- 1Department of Neurosurgery, The First Hospital of Jilin University, Changchun, China
- 2Department of Neurology, The First Hospital of Jilin University, Changchun, China
- 3Department of Intensive Care Unit, The First Hospital of Jilin University, Changchun, China
Background: In rare circumstances, patients with intracranial (dural arteriovenous fistulas) DAVFs could be complicated with brainstem engorgement, which might lead to delayed or false diagnosis and subsequent improper management.
Methods: On July 2th, 2019, a systematic search was conducted in the PubMed database for patients with intracranial DAVFs complicated with brainstem engorgement.
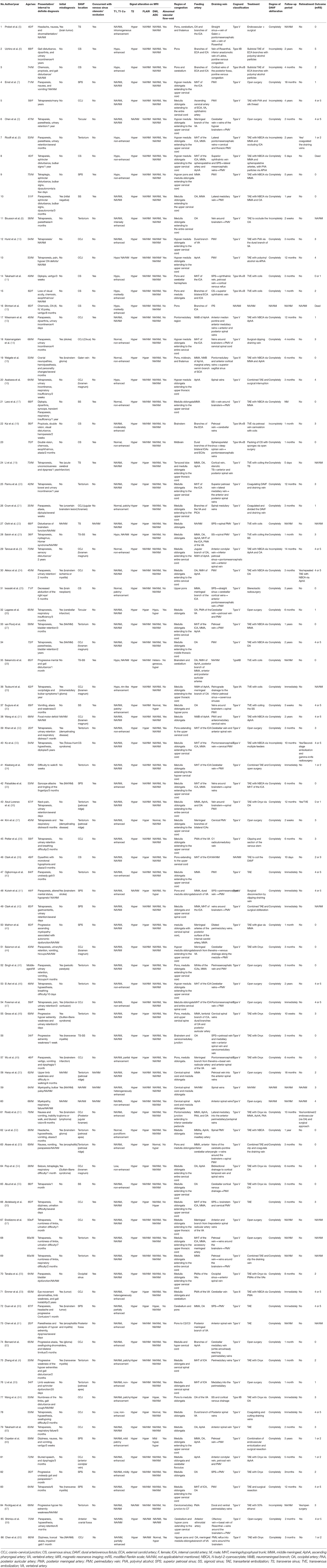
Results: Sixty-eight articles reporting of 86 patients were included for final analysis. The patients were aged from 20 to 76 years (57.10 ± 12.90, n = 82). The female to male ratio was 0.68 (35:51). Thirty-three (40.2%, 33/82) patients were initially misdiagnosed as other diseases. The specific location distributions were cranio-cervical junction, cavernous sinus, superior petrosal sinus, transverse and/or sigmoid sinus, tentorium, and other sites in 27 (32.5%), 11 (13.2%), 9 (10.8%), 10 (12.0%), 21 (25.3%), and 5 (6.0%) patients, respectively. The Cognard classification of DAVFs were II, III, IV, and V in 9 (10.7%, 9/84), 1 (1.2%, 1/84), 1 (1.2%, 1/84), and 73 (86.9%, 73/84) patients. Eighteen (22%, 18/82) patients were demonstrated to have stenosis or occlusion of the draining system distal to the fistula points. The mean follow-up period was 7.86 (n = 74, range 0–60 months) months. Fifty-four (70.1%, 54/77) patients experienced a good recovery according to the mRS score.
Conclusions: Intracranial DAVFs complicated with brainstem engorgement are rare entities. Initial misdiagnosis and delayed definite diagnosis are common in the past three decades. The treatment outcome is still unsatisfactory at present. Early awareness of this rare entity and efficiently utilizing the up to date investigations are of utmost importance.
Introduction
Dural arteriovenous fistula (DAVF) is a unique subtype of vascular malformations along the central nervous system, which is characterized by abnormal connections between meningeal/pial arteries and dural venous sinuses, meningeal veins, or cortical veins. The estimated detection rate was 0.29 per 100,000 persons per year according to a Japanese survey published in 2016 (1). In rare circumstances, patients with intracranial DAVFs could be complicated with brainstem engorgement, which might lead to delayed or false diagnosis and subsequent improper management (2–4). An illustrate case of intracranial DAVF with brainstem engorgement was presented in Figure 1. As a result of its rarity in occurrence, large case series in a single center is extremely hard to be anticipated. In order to explore the epidemiological, clinical, imaging, and prognostic characteristics of this specific entity, we conducted a systematic review of the literature.
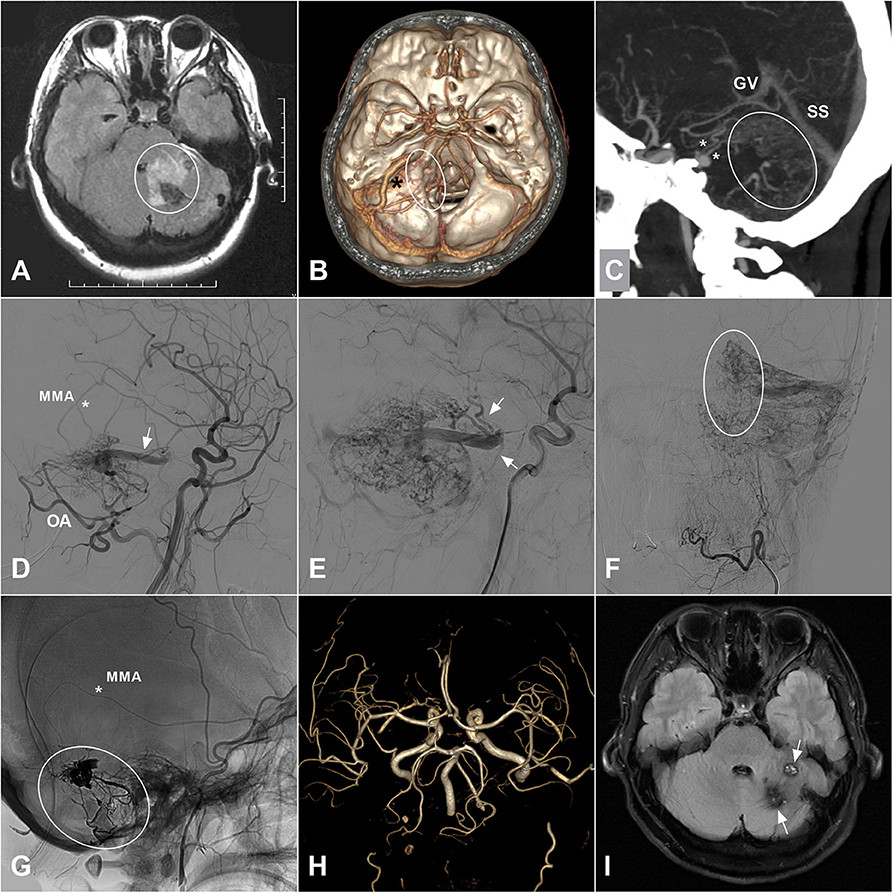
Figure 1. (A) A 35-years-old female was admitted for 3-days history of headache and vomiting. MRI on FLAIR sequence reveals a hyperintense left cerebellar lesion (white circle) with the adjacent brainstem involvement. Besides, vascular flow voids are also noted at the posterior fossa. (B) CTA shows an abnormally enlarged vein (asterisk) draining from the cerebellar surface to the brainstem. And some enlarged veins (ellipse) around the brainstem are also noted. (C) MIP of CTA shows the enlarged draining veins in the cerebellum (ellipse) and around the brainstem (asterisks). (D) Angiogram of the left ECA in lateral view shows a DAVF supplied by the MMA (asterisk) and OA and drained to the deep veins via an enlarged superficial vein (arrow). (E) Angiogram in late arterial phase shows the deep veins (arrow) around the brainstem. (F) Angiogram of the left ECA in anteroposterior view shows enlarged veins in the left cerebellar hemisphere. The ellipse indicates the midline veins. (G) Unsubtracted angiogram shows the DAVF is embolized with Onyx (ellipse) via the MMA (asterisk). (H) Follow-up MRA 1 month postoperatively shows disappearance of the DAVF. (I) Follow-up MRI on FLAIR sequence shows remission of the brainstem and cerebellar edema and deposition of hemosiderin (arrow). CTA, computed tomography angiography; DAVF, dural arteriovenous fistula; ECA, external carotid artery; FLAIR, fluid attenuated inversion recovery; MIP, maximum intensity projection; MMA, middle meningeal artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; OA, occipital artery.
Methods
On July 2th, 2019, a systematic search was conducted in the PubMed database for patients with intracranial DAVFs complicated with brainstem engorgement. Brainstem engorgement, brain stem engorgement, brainstem edema, brainstem oedema, brain stem edema, brain stem oedema, brainstem congestion, brain stem congestion, brainstem venous congestion, brain stem venous congestion, venous congestion of brain stem, venous congestion of brainstem, myelopathy, and dural arteriovenous fistula were used as key words in relevant combinations. Articles included were: (1) of which the full text could be obtained, or (2) sufficient data could be obtained from the abstract if the full text is inaccessible. Of note, studies reporting large case series were excluded from the final analysis if sufficient description of the individual clinical information was not provided. Manual searching of the reference lists of the identified articles were also performed for additional studies. We used modified Rankin Scale (mRS) for outcome assessment. An mRS score ≤ 3 was defined as good recovery.
Results
The PubMed search yielded 183 records. After a primary screening of the titles and abstracts, 97 records were excluded. After full text assessment of the 86 identified articles, 28 records were further excluded. We manually searched the reference lists of the remaining 58 articles. And 10 additional articles were identified. Finally, 68 articles reporting of 86 patients were included in the final analysis (Table 1) (2–69). The flow chart of searching strategy was presented in Figure 2. The patients were aged from 20 to 76 years (57.10 ± 12.90, n = 82). The female to male ratio was 0.68 (35:51).
Interval From Symptom Onset to Definite Diagnosis
Of the 68 cases interval from symptom onset to definite diagnosis was provided, 15 (22.1%, 15/68) patients were definitely diagnosed with intracranial DAVFs in the 1st month since symptom onset. Nineteen (28.0%, 19/68) patients were definitely diagnosed between the 2nd and 3rd months. Sixteen (23.5%, 16/68) patients were between the fourth and 6th month. Six (8.8%, 6/68) patients were between the seventh and twelfth month. Twelve (17.6%, 12/68) patients were definitely diagnosed 1 year later from symptom onset. Thirty-three (40.2%, 33/82) patients were initially misdiagnosed as other diseases.
DAVFs Characteristics
The intracranial location of DAVFs could be determined in 83 patients. The specific location distributions were anterior fossa, cranio-cervical junction, cavernous sinus, vein of Galen, occipital sinus, superior petrosal sinus, transverse/sigmoid sinus, torcular, and tentorium in 1 (1.2%), 27 (32.5%), 11 (13.2%), 1 (1.2%), 1 (1.2%), 9 (10.8%), 10 (12.0%), 2 (2.4%), and 21 (25.3%) patients, respectively (Figure 3). The Cognard classification of DAVFs were II, III, IV, and V in 9 (10.7%, 9/84), 1 (1.2%, 1/84), 1 (1.2%, 1/84), and 73 (86.9%, 73/84) patients (Figure 4). The feeding arteries were solely from the external carotid artery (ECA) in 32 (38.6%, 32/83) patients, solely from the internal carotid artery (ICA) in 14 (16.9%, 14/83) patients, solely from the vertebrobasilar artery (VBA) in 12 (14.5%, 12/83) patients, conjointly from ECA and ICA in 18 (21.7%, 18/83) patients, conjointly from ECA and VBA in 5 (6.0%, 5/83) patients, and conjointly from ECA, ICA, and VBA in 2 (2.4%, 2/83) patients.
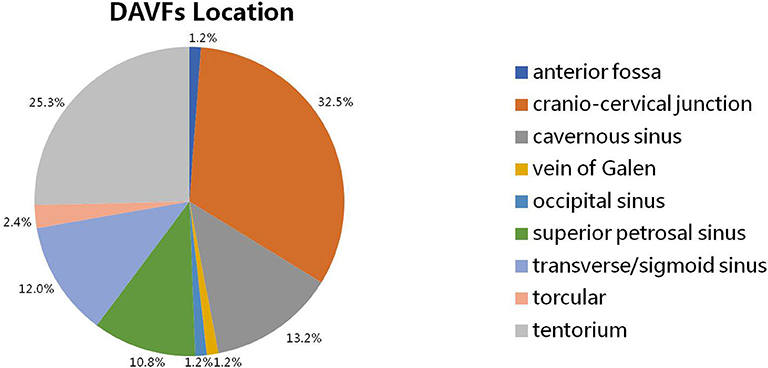
Figure 3. The specific location of intracranial DAVFs complicated with brainstem engorgement. DAVF, dural arteriovenous fistula.
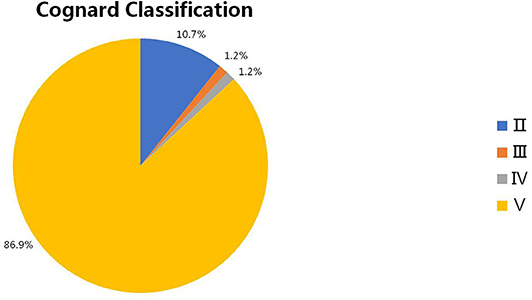
Figure 4. The Cognard classification of intracranial DAVFs complicated with brainstem engorgement. DAVF, dural arteriovenous fistula.
Findings on Imaging Modalities
Eighteen (22%, 18/82) patients were demonstrated to have stenosis or occlusion of the draining system distal to the fistula points during conventional angiography. The signals of the engorged brainstem were hypointense or normal on T1 weighted imaging (T1WI) of magnetic resonance imaging (MRI) in 15 (65.2%, 15/23) and 8 (34.8%, 8/23) patients, respectively. The engorged brainstem was enhanced on T1WI with different degrees in 37 (72.5%, 37/51) patients after gadolinium contrast. The signal was hyperintense in all of the 82 patients T2 weighted imaging (T2WI) sequence was provided. And the signal was also hyperintense for all of the 25 patients who had undergone fluid attenuated inversion recovery (FLAIR) sequence. The signals on diffusion weighted imaging (DWI) were heterogeneous, hyperintense, hypointense, and normal in 1 (10%), 3 (30%), 1 (10%), and 5 (50%) patients, respectively. All of the six patients showed hyperintensity on apparent diffusion coefficient (ADC) map. Besides, abnormal vascular flow voids could be identified in 69 (80.2%, 69/86) patients on MRI.
Treatment and Outcome
Forty-five (53.6%, 45/84) patients were treated solely with transarterial embolization, of which 7 (15.6%, 7/45) patients were incompletely embolized and 3 (6.7%, 3/45) patients experienced recurrence in spite of previous complete obliteration. Eight (9.5%, 8/84) patients underwent transvenous embolization, of which 1 (12.5%, 1/8) patient was incompletely embolized. Twenty-two (26.2%, 22/84) patients underwent open surgery, of which no recurrence was reported. One (1.2%, 1/84) patient underwent one-session successful stereotactic radiosurgery. Eight (9.5%, 8/84) patients were successfully treated conjointly with the endovascular and open surgical approaches. In general, the DAVFs were completely obliterated in 74 (89.2%, 74/83) patients during one hospitalization. Six (7.2%, 6/83) patients underwent retreatment. The mean follow-up period was 7.86 (n = 74, range 0–60 months) months. Fifty-four (70.1%, 54/77) patients experienced a good recovery according to the mRS score.
Discussion
The pathophysiology of intracranial DAVFs is still enigmatic. Though a small proportion of the DAVFs are demonstrated to be secondary to trauma, craniotomy, infection, or dural venous thrombosis, a substantial number of them are idiopathic (70). Some authors believe that progressive stenosis or thrombosis of the dural venous sinus might be the underlying mechanism of DAVF formation (61, 70). In this review, 22% of the patients with brainstem engorgement were definitely recorded to have stenosis or occlusion of the draining system distal to the fistula points. The actual occurrence of stenosis or occlusion of the draining system might be higher, as some reports did not give a detailed description of the draining system. According to a study by Luo et al. 7 (77.8%) of the nine patients with aggressive cavernous sinus DAVFs had inferior petrous sinus occlusion or stenosis, two patients (22.2%) had compartment of inferior petrous sinus-cavernous sinus (77). Hence, progressive insufficient drainage (stenosis, occlusion, or compartment) of the draining system might play an important role in the genesis of brainstem engorgement in patients with intracranial DAVFs.
The brainstem has a complex venous draining system. In general, the veins of the brainstem can be divided into the transverse and longitudinal groups, which are named on the basis of the subdivision (mesencephalon, pons, or medulla), surface (median anterior, lateral anterior, or lateral), and the direction (transverse or longitudinal) of the brainstem drained (71). From cranial to caudal, the transverse groups are peduncular vein, posterior communicating vein, vein of pontomesencephalic sulcus, transverse pontine vein, vein of pontomedullary sulcus, and transverse medullary vein. From median to lateral, the longitudinal groups are median veins (median anterior pontomesencephalic vein, median anterior medullary vein), anterolateral veins (lateral anterior pontomesencephalic vein, lateral anterior medullary vein), and lateral veins (lateral mesencephalic vein, lateral medullary and retro-olivary veins). The veins of the transverse group have extensive anastomoses with those of the longitudinal group. Besides, the terminal end of the veins draining the brainstem and cerebellum form bridging veins that are divided into three groups: (1) a galenic group draining into the vein of Galen; (2) a petrosal group draining into the petrosal sinuses; and (3) a tentorial group draining into the sinuses converging on the torcula. Hence, DAVFs in the posterior fossa or even cavernous sinus could lead to brainstem engorgement. Venous drainage of the brainstem is presented in Figure 5.
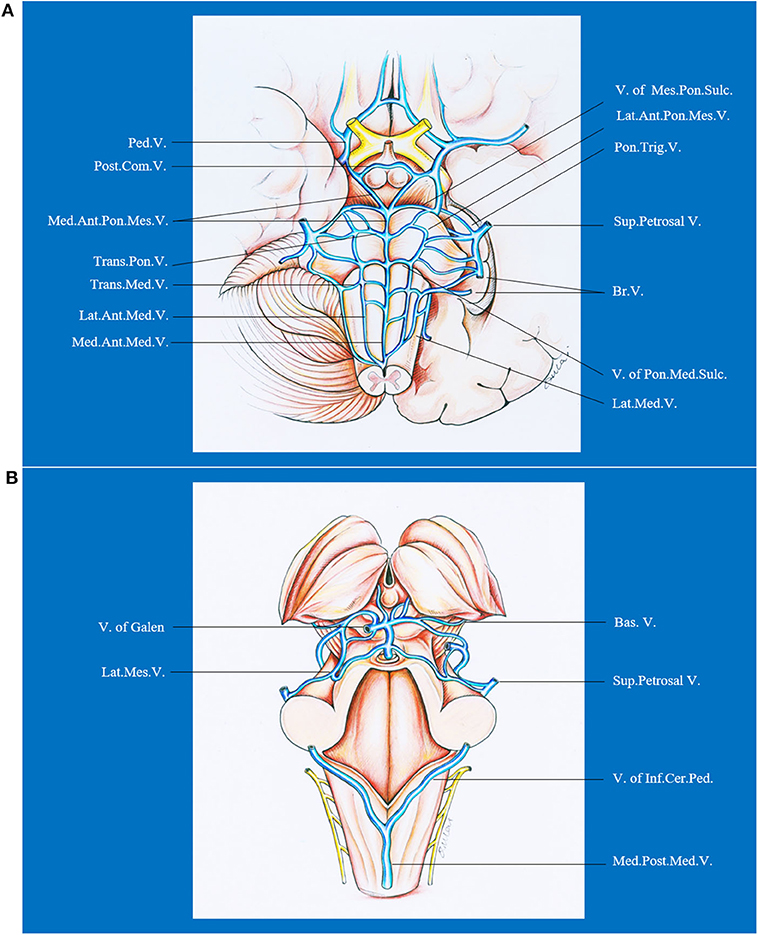
Figure 5. Ventral (A) and dorsal (B) venous drainage of the brainstem. Ant., anterior; Bas., basilar; Br., bridging; Cer., cerebellar; Com., communicating; Inf., inferior; Lat., lateral; Med., median, medullary; Mes., mesencephalic; Ped., peduncle; Pon., pontine; Post., posterior; Sul., sulcus; Sup., superior; Trans., transverse; Trig., trigeminal; V., vein.
The diagnosis of intracranial DAVFs with brainstem engorgement is still challenging. Patients that were diagnosed with neoplasm to undergo brainstem biopsy or given corticosteroids for misdiagnosing as myelitis were not uncommonly reported (4, 51). According to our analysis, 40.2% (33/82) of the patients were initially misdiagnosed as other diseases. Of note, the rate of initial misdiagnosis did not decrease in the past three decades (Figure 6). Considering the unspecific clinical manifestations of intracranial DAVFs with brainstem engorgement, meticulous and comprehensive interpretation of the auxiliary investigations is of utmost importance.
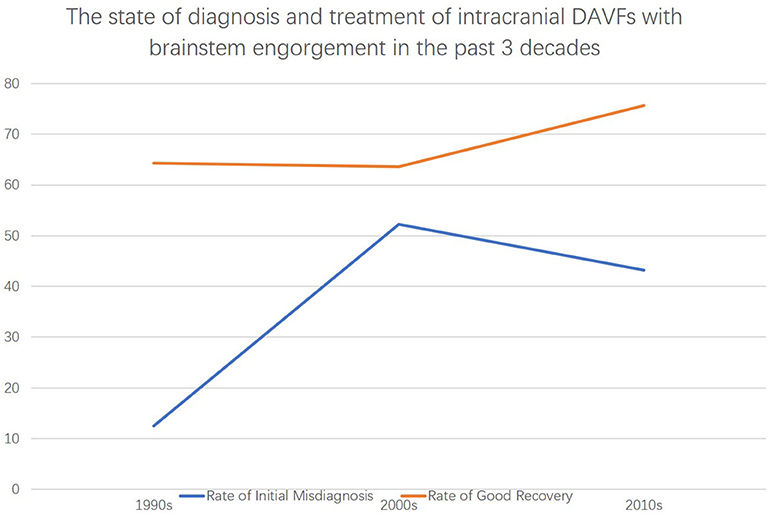
Figure 6. The state of diagnosis and treatment of intracranial DAVFs complicated with brainstem engorgement in the past three decades.
While conventional angiography is the gold standard for definite diagnosis of intracranial DAVFs, taking good advantage of different sequences of MRI data could help screen out those patients with high suspicion. Abnormal vascular flow voids on MRI are reliable evidence highly suggestive of vascular lesions. Abnormal vascular flow voids could only be identified in 80.2% (69/86) of the patients in this survey, including those identified after repeated review of the MRI or those identified during multiple investigations of MRI after symptom aggravation. T2WI or FLAIR sequence is highly sensitive (in 100% of the patients) for the engorged brainstem but with low specificity. The signals on T1WI are so polytropic that 65.2% (15/23) of the analyzed patients presented with low hypointensity and 34.8% (8/23) of the patients were normal. The engorged brainstem was enhanced on T1WI with different degrees in 72.5% (37/51) of the patients after gadolinium contrast. DWI and ADC were rarely performed in these patients. All of the six patients with ADC map showed hyperintensity which denotes the vascular origin of brainstem edema. The signal of DWI is so variable that heterogeneous, hyper, hypo, and normal intensity could be in 1 (10%), 3 (30%), 1 (10%), and 5 (50%) of the 10 identified patients, which might reflect the different degree and duration of venous congestion around the brainstem. Furthermore, contrast-enhanced dynamic magnetic resonance angiography is more sensitive to find out occult vascular abnormalities (50, 72). T2*WI and susceptibility-weighted imaging are emerging sequences of MRI that are good at detecting fine vasculature and microbleeds (73). Hypointense signal could be noticed in the engorged brainstem on T2*WI and susceptibility-weighted imaging, for long-term venous congestion might lead to intraparenchymal microbleeding in the brainstem (57, 60). Besides, some authors also demonstrated decreased cerebral blood volume and prolongation of the mean transit time on magnetic resonance perfusion in the engorged brainstem (66). Hence, advanced MRI sequences could increase the sensitivity and specificity in differential diagnosis of lesion nature and avoid delayed treatment and unnecessary conventional angiography.
There is no consensus on the treatment option for intracranial DAVFs with brainstem engorgement. Of note, premature administration of corticosteroid could be dangerous even fatal in case of undiagnosed DAVFs with brainstem or spinal cord engorgement (74, 75). Hence, precise and comprehensive diagnosis is crucial for further treatment. The treatment should be based on the specific angioarchitecture, intracranial location, and technique availability. Generally speaking, the treatment strategies for DAVFs include open surgery, endovascular embolization, and radiotherapy. As the lag time of effect could be up to 3 years (76), radiotherapy is unsuitable for patients with brainstem engorgement. With the development of endovascular technique and materials, endovascular embolization has become the first-line choice for the majority of intracranial DAVFs (63, 70). Besides, endovascular treatment can be an adjunctive step of further open surgery. For patients with difficult arterial/venous access, incomplete fistula obliteration, recanalization after embolization, open surgery can be considered. Whereas, in patients where a transfemoral approach is impaired for the tortuosity of feeding arteries or the presence of isolated sinuses, percutaneous or intraoperative puncture of perforating arteries or draining veins and venous sinuses represent a new choice to facilitate distal access to the DAVFs (70, 77). In this review, 63.1% of the patients were treated endovascularly (transarterial or transvenous), 26.2% of the patients underwent open surgery, and 9.5% of the patients were treated conjointly with endovascular and open surgical approaches.
The prognosis of patients with DAVFs associated brainstem engorgement is still unsatisfactory, though slight increase in good recovery could be noted in the past three decades (Figure 3). Only 70% of the patients experienced a good recovery (mRS score ≤ 3). A substantial number of patients can have more or less neurological deficits. Except for the peculiar location of DAVFs, angioarchitecture, and surrounding neural structures, early diagnosis is the most important factor impacting prognosis. According to this review, correct diagnosis could be achieved in only 50% of the patients in the first 3 months after symptom onset. What's more, the rate of initial misdiagnosis did not decrease in the past three decades (Figure 3). Hence, early awareness of this rare entity and efficiently utilizing the up to date investigations are of utmost importance.
Limitations
The opinion of this review was deduced from retrospective review of the published case reports or small case series. The results would be biased by many factors. Firstly, the levels in diagnosis and treatment vary greatly between different centers. Secondly, due to the reporting customs among different authors, a lot of key information was missing. Thirdly, the mean follow-up period was only 7.86 (n = 74, range 0–60 months) months, which could impair the accuracy in outcome assessment. Of note, there were two studies reporting larger case series of DAVFs with brainstem engorgement (63, 77) that were not included in this analysis because so much information was missing according to our inclusion criteria.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
JY contributed to the conception and design of the manuscript. LQ and HL performed literature review. KH and GL wrote the manuscript. KX and JY critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research received funding support from the Ninth Youth Scientific Research Funding of The First Hospital of Jilin University (jdyy92018035).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kuwayama N. Epidemiologic survey of dural arteriovenous fistulas in japan: clinical frequency and present status of treatment. Acta Neurochir Suppl. (2016) 123:185–8. doi: 10.1007/978-3-319-29887-0_26
2. Chen CJ, Chen CM, Lin TK. Enhanced cervical MRI in identifying intracranial dural arteriovenous fistulae with spinal perimedullary venous drainage. Neuroradiology. (1998) 40:393–7. doi: 10.1007/s002340050609
3. Tanoue S, Goto K, Oota S. Endovascular treatment for dural arteriovenous fistula of the anterior condylar vein with unusual venous drainage: report of two cases. AJNR Am J Neuroradiol. (2005) 26:1955–9.
4. Zhang S, Liu H, Li J. Cervical myelopathy caused by intracranial dural arteriovenous fistula with acute worsening after steroid administration. World Neurosurg. (2018) 120:328–30. doi: 10.1016/j.wneu.2018.09.029
5. Probst EN, Christante L, Zeumer H. Brain-stem venous congestion due to a dural arteriovenous fistula in the posterior fossa. J Neurol. (1994) 241:175–7. doi: 10.1007/BF00868346
6. Uchino A, Kato A, Kuroda Y, Shimokawa S, Kudo S. Pontine venous congestion caused by dural carotid-cavernous fistula: report of two cases. Eur Radiol. (1997) 7:405–8. doi: 10.1007/s003300050175
7. Ernst RJ, Gaskill-Shipley M, Tomsick TA, Hall LC, Tew JM Jr, et al. Cervical myelopathy associated with intracranial dural arteriovenous fistula: MR findings before and after treatment. AJNR Am J Neuroradiol. (1997) 18:1330–4.
8. Ricolfi F, Manelfe C, Meder JF, Arrue P, Decq P, Brugieres P, et al. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology. (1999) 41:803–12. doi: 10.1007/s002340050846
9. Bousson V, Brunereau L, Vahedi K, Chapot R. Intracranial dural fistula as a cause of diffuse MR enhancement of the cervical spinal cord. J Neurol Neurosurg Psychiatry. (1999) 67:227–30. doi: 10.1136/jnnp.67.2.227
10. Hurst RW, Bagley LJ, Scanlon M, Flamm ES. Dural arteriovenous fistulas of the craniocervical junction. Skull Base Surg. (1999) 9:1–7. doi: 10.1055/s-2008-1058166
11. Takahashi S, Tomura N, Watarai J, Mizoi K, Manabe H. Dural arteriovenous fistula of the cavernous sinus with venous congestion of the brain stem: report of two cases. AJNR Am J Neuroradiol. (1999) 20:886–8.
12. Shintani S, Tsuruoka S, Shiigai T. Carotid-cavernous fistula with brainstem congestion mimicking tumor on MRI. Neurology. (2000) 55:1929–31. doi: 10.1212/WNL.55.12.1929
13. Wiesmann M, Padovan CS, Pfister HW, Yousry TA. Intracranial dural arteriovenous fistula with spinal medullary venous drainage. Eur Radiol. (2000) 10:1606–9. doi: 10.1007/s003300000382
14. Kalamangalam GP, Bhattacharya J, Teasdale E, Thomas M. Myelopathy from intracranial dural arteriovenous fistula. J Neurol Neurosurg Psychiatry. (2002) 72:816–8. doi: 10.1136/jnnp.72.6.816
15. Weigele JB, Chaloupka JC, Lesley WS. Galenic dural arteriovenous fistula: unusual clinical presentation and successful endovascular therapy. Case report. J Neurosurg. (2002) 97:467–70. doi: 10.3171/jns.2002.97.2.0467
16. Asakawa H, Yanaka K, Fujita K, Marushima A, Anno I, Nose T. Intracranial dural arteriovenous fistula showing diffuse MR enhancement of the spinal cord: case report and review of the literature. Surg Neurol. (2002) 58:251–7. doi: 10.1016/S0090-3019(02)00861-3
17. Lanz M, Thiemann U, Grzyska U, Ebke M, Schwendemann G, Kraus JA. Transient brainstem ischemia and recurrent syncope caused by a dural arteriovenous fistula. Neurology. (2003) 61:1152–3. doi: 10.1212/WNL.61.8.1152
18. Kai Y, Hamada JI, Morioka M, Yano S, Ushio Y. Brain stem venous congestion due to dural arteriovenous fistulas of the cavernous sinus. Acta Neurochir. (2004) 146:1107–11. doi: 10.1007/s00701-004-0315-3
19. Li J, Ezura M, Takahashi A, Yoshimoto T. Intracranial dural arteriovenous fistula with venous reflux to the brainstem and spinal cord mimicking brainstem infarction–case report. Neurol Med Chir. (2004) 44:24–8. doi: 10.2176/nmc.44.24
20. Pannu Y, Shownkeen H, Nockels RP, Origitano TC. Obliteration of a tentorial dural arteriovenous fistula causing spinal cord myelopathy using the cranio-orbito zygomatic approach. Surg Neurol. (2004) 62:463–7. doi: 10.1016/j.surneu.2004.01.017
21. Crum BA, Link M. Intracranial dural arteriovenous fistula mimicking brainstem neoplasm. Neurology. (2004) 62:2330–1. doi: 10.1212/01.WNL.0000130342.68494.78
22. Oishi H, Horinaka N, Shmizu T, Ozaki Y, Arai H. A case of intracranial dural arteriovenous fistula presenting with brainstem infarction. No Shinkei Geka. (2005) 33:1095–9. doi: 10.11477/mf.1436100147
23. Satoh M, Kuriyama M, Fujiwara T, Tokunaga K, Sugiu K. Brain stem ischemia from intracranial dural arteriovenous fistula: case report. Surg Neurol. (2005) 64:341–5. doi: 10.1016/j.surneu.2004.12.029
24. Akkoc Y, Atamaz F, Oran I, Durmaz B. Intracranial dural arteriovenous fistula draining into spinal perimedullary veins: a rare cause of myelopathy. J Korean Med Sci. (2006) 21:958–62. doi: 10.3346/jkms.2006.21.5.958
25. Iwasaki M, Murakami K, Tomita T, Numagami Y, Nishijima M. Cavernous sinus dural arteriovenous fistula complicated by pontine venous congestion. A case report. Surg Neurol. (2006) 65:516–8. doi: 10.1016/j.surneu.2005.06.044
26. Lagares A, Perez-Nunez A, Alday R, Ramos A, Campollo J, Lobato RD. Dural arteriovenous fistula presenting as brainstem ischaemia. Acta Neurochir. (2007) 149:965–7. doi: 10.1007/s00701-007-1250-x
27. van Rooij WJ, Sluzewski M, Beute GN. Intracranial dural fistulas with exclusive perimedullary drainage: the need for complete cerebral angiography for diagnosis and treatment planning. AJNR Am J Neuroradiol. (2007) 28:348–51. doi: 10.1016/S0098-1672(08)70215-7
28. Sakamoto S, Ohba S, Shibukawa M, Kiura Y, Okazaki T, Kurisu K. Course of apparent diffusion coefficient values in cerebral edema of dural arteriovenous fistula before and after treatment. Clin Neurol Neurosurg. (2008) 110:400–3. doi: 10.1016/j.clineuro.2007.12.010
29. Tsutsumi S, Yasumoto Y, Ito M, Oishi H, Arai H. Posterior fossa dural arteriovenous fistula as a probable cause of congestive myelopathy. Case report. Neurol Med Chir. (2008) 48:171–5. doi: 10.2176/nmc.48.171
30. Sugiura Y, Nozaki T, Sato H, Sawashita K, Hiramatsu H, Nishizawa S. Sigmoid sinus dural arteriovenous fistula with spinal venous drainage manifesting as only brainstem-related neurological deficits without myelopathy: case report. Neurol Med Chir. (2009) 49:71–6. doi: 10.2176/nmc.49.71
31. Wang HC, Lin WC, Kuo YL, Yang TM, Ho JT, Tsai NW, et al. Factors associated with brainstem congestive encephalopathy in dural arterio-venous fistulas. Clin Neurol Neurosurg. (2009) 111:335–40. doi: 10.1016/j.clineuro.2008.11.004
32. Khan S, Polston DW, Shields RW, Jr, Rasmussen P, Gupta R. Tentorial dural arteriovenous fistula presenting with quadriparesis: case report and review of the literature. J Stroke Cerebrovasc Dis. (2009) 18:428–34. doi: 10.1016/j.jstrokecerebrovasdis.2008.12.007
33. Ko SB, Kim CK, Lee SH, Yoon BW. Carotid cavernous fistula with cervical myelopathy. J Clin Neurosci. (2009) 16:1350–3. doi: 10.1016/j.jocn.2008.12.031
34. Kleeberg J, Maeder-Ingvar M, Maeder P. Progressive cervical myelopathy due to dural craniocervical fistula. Eur Neurol. (2010) 63:374. doi: 10.1159/000292430
35. Patsalides A, Tzatha E, Stubgen JP, Shungu DC, Stieg PE, Gobin YP. Intracranial dural arteriovenous fistula presenting as an enhancing lesion of the medulla. J Neurointerv Surg. (2010) 2:390–3. doi: 10.1136/jnis.2009.001750
36. Aixut Lorenzo S, Tomasello Weitz A, Blasco Andaluz J, Sanroman Manzanera L, Macho Fernandez JM. Transvenous approach to intracranial dural arteriovenous fistula (Cognard v): a treatment option. A case report. Interv Neuroradiol. (2011) 17:108–14. doi: 10.1177/159101991101700117
37. Kim NH, Cho KT, Seo HS. Myelopathy due to intracranial dural arteriovenous fistula: a potential diagnostic pitfall. Case report. J Neurosurg. (2011) 114:830–3. doi: 10.3171/2010.5.JNS10128
38. Peltier J, Baroncini M, Thines L, Lacour A, Leclerc X, Lejeune JP. Subacute involvement of the medulla oblongata and occipital neuralgia revealing an intracranial dural arteriovenous fistula of the craniocervical junction. Neurol India. (2011) 59:285–8. doi: 10.4103/0028-3886.79153
39. Clark SW, Dang T, Toth G, Pride GL, Greenberg B, Warnack W. Carotid cavernous fistula imitating brainstem glioma. Arch Neurol. (2011) 68:256–7. doi: 10.1001/archneurol.2010.366
40. Ogbonnaya ES, Yousaf I, Sattar TM. Intracranial dural arterio-venous fistula presenting with progressive myelopathy. BMJ Case Rep. (2011) 2011:4828. doi: 10.1136/bcr.09.2011.4828
41. Kulwin C, Bohnstedt BN, Scott JA, Cohen-Gadol A. Dural arteriovenous fistulas presenting with brainstem dysfunction: diagnosis and surgical treatment. Neurosurg Focus. (2012) 32:E10. doi: 10.3171/2012.2.FOCUS1217
42. Clark CN, Saifee TA, Cowley PO, Ginsberg L. Dural arteriovenous fistula of the medulla initially mimicking Guillain-Barre syndrome. Arch Neurol. (2012) 69:786–7. doi: 10.1001/archneurol.2011.2934
43. Mathon B, Gallas S, Tuillier T, Bekaert O, Decq P, Brugieres P, et al. Intracranial dural arteriovenous fistula with perimedullary venous drainage: anatomical, clinical and therapeutic considerations about one case, and review of the literature. Neurochirurgie. (2013) 59:133–7. doi: 10.1016/j.neuchi.2013.04.009
44. Salamon E, Patsalides A, Gobin YP, Santillan A, Fink ME. Dural arteriovenous fistula at the craniocervical junction mimicking acute brainstem and spinal cord infarction. JAMA Neurol. (2013) 70:796–7. doi: 10.1001/jamaneurol.2013.1946
45. Singh D, Garg A, Gupta A, Goel G, Gupta R, Bansal A. Tentorial dural arteriovenous fistula presenting as episodic weakness mimicking periodic paralysis. J Neurointerv Surg. (2013) 5:e32. doi: 10.1136/neurintsurg-2012-010281
46. El Asri AC, El Mostarchid B, Akhaddar A, Naama O, Gazzaz M, Boucetta M. Factors influencing the prognosis in intracranial dural arteriovenous fistulas with perimedullary drainage. World Neurosurg. (2013) 79:182–91. doi: 10.1016/j.wneu.2012.09.012
47. Foreman SM, Stahl MJ, Schultz GD. Paraplegia in a chiropractic patient secondary to atraumatic dural arteriovenous fistula with perimedullary hypertension: case report. Chiropr Man Therap. (2013) 21:23. doi: 10.1186/2045-709X-21-23
48. Gross R, Ali R, Kole M, Dorbeistein C, Jayaraman MV, Khan M. Tentorial dural arteriovenous fistula presenting as myelopathy: case series and review of literature. World J Clin Cases. (2014) 2:907–11. doi: 10.12998/wjcc.v2.i12.907
49. Wu Q, Wang HD, Shin YS, Zhang X. Brainstem congestion due to dural ateriovenous fistula at the craniocervical junction. J Korean Neurosurg Soc. (2014) 55:152–5. doi: 10.3340/jkns.2014.55.3.152
50. Haryu S, Endo T, Sato K, Inoue T, Takahashi A, Tominaga T. Cognard type V intracranial dural arteriovenous shunt: case reports and literature review with special consideration of the pattern of spinal venous drainage. Neurosurgery. (2014) 74:E135–42. doi: 10.1227/NEU.0000000000000069
51. Roelz R, Van Velthoven V, Reinacher P, Coenen VA, Mader I, Urbach H, et al. Unilateral contrast-enhancing pontomedullary lesion due to an intracranial dural arteriovenous fistula with perimedullary spinal venous drainage: the exception that proves the rule. J Neurosurg. (2015) 123:1534–9. doi: 10.3171/2014.11.JNS142278
52. Le Guennec L, Leclercq D, Szatmary Z, Idbaih A, Reyes-Botero G, Delattre JY, et al. Dural arteriovenous fistula mimicking a brainstem glioma. J Neuroimaging. (2015) 25:1053–5. doi: 10.1111/jon.12220
53. Alvarez H, Sasaki-Adams D, Castillo M. Resolution of brainstem edema after treatment of a dural tentorial arteriovenous fistula. Interv Neuroradiol. (2015) 21:603–8. doi: 10.1177/1591019915591741
54. Pop R, Manisor M, Aloraini Z, Chibarro S, Proust F, Quenardelle V, et al. Foramen magnum dural arteriovenous fistula presenting with epilepsy. Interv Neuroradiol. (2015) 21:724–7. doi: 10.1177/1591019915609783
55. Abud LG, Abud TG, Nakiri GS, Queiroz RM, Abud DG. Intracranial dural arteriovenous fistula with perimedullary drainage treated by endovascular embolization. Arq Neuropsiquiatr. (2016) 74:178–9. doi: 10.1590/0004-282X20150171
56. Abdelsadg M, Kanodia AK, Keston P, Galea J. Unusual case of intracranial dural AV fistula presenting with acute myelopathy. BMJ Case Rep. (2016) 2016:bcr2016215227. doi: 10.1136/bcr-2016-215227
57. Enokizono M, Sato N, Morikawa M, Kimura Y, Sugiyama A, Maekawa T, et al. “Black butterfly” sign on T2*-weighted and susceptibility-weighted imaging: A novel finding of chronic venous congestion of the brain stem and spinal cord associated with dural arteriovenous fistulas. J Neurol Sci. (2017) 379:64–8. doi: 10.1016/j.jns.2017.05.066
58. Tanaka J, Fujita A, Maeyama M, Kohta M, Hosoda K, Kohmura E. Cognard type Vdural arteriovenous fistula involving the occipital sinus. J Stroke Cerebrovasc Dis. (2017) 26:e62–3. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.004
59. Emmer BJ, van Es AC, Koudstaal PJ, Roosendaal SD. Infratentorial dural arteriovenous fistula resulting in brainstem edema and enhancement. Neurology. (2017) 88:503–4. doi: 10.1212/WNL.0000000000003569
60. Duan SS, Liu H, Wang WL, Zhao CB. A case of intracranial dural arteriovenous fistula mimicking brainstem tumor. Chin Med J. (2017) 130:2519–20. doi: 10.4103/0366-6999.216398
61. Chen PM, Chen MM, McDonald M, McGehrin K, Steinberg J, Handwerker J, et al. Cranial Dural Arteriovenous Fistula. Stroke. (2018) 49:e332–4. doi: 10.1161/STROKEAHA.118.022508
62. Bernard F, Lemee JM, Faguer R, Fournier HD. Lessons to be remembered from a dural arteriovenous fistula mimicking medulla and high cervical cord glioma. World Neurosurg. (2018) 113:312–5. doi: 10.1016/j.wneu.2018.02.161
63. Li J, Ren J, Du S, Ling F, Li G, Zhang H. Dural arteriovenous fistulas at the petrous apex. World Neurosurg. (2018) 119:e968–e76. doi: 10.1016/j.wneu.2018.08.012
64. Wang XC, Du YY, Tan Y, Qin JB, Wang L, Wu XF, et al. Brainstem congestion due to dural arteriovenous fistula at the craniocervical junction: case report and review of the literature. World Neurosurg. (2018) 118:181–7. doi: 10.1016/j.wneu.2018.06.243
65. Takahashi H, Ueshima T, Goto D, Kimura T, Yuki N, Inoue Y, et al. acute tetraparesis with respiratory failure after steroid administration in a patient with a dural arteriovenous fistula at the craniocervical junction. Intern Med. (2018) 57:591–4. doi: 10.2169/internalmedicine.9115-17
66. Copelan AZ, Krishnan A, Marin H, Silbergleit R. Dural arteriovenous fistulas: a characteristic pattern of edema and enhancement of the medulla on MRI. AJNR Am J Neuroradiol. (2018) 39:238–44. doi: 10.3174/ajnr.A5460
67. Rodriguez Rubio R, Chae R, Rutledge WC, De Vilalta A, Kournoutas I, Winkler E, et al. Clipping of a high-risk dural arteriovenous fistula of the posterior fossa: 3d operative video. World Neurosurg. (2019) 126:413. doi: 10.1016/j.wneu.2019.03.101
68. Shimizu A, Ishikawa T, Yamaguchi K, Funatsu T, Ryu B, Nagahara A, et al. Brainstem venous congestion caused by perimedullary drainage in anterior cranial fossa dural arteriovenous fistula. World Neurosurg. (2019) 127:503–8. doi: 10.1016/j.wneu.2019.04.204
69. Chen PY, Juan YH, Lin SK. An isolated unilateral pontomedullary lesion due to an intracranial dural arteriovenous fistula mimicking a brain tumor - case and review. J Nippon Med Sch. (2019) 86:48–54. doi: 10.1272/jnms.JNMS.2019_86-9
70. Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke. (2017) 48:1424–31. doi: 10.1161/STROKEAHA.116.012784
71. Rhoton AL Jr. The posterior fossa veins. Neurosurgery. (2000) 47(Suppl.3):S69–92. doi: 10.1093/neurosurgery/47.3.S69
72. Meckel S, Maier M, Ruiz DS, Yilmaz H, Scheffler K, Radue EW, et al. MR angiography of dural arteriovenous fistulas: diagnosis and follow-up after treatment using a time-resolved 3D contrast-enhanced technique. AJNR Am J Neuroradiol. (2007) 28:877–84.
73. Di Ieva A, Lam T, Alcaide-Leon P, Bharatha A, Montanera W, Cusimano MD. Magnetic resonance susceptibility weighted imaging in neurosurgery: current applications and future perspectives. J Neurosurg. (2015) 123:1463–75. doi: 10.3171/2015.1.JNS142349
74. Zalewski NL, Rabinstein AA, Brinjikji W, Kaufmann TJ, Nasr D, Ruff MW, et al. Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol. (2018) 75:1542–5. doi: 10.1001/jamaneurol.2018.2605
75. Nasr DM, Brinjikji W, Rabinstein AA, Lanzino G. Clinical outcomes following corticosteroid administration in patients with delayed diagnosis of spinal arteriovenous fistulas. J Neurointerv Surg. (2017) 9:607–10. doi: 10.1136/neurintsurg-2016-012430
76. Soderman M, Edner G, Ericson K, Karlsson B, Rahn T, Ulfarsson E, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg. (2006) 104:867–75. doi: 10.3171/jns.2006.104.6.867
Keywords: dural arteriovenous fistula, brainstem engorgement, transarterial embolization, transvenous embolization, open surgery
Citation: Hou K, Li G, Qu L, Liu H, Xu K and Yu J (2020) Intracranial Dural Arteriovenous Fistulas With Brainstem Engorgement: An Under-Recognized Entity in Diagnosis and Treatment. Front. Neurol. 11:526550. doi: 10.3389/fneur.2020.526550
Received: 28 January 2020; Accepted: 28 August 2020;
Published: 25 September 2020.
Edited by:
Atilla Ozcan Ozdemir, Eskişehir Osmangazi University, TurkeyReviewed by:
Ashish Kulhari, JFK Medical Center, United StatesWaldo Rigoberto Guerrero, University of South Florida, United States
Copyright © 2020 Hou, Li, Qu, Liu, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlu Yu, amx5dUBqbHUuZWR1LmNu; Kan Xu, eHVrYW5qbHVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Kun Hou
Kun Hou Guichen Li2†
Guichen Li2† Kan Xu
Kan Xu Jinlu Yu
Jinlu Yu