
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 29 October 2020
Sec. Epilepsy
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.545074
This article is part of the Research Topic The Changing Face of Epilepsy Surgery: Contributions of Computational Neuroscience and Robotics to the Field View all 18 articles
Objective: Patients with medically refractory focal epilepsy can be difficult to treat surgically, especially if invasive monitoring reveals multiple ictal onset zones. Possible therapeutic options may include resection, neurostimulation, laser ablation, or a combination of these surgical modalities. To date, no study has examined outcomes associated with resection plus responsive neurostimulation (RNS, Neuropace, Inc., Mountain View, CA) implantation and we describe our initial experience in patients with multifocal epilepsy undergoing this combination therapy.
Methods: A total of 43 responsive neurostimulation (RNS) devices were implanted at UCI from 2015 to 2019. We retrospectively reviewed charts of patients from the same time period who underwent both resection and RNS implantation. Patients were required to have independent or multifocal onset, undergo resection and RNS implantation, and have a minimum of six-months for follow-up to be included in the study. Demographics, location of ictal onset, location of surgery, complications, and seizure outcome were collected.
Results: Ten patients met inclusion criteria for the study, and seven underwent both procedures in the same setting. The average age was 36. All patients had multifocal ictal onset on video electroencephalogram or invasive EEG with four patients undergoing subdural grid placement and four patients undergoing bilateral sEEG prior to the definitive surgery. Five patients underwent resection plus ipsilateral RNS placement and the remainder underwent resection with contralateral RNS placement. Two minor complications were encountered in this group. At six months follow up, there was an average of 81% ± 9 reduction in seizures, while four patients experienced complete seizure freedom at 1 year.
Conclusion: Patients with multifocal epilepsy can be treated with partial resection plus RNS. The complication rates are low with potential for worthwhile seizure reduction.
Approximately one-third of epilepsy patients have seizures that are refractory to antiepileptic medications (1, 2). Left untreated, patients are at risk of developing multiple comorbidities, and potentially death (3). For this reason, patients with medically refractory focal epilepsy should be referred to a Level 4 NAEC (National Association of Epilepsy Centers) center to be evaluated for surgical candidacy. The best outcomes are seen with temporal lobectomy for mesial temporal sclerosis (MTS) or other lesional resections, with 68% of patients with MTS becoming seizure free and 50% of neocortical extratemporal resections becoming seizure free at 2 years (4).
After being diagnosed with medically refractory epilepsy, pre-surgical evaluation includes video electroencephalography (EEG) monitoring to localize the ictal onset zone(s). If ictal onset is difficult to localize, or if bilateral or eloquent area ictal onset is suspected, the patients move on to have invasive monitoring studies including stereoelectroencephalography (sEEG) or subdural grids (SDG) to better delineate the ictal onset zone(s). Depending upon the location of the onset zone, subsequent resection, neurostimulation, or laser ablation might be performed.
However, there are limitations associated with performing resections including the inability to perform bilateral resection of the same lobe and the unfavorable functional outcomes associated with resecting eloquent regions. Therefore, patients with medically refractory epilepsy which involve multiple independent ictal onset zones or eloquent areas can be very difficult to treat surgically. Historically, when there is bilateral or multifocal ictal onset, palliative procedures such as callosotomy or neuromodulation have been performed (5–9). Unfortunately, these rarely result in complete seizure freedom. The authors describe their experience with resection of primary ictal onset zone as well as RNS implantation for additional ictal onset areas. This combination approach provides the ability to expand the treatment area outside of what is possible with resection alone. The authors demonstrate their early series of patients who underwent resection and RNS as part of their surgical management.
A total of 43 responsive neurostimulation (RNS) were placed at UCI from 2013 to 2019. To be included in the study, patients must have independent multifocal ictal onset which was demonstrated with video encephalography (vEEG) or invasive monitoring (sEEG or SDG) and undergone resection plus RNS implantation. Resection and RNS implantation were not required to be performed during the same surgical setting. Subjects must also have at least six months of follow up. Between February 2015 to January 2019, 10 patients met inclusion criteria and underwent responsive neurostimulation implantation as well as a resective procedure. Eight out of ten patients underwent phase two invasive monitoring with either SDG placement and sEEG or bilateral sEEG, while two patients underwent definitive treatment without undergoing invasive monitoring (Tables 1, 2). This study was approved by the University of California, Irvine's Institutional Review Board (IRB).
All patients completed neuroimaging, neuropsychological testing, and non-invasive vEEG as part of their phase one evaluation. They were then presented and discussed at our institution's multidisciplinary epilepsy management conference. Epileptologists, neuroradiologists, and neuropsychologists as well as the senior author (SV) were present during these conferences. The subjects were presented by their treating epileptologist, and all relevant imaging studies, vEEG clips, and neuropsychological tests were thoroughly discussed. A treatment consensus was reached amongst all members of the group. After invasive monitoring was complete, patients with complex seizure onset (i.e., bilateral, eloquent area, or multifocal ictal onset) were again discussed at epilepsy management conference and a treatment consensus was reached. Once treatment consensus was reached, the patient was scheduled for definitive surgery within two weeks. All patients in the study underwent resection plus RNS implantation, although these were not necessarily performed during the same surgery.
Patients first undergo either sEEG or SDG implantation to localize the ictal onset. If sEEG is performed and it is determined that they will require resection plus RNS, the patients are discharged home and brought back at a later date for the definitive surgery. If SDG is performed, the definitive surgery is performed upon removal of electrodes. Resections were performed after stereotactic RNS implantation to avoid risk of brain shift intraoperatively.
In patients that required depth electrode placement for RNS, stereotactic implantation of RNS was done using the Robotic Operating Surgical Assistant (ROSA) (Zimmer-Biomet, Warsaw, IN). A mayfield skull clamp was placed on the patient's head for immobilization. A curvilinear incision was marked in a location that was accessible to both leads and not interfere with reopening for future generator replacements, which served as the incision for placement of the RNS generator. The ictal onset zone identified by previously implanted sEEG electrodes were used to target RNS leads. All target and entry points were planned on the ROSA on the day of surgery (Figure 1).
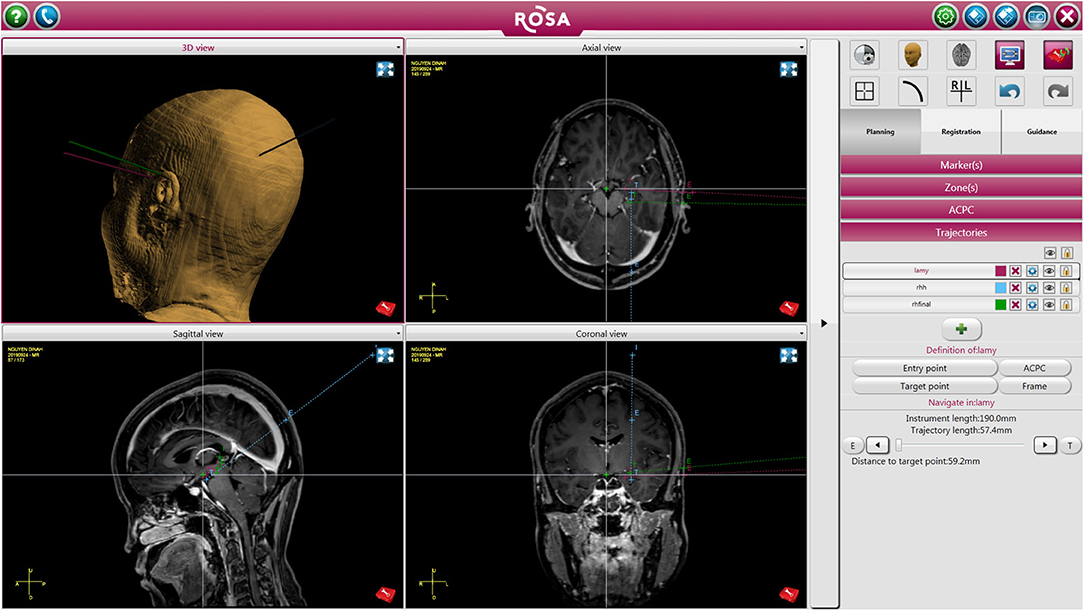
Figure 1. Patient undergoing left sided RNS placement with right temporal lobectomy. The figure depicts trajectory and target points for the RNS leads.
The skull clamp was then attached to the robot with the patient in the correct position, and registration was performed using the robot's laser registration. Following registration, the robotic arm was used to identify the two entry points. A hand drill was utilized to access the cranial vault, and the dura was carefully opened with a dural probe. The robotic arm maintained a rigid trajectory to the target while an outer cannula and subsequently the RNS lead were passed. Each lead was secured in place using a dog bone-shaped plate from a cranial plating system, which has been previously described in a separate paper (10). The curvilinear incision was then opened, and a small craniectomy was created to hold the RNS generator. The device was then connected to the implanted leads.
For patients that required strip electrode placement for RNS, stereotactic implantation was done using the Stryker (Stryker Corporation, Kalamazoo, MI) neuro-navigation system. Again, a Mayfield skull clamp was applied and the images were uploaded onto the navigation system. Target areas were selected based on patient's SDG data. Patients requiring strip electrodes most commonly underwent temporal lobectomy and RNS placement on the ipsilateral side. The electrodes can be placed once the resection is completed and secured into place by anchoring it to the craniotomy site edges. The rest of the procedure is done in the similar way as described above.
In patients undergoing both resection and RNS placement, once the RNS is implanted successfully, the resection is performed based upon the area of interest with standard surgical techniques (Figures 2A,B).
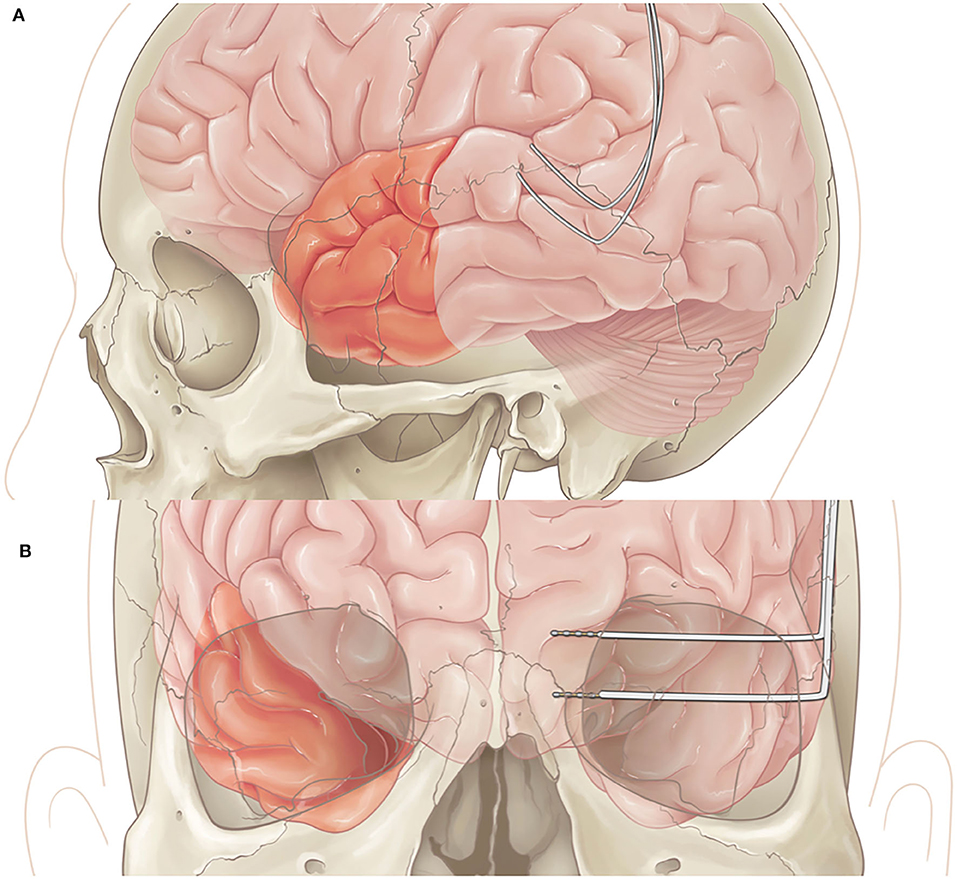
Figure 2. (A) Lateral view of left anterior temporal lobectomy with RNS placement in posterior temporal area. (B) Coronal view of right temporal lobectomy with RNS placement in left mesial structures.
An intraoperative CT is performed after the procedure and the image is fused with the planning MRI to confirm accurate placement of RNS leads.
The clinical data of subjects who underwent resection plus RNS implantation from July 2015 to January 2019 was retrospectively collected and analyzed. Data for the following variables were collected: (1) age at seizure onset; (2) number of baseline seizures per month; (3) location of ictal onsets; (4) location of RNS leads and resection; (5) postoperative outcome; (6) ECoG from RNS; and (7) surgical complications.
The age range at the time of surgery was 27 to 53 years, with a mean age of 36 years (Table 3). There were two patients that underwent definitive treatment without first undergoing invasive monitoring because their vEEG showed clear independent bilateral ictal onset in one patient and left ictal onset in another patient. Four patients underwent SDG placement as part of the invasive monitoring, while the remainder underwent sEEG. Of the four patients that underwent SDG, two initially had sEEG that lateralized to one side and therefore patient returned for SDG implantation to better localize the onset zone. Five patients underwent RNS and ipsilateral resection and the remaining patients underwent contralateral RNS and resection. The patient with bilateral ictal onset later underwent wada testing which showed language dominance on the right, therefore she underwent left temporal lobectomy with right RNS placement. The patient that had only left ictal onset, underwent left temporal lobectomy but continued to have persistent seizures and therefore underwent bilateral sEEG, which showed right sided onset as well, therefore, she underwent right RNS placement. There were two minor postoperative complications; one patient developed an epidural hematoma under the RNS generator site requiring evacuation while the second had a cerebral spinal fluid leak that require lumbar drain placement (Table 2). No long-term sequela was noted and all patients returned to baseline. At six months of follow up, there was an average of 81% ± 9 decrease in seizure frequency, with two patients becoming seizure free. Four patients continued to remain seizure-free at their one year follow up with a total of 83% ± 17 seizure reduction in the study group. Two patients remained seizure free at 2 years follow up, while the rest have not had their 2 year follow ups yet. Seizure reduction was measured by subtracting the number of seizures per month at the time of follow up divided by the number of baseline seizures per month by the number one . Baseline seizures were calculated based on number of seizures patients had for 6 months.
Epilepsy surgery offers a potential cure for patients with medically refractory focal epilepsy (6, 9). Resections are considered when the seizure focus can be removed with minimal risk of disabling neurological deficits. Thus, patients with multifocal ictal onset zones, ictal onsets in eloquent areas, or bilateral ictal onsets still pose a significant challenge and these patients can be significantly more difficult to treat. The RNS device provides us with the ability to treat patients with multifocal and eloquent region onset (5). In this case series, we were able to supplement resective surgery with RNS in patients in which resective surgery alone could not address the entire ictal onset zone, such as in eloquent areas or with bilateral onset zones.
Patients with multifocal epilepsy historically were thought to be poor surgical candidates (11–13). There have been a few case series that show that patients with bilateral temporal lobe epilepsy may benefit after unilateral surgery (14–16), however, determining which side to resect also poses a challenge. In patients that have bilateral temporal lobe epilepsy, the chance of good seizure outcome after surgery (Engel class I or II) (17) is still much lower than patients with unilateral temporal lobe epilepsy at about 25–45% depending on the study (12, 18, 19). In our patients with bilateral ictal onset, we were able to use invasive EEG to lateralize which side demonstrated greater seizure activity. Because resection has been shown to have the best seizure free outcomes (6), we chose to treat the most active region with resection, while the contralateral side underwent RNS placement.
Patients with eloquent area onset (i.e., language or motor regions) pose an even greater challenge for treatment. In these patients, the risk of neurological deficit in resecting eloquent cortex outweigh the benefits of surgery. In these patients, partial resection plus RNS on the ipsilateral side can be done. This allows us to broaden the scope of coverage from the safe margin of the resection to eloquent regions with the RNS, effectively allowing us to extend our treatment of ictal onset outside of what is safe with resection alone.
In our patient population, there was an overall reduction in clinical seizures post operatively. Despite this, at the most recent interrogation the RNS system recorded ongoing electrographic (Figures 3A,B) seizures in 60% of the patients and epileptiform discharges and patterns in all 10 patients from the remaining areas which were not able to be safely resected. Placement of the RNS system allowed for the detection and treatment of these residual activities. Partial resection of the seizure focus can help lessen the burden of ictal activities and reduce seizure frequency. However, when the complete seizure focus is not removed, there remains a propensity for seizure recurrences (6). The authors argue that supplementing resections with RNS to treat the residual epileptogenic regions can have a complementary effect and yield greater seizure reduction.
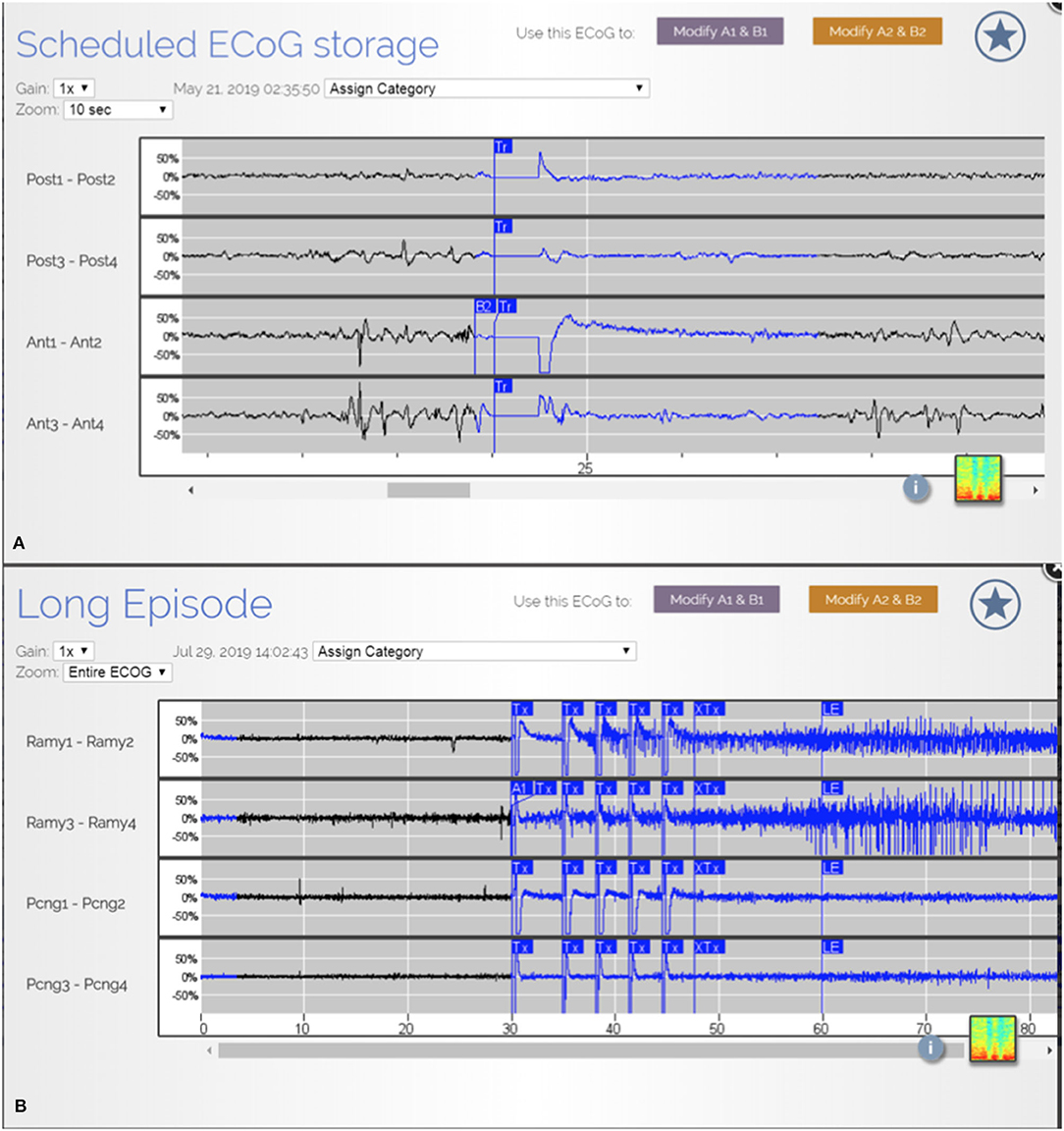
Figure 3. (A) ECoG from patient JA showing epileptiform discharges from multiple areas. (B) ECoG from patient R.R. showing detection and treatment of another electrographic seizure from right amygdala.
The results of this present study show that resection of ictal onset plus RNS implantation in another location is safe and efficacious. There were two minor complications that did not cause long-term deficits.
There are two main limitations of this study. One limitation is that the study group is not well powered with only 10 patients. Although our results provide valuable information regarding the safety and efficacy of resection plus RNS, the clinical outcomes data may not be statistically significant given the small sample. Additionally, there are only two patients included in the study who had long-term outcomes over one-year, and oftentimes, the clinical benefit from epilepsy surgery may take several years to fully manifest, especially in the setting of RNS placement. Nevertheless, neuromodulation often demonstrates improvements in seizure reduction over time, suggesting continued improvement in seizure control may be seen in this group in the future.
RNS plus resection allows us to broaden the scope of coverage in patients with multifocal or eloquent ictal onset zones. While resection alone is limited by inability to perform bilateral resections or resections of eloquent regions, RNS provides the ability to extend the coverage and aid with seizure management in this difficult to treat patient population.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Cristobal Barrios, MD, vice chair of institutional review board at University of California Irvine. Written informed consent to participate in this study was not required according to local legislation and national guidelines.
DKT: developed the concept of the work, analysis of data, drafting the work, and critically revising the manuscript. DCT: developed the concept of the work, analysis of data, drafting the work, and critically revising the manuscript. LM, JL, and FH: revising the manuscript. SV: developed the concept of the work, analysis of data, drafting the work, critically revising the manuscript, and approved the manuscript for submission. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. (2000) 342:314–9. doi: 10.1056/NEJM200002033420503
2. Sillanpaa M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain. (2006) 129:617–24. doi: 10.1093/brain/awh726
3. Luoni C, Bisulli F, Canevini MP, De Sarro G, Fattore C, Galimberti CA, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. (2011) 52:2181–91. doi: 10.1111/j.1528-1167.2011.03325.x
4. Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. (2005) 65:912–8. doi: 10.1212/01.wnl.0000176055.45774.71
5. Ben-Menachem E. Neurostimulation-past, present, and beyond. Epilepsy Curr. (2012) 12:188–91. doi: 10.5698/1535-7511-12.5.188
6. Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. (2012) 307:922–30. doi: 10.1001/jama.2012.220
7. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. (2010) 51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x
8. Kasasbeh AS, Smyth MD, Steger-May K, Jalilian L, Bertrand M, Limbrick DD. Outcomes after anterior or complete corpus callosotomy in children. Neurosurgery. (2014) 74:17–28; discussion 28. doi: 10.1227/NEU.0000000000000197
9. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. (2001) 345:311–8. doi: 10.1056/NEJM200108023450501
10. Tran DK, Paff M, Mnatsakanyan L, Sen-Gupta I, Lin JJ, Hsu FPK, et al. A novel robotic-assisted technique to implant the responsive neurostimulation system. Oper Neurosurg. (2019) 18:728–35. doi: 10.1093/ons/opz226
11. Chung MY, Walczak TS, Lewis DV, Dawson DV, Radtke R. Temporal lobectomy and independent bitemporal interictal activity: what degree of lateralization is sufficient? Epilepsia. (1991) 32:195–201. doi: 10.1111/j.1528-1157.1991.tb05244.x
12. Holmes MD, Miles AN, Dodrill CB, Ojemann GA, Wilensky AJ. Identifying potential surgical candidates in patients with evidence of bitemporal epilepsy. Epilepsia. (2003) 44:1075–9. doi: 10.1046/j.1528-1157.2003.58302.x
13. Hufnagel A, Elger CE, Pels H, Zentner J, Wolf HK, Schramm J, et al. Prognostic significance of ictal and interictal epileptiform activity in temporal lobe epilepsy. Epilepsia. (1994) 35:1146–53. doi: 10.1111/j.1528-1157.1994.tb01781.x
14. Boling W, Aghakhani Y, Andermann F, Sziklas V, Olivier A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. J Neurol Neurosurg Psychiatry. (2009) 80:533–8. doi: 10.1136/jnnp.2008.155291
15. Hirsch LJ, Spencer SS, Spencer DD, Williamson PD, Mattson RH. Temporal lobectomy in patients with bitemporal epilepsy defined by depth electroencephalography. Ann Neurol. (1991) 30:347–56. doi: 10.1002/ana.410300306
16. Sirven JI, Malamut BL, Liporace JD, O'Connor MJ, Sperling MR. Outcome after temporal lobectomy in bilateral temporal lobe epilepsy. Ann Neurol. (1997) 42:873–8. doi: 10.1002/ana.410420608
17. Jr EJ. Outcome With Respect to Epileptic Seizures, in Surgical Treatment of the Epilepsies. New York, NY: Raven Press. (1987). p. 553–71.
18. Cukiert A, Cukiert CM, Argentoni M, Baise-Zung C, Forster CR, Mello VA, et al. Outcome after cortico-amygdalo-hippocampectomy in patients with severe bilateral mesial temporal sclerosis submitted to invasive recording. Seizure. (2009) 18:515–8. doi: 10.1016/j.seizure.2009.05.003
Keywords: epilepsy, lobectomy, temporal, surgery, robotic, responsive neurostimulation (RNS)
Citation: Tran DK, Tran DC, Mnatsakayan L, Lin J, Hsu F and Vadera S (2020) Treatment of Multi-Focal Epilepsy With Resective Surgery Plus Responsive Neurostimulation (RNS): One Institution's Experience. Front. Neurol. 11:545074. doi: 10.3389/fneur.2020.545074
Received: 23 March 2020; Accepted: 31 August 2020;
Published: 29 October 2020.
Edited by:
Jorge Alvaro Gonzalez-Martinez, University of Pittsburgh, United StatesReviewed by:
Carlo Di Bonaventura, Sapienza University of Rome, ItalyCopyright © 2020 Tran, Tran, Mnatsakayan, Lin, Hsu and Vadera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diem Kieu Tran, a3R0OTY3QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.