age, sex, ethnicity, disease severity, and disease activity were employed. We included studies utilizing memantine per orem given at least 20 mg/day as the intervention compared with placebo and/or active agent/s. No restrictions in terms of concurrent or prior utilization of DMTs and other immunosuppressive drugs were implemented in this study. All trials tagged as primary researches, reported in English, and available as full-text articles were included.
• Change in Paced Auditory Serial Addition Test (PASAT) score—The PASAT is a neuropsychological test with a 0–60 scoring system with one-point increments. A positive change means improvement in information processing and sustained attention.
• Change in Ashworth Spasticity Scale (ASS) score—The ASS is a 0–4 scale with one-point increments for spasticity. A higher score signifies a higher degree of spasticity.
• Change in Modified Fatigue Impact Scale (MFIS) score—The MFIS is a 0–84 multidimensional scale used to assess perceived impact of fatigue in terms of physical, cognitive, and psychosocial aspects. A higher score means a higher perceived negative impact.
• Change in Expanded Disability Status Scale (EDSS) score—The EDSS is a 0–10 disability scale with 0.5-point increments wherein a higher score means greater degree of disability.
• Adverse drug events (ADE)—The proportion of participants who experienced any serious and non-serious adverse drug event after drug administration assessed at a defined time.
Other outcome measures that assess cognitive function and spasticity in patients with MS are summarized in the Supplementary Material.
The following electronic databases were searched for relevant trials: MEDLINE by PubMed, Cochrane Central Register for Controlled Trials (CENTRAL), Scopus, Embase, Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), ClinicalTrials.gov website, and HERDIN Database of the Philippines. The following general and MeSH term-based search strategy was employed: (memantine OR memantin OR 1,3-dimethyl-5-aminoadamantane OR 1-amino-3,5-dimethyladamantane OR namenda OR ebixa OR memantine hydrochloride OR axura OR D-145 OR D 145 OR D145) AND multiple sclerosis AND (randomized controlled trial OR control OR clinical trial OR random OR placebo OR trial OR groups OR assign OR allocation OR volunteer). Search strategies used in other databases are summarized in the Supplementary Material.
The Cochrane Collaboration Tool was used in the assessment of risk bias of the included studies.
The following details were collected and collated appropriately from the included trials: study design, participants, intervention details for the treatment group and the control/placebo group, and relevant outcomes described above.
Mean differences with 95% confidence intervals were used to measure treatment effect for the continuous outcomes, while risk ratios (RR) of benefit or harm with 95% confidence intervals were used for the dichotomous outcomes.
Syntheses of data were performed using the RevMan (computer program) (Version 5.4. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Meta-analyses were performed using the fixed-effects model. For the continuous outcomes, the inverse variance method was used, while for the dichotomous outcomes, the Mantel–Haenszel method was employed. Statistical significance was reached if the 95% CI of the mean difference did not include the number zero for the continuous outcomes. For the dichotomous outcomes, statistical significance was noted if the 95% CI of the RR did not include the number one.
Clinical heterogeneity was assessed by comparing the population, intervention, comparison, and outcome measures in all the included studies. Methodological heterogeneity was evaluated by comparing the study designs and the risk of bias in the trials. Statistical heterogeneity was evaluated using the χ2-test with a p-value < 0.10 to indicate statistically significant heterogeneity. The degree of heterogeneity was measured using I2 statistic with values 0 to 40% indicating unimportant statistical heterogeneity (28).
A total of 203 articles from major databases were retrieved. Forty-five records were identified as duplicates and were discarded. A total of 158 records were screened and 150 were excluded, of which 66 were review articles, 58 were studies that focused on diseases other than MS, 13 were conference proceedings, 11 were trials which studied drugs other than memantine, 1 was an animal study, and another was a case–control study. The remaining eight records were subjected to eligibility testing and four studies were excluded. A total of four studies were included in the qualitative and quantitative syntheses. The PRISMA flow diagram is shown in Figure 1. All the included studies employed a double-blind, randomized, placebo-controlled design (21–24). The characteristics of the included studies are summarized in Table 1, and the characteristics of the excluded studies are shown in the Supplementary Material.
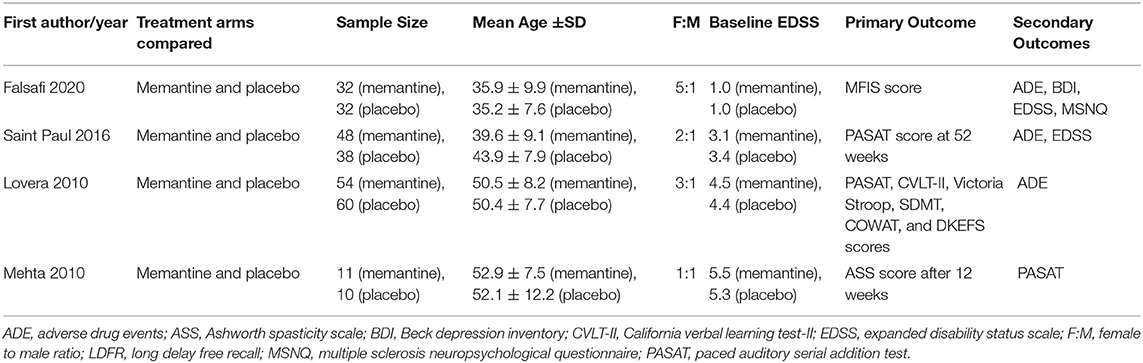
Table 1. Included studies, corresponding treatment arms compared, sample characteristics, and outcome measures.
A total of 285 patients were analyzed in the four trials. Table 2 summarizes the characteristics of the patients in the included trials. The majority were females with relapsing-remitting MS subtype. North Americans and Europeans comprise the majority of the participants. The baseline mean EDSS score ranged from 1.0 to 5.5.
All studies compared memantine and placebo. Three trials used the same memantine titration schedule: 5 mg once daily during the first week, 5 mg twice daily during the second week, 5 mg in the morning and 10 mg in the evening during the third week, and 10 mg twice daily thereafter. One trial administered memantine at 10 mg daily for the first week followed by 20 mg daily throughout the duration of the trial (24). One trial allowed dose reduction based on the maximum tolerated dose (21). One trial administered memantine for 52 weeks (22), while the rest administered the treatment for 12 weeks.
All four trials are deemed to have low risk for selection, performance, and detection biases as all of them employed a randomized double-blind design. Two studies are deemed to have unclear risk for attrition bias given the attrition rates of 28 and 38% in the placebo and treatment groups, respectively, in the study by Saint Paul et al. (22) and 25 and 50% in the placebo and treatment groups, respectively, in the study by Falsafi et al. (24). No other potential risk of bias is noted in the included trials. Figure 2 summarizes the risk of bias assessment.
Three studies were merged to evaluate the effect of memantine compared with placebo on cognitive function of MS patients using the PASAT score. No significant difference in mean difference of PASAT score is noted between the memantine and placebo groups [MD (95% CI) = −0.01 (−0.47, 0.45), p = 0.99, I2 = 0%] (see Figure 3). One study (21) utilized other outcome measures for cognitive functions: California Verbal Learning Test–II (CVLT-II), Victoria Stroop, Symbol Digit Modalities Test (SDMT), Controlled Oral Word Association Test (COWAT), and Delis–Kaplan Executive Function System (DKEFS). There were no significant differences in mean changes of scores between the memantine and placebo groups [difference −0.6 (95% CI −2, 0.8), 0.0 (−0.2, 0.2), −0.4 (−3.2, 2.3), 0.4 (−3.5, 4.3), and −0.3 (−0.9, 0.3), respectively]. The trial by Falsafi et al. (24) also did not find a significant difference in mean change of MSNQ score between the memantine and placebo groups [difference −4.3 (95% CI −10.0 to 1.5)].
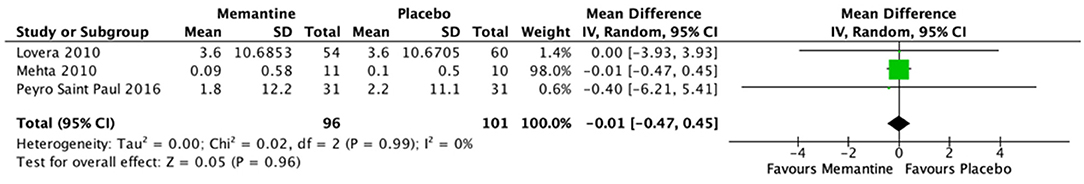
Figure 3. Forest plot of mean difference in PASAT scores for studies comparing memantine and placebo.
Of the four included studies, only the trial by Mehta et al. (23) assessed the effect of memantine on spasticity. The mean difference in ASS scores between the placebo (1.0, SD 2.67) and memantine (1.55, SD 2.81) groups before and after 12 weeks of treatment is not significantly different (p = 0.65; 95% CI −1.96, 3.05).
One trial studied the effect of memantine in MS-associated fatigue. Mean change from baseline MFIS score is not significantly different between the treatment and control groups [between-group difference = −1.9, 95% CI (−11.7 to 7.8), p = 0.702].
In the two trials that evaluated the effect of memantine on disability using EDSS, no significant difference is observed in the mean difference of the baseline and post-treatment EDSS scores between the memantine and placebo groups [adjusted mean difference = −0.28, 95% CI (−0.72, 0.17), p = 0.22, N = 86] (22). In another trial, there was also no significant difference in the mean difference of the baseline and post-treatment degree of disability between groups using the MSFC outcome measure [mean difference = −0.06, 95% CI (−0.27, 0.16), p = 0.58, N = 21] (23).
The rates of adverse drug events among patients allocated to memantine range from 0 to 27.1% (21, 22). The most common ADEs are as follows: dizziness (1.85–27.1%), headache (27.1%), bladder infection (12.96%), fatigue (11.11–12.5%), diarrhea (10.4%), cough (9.26%), agitation (8.33%), somnolence (5.56%), ataxia (5.56%), anxiety (4.17%), constipation (3.7%), spasticity (3.7%), and rash (3.7%). The rates of other rare ADEs, including speech impairment, confusion, dizziness, and nervousness, range from 1.85 to 2.08%. In one trial involving the administration of memantine for 12 weeks, the rate of ADEs in the memantine group is not significantly different from that of the control group (21). In a study involving the administration of memantine for 52 weeks, the rates of the following ADEs in the memantine group are significantly higher compared with the rates in the placebo group: dizziness (p = 0.0005), headache (p = 0.023), and fatigue (p = 0.032) (22). Forest plots of combinable data comparing memantine and placebo in terms of adverse drug events are shown in Figure 4. Pooled evidence indicates that the number of patients who experienced dizziness is significantly higher in the memantine group compared with that in the placebo group (p = 0.01; 95% CI 1.67, 103.46). The rates of fatigue and agitation in the memantine group are higher than those in the placebo group, although the differences are not statistically significant (p = 0.06; 95% CI 0.95, 8.17 and p = 0.34; 95% CI 0.41, 12.61, respectively). In terms of serious adverse events, one episode of seizure was noted in a patient in the placebo group in one trial (22). No deaths occurred in the included trials.
This review provides comprehensive evidence from pooled results of four studies on the efficacy and safety of memantine in preventing cognitive decline, reducing spasticity and fatigue, and controlling disability among adult patients with multiple sclerosis.
The pathophysiology that led to the consideration of memantine, an NMDA receptor antagonist that blocks glutamate in neurons, as a potential agent for the prevention of cognitive impairment in patients with MS arose from the hypothesis that states that cognitive impairment in MS may be related to the excessive glutamate noise in the CNS plaques (29). Recent studies suggest that disease-modifying therapies significantly reduce the effect of neurodegeneration and subsequent cognitive impairment in MS. The BENEFIT (11), AFFIRM, and STRATA (12) trials emphasize the positive effect on cognitive functioning in the long-term follow-up of MS patients treated with interferon B-1b and natalizumab. Studies investigating drugs commonly used for dementia, however, failed to demonstrate a significant benefit for cognition (13, 14). There is not enough evidence to support the role of memantine in improving cognitive function of patients with MS as measured by mean difference in PASAT scores and other measures: CVLT-II, Victoria Stroop, SDMT, COWAT, and DKEFS. One possible reason is the timing of memantine administration. Animal studies involving encephalomyelitis rodent models suggest that the protective effect of NMDA blockade for cognitive deterioration in the setting of neuroinflammatory diseases is evident if carried out in the initial stages of neuroinflammation (30). The trials failed to segregate patients into those with early disease and with relapsing or progressive course. Another possible reason why the potential benefit of memantine is not demonstrated in the pooled evidence is the lack of subpopulation analysis in terms of DMT utilization. Inflammatory mediators such as tumor necrosis factor stimulate glutamate secretion and excitotoxicity (31). Glutamate blockade alone may not be enough in preventing cognitive deterioration. This is supported by studies demonstrating the protective effects of ocrelizumab (32), alemtuzumab (33), interferon B-1b (11), and natalizumab (12) on cognition of MS patients on long-term follow-up. The authors postulate that a subgroup of patients might benefit from memantine in terms of protection against cognitive deterioration—patients with early disease and receiving DMTs. To date, there are still no studies in the literature that define early MS disease. Future studies may look into the role of 7-T magnetic resonance imaging for early diagnosis of MS (34).
It is important to note that DMT initiation can be associated with paradoxical brain pseudo-atrophy which is understood to be due to fluid shifts and resolution of edema as neuroinflammation subsides (35). This is different from true brain atrophy which is a true marker of extensive demyelination, axonal loss, and degeneration (36). True brain atrophy, specifically thinning of the fronto-parietal cortical regions, precuneus atrophy (37), and thalamic atrophy (38) are significantly correlated with cognitive impairment. To date, evidence on the difference in outcomes of memantine treatment in patients with pseudo-atrophy compared with those with true brain atrophy is still lacking. It is logical to postulate, however, that better cognitive outcomes may be expected if memantine treatment is initiated early on than if started when true atrophy from neurodegeneration is already apparent.
Another possible reason for the non-demonstration of clinical benefit is the relatively short duration of treatment. Among patients with Alzheimer's disease dementia, improvement in cognitive function is already evident as early as 12 weeks of administration of memantine (39). This scenario may not be applicable among patients with MS as their decline in cognitive functions is tethered to the number of relapses or attacks and is understood to progress more slowly compared with the steady gradual decline seen among patients with Alzheimer's disease dementia. The period of 12 to 52 weeks of memantine administration and observation may not be enough. The severity of cognitive impairment may also be very different as patients with MS may present with mild to moderate impairment in contrast to patients with Alzheimer's disease dementia who usually present with mild to severe impairment. With a sensitivity of 74% in detecting cognitive impairment (40), the PASAT measure may not be perfectly perceptive in detecting subtle changes in cognitive functioning in the mild to moderate arena of the cognitive functioning spectrum. Lastly, another possible reason can be due to non-concordance of the pathophysiology. Although high levels of glutamate noise—the target of memantine—are seen in spectroscopic studies of white matter lesions in MS, this may not be the only contributing process to cognitive impairment. Recent studies suggest other contributory pathophysiologic features such as thalamic degeneration, hippocampal changes, synaptic loss (8, 41), and reduced GABA levels (31), hence the ineffectiveness of memantine in improving cognitive function or in preventing cognitive impairment.
This review demonstrates that short-term memantine administration does not significantly reduce spasticity among MS patients. One explanation is the possibility of non-concordance in the pathophysiology. Although studies involving animal models of MS demonstrate that antagonism in the NMDA receptor reduces muscle tone (16, 42), more complex pathophysiologic processes such as dynamic changes in the levels of cytokines, prostaglandins, and reactive oxygen species have been implicated in the pathophysiology of spasticity in MS (15). Moreover, with a sensitivity in detecting spasticity of just 50% (43), the zero- to four-point ADSS may not be able to detect subtle changes in spasticity.
Pooled evidence failed to show that memantine can reduce the degree of MS-related fatigue as measured by MFIS. One reason is that fatigue is theorized to be tethered to depression and cognitive symptoms as presented in the model by Brenner and Piehl (44). Treating fatigue as just a consequence of excessive glutamate noise in MS with memantine may not be sufficient. Another reason again may be the possibility of non-concordance in pathophysiology as MS-related fatigue is not only correlated with glutamate toxicity but also with gray and white matter atrophy (18). It is important to note that memantine may actually exacerbate the symptoms of fatigue. Two of the trials in this review showed that the rate of fatigue among patients in the memantine group is higher than that in the placebo group, though this is not statistically significant (21, 22).
In terms of disability, evidence in this review failed to show benefit from short-term administration of memantine (12–52 weeks) compared with placebo. This may be due to the recruited participants. In the trial which evaluated pre- and post-treatment EDSS, the baseline level of disability is within the mild spectrum with mean EDSS score ranging from 3.1 to 3.2 (22). Furthermore, all recruited participants in the said study belong to the relapsing-remitting clinical subtype, a subgroup that generally has mild forms of disability.
The role of memantine in MS according to the 2014 National Institute of Health Care and Excellence clinical guideline on the management of MS in adults (CG186) is limited to being a second-line treatment of MS-related nystagmus and oscillopsia, second to gabapentin (45). This recommendation is backed by studies on acquired and congenital nystagmus in general using retrospective and non-randomized cross-over designs (46–48). At the time of writing, there is insufficient evidence to support the beneficial role of memantine in MS-related cognitive impairment, spasticity, fatigue, and disability.
Pooled evidence in this study shows that memantine is associated with non-serious adverse drug events such as dizziness, fatigue, and agitation. This is consistent with the findings in other studies on memantine in multiple sclerosis. In a pilot study by Villoslada and collegues (49) involving 19 patients with MS, memantine at a higher dose of 30 mg/day was associated with blurred vision, fatigue, severe headache, increased muscle weakness, walking difficulties, or unstable gait. These events led to the pre-mature termination of the trial. Conceivably, the high rate of adverse events in the pilot study is due to the quick titration of memantine, with incremental increase of 10 mg per day after 1 week. This review provides evidence demonstrating that memantine administration is not associated with any serious adverse drug events or death when administered following proper titration.
There is not enough evidence to support the role of memantine at a dose of 20 mg per day administered for 12–52 weeks among patients with MS in preventing cognitive deterioration, controlling spasticity, reducing fatigue, and improving the degree of functionality compared with placebo. Memantine administration, though associated with minor adverse drug events such as dizziness, fatigue, and anxiety, is generally safe among patients with MS.
Further researches investigating the different MS clinical subtypes, role of co-administration of memantine with DMTs, longer duration of administration, and more unified and sensitive outcome measures are needed to evaluate the potential benefit of memantine among patients with MS.
The original contributions generated in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
All authors have been sufficiently involved in this work to take responsibility for its validity and final presentation as an original publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.574748/full#supplementary-material
ADE, adverse drug events; AFFIRM, Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis; ASS, Ashworth Spasticity Scale; BENEFIT, Betaseron/Betaferon in Newly Emerging MS For Initial Treatment; BDI, Beck Depression Inventory; CENTRAL, Cochrane Controlled Register of Trials; COWAT, Controlled Oral Word Association Test; CVLT-II, California Verbal Learning Test–II; DKEFS, Delis–Kaplan Executive Function System; DMT, disease-modifying therapies; EDSS, Expanded Disability Status Scale; HERDIN, Health Research and Development Information Network; LDFR, long delay free recall; LILACS, Literatura Latino-Americana e do Caribe em Ciências da Saúde; MFIS, Modified Fatigue Impact Scale; MS, multiple sclerosis; MSFC, Multiple Sclerosis Functional Composite; MSNQ, Multiple Sclerosis Neuropsychological Questionnaire; PASAT, Paced Auditory Serial Addition Test; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SDMT, Symbol Digit Modalities Test; STRATA, Safety of TYSABRI Re-dosing and Treatment.
1. Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. (2017) 23:1123–36. doi: 10.1177/1352458517694432
2. Ramagopalan SV, Sadovnick AD. Epidemiology of multiple sclerosis. Neurol Clin. (2011) 29:207–17. doi: 10.1016/j.ncl.2010.12.010
3. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. (2014) 83:1022–4. doi: 10.1212/WNL.0000000000000768
4. Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. (2010) 9:520–32. doi: 10.1016/S1474-4422(10)70064-8
5. Guimarães J, Sá MJ. Cognitive dysfunction in multiple sclerosis. Front Neurol. (2012) 3:74. doi: 10.3389/fneur.2012.00074
6. Brochet B, Ruet A. Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol. (2019) 10:261. doi: 10.3389/fneur.2019.00261
7. Piri Cinar B, Guven Yorgun Y. What we learned from the history of multiple sclerosis measurement: expanded disease status scale. Arch Neuropsychiatry. (2018) 55(Suppl. 1):69–75. doi: 10.29399/npa.23343
8. Grzegorski T, Losy J. Cognitive impairment in multiple sclerosis—a review of current knowledge and recent research. Rev Neurosci. (2017) 28:845–60. doi: 10.1515/revneuro-2017-0011
9. Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RHB. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. (2016) 22:1327–36. doi: 10.1177/1352458515616204
10. Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurology. (2003) 61:1113–20. doi: 10.1212/01.WNL.0000090564.88719.37
11. Kappos L, Freedman MS, Polman CH, Edan G, Hartung H-P, Miller DH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. (2009) 8:987–97. doi: 10.1016/S1474-4422(09)70237-6
12. O'Connor P, Goodman A, Kappos L, Lublin F, Polman C, Rudick RA, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology. (2014) 83:78–86. doi: 10.1212/WNL.0000000000000541
13. Wiebenga OT, Hulst HE, Kooi EJ, Killestein J, Geurts JJG, Heilman KM, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. (2011) 77:1998–2000. doi: 10.1212/WNL.0b013e318239c242
14. Huolman S, Hämäläinen P, Vorobyev V, Ruutiainen J, Parkkola R, Laine T, et al. The effects of rivastigmine on processing speed and brain activation in patients with multiple sclerosis and subjective cognitive fatigue. Mult Scler J. (2011) 17:1351–61. doi: 10.1177/1352458511412061
15. Patejdl R, Zettl UK. Spasticity in multiple sclerosis: contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun Rev. (2017) 16:925–36. doi: 10.1016/j.autrev.2017.07.004
16. Tsai M-C, Chen M-L, Lo S-C, Tsai G-C. Effects of memantine on the twitch tension of mouse diaphragm. Eur J Pharmacol. (1989) 160:133–40. doi: 10.1016/0014-2999(89)90662-6
17. Minden SL, Frankel D, Hadden L, Perloff J, Srinath KP, Hoaglin DC. The Sonya Slifka longitudinal multiple sclerosis study: methods and sample characteristics. Mult Scler J. (2006) 12:24–38. doi: 10.1191/135248506ms1262oa
18. Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. (2010) 10:1437–47. doi: 10.1586/ern.10.99
19. Azevedo CJ, Kornak J, Chu P, Sampat M, Okuda DT, Cree BA, et al. In vivo evidence of glutamate toxicity in multiple sclerosis. Ann Neurol. (2014) 76:269–78. doi: 10.1002/ana.24202
20. Kutzing MK, Luo V, Firestein BL. Protection from glutamate-induced excitotoxicity by memantine. Ann Biomed Eng. (2012) 40:1170–81. doi: 10.1007/s10439-011-0494-z
21. Lovera JF, Frohman E, Brown TR, Bandari D, Nguyen L, Yadav V, et al. Memantine for cognitive impairment in multiple sclerosis: a randomized placebo-controlled trial. Mult Scler. (2010) 16:715–23. doi: 10.1177/1352458510367662
22. Saint Paul LP, Creveuil C, Heinzlef O, De Seze J, Vermersch P, Castelnovo G, et al. Efficacy and safety profile of memantine in patients with cognitive impairment in multiple sclerosis: a randomized, placebo-controlled study. J Neurol Sci. (2016) 363:69–76. doi: 10.1016/j.jns.2016.02.012
23. Mehta LR, McDermott MP, Goodman AD, Schwid SR. A randomized trial of memantine as treatment for spasticity in multiple sclerosis. Mult Scler J. (2010) 16:248–51. doi: 10.1177/1352458509355462
24. Falsafi Z, Tafakhori A, Agah E, Mojarrad M, Dehghani R, Ghaffarpour M, et al. Safety and efficacy of memantine for multiple sclerosis-related fatigue: a pilot randomized, double-blind placebo-controlled trial. J Neurol Sci. (2020) 414:116844. doi: 10.1016/j.jns.2020.116844
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
26. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
27. Carroll WM. 2017 McDonald MS diagnostic criteria: evidence-based revisions. Mult Scler J. (2018) 24:92–5. doi: 10.1177/1352458517751861
28. Higgins J, Green S editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. London: The Cochrane Collaboration (2011).
29. O'Grady KP, Dula AN, Lyttle BD, Thompson LM, Conrad BN, Box BA, et al. Glutamate-sensitive imaging and evaluation of cognitive impairment in multiple sclerosis. Mult Scler J. (2019) 25:1580–92. doi: 10.1177/1352458518799583
30. Bellizzi MJ, Geathers JS, Allan KC, Gelbard HA. Platelet-activating factor receptors mediate excitatory postsynaptic hippocampal injury in experimental autoimmune encephalomyelitis. J Neurosci. (2016) 36:1336–46. doi: 10.1523/JNEUROSCI.1171-15.2016
31. Lazo-Gomez R, Velázquez G de LL-G, Mireles-Jacobo D, Sotomayor-Sobrino MA. Mechanisms of neurobehavioral abnormalities in multiple sclerosis: contributions from neural and immune components. Clin Neurophysiol Pract. (2019) 4:39–46. doi: 10.1016/j.cnp.2019.01.004
32. Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. (2017) 376:221–34. doi: 10.1056/NEJMoa1601277
33. Riepl E, Pfeuffer S, Ruck T, Lohmann H, Wiendl H, Meuth SG, et al. Alemtuzumab improves cognitive processing speed in active multiple sclerosis-A longitudinal observational study. Front Neurol. (2018) 8:730. doi: 10.3389/fneur.2017.00730
34. Bruschi N, Boffa G, Inglese M. Ultra-high-field 7-T MRI in multiple sclerosis and other demyelinating diseases: from pathology to clinical practice. Eur Radiol Exp. (2020) 4:59. doi: 10.1186/s41747-020-00186-x
35. De Stefano N, Arnold DL. Towards a better understanding of pseudoatrophy in the brain of multiple sclerosis patients. Mult Scler J. (2015) 21:675–6. doi: 10.1177/1352458514564494
36. Andravizou A, Dardiotis E, Artemiadis A, Sokratous M, Siokas V, Tsouris Z, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Autoimmun Highlights. (2019) 10:7. doi: 10.1186/s13317-019-0117-5
37. Pravatà E, Rocca MA, Valsasina P, Riccitelli GC, Gobbi C, Comi G, et al. Gray matter trophism, cognitive impairment, and depression in patients with multiple sclerosis. Mult Scler J. (2017) 23:1864–74. doi: 10.1177/1352458517692886
38. Papathanasiou A, Messinis L, Zampakis P, Panagiotakis G, Gourzis P, Georgiou V, et al. Thalamic atrophy predicts cognitive impairment in relapsing remitting multiple sclerosis. Effect on instrumental activities of daily living and employment status. J Neurol Sci. (2015) 358:236–42. doi: 10.1016/j.jns.2015.09.001
39. Marum R. Update on the use of memantine in Alzheimer's disease. Neuropsychiatr Dis Treat. (2009) 5:237. doi: 10.2147/NDT.S4048
40. Rosti E, Hämäläinen P, Koivisto K, Hokkanen L. The PASAT performance among patients with multiple sclerosis: analyses of responding patterns using different scoring methods. Mult Scler J. (2006) 12:586–93. doi: 10.1177/1352458506070624
41. Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. (2018) 90:278–88. doi: 10.1212/WNL.0000000000004977
42. Schwarz M, Block F, Pergande G. N-methyl-D-aspartate (NMDA)-mediated muscle relaxant action of flupirtine in rats. Neuroreport. (1994) 5:1981–4. doi: 10.1097/00001756-199410000-00036
43. Malhotra S, Cousins E, Ward A, Day C, Jones P, Roffe C, et al. An investigation into the agreement between clinical, biomechanical and neurophysiological measures of spasticity. Clin Rehabil. (2008) 22:1105–15. doi: 10.1177/0269215508095089
44. Brenner P, Piehl F. Fatigue and depression in multiple sclerosis: pharmacological and non-pharmacological interventions. Acta Neurol Scand. (2016) 134:47–54. doi: 10.1111/ane.12648
45. NICE. Multiple Sclerosis in Adults: Management. NICE Clin Guidel CG186. (2014). p. 20–2. Available online at: http://www.nice.org.uk/guidance/cg186/chapter/1-recommendations#modifiable-risk-factors-for-relapse-or-progression-of-ms (accessed December 1, 2020).
46. Starck M, Albrecht H, Pöllmann W, Straube A, Dieterich M. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol. (1996) 244:9–16. doi: 10.1007/PL00007728
47. Shery T, Proudlock FA, Sarvananthan N, McLean RJ, Gottlob I. The effects of gabapentin and memantine in acquired and congenital nystagmus: a retrospective study. Br J Ophthalmol. (2006) 90:839–43. doi: 10.1136/bjo.2005.086322
48. Thurtell MJ, Joshi AC, Leone AC, Tomsak RL, Kosmorsky GS, Stahl JS, et al. Cross-over trial of gabapentin and memantine as treatment for acquired nystagmus. Ann Neurol. (2010) 67:676–80. doi: 10.1002/ana.21991
Keywords: memantine, multiple sclerosis, cognitive impairment, fatigue, spasticity
Citation: Turalde CWR, Espiritu AI and Anlacan VMM (2021) Memantine for Multiple Sclerosis: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Neurol. 11:574748. doi: 10.3389/fneur.2020.574748
Received: 21 June 2020; Accepted: 22 December 2020;
Published: 15 February 2021.
Edited by:
Philipp Albrecht, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Steffen Pfeuffer, University Hospital Münster, GermanyCopyright © 2021 Turalde, Espiritu and Anlacan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Wilson R. Turalde, Y3J0dXJhbGRlQHVwLmVkdS5waA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.