- 1Hobbs Rehabilitation, Winchester, United Kingdom
- 2Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
- 3Careggi University Hospital, Florence, Italy
- 4Department of Neuroscience, Uppsala University, Uppsala, Sweden
- 5Hematology and Bone Marrow Transplantation Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), San Raffaele Scientific Hospital, Milan, Italy
- 6Kings Health Partners, Department of Haematology, Guys Hospital, London, United Kingdom
- 7Institute of Paediatric Oncology and Haematology, N.N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
- 8Department of Neuroscience, University of Genova and Clinical Scientific Institutes Maugeri, Genoa, Italy
- 9School of Health and Related Research, University of Sheffield, Sheffield, United Kingdom
- 10Department of Brain Sciences, Imperial College London, London, United Kingdom
- 11CHU de Poitiers, Service de Médecine Interne et Maladies Infectieuses, Poitiers, France
- 12CHU de Poitiers, CIC-1402, Poitiers, France
- 13Department of Haematology and Blood Stem Cell Transplantation, St Vincent's Health Network Sydney and Senior Lecturer, St Vincent's Clinical School, University of New South Wales Medicine, Sydney, NSW, Australia
- 14Patient Advocacy Committee, EBMT Executive Office, Barcelona, Spain
Autologous haematopoietic stem cell transplantation (AHSCT) is increasingly used to treat people with multiple sclerosis (MS). Supported by an evolving evidence base, AHSCT can suppress active inflammation in the central nervous system and induce long-term changes in immune cell populations, thereby stabilizing, and, in some cases, reversing disability in carefully selected MS patients. However, AHSCT is an intensive chemotherapy-based procedure associated with intrinsic risks, including profound cytopenia, infection, and organ toxicity, accompanied by an on-going degree of immuno-compromise and general deconditioning, which can be associated with a transient increase in functional impairment in the early stages after transplantation. Although international guidelines and recommendations have been published for clinical and technical aspects of AHSCT in MS, there has been no detailed appraisal of the rehabilitation needed following treatment nor any specific guidelines as to how this is best delivered by hospital and community-based therapists and wider multidisciplinary teams in order to maximize functional recovery and quality of life. These expert consensus guidelines aim to address this unmet need by summarizing the evidence-base for AHSCT in MS and providing recommendations for current rehabilitation practice along with identifying areas for future research and development.
Introduction
Multiple Sclerosis
Multiple Sclerosis (MS) is an acquired chronic immune-mediated inflammatory disease of the central nervous system (CNS) and is the commonest cause of non-traumatic disability in adults of working age (1, 2).
Approximately 85% of people with MS present with a relapsing remitting course (RRMS) characterized by distinct episodes of new or worsening neurological dysfunction (relapses) followed by complete or partial recovery (remission) (3). In the majority of cases, patients transition into a secondary progressive phase (SPMS) 10–25 years after the disease onset, accumulating progressive disability (4). A minority of patients (10–15%) present with a progressive disease course from the onset of disease, described as primary progressive MS (PPMS) (4).
Clinical presentation varies dependent on the site of lesions within the CNS. Clinical features may include abnormalities of muscle strength, sensation, balance, coordination, vision, cognition, speech, swallowing, bladder, bowel, and sexual function as well as tremor, pain, fatigue, heat sensitivity, and changes in mood and personality (5). MS can be highly disabling with considerable personal, social, and economic consequences (6, 7).
Medical Management of Multiple Sclerosis
Currently, there is no known cure for MS. Medical treatment aims to modify the course of the disease process and control symptoms as they develop (8). In RRMS, treatment is aimed at reducing permanent damage to the CNS by decreasing inflammation and preventing relapses (8). There are now 15 licensed disease-modifying therapies (DMTs), which aim to suppress inflammation and prevent the progressive phase of disease (9). Steroids are frequently used to manage relapses in the acute setting, but have no beneficial long-term effect. The treatment options for patients with SPMS and PPMS are very limited.
DMTs have various levels of efficacy but many patients respond poorly and continue to accumulate disability (10). There is also a risk of immuno-compromise and other toxicities. In recent years increasing numbers of patients with MS have been treated with autologous HSCT (11–13). Specific EBMT guidelines have been published to assist with patient selection, advice about transplant protocols, and supportive care (14).
AHSCT and Multiple Sclerosis
In recent years an increasing evidence base and published professional guidelines have supported more widespread use of AHSCT as a treatment option in patients with highly active RRMS. MS is now the fastest growing indication for AHSCT across Europe (15).
AHSCT provides a “one-off” treatment as opposed to DMTs, which generally require on-going administration (7, 16). AHSCT, however is an intensive chemotherapy-based procedure that can result in deconditioning and which may add further to functional impairment, at least temporarily (17). AHSCT and its associated short- and long-term risks require counseling, detailed workup and an admission to specialized HSCT facilities (18). The provision of a coordinated care plan derived jointly by transplant hematologists, MS neurologists, and other allied healthcare professionals is essential throughout the procedure (19).
Although AHSCT is not thought to be directly associated with repair or regeneration of damaged myelin or nerve fibers, previous studies have demonstrated reversal of disability in some patients following treatment which could be explained by the prolonged suppression of inflammation and physiological CNS repair (19). Secondary benefits of AHSCT include reduced fatigue and improved energy reserves in contrast to profound fatigue, which is commonly associated with active inflammation (20). At present there are no studies to confirm whether neuro-rehabilitation may promote recovery following AHSCT (21), but this may be a reasonable extrapolation from standard neuro-rehabilitation practice. AHSCT has the capacity to halt the inflammatory process for an extended period of time, which may allow the CNS to acquire the capacity to repair and re-organize the damaged areas. Neuro-rehabilitation can contribute to this.
Neuro-Rehabilitation in MS
Outcomes for individuals with MS could be maximized through rehabilitation; both by optimizing a patient's physical fitness and by guiding and stimulating neuroplasticity. In MS, evidence suggests that neuroplasticity can play a role in limiting the clinical impact of damage (22). Task orientated interventions can result in the reorganizing or restoration of altered patterns of brain activity and may induce clinically meaningful re-myelination and plasticity changes (23). In MS, maladaptive plasticity can occur in the CNS following neuronal injury (23), highlighting the need for specialist therapy teams to guide rehabilitation intervention.
With AHSCT, there is a rapid and prolonged suppression of CNS inflammation associated with a sustained interval free of immunosuppressive treatments; therefore, the early administration of a neuro-rehabilitation program represents a unique opportunity to maximize its effect, possibly in association with axonal repair.
Similar to the medical management for MS, rehabilitation intervention is determined by the symptoms and clinical presentation for each patient and therefore a full clinical assessment is essential with the rehabilitation being goal directed and person centered (24, 25). This should be multi-disciplinary with involvement from neurologists, specialist nurses, rehabilitation specialists, physiotherapists, occupational therapists, speech and language therapists, and neuro-psychologists. Where possible, the rehabilitation must involve self-management and where possible family and carers to ensure a 24 h approach.
The Unmet Need: Guidelines and Recommendations for Rehabilitation of Patients Undergoing AHSCT for MS
Currently, there is no established rehabilitation pathway for patients with MS either before or after AHSCT. Although pre-habilitation and rehabilitation have been explored in malignant hematological diseases where AHSCT is routinely used (26), there is very limited evidence in relation to AHSCT in MS. In addition, there is no consensus as to how rehabilitation should be delivered and provision will vary from country to country. Now that there is a significant demand across health services in many countries for AHSCT in patients with MS (14), addressing this unmet need has become an urgent matter. Therapists need clear guidelines to advise when they should start rehabilitation, to what intensity, how to address the potential complications associated with the AHSCT procedure and how to tailor rehabilitation programs to suit each individual's symptoms and goals. Rehabilitation plans need to factor in patients' initial presentation and their positive and negative response to AHSCT particularly if they become systemically unwell and deconditioned during the treatment period.
Aims and Process for Guidelines and Recommendations
The following guidelines and recommendations have been established by consensus to support therapists with the rehabilitation of patients with MS undergoing AHSCT with the goal of optimizing management and overall outcome.
Methodology
Following approval by the processes of the EBMT Autoimmune Diseases Working Party (ADWP), an authorship group was convened from clinicians from relevant professional groups active in or associated with the ADWP and Nurses Group (NG) of the European Society for Blood and Marrow Transplantation (EBMT) with experience in AHSCT for neurological ADs as well as patient consumer representation via the EBMT Patient Advocacy Committee. As such, this group included therapists, hematologists, neurologists, specialist nurses, and patient representation, with access to patients with MS who had undergone AHSCT. An important point is that several areas of specialism have collaborated to share expertise in respective areas.
In the absence of specific studies or guidelines for rehabilitation before or after HSCT for MS, based on knowledge within the authorship group and literature searching, the aim was to produce an expert consensus as a starting point. A systematic review was considered to be premature and therefore was not undertaken. Whilst the structure of the guideline and recommendations broadly considered the principles of the AGREE process (27), this was not a systematic review nor did it address specific health question or adhere to the AGREE or similar process. The output was therefore in line with other EBMT guidelines and recommendations, including a recent “White Paper Report” (26) developed to guide rehabilitation in patients who have or are due to receive AHSCT which focused on allogeneic HSCT and graft-versus-host disease.
The target readership primarily includes specialist therapists directly involved in planning rehabilitation for patients with MS undergoing AHSCT as well as clinical teams and their members involved in the planning and delivery of AHSCT. As such the guidelines are written in a technical style for the target readership of health professionals, and, although they are not intended as a primary resource for patient information, they should be made accessible to them via their health professionals. These guidelines cover adults, young people, and children. They are intended to be holistic aiming to address physical rehabilitation as well as cognitive, communication, and psychosocial factors. Specific evidence in the area of rehabilitation in AHSCT for MS is very limited, and therefore all recommendations are consensus and based on agreed best practice within the group, whilst the need for future systematic research in specific areas is recognized.
The aim of these guidelines and recommendations is to suggest a pathway for rehabilitation, recognizing the challenges that patients face at each stage. A further aim was to generate questions for future research in this field. We have divided the rehabilitation process into four phases:
– Phase 1: Assessment and pre-habilitation 4 weeks before starting treatment,
– Phase 2: Acute rehabilitation,
– Phase 3: Sub-acute rehabilitation, and
– Phase 4: Community rehabilitation including vocational rehabilitation.
Each phase of the pathway has a different emphasis but each treatment plan must still be tailored to the individual and will change depending on the stage of a patient's rehabilitation. These guidelines therefore outline the assessment process and propose outcome measures and standardized assessment tools as well as guidance on therapeutic interventions.
Selection Criteria for AHSCT for Patients With MS
Patients with MS being considered or offered AHSCT should meet the criteria suggested in current EBMT guidelines and recommendations and further updates, and treatment should be approved by an appropriately constituted multi-disciplinary team (MDT) (14).
The Multidisciplinary Team (MDT)
In line with EBMT recommendations, care should be provided with a co-ordinated multidisciplinary approach by a team responsible for initial assessment of suitability for AHSCT, and an extended team, who facilitate referral, assessment, and delivery of rehabilitation throughout the four phases of the pathway.
The MDT membership should include:
• Neurologist(s) with an interest in MS
• Hematologist(s) with an interest in HSCT
• Specialist MS nurse(s)
• Specialist HSCT nurse(s)
• Rehabilitation specialist(s)
• Specialist therapists (acute, sub-acute and community based) including physiotherapists, occupational therapists, speech and language therapists
• Neuropsychologists and counselors
• Dieticians
• Pain management specialists
• Infectious disease specialists
• Other specialist nurses including continence and tissue viability nurses, late effects nurses
• GPs and district nursing teams.
AHSCT – The Process
For detailed general and MS specific literature on AHSCT, please refer to EBMT guidelines for AHSCT in MS and neurological diseases (14, 17, 28, 29) and recent reviews, which cover the transplant process itself and its us in MS.
The process is summarized in Figure 1 and as follows:
1. Pre-transplant workup, including wash out of DMTs Patients should be reviewed by both MS neurologists and transplant Hematologists who are experienced in using AHSCT in this context. Patients are initially assessed and provided with extensive counseling to ensure that they are familiar with the benefits and risks of AHSCT (18, 30). Both short- and long-term risks including the effect on reproductive function and other late effects need to be considered (31). The individual is usually taken off their DMT at variable time points prior to the stem cell mobilization, dependent on the specifice DMT (14).
2. Peripheral blood stem cell [PBSC] mobilization and leukapheresis In the weeks to months prior to transplant, the patient undergoes a stem cell mobilization procedure, which allows stem cells to be procured for later transplantation (29).
3. Conditioning regimen Depending on the protocol, the patient is generally hospitalized from the start of the intensive cytotoxic “conditioning” regimen, which usually includes a combination of high-dose chemotherapy and antibody-based therapy [such as anti-thymocyte globulin (ATG)] which results in ablation of haematopoietic and immune cells throughout the body (17). Usually a central venous line is inserted to facilitate administration of cytotoxic drugs and transfusions. The line may occasionally remain in situ beyond the AHSCT procedure and its presence may need consideration when planning rehabilitation.
4. The “transplant” The cryopreserved stem cells are thawed and infused through the central venous line with close monitoring of the patient in case of reaction toe the infusion.
5. Post-transplant care Following stem cell infusion, the patient remains in hospital for a period of close monitoring, supportive care for side effects of treatment, and isolation from potential sources of infection while awaiting engraftment of the haematopoietic system, usually defined as the recovery of a neutrophil count of 109/L for three days (17), which in most cases occurs 10–14 days following the infusion of the stem cells.
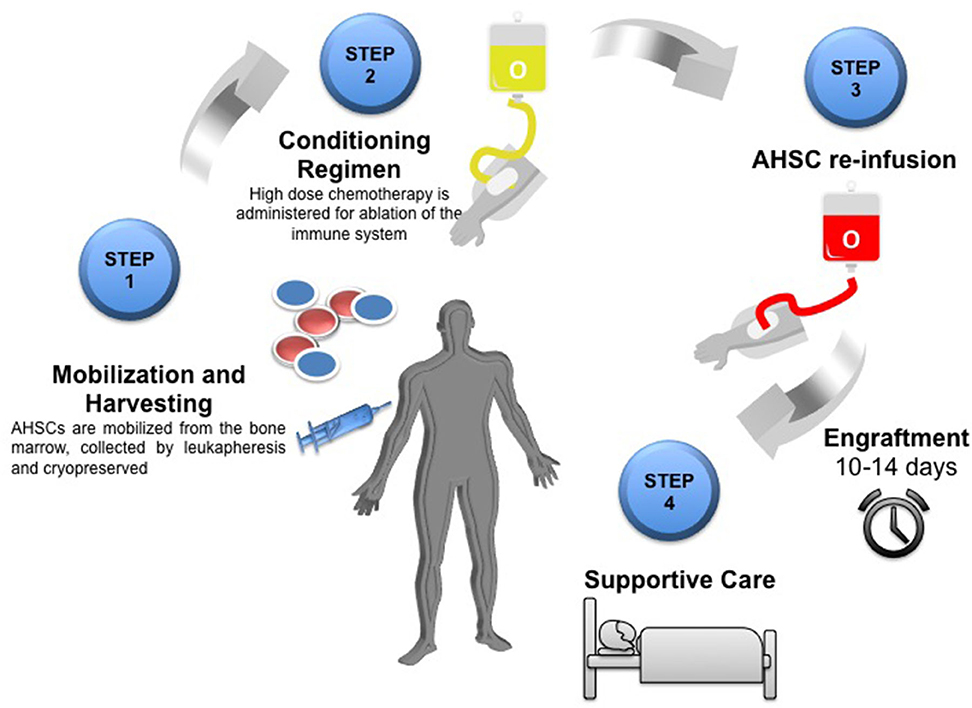
Figure 1. Schematic overview of the AHSCT process Step one: Mobilization of AHSCs from the bone marrow by leukapheresis and cryopreserved until required. Step two: Conditioning regimen using high dose chemotherapy for ablation of the immune system. Step 3: Re-infusion of autologous haematopoeitic stem cells (AHSC). Engraftment of bone marrow takes 10–14 days. Step 4: Post-transplant care and supportive therapy.
Patients are usually discharged from hospital within 4 weeks, although this depends on their baseline condition and tolerance of AHSCT. Potential early side effects include fever, infection, and sepsis in association with leukopenia, other cytopenias (anemia and thrombocytopenia) requiring blood product transfusions, and a range of other organ toxicities (alopecia, mucositis, diarrhea, vomiting, renal, and fluid balance issues and serum sickness). Worsening of existing neurological symptoms can occur often in the context of fever, drug reactions, and infection (17). Neurological deterioration in response to fever is known as the Uhthoff phenomenon, and, whilst the effect on patients during AHSCT is usually short lived and reversible, some evidence suggests the potential for prolonged deleterious effect on neurological function (32). Thus, a proactive approach is appropriate during AHSCT including prompt administration of antibiotics and steroid treatment to prevent or minimize the duration of fever (32).
Secondary complications include deconditioning, reduced caloric intake due to nausea and mucositis and limited activity in the immediate post-transplant period (32). Prolonged bed rest and isolation during AHSCT may also lead to pressure sores, thromboembolic risks, changes in spasticity and posture, acquired urinary or bowel dysfunction, bone loss, vitamin D deficiency, low mood, and fatigue (17).
Long-term effects include risks of infertility (31), autoimmune dysfunction including thyroid abnormalities and autoimmune cytopenias, endocrine, and other organ impairment and secondary malignancies (28).
Rehabilitation Within the AHSCT Pathway
Utilizing a biopsychosocial model, rehabilitation aims to optimize a patients' health, functional independence and well-being, comprising a comprehensive programme covering the physical, cognitive, psychological, and social aspects of their care. Previous recommendations have advocated the need for physical therapy before and after HSCT to promote recovery of functional capacity and improve quality of life (26). These were aimed at a wide range of hematological conditions and did not include the special considerations for neurological conditions including MS.
The paucity of evidence in the field of rehabilitation means that it is not possible to make firm recommendations. However, a summary of the basis for AHSCT alongside consensus guidance is presented to support therapists and clinicians caring for these patients to optimize collaborative care and identify areas for future development.
In these guidelines, rehabilitation for patients with AHSCT is delivered over 4 distinct phases during the pathway as detailed above and in Figure 2. Whilst traditionally rehabilitative interventions are delivered during and after treatment, these guidelines recommend an initial “pre-habilitation” phase in line with current evidence in the field of cancer rehabilitation. Pre-habilitation would occur before “beginning of acute treatment and would include physical and psychological assessments that establish a baseline functional level, identifies impairments, and provides targeted interventions that improve a patient's health to reduce the incidence and the severity of current and future impairments” (33). We suggest that pre-habilitation is a potential solution to address the needs of these MS patient to improve their outcomes.
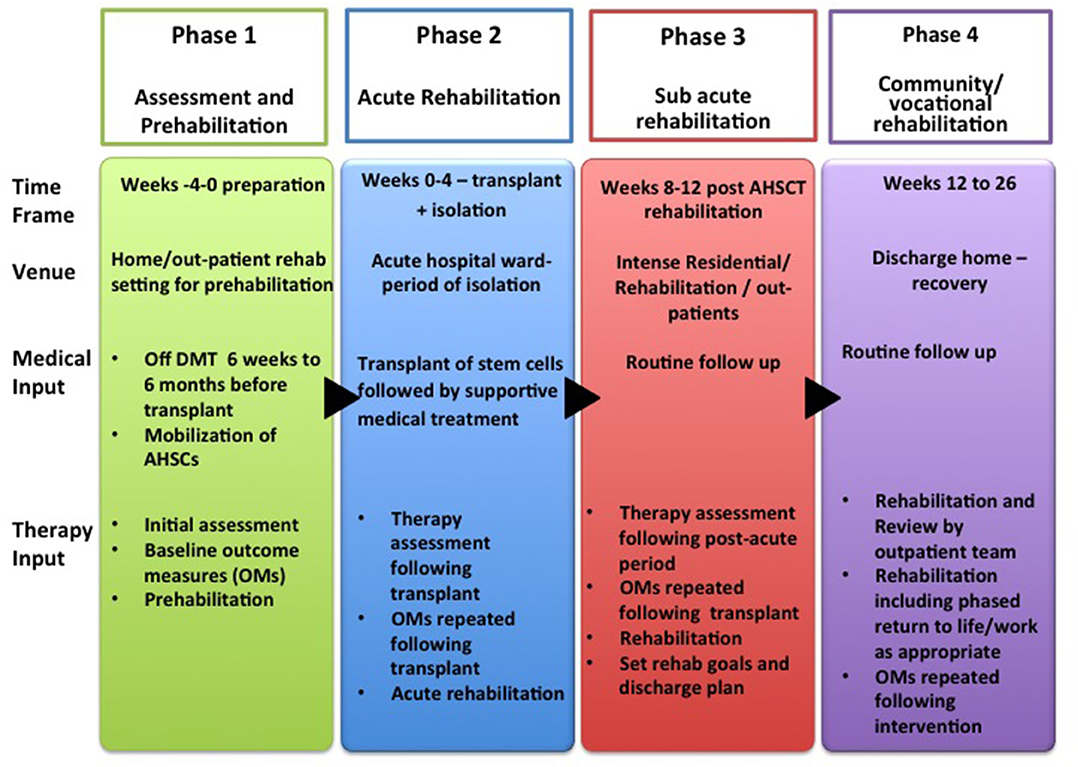
Figure 2. Rehabilitation Pathway for MS patients undergoing AHSCT (AHSCT, Autologous Haematopoietic Stem cell Transplantation; OMs, Outcome Measures; Rehab, Rehabilitation).
In general terms, patients who receive enhanced therapy whilst in acute care go home sooner (34). Rehabilitation in sub-acute and long-term follow up phases may further improve functional outcomes and reduce the burden of long-term care costs associated with dependency but further research is needed to determine the benefits (21, 34).
Rehabilitation Model
Effective rehabilitation is holistic, multi-disciplinary, goal directed, patient centered, and, where possible, evidence based, as recommended in the various international rehabilitation guidelines for patients with MS including the NICE guidelines (35), the Cochrane review of 2019 (25) and European Multiple Sclerosis Platform Rehabilitation Recommendations (36) to name a few. It is essential that the rehabilitation is adapted to patients' changing needs as they progress.
A series of reliable and validated outcome measures (OMs) should be recorded at key points throughout the patient's journey to monitor any change in key domains within the biopsychosocial model of care as outlined by the World Health Organization's International Classification of Functioning, Disability and Health (37).
The Assessment
Patients should undergo a detailed assessment (25, 35) at the start of each phase of rehabilitation not only looking at a patient physically but also their cognition, psychology, speech, function, and the utilization of standardized assessments tools and outcome measures where possible is encouraged. This will be patient specific depending on their presentation and will be phase specific.
Standardized Assessment Tools and Outcome Measures (OMs)
There are no tools or OMs specifically available for use for patients with MS receiving AHSCT and future research into this area is needed. Whilst these guidelines suggest a spectrum of tools and OMs, these are not exhaustive and must be used based on a patient's presentation and their phase of rehabilitation. For example, a patient may not be able to mobilize acutely in the initial days following their transplant, and a 10 m walk test will only be appropriate when they progress and start to mobilize.
To assess someone's cognitive function, there are a number of standardized assessment tools. For example, the Minimal Assessment of Cognitive Function in MS (MACFIMS), and the Montreal Cognitive Assessment (MoCA) have been shown to be a valuable screening tool for MS patients (38, 39). The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) was specifically designed with MS patients in mind (40). Further examples of cognitive assessments include the Loewenstein Occupational Therapy Cognitive Assessment (LOCTA) (41), the Rivermead Memory Team (42), the Behavior Assessment of the Dysexecutive Syndrome (43) and the TEA—Test of Everyday Attention which are not specific to MS (44).
For some patients, an assessment of their cognition through function may be more beneficial. For speech therapy, there are assessments for dysarthria for example the Frenchay Dysarthria Assessment (FDA-2) (45) and for language, the Mount Wilga High Level Language Assessment but neither are exclusive to the MS population.
Much like the assessment tools, a series of reliable and validated outcome measures (OMs) should be recorded at key points throughout the transplantation treatment to monitor any change in key domains within the biopsychosocial model of care as outlined by the World Health Organization's International Classification of Functioning, Disability and Health (37). In accordance with our recommendations, OMs should be used at the start and end of each phase and when there is significant change.
The National Multiple Sclerosis Society suggested a number of measures (46) but Cohen et al. described a clinical decision making process when selecting outcome measures (47). However, to date, no research has been conducted to recommend specific OMs for the MS population undergoing AHSCT. Therefore, our recommendations cover those OM with evidence base specifically in patients with MS. The Academy of Physical Therapy in 2012 (48–50) suggested the following OMs in patients with MS:
– 10 m walk test
– Berg balance
– Modified fatigue impact scale
– Rivermead Mobility index
– MS QOL 54 measure
– Dynamic gait index
– Activities of daily living (ADL)
– Nine hole peg test.
Rehabilitation During Phase 1: Assessment and Pre-Habilitation−4 Weeks to Start of Treatment
Assessment
Prior to the transplant, it is recommended that baseline level of impairment function and participation is ascertained through a formal MDT assessment process. This should include a full medical and physical assessment, including a medication and social history A physical assessment should include joint range of movement, muscle strength, length and tone, balance, sensation, cerebellar signs, proprioception, movement analysis, and endurance including respiratory function. The patient's cognition, swallowing, communication, nutritional status, functional independence, and endurance should also be assessed including determining how they transfer, mobilize, and complete personal activities of daily living including washing, dressing, feeding as well as their ability to do domestic tasks. A range of standardized outcome measures normally form part of the full assessment (see above).
Risk Assessment
It is important that any risk factors for potential deterioration are identified. These may include fatigue, existing muscle weakness or spasticity, reduced respiratory function, poor cough, contractures, pressure sores, postural changes, urinary tract infections and other infections, constipation, deconditioning, compromised nutrition, and pain (26). As well as MS, there may be co-morbidities that heighten the risk of these potential complications.
Advice and Information
In Phase 1, the key focus is optimizing the patients' physical, social, and emotional functioning and well-being prior to them undergoing AHSCT. Patients should be given individualized advice and education to help them self-manage their rehabilitation and prevent risks occurring. Advice leaflets provide guidance on exercise, nutrition, and any necessary lifestyle changes supporting a self-care and self-management paradigm. If appropriate, carers and family members should also be provided with advice and education to support the individual. Specialist consultation may be necessary/beneficial in some cases.
The Rehabilitation— “Pre-habilitation”
During phase 1, “Pre-habilitation,” can be provided as part of an intensive community rehabilitation package or in an outpatient setting. Residential rehabilitation may be required based on a patient's presentation. Pre-habilitation during this phase will be patient centered based on a full assessment.
The aims of this stage are not only to enhance neuromuscular systems and respiratory function but also to reduce the risk of secondary complications. The following should be considered, as appropriate:
– Breathing exercises and the use of respiratory adjuncts to optimize respiratory function (51).
– Cardiovascular exercise. The evidence suggests that adults with MS should engage in at least 30 min of moderate intensity aerobic activity twice a week as well as strength training of major muscle groups biweekly to optimize fitness (52). High impact weight bearing exercises such as hopping, jumping, combination exercises should also be considered if appropriate as these have a positive impact on bone mass density (53). Pre-transplant fitness can have a positive influence on recovery (34). Access to appropriate gym equipment such as a cross-trainer or static bike to enhance physical fitness may be required. Care should be taken to ensure that the level of exercise prescribed reflects the patient's clinical presentation, over-exertion could have a negative impact. The extent of cardiovascular exercise and the patient's ability to use equipment will depend on their presentation and pre-morbid level of function (54). Stairs or mobility may provide adequate cardiovascular exercise at this stage.
– Strengthening and stretching programmes (55)
– Spasticity management (35)
– The use of neuro-technologies (56)
– Cognitive rehabilitation (35, 57, 58),
– Nutrition (35),
– Relaxation (36),
– Fatigue management (35),
– Pain management (35)
– Providing strategies to accomplish transfers and ADL (34) and
– The provision of equipment including aids and orthotics as appropriate.
All activities should be underpinned by a self-management approach, where possible.
Rehabilitation During Phase 2: Acute Rehabilitation
Rehabilitation during this phase will be goal directed and patient centered helping prevent secondary problems through gentle mobilization. As such it aims to prevent cachexia and optimize respiratory function. At this stage, the provision of appropriate levels of exercise will be influenced by platelet count and fatigue. In most cases platelet count will have recovered by day +15 post-transplant although secondary immune thrombocytopenia may occur in 5–7% of patients later on. Mental health and well-being should be monitored throughout this phase.
The main consideration for rehabilitation at phase 2 is the individual's immunity. The immune system begins to recover around 2 weeks after infusion of the blood stem cells, during which patients are likely to remain in hospital for close monitoring and continuation of supportive treatment such as antibiotics. Even with neutrophil recovery, the immune system remains suppressed for several months. Any infections may adversely affect patients physically and cognitively (17). Infection control measures should be observed. As this patient group is at risk of urinary dysfunction, there may be a risk of urosepsis and rarely haemorrhagic cystitis and patients may require urinary catheterisation (59).
Contraindications and Precautions to Rehabilitation
Following AHSCT, patients invariably have cytopenias increasing the risk of infection and bleeding. At this time, physical function does need to be monitored so it is essential that skilled therapists are involved in guiding rehabilitation.
Thrombocytopenia is sometimes considered a contraindication to exercise and therefore platelet count should be monitored and exercise prescribed as appropriate. A previous consensus publication (26) has proposed the following scale for general HSCT patients.
– <20 × 109/L contraindication to exercise
– 20–30 × 109/L—gentle non-resistant exercises
– 30–50 × 109/L—minimal resistance (0.5–1 kg exercises)
– 50–150 × 109/L—progressive resisted exercises
– >150 × 109/L—no restrictions.
Assessment
Following the transplant and when medically appropriate, the individual is reassessed in line with the WHO ICF (37) by an appropriate MDT. Any potential areas of risk should be communicated to the specialist transplant therapy team before discharge. Any deterioration must be identified. If a patient's swallow has worsened, a bedside videofluoroscopy may be required.
Advice and Information for Self-Management
Following the assessment, carers including nursing staff and family should be provided with manual handling advice to facilitate safe transfers and mobility whilst encouraging independence, provided there is no negative impact. They should also be provided with any positioning or seating guidance to optimize respiratory function and posture and to aid spasticity management if this continues to be a problem. These patients will also need advice on minimizing the risk of infections, which may include breathing exercises and be on an appropriate diet.
Rehabilitation
The following should be assessed by an MDT as appropriate:
• Posture—in lying, sitting, standing, walking
• Seating
• Provision of any specialist equipment required
• Respiratory status
• Transfers and mobility
• Endurance
• Swallow
• Communication
• Cognition
• Personal activities of daily living and participation.
For specific symptom management, please see Multiple Sclerosis in Adults: Management (35). The following should be considered as part of rehabilitation:
– Cardiovascular exercise—many post-AHSCT patients have reduced aerobic capacity and decreased physical activity. Physical activity can restore exercise tolerance and improve function in patients after AHSCT (60).
– Progressive strengthening training can improve walking and balance but should be carefully prescribed for individual patients (53)
– Stretching programme as appropriate (55)
– Positioning and seating
– Spasticity management—commenced or continued with specific exercises, medication, and splinting as appropriate (35)
– Handling, facilitation, and specific exercise techniques for postural realignment
– Mobility progression (35)
– Balance exercises (35)
– Functional task practice
– Swallow guidance (35)
– Communication strategies (35)
– Cognitive rehabilitation (35, 57, 58)
– Fatigue management—please note that graded physical therapy has been shown to be effective in minimizing post-HSCT fatigue (61)
– Pain management (35).
Intensity
During this phase, the patients need to be seen every day by a physiotherapist to ensure that their respiratory function is monitored and optimized and their physical function is assessed as well as receiving rehabilitation specific to their needs. Each patient will respond and react differently to the transplant process so each rehabilitation package is bespoke. Regular occupational therapy input is also required to ensure that independent function is optimized and speech and language therapy is provided as required.
Rehabilitation During Phase 3: Sub-Acute Rehabilitation
Following the transplant and when medically stable, the individual should receive a period of intense inpatient or outpatient rehabilitation to optimize physical fitness, independence, and the outcome of the transplant. The timing of this will depend on the patient. This will likely be 8 weeks following the transplant, as prior to this, patients will be too weak to fully participate and are at the highest risk of EBV/CMV reactivation. Even with neutrophil recovery, the immune system remains suppressed for several months and patients may need readmission for infections and other complications. Therapists and patients also need to be aware that there is a risk of “late effects” associated with AHSCT. These may be the result of the transplant regimen and altered post-transplant immune reconstitution, but may also be driven by pre-treatment of the underlying neurological disease (19).
The rehabilitation in this phase should be aimed at treating both the neurological presentation and addressing the neuromuscular weakness and other disabilities now that the active inflammation has subsided.
Rehabilitation should include a full assessment and a treatment programme as above and should aim to further progress the patient. Therapy should consider cardiovascular workout equipment as well as the use of neuro-technologies where possible to optimize the effects of the AHSCT. Neuro-technology enables repetitive task practice. More evidence is emerging with regards to their benefits in neuro-rehabilitation (56). Neuro-technology can include the use of robotic gait training, upper limb devices, strength training, and balance devices as well as functional electrical stimulation and virtual rehabilitation (56). Care should always be taken to tailor these interventions to the individual patient and to monitor the impact of these technologies as our understanding of the long term impacts remain unclear.
Overall rehabilitation should follow a biopsychosocial, multi-disciplinary model with the patient having an allocated key worker, accessing the health care professionals required with a structured rehabilitation approach including access to groups and rehabilitation assistance sessions where appropriate. Utilizing a patient centered approach, patients and their carers should be involved with their goal setting. There should also be family meetings if required and opportunities for education to fully engage the patient in their rehabilitation. Following a self-management and self-care approach will ensure motivation, self-efficacy and the sustainability of behavior change once the patient has been discharged from formal rehabilitation.
Intensity
The rehabilitation programme provided in this phase should be intensive. The intensity will vary depending on the patient and their pre-morbid and post-transplant presentation. For active individuals who tolerated the transplant procedure well, up to 4 h of therapy intervention could be received in a typical working day with input from physiotherapy, occupational therapy, speech and language therapy, and psychology as appropriate. Therapy groups can be considered providing immune status is taken into account.
Advice and Information
Manual handling advice as well as seating and positioning programmes should be reviewed and any changes communicated as appropriate. As well as their therapy sessions, patients should be provided with exercise programmes to complete in their own time as part of their self-management programme. Patients should also be provided with advice regarding rest periods and fatigue management. The discharge planning and links into the community are made at this time. This includes exploring any on-going community support and return to work programmes.
Rehabilitation During Phase 4: Community Rehabilitation Including Vocational Rehabilitation
This phase of rehabilitation occurs when the individual has been discharged home. Therapists and individuals need to be aware of late-effects of the AHSCT. A fever episode might cause deterioration in function but patients should be made aware that such conditions are usually fully reversible. The focus of rehabilitation during this phase is a continuum of the inpatient goals within their normal home environment, integrating the patient back into their home life and promoting independence. It is important that patients continue with their individualized rehabilitation programmes and progress their mobility and independence as able.
Returning to work in a timely manner is important for this patient group as many are in employment and may have dependants. Patients are generally not encouraged to return to work until 3 months post-transplant due to infection risks and in some cases delayed up until 6 months post-transplant depending on immune recovery. When and as appropriate, patients should be referred to appropriate vocational rehabilitation services for support. Specialist occupational therapists are crucial to carry out pre-employment physical and functional testing and liaising with the patient and their employer about returning to work (62). A specific return to work programme should be designed for the patient and progression monitored. Education of the employer is important as is linking into occupational health departments (62).
Advice and Information
It is important that on-going support and guidance is available after the patient has returned home. This may require referrals to appropriate community-based teams including therapy teams, specialists (e.g., continence, sexual function, tissue viability) or social services. If appropriate, carers and family members should also be provided with advice and education to assist the individual going home. This may include advice on manual handling including the use of equipment, positioning, seating, and nutritional or feeding advice.
Comprehensive Review
Regaining neurological function can continue in the first couple of years following AHSCT and it is important to optimize this period of time with a tailored, comprehensive rehabilitation programme. Patients who have undergone AHSCT should have a comprehensive review of all aspects of their care at least once a year, which needs to be carried out by healthcare professionals with expertise in MS and its complications. The review process and follow up treatments must be individually tailored to need. It may result in onward referral for further specialist support e.g., spasticity management, orthotics or specialist equipment available as required. Hydrotherapy can be considered providing immune status is taken into account by transplant physician and specialist hydrotherapist.
Conclusions
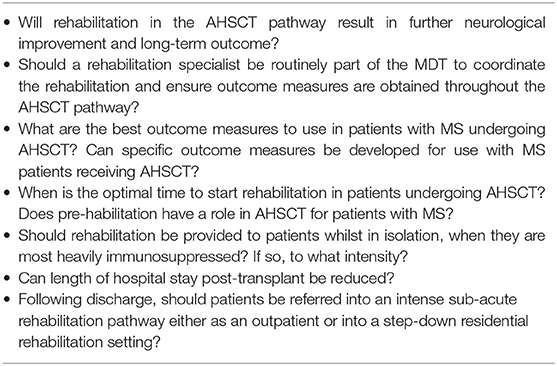
The consensus recommendations are summarized in Table 1. Rehabilitation should be considered at each stage of the pathway on an individualized basis via a proper MDT assessment, with the aim of preventing secondary problems, reducing length of hospital stay and maximizing recovery and long-term outcomes. There is also potential to reduce long-term care costs. The lack of evidence-base to support the benefits (and risks) of rehabilitation in MS patients undergoing AHSCT is highlighted and further research is warranted. Table 2 summarises the future health research questions. The proposed framework for rehabilitation can be factored into clinical trials and future practice can be refined to reflect emerging evidence.
Author Contributions
FR, HH, JS, BS, and HJ conceived the proposal and developed and wrote the manuscript with the other co-authors after approval by the EBMT Autoimmune Diseases Working Party (ADWP, Chair JS). FR, HH, CB, and SM are co-opted into the ADWP as physiotherapists with established experience and interaction with EBMT transplant centers and their patients. BS, JB, GM, PM, and AI are neurologists specialized in MS, including experience in AHSCT. JS, RS, MK, RG, KK, BW, and MP are hematologists experienced in AHSCT for MS. HJ is the EBMT Nurses Group representative on the ADWP. BV is the Chair of the EBMT Patient Advocacy Group, and thereby provided patient representation. FR and AI created the figures. All authors reviewed the final manuscript.
Funding
Other than EBMT support there is no funding body supporting these guidelines, commercial or otherwise.
Conflict of Interest
FR and HH work for Hobbs Rehabilitation, JS declares honoraria for speaking at educational meetings from Sanofi, Jazz, Janssen, Gilead, and Mallinckrodt, BV declares honoraria for speaking at educational meetings and consultancy from Janssen, Takeda, and Amgen. PM reports travel support and speaker honoraria from unrestricted educational activities organized by Novartis, Bayer HealthCare, Bayer Pharma, Biogen Idec, Merck-Serono, and Sanofi Aventis. He also discloses consulting to Magenta Therapeutics and Jasper Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EP declared a shared affiliation, with no collaboration, with two of the authors RS and CB to the handling Editor.
The Handling Editor declared a past co-authorship with one of the authors GM.
Acknowledgments
BS, JS, and AI acknowledge the support of the NIHR Sheffield Biomedical Research Centre, Sheffield Hospitals Charity, and Clinical Trials Research Unit.
Abbreviations
AD, Autoimmune Disease; ADL, Activities of Daily Living; ADWP, Autoimmune Disease Working Party; AHSCT, Autologous Haematopoietic Stem Cell Transplantation; MDT, Multi-Disciplinary Team; MS, Multiple Sclerosis; OMs, Outcome Measures.
References
1. Compston A, Coles A. Multiple sclerosis. Lancet. (2002) 359:1221–31. doi: 10.1016/S0140-6736(02)08220-X
2. Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. (2010) 9:520–32. doi: 10.1016/S1474-4422(10)70064-8
3. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
4. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. (2000) 343:1430–8. doi: 10.1056/NEJM200011163432001
5. Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. (2017) 389:1336–46. doi: 10.1016/S0140-6736(16)30959-X
6. Ernstsson O, Gyllensten H, Alexanderson K, Tinghog P, Friberg E, Norlund A. Cost of illness of multiple sclerosis - a systematic review. PLoS ONE. (2016) 11:e0159129. doi: 10.1371/journal.pone.0159129
7. Moccia M, Palladino R, Lanzillo R, Carotenuto A, Russo CV, Triassi M, et al. Healthcare costs for treating relapsing multiple sclerosis and the risk of progression: a retrospective italian cohort study from 2001 to 2015. PLoS ONE. (2017) 12:e0169489. doi: 10.1371/journal.pone.0169489
8. Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of british neurologists: revised 2015 guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. (2015) 15:273–9. doi: 10.1136/practneurol-2015-001139
9. Lucchetta RC, Tonin FS, Borba HHL, Leonart LP, Ferreira VL, Bonetti AF, et al. Disease-Modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. CNS Drugs. (2018) 32:813–26. doi: 10.1007/s40263-018-0541-5
10. Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. (2015) 72:152–8. doi: 10.1001/jamaneurol.2014.3537
11. Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. (2015) 84:981–8. doi: 10.1212/WNL.0000000000001329
12. Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysisA. Neurology. (2017) 88:2115–22. doi: 10.1212/WNL.0000000000003987
13. Mancardi G, Sormani MP, Muraro PA, Boffa G, Saccardi R. Intense immunosuppression followed by autologous haematopoietic stem cell transplantation as a therapeutic strategy in aggressive forms of multiple sclerosis. Mult Scler. (2018) 24:245–55. doi: 10.1177/1352458517742532
14. Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. (2019) 55:283–306. doi: 10.1038/s41409-019-0684-0
15. Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. (2019) 54:1525–52. doi: 10.1038/s41409-019-0516-2
16. Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA, et al. Hematopoietic stem cell therapy for autoimmune diseases - clinical experience and mechanisms. J Autoimmun. (2018) 92:35–46. doi: 10.1016/j.jaut.2018.06.002
17. Ismail A, Sharrack B, Saccardi R, Moore JJ, Snowden JA. Autologous haematopoietic stem cell therapy for multiple sclerosis: a review for supportive care clinicians on behalf of the autoimmune diseases working party of the european society for blood and marrow transplantation. Curr Opin Support Palliat Care. (2019) 13:394–401. doi: 10.1097/SPC.0000000000000466
18. Jessop H, Farge D, Saccardi R, Alexander T, Rovira M, Sharrack B, et al. General information for patients and carers considering haematopoietic stem cell transplantation (HSCT) for severe autoimmune diseases (ADs): A position statement from the EBMT Autoimmune Diseases Working Party (ADWP), the EBMT Nurses Group, the EBMT Patient, Family and Donor Committee and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant. (2019) 54:933–42. doi: 10.1038/s41409-019-0430-7
19. Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. (2017) 13:391–405. doi: 10.1038/nrneurol.2017.81
20. Bose G, Atkins HL, Bowman M, Freedman MS. Autologous hematopoietic stem cell transplantation improves fatigue in multiple sclerosis. Mult Scler. 25:1764–72. doi: 10.1177/1352458518802544
21. Keen C, Skilbeck J, Ross H, Smith L, Collins K, Dixey J, et al. Is it feasible to conduct a randomised controlled trial of pretransplant exercise (prehabilitation) for patients with multiple myeloma awaiting autologous haematopoietic stem cell transplantation? Protocol for the PREeMPT study. BMJ Open. (2018) 8:e021333. doi: 10.1136/bmjopen-2017-021333
22. Prosperini L, Di Filippo M. Beyond clinical changes: Rehabilitation-induced neuroplasticity in MS. Mult Scler. (2019) 25:1348–62. doi: 10.1177/1352458519846096
23. Lipp I, Tomassini V. Neuroplasticity and motor rehabilitation in multiple sclerosis. Front Neurol. (2015) 6:59. doi: 10.3389/fneur.2015.00059
24. Catalan M, De Michiel A, Bratina A, Mezzarobba S, Pellegrini L, Marcovich R, et al. Treatment of fatigue in multiple sclerosis patients: a neurocognitive approach. Rehabil Res Pract. (2011) 2011:670537. doi: 10.1155/2011/670537
25. Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of cochrane reviews. Cochrane Database Syst Rev. (2019) 1:CD012732. doi: 10.1002/14651858.CD012732.pub2
26. Mohammed J, Smith SR, Burns L, Basak G, Aljurf M, Savani BN, et al. Role of physical therapy before and after hematopoietic stem cell transplantation: white paper report. Biol Blood Marrow Transplant. (2019) 25:e191–e8. doi: 10.1016/j.bbmt.2019.01.018
27. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med. (2010) 51:421–4. doi: 10.1016/j.ypmed.2010.08.005
28. Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. (2017) 74:459–69. doi: 10.1001/jamaneurol.2016.5867
29. Das J, Sharrack B, Snowden JA. Autologous haematopoietic stem cell transplantation in multiple sclerosis: a review of current literature and future directions for transplant haematologists and oncologists. Curr Hematol Malig Rep. (2019) 14:127–35. doi: 10.1007/s11899-019-00505-z
30. Snowden JA, Sharrack B, Akil M, Kiely DG, Lobo A, Kazmi M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin Med. (2018) 18:329–34. doi: 10.7861/clinmedicine.18-4-329
31. Snarski E, Snowden JA, Oliveira MC, Simoes B, Badoglio M, Carlson K, et al. Onset and outcome of pregnancy after autologous haematopoietic SCT (AHSCT) for autoimmune diseases: a retrospective study of the EBMT autoimmune diseases working party (ADWP). Bone Marrow Transplant. (2015) 50:216–20. doi: 10.1038/bmt.2014.248
32. Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. (2009) 8:244–53. doi: 10.1016/S1474-4422(09)70017-1
33. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. (2013) 92:715–27. doi: 10.1097/PHM.0b013e31829b4afe
34. Wallek S, Senn-Malashonak A, Vogt L, Schmidt K, Bader P, Banzer W. Impact of the initial fitness level on the effects of a structured exercise therapy during pediatric stem cell transplantation. Pediatr Blood Cancer. (2018) 65. doi: 10.1002/pbc.26851
35. National Institute for Health and Care Excellence. Multipe Sclerosis in Adults: Management. Clinical Guideline [CG186]. (2019). Available online at: https://www.nice.org.uk/guidance/cg186. (accessed November 11, 2019)
36. European Multiple Sclerosis Platform. Recommendations-on-MS-Rehabiltation-RIMS-EMSP-2012. (2012). Available online at: https://www.eurims.org/News/recommendations-on-rehabilitation-services-for-persons-with-multiple-sclerosis-in-europe.html (accessed November 14, 2020).
37. World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization (WHO).2001 (2001).
38. Benedict RHB, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, et al. Validiity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. (2006) 122:549–58. doi: 10.1017/s1355617706060723
39. Dagenais E, Rouleau I, Demers M, Jobin C, Roger E, Chamelian L, et al. Value of the MoCA test as a screening instrument in multiple sclerosis. Can J Neurol Sci. (2013) 40:410–5. doi: 10.1017/S0317167100014384
40. Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler J. (2012) 18:891–8. doi: 10.1177/1352458511431076
41. Katz N, Itzkovich M, Averbuch S, Elazar B. Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) battery for brain-injured patients: reliability and validity. Am J Occup Ther. (1989) 43:184–92. doi: 10.5014/ajot.43.3.184
42. Efklides A, Yiultsi E, Kangellidou T, Kounti F, Dina F, Tsolaki M. Wechsler memory scale, rivermead behavioral memory test, and everyday memory questionnaire in healthy adults and alzheimer patients. Eur J Psychol Assess. (2002) 18:63–77. doi: 10.1027//1015-5759.18.1.63
43. Canali F, Brucki S, Bueno O. Behavioural assessment of the dysexecutive syndrome (BADS) in healthy elders and Alzheimer's disease patients: preliminary study. Dement Neuropsychol. (2007) 1:154–60. doi: 10.1590/s1980-57642008dn10200007
44. Robertson I, Ward T, Ridgeway VN-SI. The Test of Everyday Attention Assessment. London: Pearson (1994).
45. Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. 2nd ed. St. Louis, Mo: Elsevier (2005). p. 578.
46. Multiple Sclerosis Outcome Measures Taskforce. Compendium of Instructions for Outcome Measures. (2011). Available online at: https://www.neuropt.org/docs/ms-edge-documents/final-ms-edge-document.pdf?sfvrsn=913a970b_4
47. Cohen ET, Potter K, Allen DD, Bennett SE, Brandfass KG, Gail MS, et al. Selecting rehabilitaiton outcome measures for people with multiple sclerosis. Int J MS Care. (2015) 17:181–9. doi: 10.7224/1537-2073.2014-067
48. Academy of Neurologic Physical Therapy (ANPT). Academy of Neurologic PT Outcome Measures Recommendations. Outcome Measures for In and Out-Patient Rehabilitation. Available online at: https://www.neuropt.org/practice-resources/neurology-section-outcome-measures-recommendations/multiple-sclerosis (accessed November 14, 2020).
49. Academy of Neurologic Physical Therapy (ANPT). Academy of Neurologic Physical Therapy (ANPT). Academy of Neurologic PT Outcome Measures Recommendations. Outcome Measures for In and Out-Patient Rehabilitation. (2012). Available online at: http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations.MS-EDGE (accessed November 14, 2020).
50. Academy of Neurologic Physical Therapy (ANPT). Academy of Neurologic PT Outcome Measures Recommendations. Outcome Measures for Research. Available online at: http://www.neuropt.org/professional-resources/neurology-section-outcome-measures-recommendations.MS-EDGE
51. Westerdahl E, Wittrin A, Kånåhols M, Gunnarsson M, Nilsagård Y. Breathing exercises for patients with multiple sclerosis - a randomized controlled trial. Eur Respir J. (2014) 44(Suppl 58): 4676
52. Latimer-Cheung A E, Pilutti LA, Hicks AL, Martin Ginis K A, Fenuta AM, MacKibbon K A, et al. Effects of exercise training on fitness, mobility, fatigue and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. (2013) 94:1800–28. doi: 10.1016/j.apmr.2013.04.020
53. Tweedy SM, Beckman EM, Geraghty TJ, Theisen D, Perret C, Harvey LA, et al. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport. (2017) 20:108–15. doi: 10.1016/j.jsams.2016.02.001
54. Wood WA, Phillips B, Smith-Ryan AE, Wilson D, Deal AM, Bailey C, et al. Personalized home-based interval exercise training may improve cardiorespiratory fitness in cancer patients preparing to undergo hematopoietic cell transplantation. Bone Marrow Transplant. (2016) 51:967–72. doi: 10.1038/bmt.2016.73
55. Halabchi F, Alizadeh Z, Sahraian MA, Abolhasan M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. (2017) 17:185. doi: 10.1186/s12883-017-0960-9
56. Coscia M, Wessel MJ, Chaudary U, Millan JDR, Micera S, Guggisberg A, et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. (2019) 142:2182–97. doi: 10.1093/brain/awz181
57. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. (2008) 7:1139–51. doi: 10.1016/S1474-4422(08)70259-X
58. Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. (2014) CD009131. doi: 10.1002/14651858.CD009131.pub3
59. Motavasseli D, Chesnel C, Charlanes A, Menoux D, Charoenwong F, Le Breton F, et al. Adherence to anticholinergic therapy and clean intermittent self-catheterization in patients with multiple sclerosis. Int Neurourol J. (2018) 22:133–41. doi: 10.5213/inj.1836054.027
60. Baumann FT, Kraut L, Schule K, Bloch W, Fauser AA. A controlled randomized study examining the effects of exercise therapy on patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. (2010) 45:355–62. doi: 10.1038/bmt.2009.163
61. Oberoi S, Robinson PD, Cataudella D, Culos-Reed SN, Davis H, Duong N, et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. (2018) 122:52–9. doi: 10.1016/j.critrevonc.2017.12.011
62. The Chartered Society of Physiotherapy 2019. Download Physiotherapy Works for Fitness for Work. (2019). Available online at: http://www.csp.org.uk/publications/download-physiotherapy-works-fitness-work (accessed November 14, 2020).
Keywords: autoimmune diseases, autologous haematopoietic stem cell transplantation, neurological diseases, multiple sclerosis, rehabilitation, physical therapy, exercise
Citation: Roberts F, Hobbs H, Jessop H, Bozzolini C, Burman J, Greco R, Ismail A, Kazmi M, Kirgizov K, Mancardi G, Mawson S, Muraro PA, Puyade M, Saccardi R, Withers B, Verhoeven B, Sharrack B and Snowden JA (2020) Rehabilitation Before and After Autologous Haematopoietic Stem Cell Transplantation (AHSCT) for Patients With Multiple Sclerosis (MS): Consensus Guidelines and Recommendations for Best Clinical Practice on Behalf of the Autoimmune Diseases Working Party, Nurses Group, and Patient Advocacy Committee of the European Society for Blood and Marrow Transplantation (EBMT). Front. Neurol. 11:556141. doi: 10.3389/fneur.2020.556141
Received: 06 July 2020; Accepted: 06 November 2020;
Published: 11 December 2020.
Edited by:
Maria Pia Amato, University of Florence, ItalyReviewed by:
Mattia Fonderico, University of Florence, ItalyEmilio Portaccio, Careggi University Hospital, Italy
Copyright © 2020 Roberts, Hobbs, Jessop, Bozzolini, Burman, Greco, Ismail, Kazmi, Kirgizov, Mancardi, Mawson, Muraro, Puyade, Saccardi, Withers, Verhoeven, Sharrack and Snowden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John A. Snowden, am9obi5zbm93ZGVuMUBuaHMubmV0
 Fiona Roberts
Fiona Roberts Helen Hobbs1
Helen Hobbs1 Cristina Bozzolini
Cristina Bozzolini Raffaella Greco
Raffaella Greco Majid Kazmi
Majid Kazmi Gianluigi Mancardi
Gianluigi Mancardi Paolo A. Muraro
Paolo A. Muraro Riccardo Saccardi
Riccardo Saccardi Basil Sharrack
Basil Sharrack John A. Snowden
John A. Snowden