- 1Unit of Clinical Epidemiology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
- 2Department of Laboratory Medicine and Pathological Anatomy, Ospedale Civile S. Agostino Estense, Modena, Italy
- 3Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 4Neurorehabilitation Unit, Department of Neuroscience and Rehabilitation, G. Brotzu Hospital, Cagliari, Italy
- 5IRCCS Istituto Ortopedico Galeazzi, Scientific Director, Milan, Italy
- 6Università Vita e Salute San Raffaele, Milan, Italy
- 7Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
Background: Muscular dystrophy causes weakness and muscle loss. The effect of muscular exercise in these patients remains controversial.
Objective: To assess the effects of muscular exercise vs. no exercise in patients with muscular dystrophy.
Methods: We performed a comprehensive systematic literature search in the Medline, Embase, Web of Science, Scopus, and Pedro electronic databases, as well as in the reference literature. We included randomized clinical trials (RCTs) that reported the effect of muscular exercise on muscle strength, endurance during walking, motor abilities, and fatigue. Data were extracted independently by two reviewers. Mean difference (MD) and 95% confidence intervals (CI) were used to quantify the effect associated with each outcome. We performed pairwise meta-analyses and trial sequential analyses (TSA) and used GRADE to rate the overall certainty of evidence.
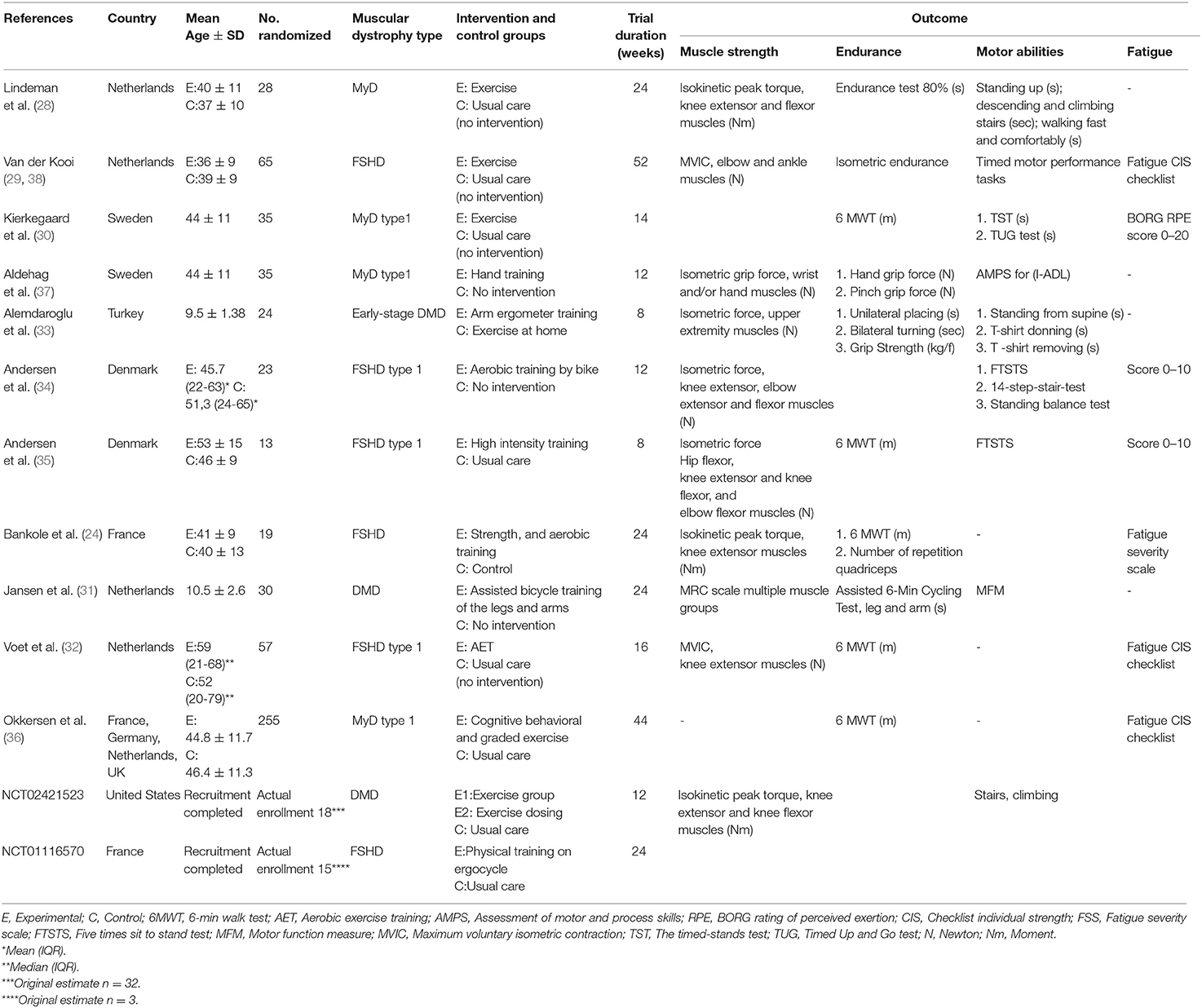
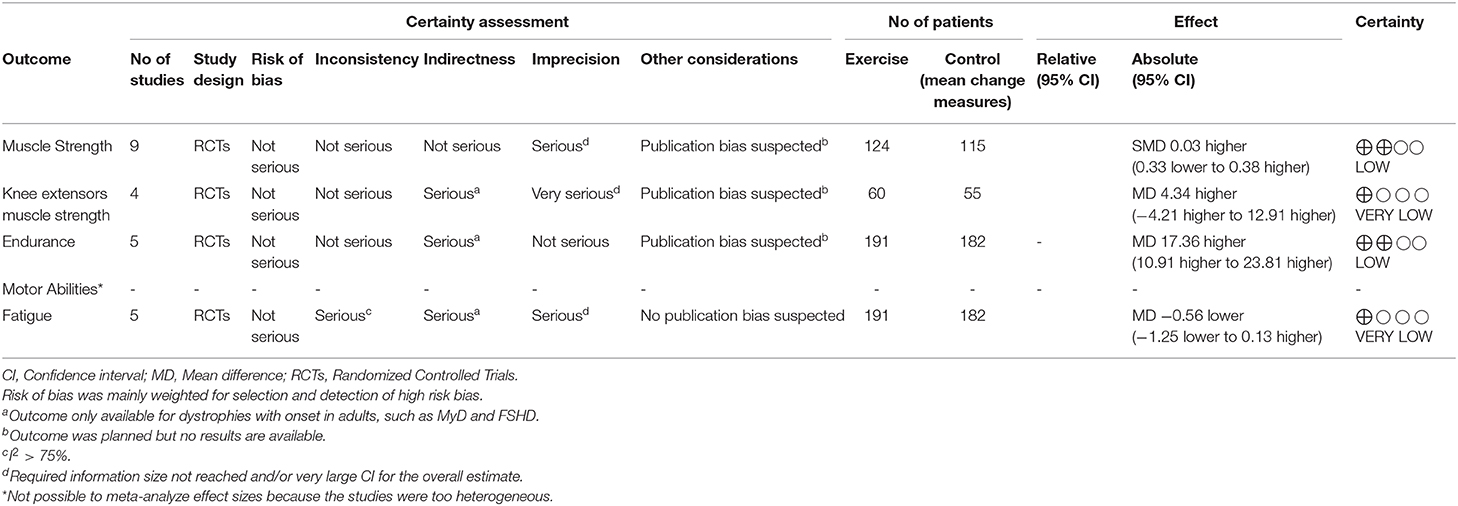
Results: We identified 13 RCTs involving 617 patients. The median duration of exercise interventions was 16 weeks [interquartile range [IQR] 12–24]. In the patients with facio-scapulo-humeral dystrophy and myotonic dystrophy, no significant difference in extensor muscle strength was noted between the exercise and the control groups [four studies, 115 patients, MD 4.34, 95% CI −4.20 to 12.88, I2 = 69%; p = 0.32; minimal important difference [MID] 5.39 m]. Exercise was associated with improved endurance during walking [five studies, 380 patients, MD 17.36 m, 95% CI 10.91–23.81, I2 = 0; p < 0.00001; MID 34 m]. TSA excluded random error as a cause of the findings for endurance during walking. Differences in fatigue and motor abilities were small. Not enough information was found for other types of dystrophy.
Conclusions: Muscular exercise did not improve muscle strength and was associated with modest improvements in endurance during walking in patients with facio-scapulo-humeral and myotonic dystrophy. Future trials should explore which type of muscle exercise could lead to better improvements in muscle strength.
PROSPERO: CRD42019127456.
Introduction
Muscular dystrophies are a heterogeneous group of progressive, inherited diseases caused by mutations in genes involved in muscle function (1). Though they can vary widely in etiology and presentation, nearly all forms of muscular dystrophy cause muscle weakness and muscle loss, which may result in limitations of daily activities, and fatigability (2, 3).
Currently, there is no cure for muscular dystrophies. Treatment consists of medication, surgery and/or rehabilitation, including physical and muscle training, aerobic capacity training or aids and adaptations such as arm supports to enable performance of daily activities (4).
Whether patients with muscular dystrophies can benefit from muscular exercise remains debated. Physical exercise can have numerous psychological and physiological positive effects for the general population, such as improvements in self-estimate and plasma endorphin concentrations (5, 6). But because of muscle degeneration in muscular dystrophy, there may be the risk of exercise-induced adverse effects such as overwork weakness following supramaximal, high-intensity exercise (7). Guidelines for the prescription of physical exercise are based on low-quality evidence, which limits their confidence in strengthening and aerobic fitness training programs (7).
Randomized clinical trials (RCT) assessing the efficacy of muscular exercise for muscular dystrophies have been limited and inconclusive to date. These diseases are rare, and research possibilities are limited (8); nevertheless, recent trials have added data to the evidence base. We conducted an updated systematic review and meta-analysis of existing RCTs to further explore the effect of muscular exercise in patients with muscular dystrophy.
Methods
Registered Protocol and Reporting Guidelines
The systematic review protocol was registered with the International Prospective Register of Systematic Reviews database (PROSPERO identifier: CRD42019127456). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines for reporting (9) (Supplementary Material).
Eligibility Criteria
For this systematic review we included only RCTs (both parallel-group and cross-over design RCTs) that enrolled patients with Duchenne's muscular dystrophy (DMD), Becker's muscular dystrophy (BMD), limb-girdle dystrophy (LD), facio-scapulo-humeral dystrophy (FSHD), and myotonic dystrophy (MyD). For the exercise intervention, we were interested in any muscular exercise as the core intervention, assessed on the basis of muscle strengthening or physical capacity and expressed as peak torque of strength, endurance during walking, motor abilities, and fatigue. All kinds of strength training, including aerobic cycling, fitness, weights, weight machines or elastic cords, were eligible for inclusion (10).
As control, trials were eligible irrespective of the type of control, with the caveat that any type of exercise or other type of intervention that would have limited our ability to separate and understand the role of muscular exercise was excluded. Control categories encompassed no intervention at all and usual care. We excluded studies in which the non-exercised limb was a control so as to avoid the cross-education phenomenon, since exercise of one side of the body can increase the voluntary strength of the contralateral side (11). Eligibility was not restricted by language, type of publication or patient age.
Search Strategy
Two methodologists conducted the search strategy in the electronic databases: Medline (since 1966), Embase (since 1974), Web of Science (since 1950), Scopus (since 1996), and Pedro (since 1999). The last search was run in February 25 2019 and it was up to date in February 4, 2020. Reference lists of relevant studies were screened for further publications. In addition, www.ClinicalTrials.gov was investigated for ongoing trials.
Outcomes
The primary outcomes were changes in muscle strength and endurance during walking. Muscle strength, measured with a dynamometer, is considered a measure of efficacy that captures changes in specific muscle groups and reflects optimal test conditions (12). Where authors reported outcome data for more than one muscle group, we extracted data according to a priority list: knee extensors, knee flexors, elbow flexors, elbow extensors, wrist flexors, and wrist extensors. Because this might be seen as a narrow outcome with limited everyday life value, we included endurance during walking as an outcome since it better represents the potential effectiveness of exercise training in ambulant dystrophic patients and mimics daily real-world conditions (13). Endurance during walking, as measured by tests such as the Six Minute Walking Test (6 MWT), was defined as the ability of a muscle to maintain its function or performance capacity over time and multiple contractions (14).
Secondary outcomes were motor abilities and fatigue. Motor ability was defined as the successful performance of motor skill based on a unit measure such as time or score (e.g., standing from supine) (15). Fatigue was defined as the inability of a muscle to generate force or power. It is an important limiting factor of exercise performance and muscle functional capacity (e.g., BORG scale) (16).
The end of treatment and follow-up assessment were used for each trial in the meta-analysis. All adverse events reported by the studies were recorded. When we identified relevant missing or unpublished data, we contacted the corresponding author of the primary study and requested the information.
Data Collection and Extraction
Two researchers independently screened the studies for eligibility by title and abstract. Full texts were then evaluated for inclusion. Two authors independently extracted and entered the data from the studies onto data extraction forms. The information was reported in a table of main features: (i) characteristics of trial participants (age, type of dystrophy, disease stage, and muscle involved); (ii) characteristics of studies (study design, study year, country, sample size calculation, funding); (iii) outcomes (muscular strength, endurance during walking, motor abilities, and fatigue). A description of key elements of interventions, taken from the Template for Intervention Description and Replication (TIDieR) checklist, was recorded (17). Disagreement between reviewers was resolved by consensus; a third author was consulted if no agreement could be reached.
Risk of Bias Assessment
The Cochrane Collaboration's tool was used to assess the risk of bias in the RCTs for random sequence generation, allocation concealment, blinding of participants and health care personnel, blinded outcome assessment, incomplete outcome data, and selective reporting.
Overall Certainty of Evidence
Two authors independently assessed the certainty of evidence for the primary outcomes using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) framework methodology (18). Five GRADE domains—study limitations, consistency of effect, imprecision, indirectness, and publication bias—were analytically assessed. For imprecision, our assessment was informed by the findings of trial sequential analysis (TSA) (19). The final judgement on certainty was revised and downgraded by one or two levels as appropriate, reflecting the extent of bias in important quality domains. All reasons for rating down are reported in detail in Table 2. We used GRADEpro software (Tool) to present the study findings1.
Statistical Analysis
To quantify the effect associated with each outcome, we used the mean difference (MD) or standardized mean difference (SMD) with 95% confidence intervals (CIs). We used the mean change from baseline scores since muscular dystrophies are rare diseases and we expected to encounter studies with small sample size and small effects. Imbalances between groups at baseline are therefore possible, even when randomization is adequately implemented (20). Positive effect measures indicated that muscle exercise is favored over no exercise for strength, endurance during walking, and motor abilities, while for fatigue, a negative effect measure indicated that the treatment was associated with less fatigue.
Meta-analyses were developed according to type of dystrophy (e.g., DMD and BMD split from the others). Heterogeneity was evaluated using the I2 statistic (21). To investigate potentially different effects on strength and endurance during walking, the studies were sub-grouped by group of muscles exercised (e.g., knee extensors). However, the overall interpretation of results, GRADE assessment of certainty, and TSA were derived from the overarching meta-analysis of all studies that assessed the outcome of interest.
We conducted a sensitivity analysis of the primary outcomes based on risk of bias for blinding of outcome assessor (detection bias: high risk vs. low and intermediate risk studies) and only published data. All analyses were performed using Review Manager (RevMan5) software version 5.32.
We performed TSA to limit the risk of potential spurious conclusions from underpowered meta-analysis and repetitive significance testing. In detail, we can control the risk of I type and II type errors and introduce the calculation of a required information size by applying trial sequential monitoring boundaries, which can inform about the research needed to achieve conclusive evidence (22). TSA for continuous outcomes is possible, however, only when studies that use the same outcome measure and effect sizes are cumulated using MD, since expected standardized mean differences are prone to providing unrealistic information size (23). For muscle strength, we arbitrarily selected the peak torque measurement of knee extensor muscles to generate inference on the conclusiveness of findings. We estimated the diversity adjusted required information size (DARIS) based on the standard deviation observed in the control group of trials with low risk of bias (alpha 5%, beta 20%), and the observed diversity in the trials in the meta-analysis. We selected the highest quality trial (24), assuming a minimal important difference (MID) of 5.39 N, estimated by multiplying the effect size of 0.5 by the pooled standard deviation between groups (25). For endurance during walking, in order to avoid clinical heterogeneity, we performed a meta-analysis and a TSA only for the 6 MWT, the most common, reliable and feasible test in clinical trials (26). The 6 MWT measures the distance in meters walked in 6 min, wherein a greater distance indicates better performance. We estimated the DARIS by selecting a commonly reported MID of 34.3 m (27); we assumed a diversity of 50% among the meta-analyzed trials. We used TSA software beta version 0.9.5.5 (23).
Results
The literature search identified 5,528 references, excluding duplicates. After screening and selection, 10 parallel group trials were included in the analysis (24, 28–36) and 1 cross-over trial (37). Two additional ongoing trials were also identified that were described only qualitatively. The study selection flow is illustrated in eFigure S1. The list of excluded published and ongoing studies are reported in eTables S1a,b.
Overall, 584 participants were involved (range 13–255). The majority (60.4%) had MyD, followed by FSHD (30.3%), and DMD (9.2%). All trials were conducted in Western countries between 1995 and 2018. A description of the interventions is presented in eTable S2. The duration of exercise intervention ranged from 8 to 52 weeks (median 16 weeks). Table 1 presents the characteristics of the trials. Most were judged as having a low risk of bias. Details are presented in eFigure S2.
Primary Outcomes
In order to complete the overall meta-analyses, we obtained unpublished data for several trials from the corresponding authors (30, 31, 34, 35, 39).
Albeit with low quality of evidence (Table 2), the effect of muscular exercise on global muscle strength (MyD, FSHD, DMD) was not statistically different between the groups (7 studies, 239 patients, median follow-up of 16 weeks with IQR 12–24, MD 0.65; 95% CI −1.33–2.63, I2 = 45%; p = 0.52, Figure 1). Subgroup analysis, with very low quality of evidence (Table 2), showed no significant difference in extensor muscle strength between the groups (4 studies, 115 patients with FSHD and MyD, median follow-up of 20 weeks with IQR 15–24, MD 4.34; 95% CI −4.20 to 12.88, I2 = 69%; p = 0.32, eFigure S3). Sensitivity analysis, which excluded unpublished data, revealed no statistically significant differences between the groups (eFigure S4). TSA showed that the required information size of 594 patients was not achieved and that the cumulative z-curve did not cross any boundaries in favor of muscular exercise (eFigure S5). No statistically significant differences were found for knee flexors and elbow flexors (eFigures S6, S7).
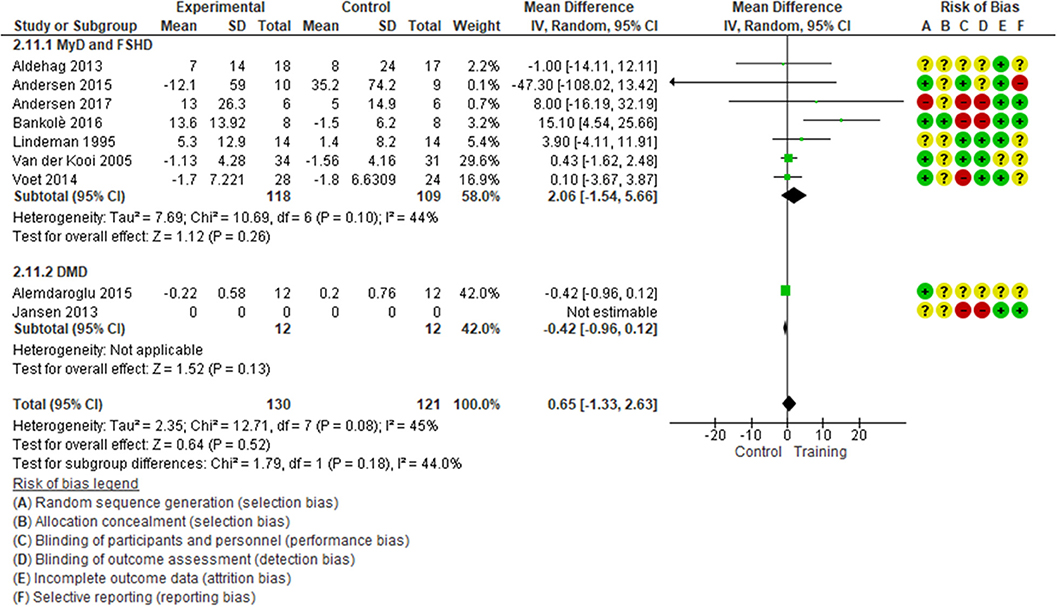
Figure 1. Global muscle strength, mean change in SMD for FSHD, MyD, and DMD patients. Contribution: knee extensors: Andersen et al. (34), Bankolè et al. (24), Lindeman et al. (28), Voet et al. (32); elbow flexors: Van der Kooi (40), Alemdaroglu et al. (33); wrist flexors: Aldehag et al. (37). *Alemdaroglu et al. (33) compared two training regimes (supervised training vs. unsupervised training at home).
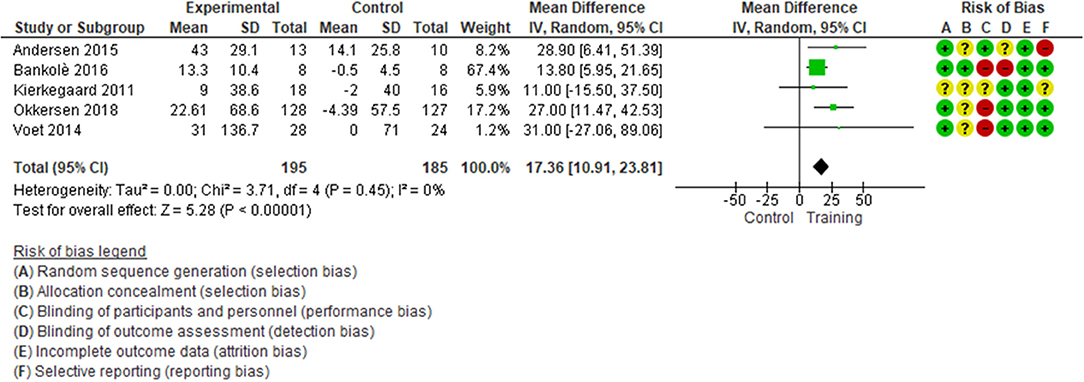
With low quality of evidence (Table 2), we found a statistically significant difference in favor of muscular exercise for improving endurance during walking (5 studies, 380 patients with FSHD and MyD, median follow-up of 16 weeks, with IQR 14–24, MD 17.36; 95% CI 10.91–23.81, I2 = 0; p < 0.0001, Figure 2), TSA showed that the required information size of 110 patients was achieved; the cumulative z-curve crossed the required information size after the second study and crossed the conventional boundary at the fourth study in favor of exercise (eFigure S8). Sensitivity analysis performed only on a low risk of bias assessment for the detection of bias reinforced the statistically significant difference in favor of the exercise group (2 studies, 289 patients, MD 22.75; 95% CI 8.90–36.60, I2 = 0; p < 0.0001, eFigure S9). The magnitude of benefits did not reach the MID threshold set at 34.3 m.
Secondary Outcomes
Clinical heterogeneity precluded comparison of cumulative effect size across studies on motor abilities as the tests evaluated different constructs. With very low quality of evidence, muscular exercise was not statistically significant at the end of treatment in reducing fatigue when compared to controls (five studies, 373 patients, median follow-up of 16 weeks with IQR 14–24, SMD −0.56; 95% CI −1.25–0.13, I2 = 83; p = 0.11, eFigure S10).
Adverse Events
Three studies did not report information on adverse events (24, 33, 35). Six studies reported no serious adverse events (28–31, 34, 37). One study reported mild adverse effects that according to the authors did not influence the effect of the interventions (32). A multicenter trial reported 47 serious adverse events involving 34 out of 255 patients during the study (24 classified as serious in the training group and 23 in the control group), the most common of which were gastrointestinal or cardiac in nature (36).
Discussion
In 2013 we reviewed the evidence for the possible beneficial effects of muscular exercise on patients with muscular dystrophy. At the time, we were unable to provide a clear answer due to the paucity of trials and the overall effect being equally positive or negative. Exercise might have been useful, not useful or even detrimental. The present update includes the previously reviewed studies plus later studies. We found that while muscular exercise is not associated with an improvement in strength it does improve endurance during walking compared to no treatment in patients with FSHD and MyD. The magnitude of benefit does not reach a clinically relevant threshold. However, judging the clinical meaningfulness and effect size of the mean differences (MDs) was not straightforward, since there are no internationally agreed standards (41). No conclusions can be drawn whether exercise improves motor ability since different studies used different clinical evaluations. Finally, exercise was not associated with improvements in reducing fatigue. The currently available evidence overwhelmingly suggests that exercise, while not conferring muscular protection, does not seem to be counterproductive.
The role of muscular exercise is controversial. The question whether exercise should be recommended in people with muscular dystrophy has two biologically plausible yet conflicting answers. Since a hallmark of this degenerative disease is the progressive loss of motor unit constituents, muscular exercise may be considered harmful because it can induce extensive damage, inflammation, and failure of skeletal muscle to repair itself (42). By the same token, the lack of physical activity, common in patients with muscular dystrophy, may lead to functional deconditioning, overweight, fatigue, and reduced muscle strength that could be countered.by regular physical exercise (43). Additional benefits of exercise include improvement in body composition, metabolism, cardiorespiratory performance, and mental well-being (44).
In light of current evidence and the controversy surrounding physical activity, muscle exercises should be planned neither with the aim of improving strength nor of reducing fatigue. Physiotherapists and physicians should focus their efforts on endurance training during walking. We found the similar amount of improvement reported in other degenerative disease such as the late-onset Pompe's disease (45) and mitochondrial myopathy (46).
The primary patient population is people with FSMS and MyD, probably because less impaired when performing this motor ability (13). No evidence was found for endurance training during walking in patients with DMD, however, probably because it is harder to involve this population in trials exploring this specific skill. If there are benefits, they are likely to be small. In brief, the risk of potentially detrimental effects associated with exercise is low if exercise is paced and performed gradually.
Two important issues concerning muscular exercise were not addressed in any of the studies in this review. First, exercise dose and intensity and session duration were not described accurately. These dimensions need to be carefully considered in order to fully understand how specific effects are produced, similar to when medications are prescribed. As major individual differences at different training intensities and at different dose-tolerated levels of exercise among patients with other muscle diseases have been revealed (47), new research hypotheses addressing optimal exercise prescription should be pursued in patients with muscular dystrophy, as well (48). Second, muscle exercise is rarely described as part of well-coordinated multidisciplinary program (48): current treatments usually include drugs, ventilation, and surgery, but muscle exercise is never offered within an integrated model. New research investigating multidisciplinary approaches need to include muscular exercise as a valid support to medical care.
A recent review on the topic (41) included studies perfectly overlapping ours, except for only one study (36). The quality of evidence was low in both reviews. The effects of exercise on strength remained uncertain, while there was some evidence for improved endurance during walking, which is shared by our findings.
Limitations
This review has several limitations. First, we investigated outcome measures (endurance and strength) that capture only a snapshot of the progression of the disorder and not are applicable to all disease stages, thus limiting the number of patients included (49). Second, most of the studies had a small sample, which raises the risk of overestimating treatment effects and reducing precision. Third, some of the studies reported high drop-outs rates and low adherence to the exercise intervention, possibly reducing its treatment effects. Fourth, additional benefits derived from regular interaction with physiotherapists might have induced further distortion of treatment effects compared to the no-treatment groups. Fifth, MIDs were rarely reported, limiting the clinical interpretation of the effects of muscular exercise.
Conclusion
Muscular exercise can be recommended to improve endurance during walking in most patients with muscular dystrophy. It is important, however, that the patient understands that the benefits might be only marginal. Indeed, realistic expectations are key to fostering cooperation between the patient and the health care staff. Muscular exercise is not recommended for strength improvement, management of motor abilities or fatigue reduction. Uncertainties in muscular exercise prescription and planning, as well as its role within multidisciplinary approaches remain. Future trials should explore which type of muscle exercise could lead to better improvements in muscle strength besides, which type of exercise lead to improvements in endurance and aerobic capacity. Well-designed trials are desirable to clarify these open issues.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found below: https://osf.io/tx9d7.
Author Contributions
SG conceived and drafted the manuscript, carried out the literature search, conducted screenings, extracted data, completed the risk of bias assessment, and performed the statistical analyses. VP provided a critical revision of the manuscript, conducted screenings, extracted data, completed the risk of bias assessment, and performed the statistical analyses. GC provided a critical revision of the manuscript, performed the statistical analyses, and assisted in writing the discussion. MM and GB interpreted the data and revised the manuscript for important intellectual content. LM conceived, interpreted the data, provided a critical revision of the manuscript, and he is the guarantor of the review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Italian Ministry of Health Revisioni sistematiche della letteratura scientifica in ortopedia, traumatologia e riabilitazione funzionale. The funding sources had no controlling role in the study design, data collection, analysis, interpretation, or report writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Franco Molteni, Rehabilitation Presidium of Valduce Ospedale Villa Beretta, Lecco, Italy for his useful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00958/full#supplementary-material
Abbreviations
BMD, Becker's Muscular Dystrophy; CI, Confidence Intervals; DARIS, Diversity Adjusted Required Information Size; DMD, Duchenne's Muscular Dystrophy; FSHD, Facio-Scapulo-Humeral Dystrophy; LD, Limb-girdle Dystrophy; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; IQR, Inter Quartile Range; MD, Mean Difference; MID, Minimal Important Difference; MyD, Myotonic Dystrophy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized Clinical Trials; TIDieR, Template for Intervention Description and Replication TSA, Trial Sequential Analyses; SMD, Standardized Mean Difference; 6MWT, Six Minute Walking Test.
Footnotes
1. ^Tool GGD. GRADEpro GDT. McMaster University (Developed by Evidence Prime, Inc.) (2015). Available online at: https://gradepro.org/cite.html (accessed: March 7, 2019).
2. ^“Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014). Available online at: http://community.cochrane.org/tools/review-production-tools/revman-5/about-revman-5 (accessed: March 7, 2019).
References
1. Rahimov F, Kunkel LM. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol. (2013) 201:499–510. doi: 10.1083/jcb.201212142
2. Emery AE. The muscular dystrophies. Lancet. (2002) 359:687–95. doi: 10.1016/S0140-6736(02)07815-7
3. Janssen MM, Bergsma A, Geurts AC, de Groot IJ. Patterns of decline in upper limb function of boys and men with DMD: an international survey. J Neurol. (2014) 261:1269–88. doi: 10.1007/s00415-014-7316-9
4. Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, Munneke M, van Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil. (2007) 88:1452–64. doi: 10.1016/j.apmr.2007.07.024
5. Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. (1990) 22:417–28.
6. Leith L. Foundations of Exercise and Mental Health. Morgantown, WV: Fitness Information Technology. p. 1744 (1994).
7. Narayanaswami P, Weiss M, Selcen D, David W, Raynor E, Carter G, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. (2014) 83:1453–63. doi: 10.1212/WNL.0000000000000892
8. Gianola S, Pecoraro V, Lambiase S, Gatti R, Banfi G, Moja L. Efficacy of muscle exercise in patients with muscular dystrophy: a systematic review showing a missed opportunity to improve outcomes. PLoS ONE. (2013) 8:e65414. doi: 10.1371/journal.pone.0065414
9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
10. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
11. Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. (2007) 37:1–14. doi: 10.2165/00007256-200737010-00001
12. Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond). (2011) 1:1217–35. doi: 10.4155/cli.11.113
13. Lerario A, Bonfiglio S, Sormani M, Tettamanti A, Marktel S, Napolitano S, et al. Quantitative muscle strength assessment in duchenne muscular dystrophy: longitudinal study and correlation with functional measures. BMC Neurol. (2012) 12:91. doi: 10.1186/1471-2377-12-91
14. Gacesa JZ, Klasnja AV, Grujic NG. Changes in strength, endurance, and fatigue during a resistance-training program for the triceps brachii muscle. J Athl Train. (2013) 48:804–9. doi: 10.4085/1062-6050-48.4.16
16. Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol (Lond). (2008) 586:11–23. doi: 10.1113/jphysiol.2007.139477
17. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
18. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
19. Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Syst Rev. (2018) 7:110. doi: 10.1186/s13643-018-0770-1
20. Banerjee S, Wells G, Chen L. Caveats in the Meta-analyses of Continuous Data: A Simulation Study. Ottawa: Canadian Agency for Drugs and Technologies in Health (2008).
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. (2008) 61:64–75. doi: 10.1016/j.jclinepi.2007.03.013
23. Thorlund K, Wetterslev J, Brok J, Imberger G, Gluud G. Trial Sequential Analysis (TSA) manual. Copenaghen. (2011). Available online at: http://www.ctu.dk/tsa/files/tsa_manual.pdf
24. Bankole LC, Millet GY, Temesi J, Bachasson D, Ravelojaona M, Wuyam B, et al. Safety and efficacy of a 6-month home-based exercise program in patients with facioscapulohumeral muscular dystrophy: a randomized controlled trial. Medicine (Baltimore). (2016) 95:e4497. doi: 10.1097/MD.0000000000004497
25. Armijo-Olivo S, Warren S, Fuentes J, Magee DJ. Clinical relevance vs. statistical significance: using neck outcomes in patients with temporomandibular disorders as an example. Man Ther. (2011) 16:563–72. doi: 10.1016/j.math.2011.05.006
26. Kierkegaard M, Tollback A. Reliability and feasibility of the six minute walk test in subjects with myotonic dystrophy. Neuromuscul Disord. (2007) 17:943–9. doi: 10.1016/j.nmd.2007.08.003
27. Eichinger K, Heatwole C, Heininger S, Stinson N, Matichak Stock C, Grosmann C, et al. Validity of the 6 minute walk test in facioscapulohumeral muscular dystrophy. Muscle Nerve. (2017) 55:333–7. doi: 10.1002/mus.25251
28. Lindeman E, Leffers P, Spaans F, Drukker J, Reulen J, Kerckhoffs M, et al. Strength training in patients with myotonic dystrophy and hereditary motor and sensory neuropathy: a randomized clinical trial. Arch Phys Med Rehabil. (1995) 76:612–20. doi: 10.1016/s0003-9993(95)80629-6
29. van der Kooi EL, Vogels OJ, van Asseldonk RJ, Lindeman E, Hendriks JC, Wohlgemuth M, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology. (2004) 63:702–8. doi: 10.1212/01.wnl.0000134660.30793.1f
30. Kierkegaard M, Harms-Ringdahl K, Edstrom L, Widen Holmqvist L, Tollback A. Feasibility and effects of a physical exercise programme in adults with myotonic dystrophy type 1: a randomized controlled pilot study. J Rehabil Med. (2011) 43:695–702. doi: 10.2340/16501977-0833
31. Jansen M, van Alfen N, Geurts AC, de Groot IJ. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial “no use is disuse.” Neurorehabil Neural Repair. (2013) 27:816–27. doi: 10.1177/1545968313496326
32. Voet N, Bleijenberg G, De Groot I, Padberg G, Van Engelen B, Geurts A. (2014). Both aerobic exercise training and cognitive behavior therapy reduce chronic fatigue in patients with facioscapulohumeral muscular dystrophy: a randomized controlled trial. Ann Phys Rehabil Med 57:e96. doi: 10.1016/j.rehab.2014.03.329
33. Alemdaroglu I, Karaduman A, Yilmaz OT, Topaloglu H. Different types of upper extremity exercise training in Duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle Nerve. (2015) 51:697–705. doi: 10.1002/mus.24451
34. Andersen G, Prahm KP, Dahlqvist JR, Citirak G, Vissing J. Aerobic training and postexercise protein in facioscapulohumeral muscular dystrophy: RCT study. Neurology. (2015) 85:396–403. doi: 10.1212/WNL.0000000000001808
35. Andersen G, Heje K, Buch AE, Vissing J. High-intensity interval training in facioscapulohumeral muscular dystrophy type 1: a randomized clinical trial. J Neurol. (2017) 264:1099–106. doi: 10.1007/s00415-017-8497-9
36. Okkersen K, Jimenez-Moreno C, Wenninger S, Daidj F, Glennon J, Cumming S, et al. Cognitive behavioural therapy with optional graded exercise therapy in patients with severe fatigue with myotonic dystrophy type 1: a multicentre, single-blind, randomised trial. Lancet Neurol. (2018) 17:671–80. doi: 10.1016/S1474-4422(18)30203-5
37. Aldehag A, Jonsson H, Lindblad J, Kottorp A, Ansved T, Kierkegaard M. Effects of hand-training in persons with myotonic dystrophy type 1–a randomised controlled cross-over pilot study. Disabil Rehabil. (2013) 35:1798–807. doi: 10.3109/09638288.2012.754952
38. van der Kooi EL, Kalkman JS, Lindeman E, Hendriks JC, van Engelen BG, Bleijenberg G, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol. (2007) 254:931–40. doi: 10.1007/s00415-006-0432-4
39. Voet NB, Bleijenberg G, Padberg GW, van Engelen BG, Geurts AC. Effect of aerobic exercise training and cognitive behavioural therapy on reduction of chronic fatigue in patients with facioscapulohumeral dystrophy: protocol of the FACTS-2-FSHD trial. BMC Neurol. (2010) 10:56. doi: 10.1186/1471-2377-10-56
40. van der Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. (2005) CD003907. doi: 10.1002/14651858.CD003907.pub2
41. Voet NB, van der Kooi EL, van Engelen BG, Geurts AC. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. (2019) 12:CD003907. doi: 10.1002/14651858.CD003907.pub5
42. Kostek MC, Gordon B. Exercise is an adjuvant to contemporary dystrophy treatments. Exerc Sport Sci Rev. (2018) 46:34–41. doi: 10.1249/JES.0000000000000131
43. Fossmo HL, Holtebekk E, Giltvedt K, Dybesland AR, Sanaker PS, Orstavik K. Physical exercise in adults with hereditary neuromuscular disease. Tidsskr. Nor. Laegeforen. (2018) 138. doi: 10.4045/tidsskr.17.1024
44. Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. (2012) 167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x
45. van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. (2010) 362:1396–406. doi: 10.1056/NEJMoa0909859
46. Cejudo P, Bautista J, Montemayor T, Villagomez R, Jimenez L, Ortega F, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle Nerve. (2005) 32:342–50. doi: 10.1002/mus.20368
47. Dahlqvist JR, Vissing J. Exercise therapy in spinobulbar muscular atrophy and other neuromuscular disorders. J Mol Neurosci. (2016) 58:388–93. doi: 10.1007/s12031-015-0686-3
48. Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. (2017) 12:79. doi: 10.1186/s13023-017-0631-3
Keywords: exercise, muscular dystrophy, randomized controlled trial, systematic review, meta-analysis, physical therapy, rehabilitation, clinical decision-making
Citation: Gianola S, Castellini G, Pecoraro V, Monticone M, Banfi G and Moja L (2020) Effect of Muscular Exercise on Patients With Muscular Dystrophy: A Systematic Review and Meta-Analysis of the Literature. Front. Neurol. 11:958. doi: 10.3389/fneur.2020.00958
Received: 10 June 2020; Accepted: 23 July 2020;
Published: 12 November 2020.
Edited by:
Stefano Tamburin, University of Verona, ItalyReviewed by:
Mark Tarnopolsky, McMaster Children's Hospital, CanadaMichelangelo Bartolo, Casa di Cura Habilita SpA, Italy
Copyright © 2020 Gianola, Castellini, Pecoraro, Monticone, Banfi and Moja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Gianola, c2lsdmlhLmdpYW5vbGFAZ3J1cHBvc2FuZG9uYXRvLml0
 Silvia Gianola
Silvia Gianola Greta Castellini1
Greta Castellini1 Marco Monticone
Marco Monticone