- 1Faculty of Medicine, Institute of Physical Medicine and Rehabilitation, University of São Paulo, São Paulo, Brazil
- 2Serviço de Reabilitação de adultos, Centro de Medicina de Reabilitacao do Alcoitão, Alcabideche, Portugal
- 3Department of Neurology, Azienda Ospedaliera S.Maria, Terni, Italy
- 4Institute of Neurology, University College London, London, United Kingdom
- 5Global Medical Affairs, Ipsen, Boulogne Billancourt, France
- 6Chulalongkorn Centre of Excellence for Parkinson's Disease & Related Disorders, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand
Botulinum toxin-A (BoNT-A) is an effective treatment for cervical dystonia (CD) and spastic paresis (SP), but it requires in-depth knowledge of anatomy and injection techniques. The Ixcellence Network® is an educational programme to provide neurology, neuropaediatrics, and physical medicine and rehabilitation (PMR) specialists with access to best clinical practices and innovations regarding SP and CD management with BoNT-A. To assess the benefits of such educational programmes and identify unmet needs, a multidisciplinary scientific committee designed INPUT (INjection Practice, Usage & Training), an international multicentric survey describing training and practices among this trained and experienced population. A self-completed questionnaire was sent online to 553 trainees and 14 trainers from the Ixcellence Network®. Among the 131 respondents, 92% specialized in PMR (48%) or neurology (44%), with a mean experience of 15.5 years in their clinical fields and 10.9 years of BoNT-A injection. Most of them (98%) reported having received training before performing their first BoNT-A injection and attending specific courses on how to perform it without any instrumental guidance (76%), and with ultrasound (73%), electrical stimulation (44%) or electromyography (41%). In terms of practices, 92% of respondents reported using at least one guidance technique while injecting, with ultrasound being the most used technique (48%). Attending specific courses was significantly associated with greater self-confidence and use, e.g. for injection with ultrasound, mean self-confidence, on a scale from 1 (not confident) to 10 (fully confident), was 7.9 for trained respondents (vs 4.0 for untrained respondents, p < 0.001) of whom 70% stated that they used this technique regularly or systematically (vs. 11% of untrained healthcare professionals (HCPs), p < 0.0001). Moreover, 84% of respondents reported having trained colleagues, residents or fellows through theoretical (70%) or practical teaching in individuals (80%) or in small groups (65%). Overall, 86% of respondents reported a notable increase over the past 5 years of the number of patients treated with BoNT-A. INPUT is the first international survey describing training and practices in SP and CD management of physicians who attended a dedicated educational programme. The results highlighted the importance of training for self-confidence, and the use of specific techniques and new approaches.
Introduction
Botulinum toxin-A (BoNT-A), a neurotoxin produced by an anaerobic bacterium Clostridium botulinum, is a well-established and effective treatment for a number of neurological movement disorders associated with muscle hyperactivity, including adult spastic paresis (SP) from stroke, multiple sclerosis, traumatic brain or spinal cord injury, pediatric SP from cerebral palsy and adult cervical dystonia (CD) (1–6).
BoNT-A is administered by local injections into the muscles involved in the movement disorder. Its effectiveness depends on several factors including the injector's skills and knowledge, since accurate muscle selection, injection site selection and adequate drug dosage are key to achieving the best results (7–10). Proper clinical examination is required, as SP and CD present a wide diversity of patterns (11, 12). The localization technique can also affect BoNT-A outcomes. Palpation or instrumentally guided injection with ultrasound (US), electrical stimulation (ES) or electromyography (EMG) are commonly used to identify targeted muscles for injection in SP and CD treatment (12). Use of these targeting techniques is very much dependent on injector's prior training, confidence and the availability of equipment.
BoNT-A treatment should be tailored individually to each patient. When selecting which muscles to inject, the disease pattern, as well as patient and carer needs, should be taken into account to optimize management and outcomes (9, 13, 14). A pre-treatment evaluation and discussion with both the patient and the carer is essential to set optimal individual therapeutic goals. This step allows treatment efficacy to be measured and the strategy to be reassessed at each injection cycle to plan further treatment (14, 15).
Optimal BoNT-A treatment requires not only specific training in SP and CD management, but also in-depth knowledge of muscle anatomy and BoNT-A injection techniques, which are skills acquired only with time and commitment (9, 15, 16). However, there is no widely accepted standardized training for BoNT-A treatment. Although there are guidelines describing the diagnosis and treatment of SP and CD, there are no clear recommendations on how to perform the injections in different patients and conditions (16, 17). Physicians looking for experience and additional expertise on BoNT-A treatment are usually mentored by their peers under variable degrees of supervision (1). This may explain the wide variety of practices observed, with healthcare professionals (HCPs) choosing their approach to muscle selection, injection technique and follow-up therapy according to their preference, experience, confidence, and access (8, 18).
A steering committee of six international experts in SP and CD management developed the Ixcellence Network®, an international educational programme to provide physicians specialized in SP and CD with an opportunity to access best clinical practices and innovations concerning treatment with BoNT-A (19). Ten training centers were selected by the steering committee based on their expertise (five on SP, three on CD and two on pediatric SP). The training approach combines advanced theoretical courses and practical training, and addresses innovative methods and concepts concerning patient diagnosis, clinical and instrumental evaluation, muscle identification, goal setting, tailored treatment administration, and rehabilitation methods (e.g. Goal Attainment Scaling (GAS), Gait analysis, Col-Cap concept, guidance techniques, etc.). HCPs (the vast majority of whom were medical practitioners) participating in these courses were selected based on their experience in the management of adult and/or pediatric SP and/or CD, with at least 2 years of practical experience with regular botulinum toxin clinics.
The INjection Practice, Usage & Training (INPUT) survey was designed to describe training, practices regarding patient management with BoNT-A and how they evolved in this trained and experienced population, with the aims of evaluating the benefits of training and identifying unmet needs.
Methods
The INPUT survey is an international, observational and multicentric study conducted among practitioners specialized in neurology, neuropaediatrics, or physical medicine and rehabilitation (PMR), and trained through the Ixcellence Network® programme.
Survey Design
A multidisciplinary scientific committee designed a self-completed questionnaire including 41 items divided into two sections: a general part with 19 questions on experience, training and clinic organization, and a specific part customized for each of the three indications (SP: 9 questions, pediatric SP: 10 questions, CD: 9 questions). The respondents were asked to answer a minimum of 28 (e.g., HCPs exclusively managing adults with SP) to a maximum of 41 questions (HCPs treating all three indications). The questionnaire included multiple-choice and open-ended questions. None of the questions was compulsory.
Recipients
Between 1 June 2018 and 26 February 2019, an online version of the questionnaire was sent by email to 553 HCPs managing patients with SP and/or CD, who attended at least one Ixcellence Network® training session between 2012 and 2018, and 14 programme trainers. Three reminders were sent on a weekly basis. Recipients were instructed to answer the online questionnaire anonymously, without opening their patient files or looking at their activity statistics.
Data Collection
The following parameters were collected: (1) general information: country of practice, experience in their field and in BoNT-A injection, training on different approaches/techniques, confidence in using these approaches, trainer activity; (2) clinic organization: time dedicated to BoNT-A injection, number of injectors in the department, waiting time for appointments, usage of guidance techniques for BoNT-A injection; (3) patient profile and management: type of patients treated and specific information related to each indication (e.g., main challenges for BoNT-A injection, time dedicated to discussion with patients, frequency of setting up a rehabilitation programme in combination with BoNT-A injections, etc.); (4) how their clinical practice evolved over the past 5 years (Supplementary Figure 1).
For patient profile and management results, only adult SP and CD data were reported in this publication, which focuses on adult SP and CD management.
Data Analysis
Data were analyzed using the Statistical Analysis Software SAS®. Results were described using numbers and percentages for qualitative variables, and means and standard deviations for quantitative variables. Continuous data were analyzed using a Student's t-test (following a Student's t distribution) and categorical data using Fisher's exact test (based on a hypergeometric distribution). The analysis was completed with subgroup analysis according to respondents' specialty (neurology vs. PMR), experience in the field (<15 years vs. ≥ 15years), experience in BoNT-A injection (<10 years vs. ≥10 years) and time allocated to BoNT-A injections (one half day vs. > one half day per week).
Results
Among the 553 trainees and 14 trainers who received the questionnaire, 131 HCPs answered the survey (i.e., response rate of 23.1%).
Respondents' Profile (Table 1)
Respondents represented 38 countries, located in Europe (60%), Latin America (25%), Africa/Middle East (12%), Asia/Oceania (3%), and specialized in PMR (48%), neurology (44%), neuropediatrics (5%), orthopedics (2%) or other specialties (1%). The mean duration of respondents' experience was 15.5 years in their clinical field (range 1–41 years) and 10.9 years of BoNT-A injection experience (range 1–29 years).
Type of Patients Treated by Respondents
Respondents reported managing adult SP (72%), CD (48%), and pediatric SP (38%). Indications treated were significantly different according to speciality, with a higher number of PMR specialists managing SP (adult: 86 vs. 64% for neurologists, p = 0.006; pediatric: 59 vs. 7% for neurologists, p < 0.0001), and a higher number of neurologists treating CD (91 vs. 14% for PMR specialists, p < 0.0001).
Clinic Organization
Respondents reported seeing monthly, on average, 23 ± 20 adult outpatients with SP for consultation and 13 ± 11 for BoNT-A injection; 28 ± 38 children outpatients with SP for consultation and 11 ± 14 for BoNT-A injection; 12 ± 18 CD outpatients for consultation and 17 ± 34 for BoNT-A injection. The mean number of injectors in the respondents' department was 2.9 ± 1.6 (range 1–10). On average, respondents allocated 1.8 ± 1.2 half-days per week to BoNT-A injection. New patients requiring BoNT-A injection waited an average of 5.7 ± 5.3 weeks (range 1–30 weeks) to get their first appointment with the HCPs. The planning of the next injection's session was predominantly flexible, according to the patient's needs (57% of respondents), with an average waiting time of 19.1 ± 23.5 days (range 0–100 days) and predominantly fixed for 43% of respondents, with an average interval of 12.3 ± 5.4 weeks (range 1–24 weeks).
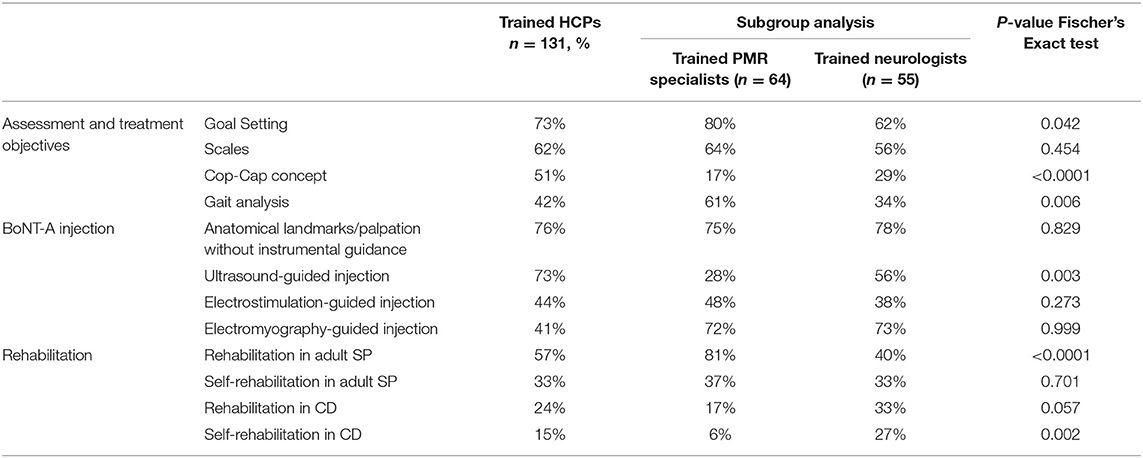
Training (Table 2)
Patient's Assessment and Treatment Objectives
Seventy three percentage of respondents attended courses dedicated to goal setting, 62% on scales, 51% on the Col-Cap concept and 42% on GAIT analysis, with significant differences between specialties: more PMR specialists were trained on goal setting and GAIT analysis compared to neurologists (80 vs. 62%, p = 0.0418 and 61 vs. 34%, p = 0.0057, respectively), while more neurologists were trained on the Col-Cap concept (69 vs. 17% for PMR specialists, p > 0.0001). On average, respondents attended 2.3 out the 4 courses dedicated to assessment and treatment objectives (2.3 for PMR specialists and 2.2 for neurologists, NS).
BoNT-A Injection
Almost all respondents (98%) reported that they were trained before performing their first BoNT-A injection. This initial training was done under a colleague tutelage (77%), practical sessions (65%) and theoretical courses (65%) with 42% of respondents receiving these three types of initial training. During their medical residency, most of the respondents were trained on how to perform the injection without any instrumental guidance (76%) with US (73%), with ES (44%) or with EMG (41%). Neurologists were significantly more trained in using EMG than PMR specialists (56 vs. 28%, respectively, p = 0.003). Regarding guidance technique usage, on average, respondents were trained on 1.6 out the 3 topics (1.5 for PMR specialists and 1.7 for neurologists, NS).
Rehabilitation Approaches
Respondents reported specific training sessions on rehabilitation for adult SP (57%), self-rehabilitation for adult SP (33%), rehabilitation for CD (24%) and self-rehabilitation for CD (15%). Subgroup analysis showed a higher number of PMR specialists trained in rehabilitation for adult SP compared with neurologists (81 vs. 40%, p < 0.0001). Contrarily, more neurologists were trained in self-rehabilitation for CD (27 vs. 6%, p < 0.0023). On average, respondents attended training on 1.4 out the three topics related to rehabilitation (1.5 for PMR specialists and 0.8 for neurologists, NS), and on 0.6 out the three topics related to self-rehabilitation (0.6 for PMR specialists and neurologists, NS).
Training of Colleagues
Among the respondents, 84% stated that they had opportunities to train some colleagues, residents or fellows through at least one of the following formats: individual practical teaching (80%), theoretical teaching (70%) or practical teaching in small groups (65%).
BoNT-A Injection in Clinical Practice
With Palpation Only
BoNT-A injection was performed without using anything except palpation and anatomical references, regularly or systematically (48%), scarcely (35%), and never (17%), without any difference between PMR specialists and neurologists.
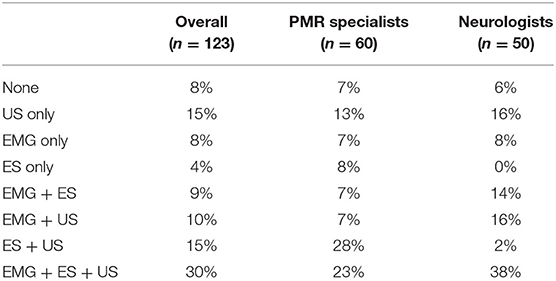
With Localization Devices (Table 3)
Almost all respondents (92%) reported using at least one guidance device scarcely, regularly or systematically to inject BoNT-A. US was the most used with 48% of respondents using it regularly or systematically vs. 40% for ES and 41% for EMG. Most of the respondents (64%) declared using at least two devices and 30% used all three localization devices. There was no significant difference between specialties, but some trends were observed, for example, ES was used by more PMR specialists while EMG seemed to be predominately used by neurologists.
SP Patient Management
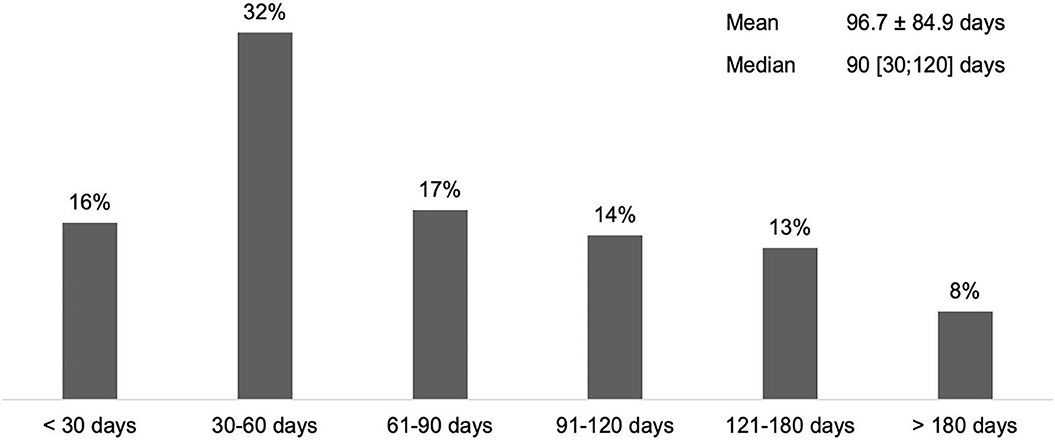
According to 65% of respondents who manage adults with SP (n = 94), the average time to first BoNT-A treatment after stroke was <90 days (Figure 1).
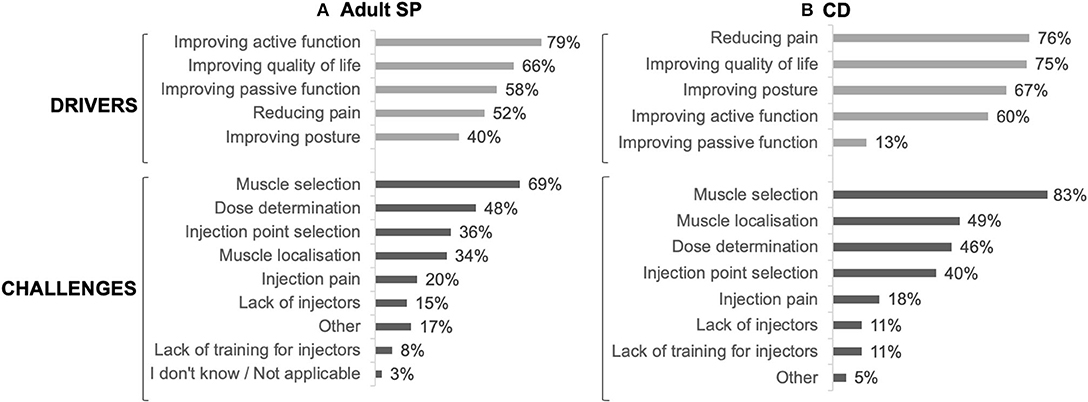
The main drivers for prescribing BoNT-A were to improve active function (79%), quality of life (66%) and passive function (58%), whereas muscle selection (69%), dose determination (48%), and injection point selection (36%) were considered challenging by the respondents (Figure 3A).
Among respondents treating adult SP (n = 89), 94% reported using localization devices at least scarcely (22% only one type, 35% 2 types, 37% all 3 types). US was the most used guidance device with 51% of respondents using it regularly or systematically vs. 46% for ES and 40% for EMG (Table 3).
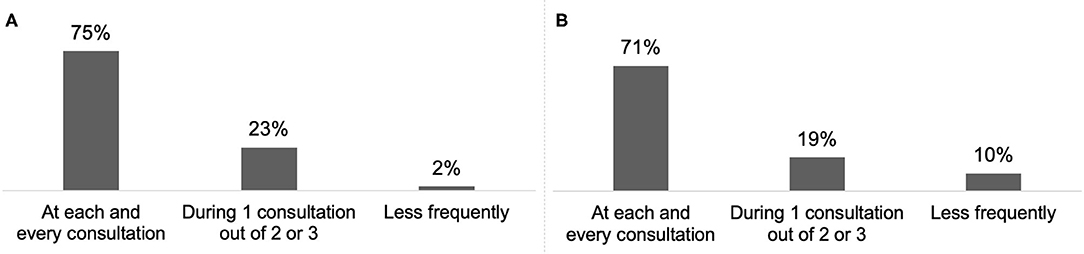
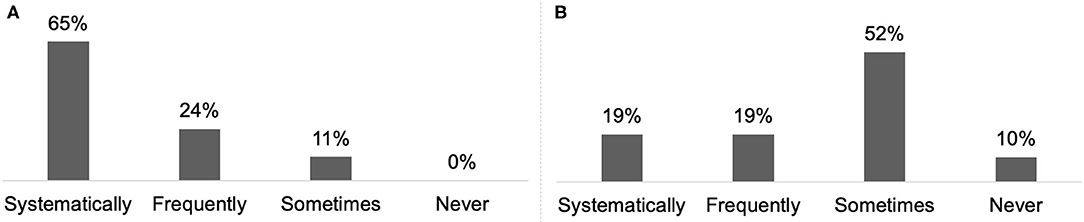
Almost all respondents (98%) reported discussing treatment goals with their patient or the carer/family at least once every two or three consultations (Figure 4A) and 65% reported combining systematically BoNT-A treatment with a rehabilitation programme (Figure 5A).
CD Patient Management
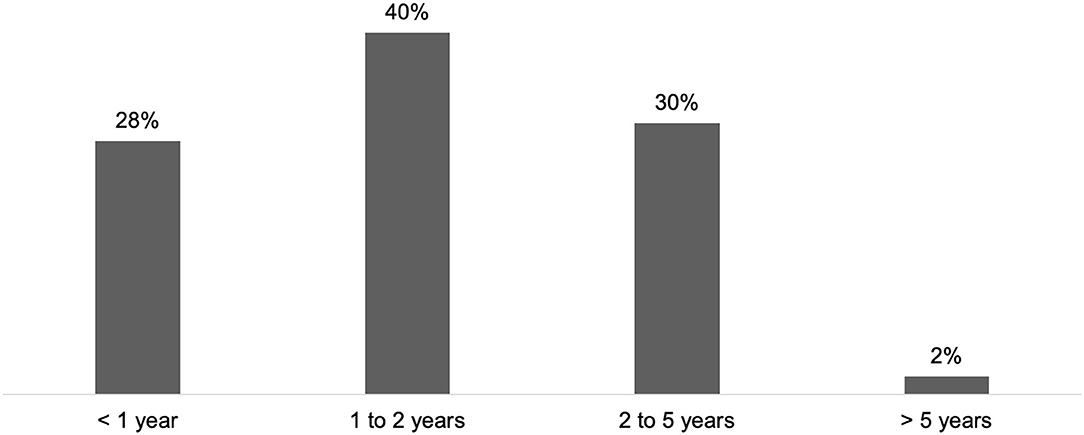
In the practice of HCPs managing CD (n = 68), the first consultation occurred during the first year after symptom onset for 28% of respondents, and between 1 and 2 years for 40% of respondents (Figure 2). According to 32% of respondents, the average time was higher than 2 years (2 to 5 years: 30%; > 5 years: 2%).
In CD patients, the main drivers for BoNT-A treatment were reducing pain (76%), improving quality of life (75%) and improving posture (67%), whereas muscle selection (83%), muscle localization (49%) and dose determination (46%) were considered challenging (Figure 3B).
Subgroup analysis showed that 92% of respondents managing CD (n = 60) performed BoNT-A with the help of a guidance technique at least scarcely (25% used only one type, 30% 2 types, 37% all three types). EMG was the most used localization device with 50% of respondents using it regularly or systematically vs. 42% for US and 37% for ES (Table 3).
For 70% of respondents, treatment goals were discussed with CD patients and their carer/family at each consultation (Figure 4B) and 19% reported combining systematically BoNT-A treatment with a rehabilitation programme (Figure 5B).
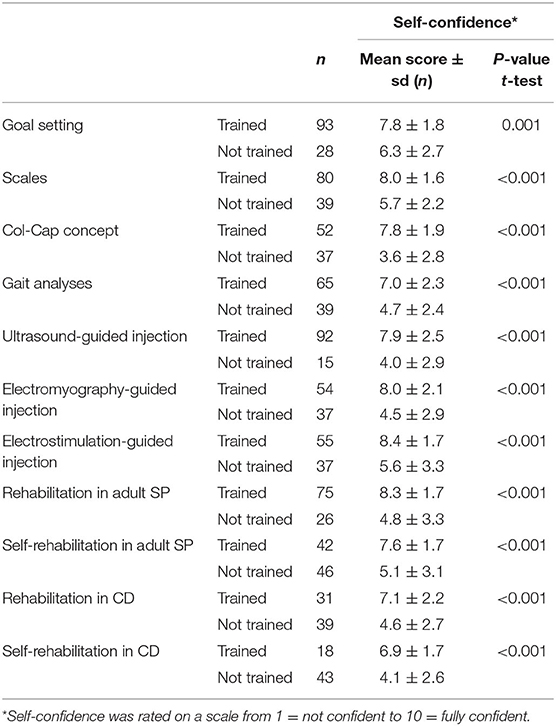
Impact of Training on Self-Confidence (Table 4)
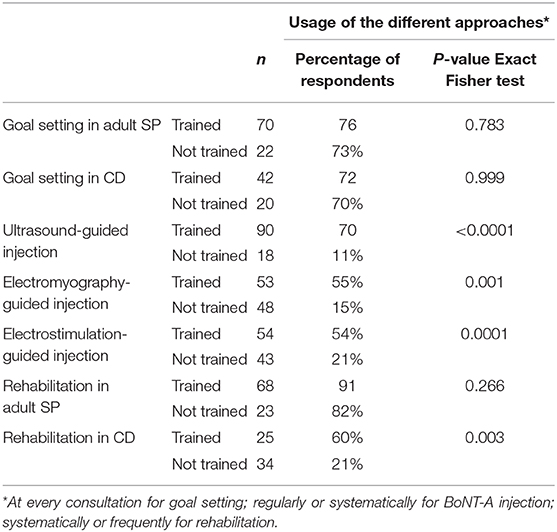
Self-confidence in using specific approaches or techniques was measured on a scale from 1 (not confident) to 10 (fully confident). Comparing the scores amongst the HCPs who declared having been trained vs. the ones who did not allowed an estimation of the influence of training on self-confidence. Data reported in Table 4 highlighted a significant positive effect of training. Overall, HCPs who followed specific training on goal setting, injection using instrumental guidance and rehabilitation approaches reported significantly greater self-confidence compared to those who did not.
Impact of Training on Practice (Table 5)
To assess the impact of training on practice, the frequency of the different approaches and techniques was compared between respondents who followed dedicated courses and those who did not. Regarding goal setting, no significant difference was observed in term of usage/prescription between specifically trained or untrained HCPs. Contrarily, the use of guidance technique was significantly higher among HCPs who received specific training: US, EMG and ES were used regularly or systematically by 70, 55, and 54% of trained HCPs, respectively, vs. 11% (p < 0.0001), 15% (p = 0.0015), and 21% (p = 0.0001) of untrained HCPs.
The frequency of rehabilitation approaches did not differ among respondents managing adult SP who attended dedicated courses and those who did not. However, training had a significant positive impact on respondents prescribing rehabilitation in CD with 60% of trained HCPs vs. 21% of untrained ones combining rehabilitation with BoNT-A treatment systematically (p = 0.0028).
Evolution of Practice Over the Five Past Years
The majority of respondents (86%) reported a notable increase of the number of patients treated with BoNT-A over the past 5 years. Regarding the technique employed, while most did not report a change in BoNT-A injection with palpation only, or with EMG or with ES, 83% stated that their usage of US increased over the five past years. Accordingly, increase in the usage of goal setting, scales, Col-Cap concept and GAIT analysis was reported by 70, 51, 73, and 53% of respondents, respectively. Rehabilitation approaches are also more frequently included in patient management with a reported increase in the prescription of physiotherapy in combination with BoNT-A treatment (53%) and of self-rehabilitation programmes (65%).
Discussion
The aim of the INPUT survey was to describe training and practices of former Ixcellence Network® attendees in order to assess the impact of the programme and identify points for improvement.
Survey participants were representative of the Ixcellence Network® trainees. Among the 728 HCPs who attended courses between 2012 and 2017, 86% were PMR and neurology specialists, and 72% came from European countries (20). Most of the respondents were specialized in PMR and neurology (92%), and from Europe (60%). In addition, the number of respondents per indication was consistent with the number of dedicated courses proposed in Ixcellence Network® (five for adult SP, two for pediatric SP and three for CD). Respondent characteristics also confirmed that the attendee profiles matched the programme selection criteria. HCPs had to have at least 2 years of experience in treating adult SP, pediatric SP or CD with BoNT-A to attend an Ixcellence Network® training course. Respondents reported an extensive experience in both their field and BoNT-A injection, with an average practice of over 10 years.
The Ixcellence Network® programme targets specialized practitioners, keen to develop further their expertise with training and innovative approaches. Results from the INPUT survey confirmed that this population is well-trained, but remains keen to improve further their knowledge and skills. Almost all respondents received training before performing BoNT-A injection alone and half of them by different methods (theoretical courses, practical sessions and colleague tutelage). Most of them also completed their medical residency with dedicated training on specific approaches and techniques covering patient assessment, treatment goals, BoNT-A treatment and rehabilitation approaches. Interestingly, the most attended courses were goal setting, BoNT-A injection without instrumental guidance or with US (three of every four HCPs), while less attended courses included BoNT-A injection with EMG or ES and rehabilitation approaches.
Regarding daily practice, respondents were well aware of innovative approaches and applied them frequently. Almost all HCPs used at least one guidance device and one in every three HCPs used all three devices (US, EMG and ES). Published data indicate improved accuracy of injection with instrumental guidance for SP and CD, compared with palpation only (21–23). For both SP and CD, goals were discussed at every consultation by around three quarters of HCPs. This step is essential to define achievable, realistic and relevant goals with the patient (1). Results from the ULIS-II study, a large international observational cohort study of real-life practice and outcomes in post-stroke treatment of upper limb spasticity with BoNT-A, showed that 80% of patients achieved their previously set treatment goals using Goal Attainment Scaling (GAS), mainly in terms of passive and active functions, thus improving their daily life (18). Combination of BoNT-A treatment with rehabilitation and/or self-rehabilitation, whose benefits were shown in both SP and CD treatment, have been reported to be included in patient management (24–26). BoNT-A treatment after stroke within the first 3 months was frequent among respondents (63%). This approach is supported by recent studies that show that early intervention (within the first 12 weeks) may modify the disease course and delay the presentation of symptoms (27, 28). In the literature, the average delay for CD diagnosis varies from 44 months to 6.8 years and is associated with a negative impact on quality of life (29, 30). Within the INPUT survey population, this delay appeared to be <2 years.
The proportion of PMR specialists and neurologists was well-balanced in the sample, which allowed comparison of both groups. Subgroup analysis revealed significant differences in terms of type of patients treated, and training and trends regarding the BoNT-A injection technique. PMR specialists, who mostly manage SP, are more likely to be trained in goal setting, GAIT analysis (used for both SP diagnosis and assessment) and rehabilitation in SP. This group mostly uses US and ES for BoNT-A injection. Contrarily, neurologists mostly manage CD and focus their training on the Col-Cap concept (used for CD diagnosis and assessment), in BoNT-A injection with EMG and, more recently, US.
The INPUT survey also highlighted interesting differences between SP and CD management. Although most of the respondents reported discussing goals with patients and carers/family for both indications in at least one in every three visits, only 2% of HCPs treating SP did so less frequently vs. 10% of HCPs treating CD. Rehabilitation is also more frequently prescribed for SP patients compared to CD patients. Treatment goals for BoNT-A injection also differed, with pain reduction being more important for CD than SP. This is consistent with the results from an international survey of 1,071 CD patients showing that pain is frequent in this population (reported by 66% of respondents) and its reduction is highly expected (62% of respondents) (31).
In our study, attending specific courses was always significantly associated with greater self-confidence. However, the impact of training on practice differed depending on the approach or technique. Trained HCPs performed BoNT-A injection with a localization device and prescribed rehabilitation for CD more regularly than untrained respondents, but training had no significant impact on goal setting usage or rehabilitation prescription for adult SP. Attending dedicated courses seemed more important for usage of techniques requiring specific skills and knowledge, or new approaches. In a recent survey conducted by the American Academy of Neurology (AAN) among graduating adult and child neurology residents in the USA, two of three respondents reported a desire for additional residency training in BoNT-A injections (32).
Although attendee satisfaction and changes in self-confidence and practices are evaluated within the programme immediately after each course and 6 months later, the aim of this study was to better evaluate the overall programme impact after 5 years. Results confirmed that the programme has a positive impact on the former attendees. Respondents reported changing their practices regarding BoNT-A treatment over the past 5 years with more patients treated and a growing use of US. Patient assessment and treatment goal-setting approaches were also more frequently used, especially goal setting, Col-Cap concept and rehabilitation, which are more recently introduced approaches. Interestingly, BoNT-A injection with US (three of four HCPs) was among the most attended training courses, while less popular courses were BoNT-A injection with EMG or ES and new rehabilitation approaches. This confirmed the development of these approaches in recent years. Moreover, 84% of the INPUT survey respondents reported teaching others, which is consistent with the data collected within the programme. A total of 82% of trainees reported having shared the information learned during the courses with colleagues/peers and 89% said they would consider training their colleagues (20). This suggests that, through the different courses offered, Ixcellence Network® influences a great number of physicians.
The results of the INPUT survey confirmed that Ixcellence Network® attendees are well-trained and motivated specialists. However, some skills can be optimized, e.g., goal setting in CD, adjunct therapies to BoNT-A. New centers may be opened in the future to provide dedicated courses on these developing topics.
Before injecting BoNT-A for the first time, many of the respondents learned from peers. It is important to set up specific courses for HCPs who are not specialized in the field and to develop a standard for national/international programmes.
There are limitations in the survey methodology of this study. Firstly, the response rate was relatively low (23%), which highlights the difficulties in organizing surveys among specialist physicians who are a professional group with low survey response rates in general (32). In our study, the response rate may be influenced by a number of factors, such as the number of items in the questionnaire and the lag period between the training and the survey invitation. Although consistent with similar surveys found in the literature (RR usually around 20–30%) (33) this makes the extrapolation of our results to real life only approximate. Nevertheless, to the best of our knowledge, INPUT is the first international survey characterizing a clinical training programme in order to assess its impact and identify improvement opportunities. The few survey results published describe general or local practices or a specific aspect of management (33–38). Respondents are probably the most motivated to undertake training and innovative approaches, and the most involved in the Ixcellence Network® programme. A direct link cannot be established between the Ixcellence Network® and the impact of training on self-confidence and practice changes because, for example, respondents may have attended courses outside of the programme. The questionnaire design did not allow comparison between practices in terms of BoNT-A treatment for SP and CD. The questions on BoNT-A injection technique were included only in the general question section. Since a significant number of respondents reported treating both indications, it was not possible to statistically compare both subpopulations.
Conclusion
INPUT is the first international survey describing the training and practices in SP and CD management by physicians who attended a dedicated training programme. The results highlighted the importance of training for self-confidence, and the use of specific techniques and new approaches. The Ixcellence Network® may have a positive impact on the daily practice of physicians already using BoNT-A, which is consistent with its aim to upgrade the skills of specialized and experienced HCPs. This study also helped identify areas for improvements, such as the opening of new centers dedicated to emerging approaches in SP and CD management.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors developed the concept and the methodology of this study, including the design of the questionnaire, under the supervision of RB. Data analysis and writing of the original draft were conducted by TC and RB. JT, CC, KB, and LJ reviewed, edited, and approved the manuscript.
Funding
This research was sponsored by Ipsen.
Conflict of Interest
The training courses and the development of this manuscript were supported by Ipsen Pharma. Ixcellence Network® is a registered trademark owned by Ipsen Pharma. All authors except JT received honoraria, and a speaker and consultancy fee from Ipsen Pharma. TC has not personally received any research funding and has no financial interest in BoNT and received honoraria from Ipsen Pharma as a speaker at symposia, training courses and participation in the advisory board, and has participated in several clinical studies sponsored by Ipsen Pharma. LJ received research grants and honoraria as a scientific advisor, lecturer and peer trainer from Allergan and Merz. CC received grant support as a speaker and advisory board committee member from Ipsen Pharma, Merz Pharma, Zambon Pharma, Sunovion Pharmaceuticals, and Bial. KB received personal compensation for activities with UCB Pharma and from Novartis as a speaker. JT is an employee of Ipsen Pharma. RB received honoraria from Boehringer Ingelheim, GlaxoSmithKline, Abbott, Novartis, Roche, Lundbeck Pharmaceuticals, and grants from the Thailand Research Fund, a Ratchadapiseksompoj faculty grant from Chulalongkorn University, a research grant from the Neurological Society of Thailand, a research unit grant from Chulalongkorn University, a Parkinson's Disease Center Development Grant from the Thai Red Cross Society, the Newton Fund (UK) and intellectual property rights from the Laser cane for Parkinson disease, Electronic Parkinson's disease diary and Parkinson's glove.
Acknowledgments
The authors would like to sincerely thank the Ixcellence Network® group including the trainers: Alberto Albanese, Alexandra Botsina, Anna Castagna, Santiago Catania, Luís Gonçalves, Wolfgang Jost, Svetlana Khatkova, Serdar Koçer, Mercedes Martínez Moreno, Peter Misra, Samuel Ignacio Pascual Pascual, Arquimedes Ramos, and Jose Javier Zorilla Sanchez; and the attendees that took time to answer the questionnaire: Soumya Ghosh, Martin Boxill, Peter Valkovic, Sagastegui Rodríguez José Alberto, Lupescu Tudor, Maria Graciela Borelli Cattaneo, Vladimír Zapletal, Almeida João Eduardo, Regina Helena Fornari Chueire, Francisco Muñoz Escudero, Zadori Denes, Tibre Vasile, Victor-Alexandru Constantinescu, Maria José Festas, Shikhkerimov Rafiz, Oshibnataj Ali, Søren Thinus Just Christensen, Suely Kuwae Mitiko, Sana Maiz, Michele Bertoni, Bijan Forogh, Maia Debora, Ahmed Amine El Oumri, Vargas Roger, Sandro Rachevsky Dorf, Allen Hermosillo Lucia, Mohamed Ahmed, Streitova Hana, Inna Rubanovitsh, Fernando Paredes, Maria José Zarco-Periñan, Lucica Ghita, Lucia Dias, Stanislaw Ochudlo, Guiotti Murilo, Youness Abdelfettah, Samia Karkouri, Marina Ramella, Flavio Ribas, Szmurlo Malgorzata, Malgorzata Dec, Heinsoo Maris, Redmond Helen, Juste Diaz Jorge, Cristina el Prete, Sören Bruno Elmgreen, Agustin Torrequebrada Gimenez, Ana Maria Ionescu, Tadeja Hernja Rumpf, Maria Lourenço, Maria Fernanda Toro, Michal Schinwelski, Caroline Giordana, Brahimi Amine, Conde Realpe Oscar Fernando, Dusan Flisar, Jorge Hernández, Dragana Stefanovic, Echeverry Diaz Jennifer, Rios Quevedo Marianella, Hynek Lachmann, Redmond Helen, Maurits Hoonhorst, Tomas Enrique Racines Molina and the 64 respondents who chose not to disclose their name. The authors thank Medical Education Corpus (Levallois-Perret, France) for providing medical writing support, which was sponsored by Ipsen (Boulogne-Billancourt, France) in accordance with Good Publication Practice guidelines.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.570671/full#supplementary-material
Supplementary Figure 1. INPUT survey questionnaire.
References
1. Albanese A, Abbruzzese G, Dressler D, Duzynski W, Khatkova S, Marti MJ, et al. Practical guidance for CD management involving treatment of botulinum toxin: a consensus statement. Neurol. (2015) 262:2201–13. doi: 10.1007/s00415-015-7703-x
2. Baguley IJ, Nott MT, Turner-Stokes L, De Graaff S, Katrak P, McCrory P, et al. Investigating muscle selection for botulinum toxin-A injections in adults with post-stroke upper limb spasticity. J Rehabil Med. (2011) 43:1032–7. doi: 10.2340/16501977-0885
3. Strobl W, Theologis T, Brunner R, Kocer S, Viehweger E, IPascual-Pascual I, et al. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. J Rehabil Med. (2011) 43:1032–7. doi: 10.3390/toxins7051629
4. van Kuijk AA, Hendricks HT, Pasman JW, Kremer BH, Geurts AC. Are clinical characteristics associated with upper-extremity hypertonia in severe ischaemic supratentorial stroke? J Rehabil Med. (2007) 39:33–7. doi: 10.2340/16501977-0009
5. Milinis K, Young CA. Trajectories of Outcome in Neurological Conditions (TONiC). Systematic review of the influence of spasticity on quality of life in adults with chronic neurological conditions. Disabil Rehabil. (2016) 38:1431–41. doi: 10.3109/09638288.2015.1106592
6. Verplancke D, Snape S, Salisbury CF, Jones PW, Ward AB. A randomized controlled trial of botulinum toxin on lower limb spasticity following acute acquired severe brain injury. Clin Rehabil. (2005) 19:117–25. doi: 10.1191/0269215505cr827oa
7. Chan AK, Finlayson H, Mills PB.Does the method of botulinum neurotoxin injection for limb spasticity affect outcomes? A systematic review. Clin Rehabil. (2017) 31:713–21. doi: 10.1177/0269215516655589
8. Castagna A, Albanese A. Management of cervical dystonia with botulinum neurotoxins and EMG/ultrasound guidance. Neurol Clin Pract. (2019) 9:64–73. doi: 10.1212/CPJ.0000000000000568
9. Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med. (2009) 41:13–25. doi: 10.2340/16501977-0303
10. Jinnah HA, Goodmann E, Rosen AR, Evatt M, Freeman A, Factor S.Botulinum toxin treatment failures in cervical dystonia: causes, management and outcomes. J Neurol. (2016) 263:1188–94. doi: 10.1007/s00415-016-8136-x
11. Jost WH Tatu L. Selection of muscles for botulinum toxin injections in cervical dystonia. Mov Disord Clin Pract. (2015) 2:224–6. doi: 10.1002/mdc3.12172
12. Kaku M, Simpson DM. Spotlight on botulinum toxin and its potential in the treatment of stroke-related spasticity. Drug Des Devel Ther. (2016) 10:1085–99. doi: 10.2147/DDDT.S80804
13. Truong D, Dressler D, Hallett M, Zachary C. (editors.). Manual of Botulinum Toxin Therapy. Cambridge: Cambridge University Press. (2014). doi: 10.1017/CBO9781139178068
14. Olver J, Esquenazi A, Fung VSC, Singer BJ, Ward AB. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol. (2010) 17(Suppl. 2):57–73. doi: 10.1111/j.1468-1331.2010.03128.x
15. Ward AB, Molenaers G, Colosimo C, Berardelli A.Clinical value of botulinum toxin in neurological indications. Eur J Neurol. (2006) 13(Suppl. 4):20–6. doi: 10.1111/j.1468-1331.2006.01650.x
16. RCP London. Spasticity in Adults: Management Using Botulinum Toxin. (2018). Available online at: https://www.rcplondon.ac.uk/guidelines-policy/spasticity-adults-management-using-botulinum-toxin (accessed July 2, 2019).
17. Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. (2011) 18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x
18. Turner-Stokes L, Fheodoroff K, Jacinto LJ, Maisonobe J. Results from the upper limb international spasticity study-II (ULIS-II): a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open. (2013) 3:e002771. doi: 10.1136/bmjopen-2013-002771
19. Fheodoroff K, Bhidayasiri R, Jacinto J, Chung TM, Bhatia K, Landreau T, et al. Ixcellence network: an international educational network to improve current practice in the management of cervical dystonia or spastic paresis by botulinum toxin injection. Funct Neurol. (2017) 32:103–10. doi: 10.11138/FNeur/2017.32.2.103
20. Colosimo C, Bhidayasiri R, Fheodoroff K, Bhatia K, Chung TM, Landreau T, et al. Management of spastic paresis and cervical dystonia: access to therapeutic innovations through an international program of practical courses. Clin Ther. (2019) 41:2321–30.e4. doi: 10.1016/j.clinthera.2019.09.007
21. Grigoriu AI, Dinomais M, Rémy-Néris O, Brochard S. Impact of injection-guiding techniques on the effectiveness of botulinum toxin for the treatment of focal spasticity and dystonia: a systematic review. Arch Phys Med Rehabil. (2015) 96:2067–78.e1. doi: 10.1016/j.apmr.2015.05.002
22. Picelli A, Lobba D, Midiri A, Prandi P, Melotti C, Baldessarelli S, et al. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: a randomized controlled trial comparing three injection techniques. Clin Rehabil. (2014) 28:232–42. doi: 10.1177/0269215513497735
23. Turna IF, Erhan B, Gunduz NB, Turna O. The effects of different injection techniques of botulinum toxin a in post-stroke patients with plantar flexor spasticity. Acta Neurol Belg. (2020) 120:639–43. doi: 10.1007/s13760-018-0969-x
24. Hong Z, Sui M, Zhuang Z, Liu H, Zheng X, Cai C, et al.Effectiveness of neuromuscular electrical stimulation on lower limbs of patients with hemiplegia after chronic stroke: a systematic review. Arch Phys Med Rehabil. (2018) 99:1011–22.e1. doi: 10.1016/j.apmr.2017.12.019
25. Hu W, Rundle-Gonzalez V, Kulkarni SJ, Martinez-Ramirez D, Almeida L, Okun MS, et al. A randomized study of botulinum toxin versus botulinum toxin plus physical therapy for treatment of cervical dystonia. Parkinsonism Relat Disord. (2019) 63:195–8. doi: 10.1016/j.parkreldis.2019.02.035
26. Orsini M, Araujo Leite MA, Chung TM, Bocca W, de Souza JA, Gameiro de Souza O, et al. Botulinum neurotoxin type a in neurology: update. Neurol Int. (2015) 7:5886. doi: 10.4081/ni.2015.5886
27. Rosales RL, Balcaitiene J, Berard H, Maisonobe P, Goh KJ, Kumthornthip W, et al. Early abobotulinumtoxina (dysport®) in post-stroke adult upper limb spasticity: ONTIME pilot study. Toxins. (2018) 10:253. doi: 10.3390/toxins10070253
28. Teasell R, Cotoi A, Chow J, Wiener J, Iliescu A, Hussein N, et al. Evidence-Based Review of Stroke Rehabilitation (18th Edition). (2016). Available online at: http://www.ebrsr.com/sites/default/files/documents/v18-SREBR-ExecutiveSummary-2.pdf (accessed September 26, 2019).
29. Bertram KL, Williams DR. Delays to the diagnosis of cervical dystonia. J Clin Neurosci. (2016) 25:62–4. doi: 10.1016/j.jocn.2015.05.054
30. Tiderington E, Goodman EM, Rosen AR, Hapner ER, Johns MM 3rd, Evatt ML, et al. How long does it take to diagnose cervical dystonia? J Neurol Sci. (2013) 335:72–4. doi: 10.1016/j.jns.2013.08.028
31. Comella C, Bhatia K. An international survey of patients with cervical dystonia. J Neurol. (2015)262:837–48. doi: 10.1007/s00415-014-7586-2
32. Cunningham CT, Quan H, Hemmelgarn B, Noseworthy T, Beck CA, Dixon E, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. (2015) 15:32. doi: 10.1186/s12874-015-0016-z
33. Jordan JT, Mayans D, Schneider L, Adams N, Khawaja AM, Engstrom J.Education research: neurology resident education: trending skills, confidence, and professional preparation. Neurology. (2016) 86:e112–7. doi: 10.1212/WNL.0000000000002463
34. Cusick A, Lannin N, Kinnear BZ. Upper limb spasticity management for patients who have received botulinum toxin A injection: australian therapy practice. Aust Occupational Ther J. (2015) 62:27–40. doi: 10.1111/1440-1630.12142
35. Holmes RJ, Connell LA. A survey of the current practice of intramuscular botulinum toxin injections for hemiplegic shoulder pain in the UK. Disabil Rehabil. (2019) 41:720–6. doi: 10.1080/09638288.2017.1400596
36. Kerkemeyer L, Lux G, Walendzik A, Wasem J, Neumann A. Medical care of patients with spasticity following stroke: evaluation of the treatment situation in Germany with focus on the use of botulinum toxin. Nervenarzt. (2017) 88:919–28. doi: 10.1007/s00115-017-0312-4
37. Fehlings D, Narayanan U, Andersen J, Beauchamp R, Gorter JW, Kawamura A, et al. Botulinum toxin-A use in paediatric hypertonia: canadian practice patterns. Can J Neurol Sci. (2012) 39:508–15. doi: 10.1017/S0317167100014049
Keywords: Botulinum toxin-A, cervical dystonia, patient management, spastic paresis, rehabilitation, continuous medical education (CME), practices
Citation: Chung TM, Jacinto LJ, Colosimo C, Bhatia KP, Tiley J and Bhidayasiri R (2020) Botulinum Neurotoxin-A Injection in Adult Cervical Dystonia and Spastic Paresis: Results From the INPUT (INjection Practice, Usage and Training) Survey. Front. Neurol. 11:570671. doi: 10.3389/fneur.2020.570671
Received: 08 June 2020; Accepted: 13 August 2020;
Published: 16 September 2020.
Edited by:
Pedro J. Garcia-Ruiz, University Hospital Fundación Jiménez Díaz, SpainReviewed by:
Araceli Alonso-Canovas, Ramón y Cajal University Hospital, SpainJuan Carlos Martinez Castrillo, Ramón y Cajal University Hospital, Spain
Copyright © 2020 Chung, Jacinto, Colosimo, Bhatia, Tiley and Bhidayasiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roongroj Bhidayasiri, cmJoQGNodWxhcGQub3Jn
†ORCID: Roongroj Bhidayasiri orcid.org/0000-0002-6901-2064
Carlo Colosimo orcid.org/0000-0002-2216-3973
Luis Jorge Jacinto orcid.org/0000-0002-3428-2312
Kailash P. Bhatia orcid.org/0000-0001-8185-286X
 Tae Mo Chung1
Tae Mo Chung1 Carlo Colosimo
Carlo Colosimo Julie Tiley
Julie Tiley