
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 17 September 2020
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.01052
This article is part of the Research Topic Consequences of the COVID-19 Pandemic on Care for Neurological Conditions View all 77 articles
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases were first reported in Wuhan, Hubei province of China in December, 2019. SARS- COV-2 primarily affects the cardio-respiratory system. Over the last few months, several studies have described various neurological sequelae of SARS-COV-2 infection. Neurological complications are more frequent in patients with severe respiratory infections. In this review, we have analyzed the current literature on neuromuscular complications associated with SARS-COV-2 and highlighted possible mechanisms of neuromuscular invasion. We reviewed 11 studies describing 11 cases of Guillain Barre syndrome (GBS), and 1 case each of Miller Fisher syndrome, Polyneuritis Cranialis, Acute myelitis, Oculomotor paralysis and Bell's Palsy associated with SARS-COV-2 infection. Mean age of patients with GBS was 61.54 years, with standard deviation (SD) 14.18 years. Majority patients had fever and cough as the first symptom of SARS COV-2 infection. Mean time for onset of neurological symptoms from initial symptoms in 11 patients was 8.18 days, with SD of 2.86 days. Mean time to performing electrodiagnostic study from onset of neurological symptom was 6 days with standard deviation of 3.25. Six patients had demyelinating pattern, three had acute sensory motor axonal neuropathy, and one had acute motor axonal neuropathy on electrodiagnostic studies.
In December 2019, several reports of patients with severe pneumonia of unknown causes emerged from Wuhan, Hubei province of China (1). In February 2020, the International Committee on Taxonomy of Viruses officially renamed the novel coronavirus responsible for this outbreak as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2). The World Health Organization declared SARS- COV-2 as pandemic on March 11, 2020 (3). Since then, the epicenter of the pandemic has moved from China to Europe then to North America and Asia.
The first neurological complication from SARS-CoV-2 was reported as a case of viral encephalitis on March 4, 2020, at Beijing Ditan Hospital (4). In a retrospective case series of 214 patients with SARS-COV-2 infection from Wuhan, neurologic symptoms were seen in 36.4% of patients and were more common in patients with severe respiratory infections; these included acute cerebrovascular events, impaired consciousness, and muscle injury (5).
Neuromuscular complications such as critical illness myopathy, polyneuropathy, and guillain barre syndrome (GBS) have been reported with prior SARS outbreaks of 2003 and Middle East respiratory syndrome (MERS) (6, 7). Over the last few months, several studies have described numerous neuromuscular complications in patients with SARS-COV-2 infection. This article presents a narrative review of the current literature on neuromuscular complications associated with SARS- COV 2 infection and describes the possible underlying mechanism of neuro-muscular invasion.
We searched Medline, Google Scholar, and Pubmed using keywords; “Neurological,” “Neurology,” “Neuromuscular,” “complications,” “SARS COV-2,” “COVID-19.” Search was limited to English language manuscript only. The literature search was last done on 31st May, 2020. At the time of writing this article, we identified 53 research literature describing neurological complications in SARS-COV-2, out of these, 11 described neuromuscular complications in SARS- COV-2 (8–18). Out of these 11, seven studies described 11 cases of GBS, one described Miller Fisher syndrome and Polyneuritis Cranialis, one described Acute myelitis, one described Oculomotor paralysis and 1 described Bell's Palsy (8–18).
Table 1 describes the demographic data, time to onset of neurological symptoms, diagnostic criteria, intervention and outcomes from 11 reported studies with neuromuscular complications associated with SARS- COV-2 infection.
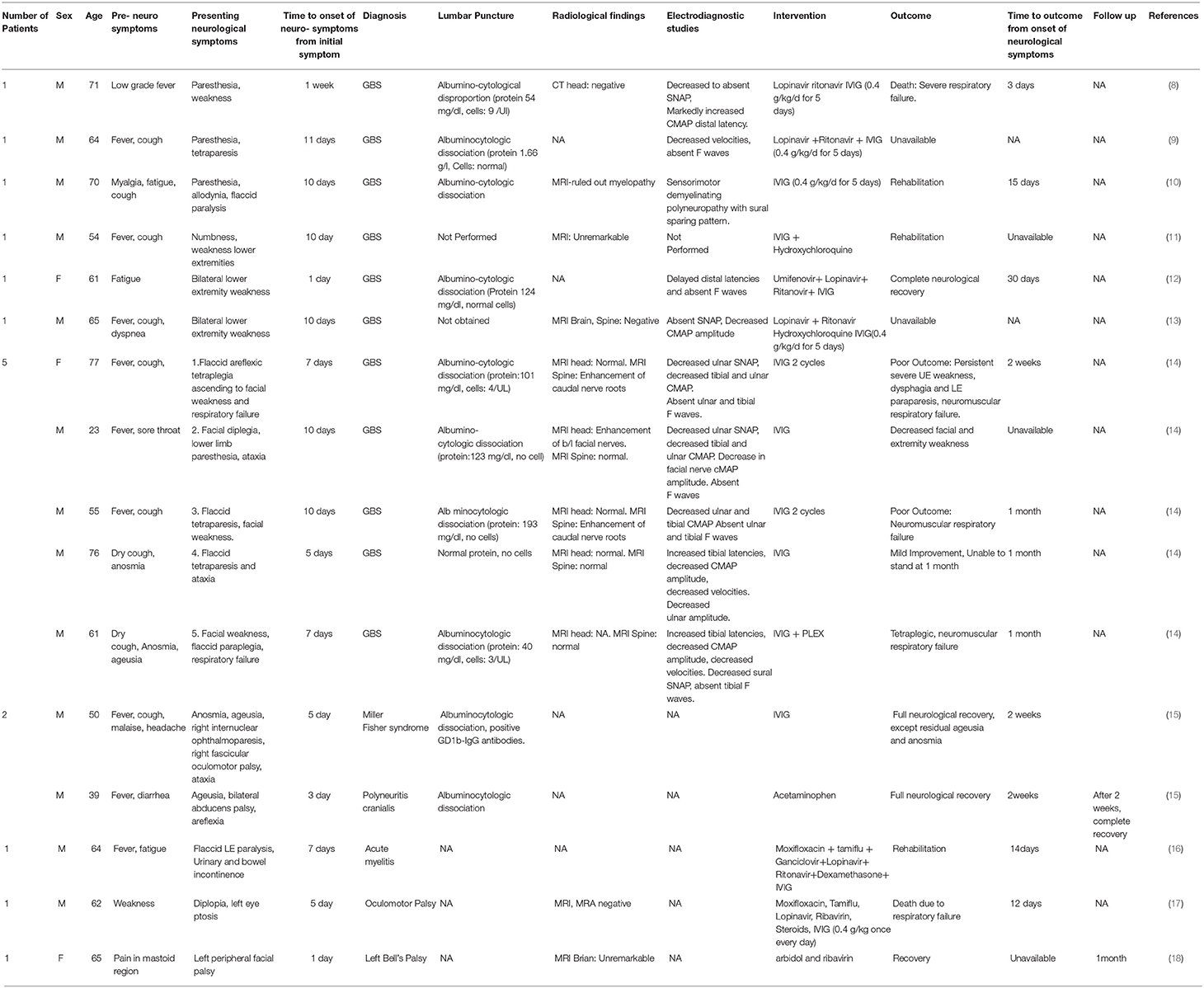
Table 1. Description of demographic data, time to onset of neurological symptoms, diagnostic criteria, intervention and outcomes from 11 reported studies with neuromuscular complications associated with SARS- COV-2 infection.
Seven studies have described a total of 11 cases of GBS (8–14). Out of 11 patients, nine were male, and two was female (8–14). Mean age of these 11 patients was 61.54 years, with standard deviation (SD) 14.18 years. Seven out of 11 patients had fever as the first symptom of SARS COV-2 infection (8–14). Six out of those seven had cough as an accompanying symptom (8–14). Two out of 11 patients had fatigue and myalgia as the first symptom of SARS COV-2 infection (8–14). Remaining two out of 11 patients had cough and anosmia as the first symptom of SARS COV-2 infection (8–14).
Mean time for onset of neurological symptoms from initial symptoms in 11 patients was eight. 18 days, with SD of 2.86 days.
Out of 11 patients, nine underwent lumbar puncture out of which 8 showed albuminocytological disproportion on cerebrospinal fluid analysis. One showed normal protein and no cells. Lumbar puncture was not performed in two patients.
Imaging studies including computed tomography (CT) head scan, Magnetic resonance imaging (MRI) brain and spine were obtained in nine out of 11 patients. Two patients had MRI evidence of caudal nerve root enhancement on MRI spine, one patient had bilateral facial nerve enhancement. In the remaining six patients, imaging studies were unremarkable.
Electrodiagnostic studies were obtained in 10 out of 11 patients. Mean time to performing electrodiagnostic study from onset of neurological symptom was 6 days with standard deviation of 3.25. Six out of 10 patients had demyelinating patterns (prolonged motor latencies, severe conduction velocity slowing, and conduction blocks) (8–14). Three patients had acute sensory motor axonal neuropathy and remaining one had acute motor axonal neuropathy (8–14). None of the patients had follow up electrodiagnostic study.
Table 2 describes details of electrodiagnostic studies from 10 reported cases of Guillain Barre syndrome associated with SARS- COV-2 infection.
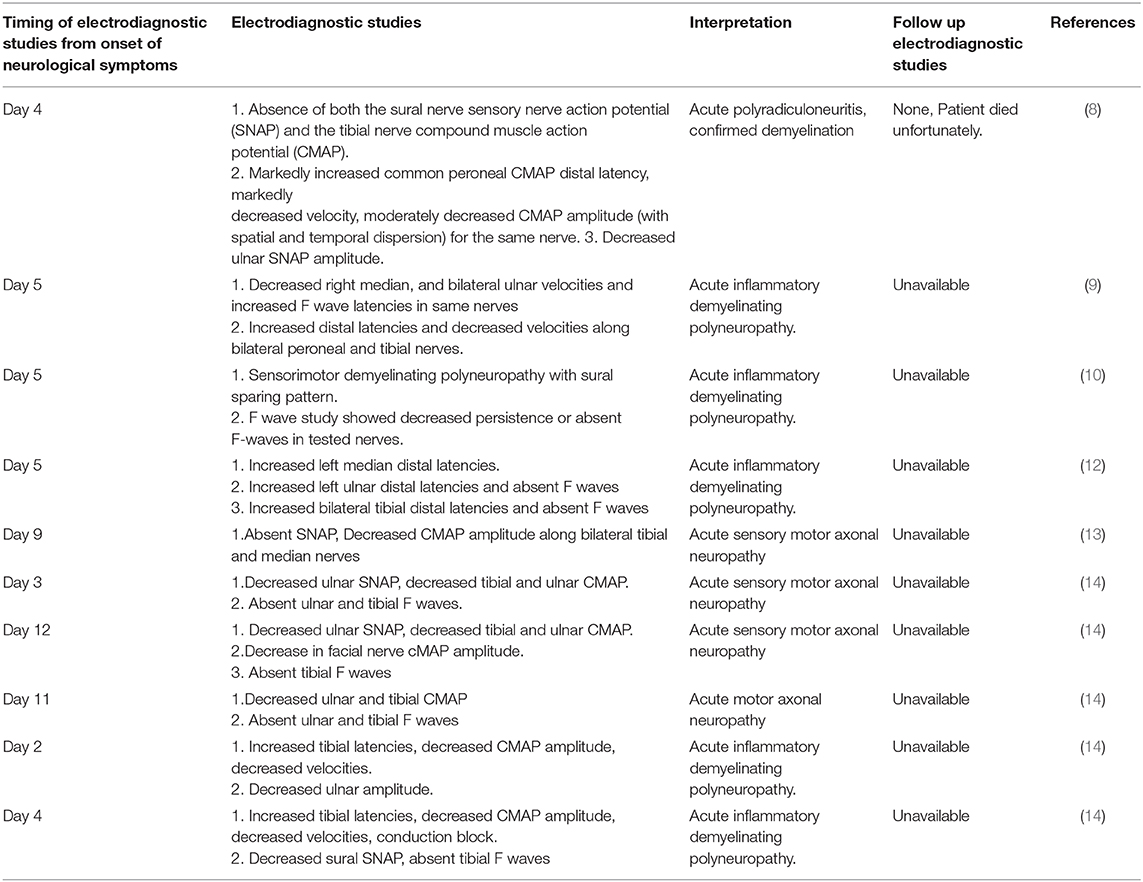
Table 2. Description of electrodiagnostic studies from 10 reported cases of Guillain Barre syndrome associated with SARS- COV-2 infection.
All 11 patients received IVIG treatment in combination with various antivirals, antibiotics, and immunosuppressive agents. Majority cases used a standard dose of IVIG; 0.4 mg/kg/day for 5 days. None of the studies mentioned any complications associated with IVIG therapy.
Death was reported as an outcome in one of 11 patients. Final outcomes were unavailable/not reports for three patients. Poor outcome defined as persistent/ worsening of symptoms was reported in two patients. One had complete neurological recovery. Remaining four patients had improvement of symptoms with decreased weakness. Though none of the studies mentioned dysautonomia, one patient had hemodynamic disturbances with severe drug-resistant hypertension, suggesting possible autonomic nervous system involvement (8). Four out of 11 patients developed neuromuscular respiratory failure. One patient developed respiratory failure, 3 days from onset of neurological symptoms, one at 2 weeks days from onset of neurological symptoms and remaining two at a month after onset of neurological symptoms.
Ortiz et al. reported the first case of Miller fisher syndrome in a 50-year-old man who presented with anosmia, ageusia, right internuclear ophthalmoparesis, right fascicular oculomotor palsy, ataxia, areflexia, 5 days after developing cough, malaise, headache, low back pain, and fever (15). Workup was remarkable for albuminocytologic dissociation and positive testing for GD1b-IgG antibodies (15). SARS- COV-2 infection was confirmed by qualitative real-time reverse-transcriptase–polymerase-chain-reaction assay utilizing oropharyngeal swab (15). The patient was successfully treated with IVIG and achieved complete neurological recovery after 2 weeks, with exception of residual ageusia and anosmia (15).
Ortiz et al. also described the first case of polyneuritis cranialis in a 39-year-old male who presented with ageusia, bilateral abducens palsy, areflexia, 3 days after developing diarrhea, a low-grade fever, and a poor general condition (15). Workup was remarkable for albuminocytologic dissociation (15). SARS- COV-2 infection was confirmed by qualitative real-time reverse-transcriptase–polymerase-chain-reaction assay utilizing oropharyngeal swab (15). The patient had normal respiratory, cardiovascular and abdominal examination and therefore was treated symptomatically with acetaminophen only which resulted in full neurological recovery after 2 weeks (15). This case indicates that patients with normal cardio-respiratory exam may have better neurological outcomes.
Zhao et al. reported the first case of acute myelitis in a 66 year old male who developed flaccid weakness of bilateral lower extremity with bowel and bladder incontinence and sensory level at T 10, 7 days after developing fever (16). SARS- COV-2 infection was confirmed by nucleic testing utilizing nasopharyngeal swab (16). Lumbar puncture and MRI studies were not performed given pandemic related reasons (16). The patient was treated with a combination of moxifloxacin, tamiflu, ganciclovir, lopinavir, ritonavir, dexamethasone, and IVIG (15 g once daily × 7 days) (16). The patient achieved improvement in bilateral upper and lower extremity strength and was eventually discharged to a rehabilitation facility (16).
Wei et al. described the first case of oculomotor paralysis in a 65 year old male who presented with 5 days history of persistent diplopia, and left eyelid droop (17). The patient had complete ptosis of left eye and the left eye was down and out at rest (17). MRI and Magnetic resonance angiography (MRA) were unremarkable however, CT chest showed diffuse ground glass opacities (17). SARS- COV-2 was detected in the throat swab (17). The patient was treated with a combination of moxifloxacin, tamiflu, ribavirin, lopinavir, methylprednisolone, and IVIG (0.4 g/kg once every day). Unfortunately, he developed respiratory failure and died on day 12 of admission (17).
Wan et al. described the first case of Bell's Palsy in a 65 year old female who presented with left lower motor neuron facial paralysis, 2 days after developing pain in the mastoid region (18). Interestingly, this patient had no other symptoms of viral illness. MRI brain showed no abnormality however, computed tomography (CT) chest showed patchy areas of ground-glass shadows in the right lower lung raising suspicion for SARS-COV 2 (18). SARS- COV-2 infection was confirmed by real-time reverse-transcriptase–polymerase-chain-reaction assay utilizing throat swabs (18). The patient was successfully treated with arbidol and ribavirin and achieved resolution of neurological symptoms and lung shadows after 1 month (18).
The first major target of SARS- COV-2 is the ACE-2 receptor located on epithelial cells of the respiratory tract (19). This binding results in downregulation of ACE-2 expression as well as the viral entry and replication (20). Loss of ACE-2 expression leads to dysregulation of the renin-angiotensin system which causes an elevated production of angiotensin II (21). The overproduction of angiotensin II results in a cascade of interactions that eventually leads to severe acute lung injury (21).
ACE2 receptors are present widespread throughout the brain, including cardio-respiratory neurons of the brainstem (dorsal vagal complex), endothelial cells, glial cells, basal ganglia, motor cortex, and raphe (21–23). Once in blood circulation, SARS-COV-2 can travel via hematogenous route to infect the endothelial cells of the blood–brain barrier and then accumulate in ACE-2 rich brain regions causing neurological sequelae (21, 24). Respiratory distress experienced during SAR-CoV2 infection may result from compromise of the brainstem's cardiorespiratory center (21, 25, 26).
The anatomical organization of olfactory nerve and olfactory bulb in the nasal cavity provides a direct portal for entry of SARS-COV-2 from periphery to CNS (21, 27). After infecting nasal cells, COV can reach the brain and cerebrospinal fluid through the olfactory nerve and olfactory bulb within 7 days and cause inflammation and demyelinating reaction (4).
The major mechanism by which viruses cause neuromuscular complications involves the entry through peripheral nerve endings located in the skin and mucosa (28, 29). This process is followed by an endogenous neuronal mechanism causing retrograde axonal transport of viruses from the cell periphery to the neuronal cell body (28, 29). Multiple other COV-viruses are known to exhibit transsynaptic transfer properties including HCoV-OC43, HEV 67N (21, 30–32). HEV 67N shares more than 91% homology with the novel SARS-CoV-2 thus further consolidating the hypothesis of retrograde transfer as a possible mechanism of neuro-muscular invasion in SARS-COV-2 (21, 30–32).
Cytokine storm is an immune-mediated life-threatening disease, which is caused by impaired natural killer and cytotoxic T-cell function (33, 34). Viral infection is the most frequent trigger, either as a primary infection in healthy people or after reactivation in immunosuppressed patients (33, 34). Cytokine storm is associated with an exaggerated inflammatory response caused by hypersecretion of proinflammatory cytokines such as interferon γ, tumor necrosis factor α (TNFα), interleukin 1, 4, 6, 8, 10, and 18 which causes tissue damage and progressive systemic organ failure (33–38). Experimental studies infecting in vitro cultured glial cells (including microglia, astrocytes and oligodendrocytes) with COV noted enormous production of inflammatory factors such as IL-6, IL-12, IL-15, and TNF-α (4, 39, 40). Interleukin (IL)-6, is positively correlated with the severity of COVID-2019 symptoms (33–40). Exaggerated immune responses with SARS-COV-2 might contribute to development of acute inflammatory demyelinating poly radiculo-neuropathies.
All the above described mechanisms were not based on studies on peripheral neurons or Schwann cells, therefore the exact mechanism remains unknown. Further dedicated studies are required to undermine the exact cause of neuromuscular invasion of SARS-COV 2 virus.
Neurological complications with SARS-COV-2 are being reported exponentially. Majority literature is anecdotal and reported in the form of isolated case reports and case series. In order to better understand the causal relationship, and underlying pathophysiology, meta-analysis of these studies is warranted. Physicians must familiarize themselves with the rapidly evolving literature to provide uptodate care to the affected patients.
NK, NN, and SA contributed to literature research, manuscript writing. RG made revisions and helped in editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley B, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. (2020) 92:602–611. doi: 10.1002/jmv.25731
3. WHO Director-General's opening remarks at the media briefing on COVID-19 (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessecd March 11, 2020).
4. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. (2020) S0889-1591(20)30357-3.
5. Mao L, Wang MD, Chen SH, He QW, Chang J, Hong CD, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv. (2020). doi: 10.2139/ssrn.3544840
6. Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. (2017) 13:227–33. doi: 10.3988/jcn.2017.13.3.227
7. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. (2005) 14:113−9.
8. Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e741. doi: 10.1212/NXI.0000000000000741
9. Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain-Barré syndrome. Rev Neurol (Paris). (2020) 176:516–518. doi: 10.1016/j.neurol.2020.04.003
10. Coen M, Jeanson G, Culebras Almeida LA, Hubers A, Stierlin F, Najjar I, et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. (2020) 87:111–112. doi: 10.1016/j.bbi.2020.04.074
11. Virani A, Rabold E, Hanson T, Haag A, Elrufay R, Cheema T, et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. (2020) 20:e00771. doi: 10.1016/j.idcr.2020.e00771
12. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence?. Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
13. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. (2020) 76:S0967-5868(20)30882-1. doi: 10.1016/j.jocn.2020.04.062
14. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. (2020) 382:2574–76 doi: 10.1056/NEJMc2009191
15. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Ma, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) 95:e601–5. doi: 10.1212/WNL.0000000000009619
16. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv. (2020). doi: 10.1101/2020.03.16.20035105
17. Wei H, Yin H, Huang M, Guo Z. The 2019 novel coronavirus pneumonia with onset of oculomotor nerve palsy: a case study. J Neurol. (2020) 267:1550–3. doi: 10.1007/s00415-020-09773-9
18. Wan Y, Cao S, Fang Q, Wang M, Huang Y. Coronavirus disease 2019 complicated with Bell's palsy: a case report. Neurology. doi: 10.21203/rs.3.rs-23216/v1
19. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2020) 12:14. doi: 10.3390/v12010014
20. Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med. (2006) 84:814–20. doi: 10.1007/s00109-006-0094-9
21. Iroegbu JD, Ifenatuoha CW, Ijomone OM. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol Sci. (2020) 41:1329–37. doi: 10.1007/s10072-020-04469-4
22. Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. (2008) 107:1482–94. doi: 10.1111/j.1471-4159.2008.05723.x
23. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol - Regul Integr Comp Physiol. (2007) 292:R373–81. doi: 10.1152/ajpregu.00292.2006
24. Desforges M, Favreau DJ, Brison É, Desjardins J, Meessen-Pinard M, Jacomy H, et al. Human coronaviruses: respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents. In: Singh SK, Ruzek D, editors. Neuroviral Infections. Boca Raton: CRC Press (2013). p. 93–121. doi: 10.1201/b13908-7
25. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. (2020) 9:552–5. doi: 10.1002/jmv.25728
26. Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. (2016) 59:163–9. doi: 10.1159/000453066
27. Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. (2013) 13:379-393. doi: 10.1016/j.chom.2013.03.010
28. Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. (2000) 8:465–72. doi: 10.1016/S0966-842X(00)01824-2
29. Berth SH, Leopold PL, Morfini G. Virus-induced neuronal dysfunction and degeneration. Front Biosci. (2009) 14:5239–59. doi: 10.2741/3595
30. Dubé M, Le Coupanec A, Wong AH, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. (2018) 92:e00404–e00418. doi: 10.1128/JVI.00404-18
31. Li Z, He W, Lan Y, Zhao K, Lv X, Lu H, et al. The evidence of porcine hemagglutinating encephalomyelitis virus induced nonsuppurative encephalitis as the cause of death in piglets. PeerJ. (2016) 4:e2443. doi: 10.7717/peerj.2443
32. Gonzalez J, Gomez-Puertas P, Cavanagh D, Gorbalenya A, Enjuanes L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch Virol. (2003) 148:2207–35. doi: 10.1007/s00705-003-0162-1
33. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult hemophagocytic syndrome. Lancet. (2014) 383:1503–16. doi: 10.1016/S0140-6736(13)61048-X
34. Scott RB, Robb-Smith AH. Histiocytic medullary reticulocytosis. Lancet. (1939) 2:194–8. doi: 10.1016/S0140-6736(00)61951-7
35. Sumegi J, Barnes MG, Nestheide SV, Lee SM, Villanueva J, Zhang K, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. (2011) 117:E151–60 doi: 10.1182/blood-2010-08-300046
36. Milner JD, Orekov T, Ward JM, Cheng L, Velez FT, Junttila I, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. (2010) 116:2476–83. doi: 10.1182/blood-2009-11-255174
37. Chiossone L, Audonnet S, Chetaille B, Chasson L, Farnarier C, Haddad YB, et al. Protection from inflammatory organ damage in a murine model of hemophagocytic lymphohistiocytosis using treatment with IL-18 binding protein. Front Immunol. (2012) 3:239 doi: 10.3389/fimmu.2012.00239
38. Wan SX, Yi QJ, Fan SB, Lv JL, Zhang XX, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv. (2020). doi: 10.1101/2020.02.10.20021832
39. Bohmwald K, Galvez NMS, Rios M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. (2018) 12:386. doi: 10.3389/fncel.2018.00386
Keywords: COVID 19, SARS-CoV-2, neuromuscular, neurology, complications, pathophysiology
Citation: Katyal N, Narula N, Acharya S and Govindarajan R (2020) Neuromuscular Complications With SARS-COV-2 Infection: A Review. Front. Neurol. 11:1052. doi: 10.3389/fneur.2020.01052
Received: 19 June 2020; Accepted: 11 August 2020;
Published: 17 September 2020.
Edited by:
Tomohisa Nezu, Hiroshima University, JapanReviewed by:
Kalliopi Pitarokoili, Ruhr University Bochum, GermanyCopyright © 2020 Katyal, Narula, Acharya and Govindarajan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nakul Katyal, a2F0eWFsLm5ha3VsQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.