- 1Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Nephrology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 4Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 5Department of Intensive Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 6Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 7Department of Medical Intensive Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 8Department of Infectious Disease, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 9Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 10School of Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The complications of coronavirus disease 2019 (COVID-19) involved multiple organs or systems, especially in critically ill patients. We aim to investigate the neurological complications in critically ill patients with COVID-19.
Methods: This retrospective single-center case series analyzed critically ill patients with COVID-19 at the intensive care unit of Tongji Hospital, Wuhan, China from February 5 to April 2, 2020. Demographic data, clinical and laboratory findings, comorbidities and treatments were collected and analyzed.
Results: Among 86 patients with confirmed COVID-19, 54 patients (62.8%) were male, and the mean (SD) age was 66.6 (11.1) years. Overall, 65% patients presented with at least one neurological symptom. Twenty patients (23.3%) had symptoms involving the central nervous system, including delirium, cerebrovascular diseases and hypoxic-ischemic brain injury, while 6 patients (7%) had neuromuscular involvement. Seven of 86 patients exhibited new stroke and 6 (7%) cases were ischemic. A significantly higher prevalence of antiphospholipid antibodies was observed in patients with ischemic stroke than in those without stroke (83.3 vs. 26.9%, p < 0.05). Patients with ischemic stroke were more likely to have a higher myoglobulin level, and a lower hemoglobin level.
Conclusions: The clinical spectrum of neurological complications in critically ill patients with COVID-19 was broad. Stroke, delirium and neuromuscular diseases are common neurological complications of COVID-19. Physicians should pay close attention to neurological complications in critically ill patients with COVID-19.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) began in Wuhan, Hubei Province in December 2019 and has rapidly spread throughout China (1–3). It is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1), which is similar to the zoonotic SARS-CoV from 2002 and the Middle East respiratory syndrome coronavirus (MERS-CoV) from 2012 (4). In a short time, COVID-19 has spread worldwide. On March 11, 2020, the World Health Organization (WHO) characterized COVID-19 as a pandemic (5, 6).
The clinical spectrum of the SARS-CoV-2 infection, COVID-19, appears to be wide, including asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death (2). Furthermore, various complications beyond the respiratory system, such as acute myocardial injury, acute kidney injury and gastrointestinal complications, have been investigated (2–4, 7–12).
With the increasing number of confirmed cases and accumulating clinical data, neurological complications associated with COVID-19 have been a challenge for clinical management and have generated considerable concern. Recent data from Wuhan, China, reported neurological complications in 36% of 214 COVID-19 patients (13). The neurological manifestations can vary from mild and unspecific symptoms, such as headache and hyposmia, to catastrophic symptoms, including stroke, acute hemorrhagic necrotizing encephalopathy, encephalitis/meningitis and Guillain-Barré syndrome (13–25). However, neurological features of COVID-19 infection in critically ill patients, have not been fully investigated. Herein, we conducted a retrospective study to analyze the neurological manifestations of critically ill patients with COVID-19 in intensive care units (ICU) to explore various pathophysiological mechanisms that could contribute to neurological complications in these patients.
Materials and Methods
Participants and Study Design
This is a single-center, retrospective, observational study performed at the Tongji Hospital, Wuhan, China. A designated ICU was established and specialized for critically ill patients with COVID-19 and was managed by the National Medical Team from Peking Union Medical College Hospital, Beijing, China. We retrospectively analyzed patients with COVID-19 who were diagnosed according to the criteria for critically ill patients with confirmed COVID-19 in our ICU from February 5, 2020 to April 2, 2020. All patients included were confirmed cases with positive reverse transcription-polymerase chain reaction (RT-PCR) results for SARS-CoV-2 before admission or positive serological tests for anti-SARS-CoV-2 specific immunoglobulin (Ig) M and G during hospitalization. The diagnosis and classification of disease severity of COVID-19 were made according to Chinese Management Guidance for COVID-19 Diagnosis and Treatment (7th version) (26). Patients who met one of the following conditions were classified as critically ill: (1) Respiratory failure requiring mechanical ventilation (MV). (2) Shock. (3) Patients complicated with other organ failure who required ICU monitoring and treatment.
All individual-level medical information, including demographic characteristics, medical history, clinical, radiological and laboratory findings, treatments and outcome data, were retrieved from the electronic medical records.
This study was approved by the institutional review board of Peking Union Medical College Hospital (No. S-K1151). Written informed consent was waived as this retrospective study was carried out to investigate an emerging infectious disease. The study was performed in accordance with the Declaration of Helsinki.
Laboratory and Neuroimaging Evaluation
Head CT scans were performed for patients with severe neurological complications after February 28, 2020 using a transport ventilator. Antiphospholipid syndrome (APS) panels, including serum levels of anticardiolipin IgG, IgM and IgA, and anti-β2-glycoprotein 1 (aβ2GP1) IgG, IgM and IgA were determined using a chemiluminescence assay (QUANTA Flash® assays, Inova) according to the manufacturer's instructions. Metagenomic next-generation sequencing of patients CSF samples was performed according to a standard flow, which has been described elsewhere (27, 28).
Definitions
Lymphocytopenia was defined as a lymphocyte count <1.1 × 109/L. Coagulopathy was defined as a 3-s extension of prothrombin time or a 10-s extension of activated partial thromboplastin time. Delirium was defined according to Diagnostic and Statistical Manual of Mental Disorders, 6th edition. Flaccid paralysis was defined as bilateral paralysis with the loss of muscle tone and absence of tendon reflexes. Stroke was defined as a syndrome of rapidly emerging clinical signs of focal or global disturbance of cerebral function lasted at least 24 h, or detection of cerebral lesions in accordance with vascular origin on neuroimaging examination. Strokes were further verified and classified into ischemic stroke or spontaneous intracerebral hemorrhage based on neuroimaging results. Hypoxic ischemic brain injury is used to describe diffuse brain injury as a result of hypoxia or reduction of oxygen. The outcome is defined as the condition evaluated on April 2, 2020.
Statistical Analysis
Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 17.0 and EXCEL 1810. Data are expressed as medians with the interquartile range (IQR) or means ± standard deviation (SD) according to the distribution. Analysis of variance (ANOVA), Student's t-test, or the Mann–Whitney test (non-normal distributions) were used to analyze continuous variables. Pearson χ2 test or a Fisher's exact test were used to analyze categorical variables. A two-tailed p < 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
We finally included 86 critically ill patients with confirmed COVID-19 after excluding 10 patients without available key information, 11 patients with suspected COVID-19, and two patients with a mild or moderate disease course. Of 86 patients, 54 (62.8%) were male, and the mean (SD) age was 66.6 (11.1) years old. The demographic and clinical features of these patients are summarized in Table 1.
Most of these patients presented with fever (87.2%) and cough (75.6%). Fifty-six (65.1%) patients presented with at least one type of neurological symptom (headache, dizziness, myalgia, fatigue or hyposmia), including 15 patients with myalgia, 46 patients with fatigue, 8 patients with headache, 6 patients with dizziness, and none patient complaining of hyposmia.
Underlying cardiovascular diseases, including hypertension, diabetes, coronary artery disease, and stroke, as well as smoking were prevalent in critically ill patients with COVID-19, while hypertension was the most common comorbidity and occurred in 44 (51.1%) patients. A total of 12 (14.0%) patients had a past medical history of stroke, including 7 cases of ischemic stroke, 4 cases of intracranial hemorrhage and 1 case of subarachnoid hemorrhage. One patient reported a medical history of myasthenia gravis; and one patient reported a medical history of Alzheimer disease.
The complications of COVID-19 involved multiple organs or systems, including the lymphohematopoietic system, kidney, liver and heart. Coagulopathy was common and occurred in 49 (57.0%) patients. Sixteen (18.6%) patients were complicated with atrial fibrillation during the disease course of COVID-19.
Of the 86 patients, 70 (81.4%) received invasive MV, 5 (5.8%) received extracorporeal membrane oxygenation, and 16 (18.6%) received continuous renal replacement therapy. Most critically ill patients received antiviral therapy (77.9%) and immunotherapy (81.4% received intravenous immunoglobulin and 82.6% received steroids). Forty-eight (55.8%) patients received anticoagulation therapy because of underlying coagulopathy or thromboembolic events. The fatality rate was high; 55 (64.0%) patients died through April 2, 2020 (the median follow-up duration was 35 days).
Laboratory Findings on ICU Admission
The laboratory findings of the patients are summarized in Table 2. Lymphocytopenia was common and occurred in 77 (89.5%) patients. Lactate dehydrogenase was elevated in 78 (90.7%) patients. Creatine kinase was elevated in 29 (33.7%) patients and myoglobulin elevation was documented in 26 (30.2%) patients. N terminal pro B type natriuretic peptide (NT-proBNP) was elevated in 60 (69.8%) patients, and cardiac troponin I (cTnI) was elevated in 48 (55.8%) patients. D-dimer was elevated in 55 (64.0%) patients. High-sensitive C-reactive protein was elevated in 80 (93.0%) patients. Interleukin−6 was elevated in 66 (76.7%) patients. Twelve of the 32 (37.5%) tested patients were positive upon APS panel testing.
Neurological Complications During the Disease Course
Neurological complications involving the central nervous system (CNS) were common, and 20 (23.3%) patients had at least one neurological complication of the CNS (delirium, acute ischemic stroke, intracerebral hemorrhage and hypoxic-ischemic brain injury). Delirium was presented in 11 (12.8%) patients, which was a reason that patients could not tolerate non-invasive MV and were admitted to the ICU for invasive MV. Two patients had hypoxic-ischemic brain injury, and one of these patients received cardiopulmonary resuscitation.
Acute ischemic stroke (AIS) occurred in six patients (7.0%, Figure 1, Table 3, Supplementary Figures 1–10), and intracranial hemorrhage occurred in one case. Additionally, two patients presented with acute focal neurologic deficit without neuroimaging evaluation. Of the six patients with AIS, two were deeply sedated, and infarctions were first revealed by head CT in these patients. Five patients were male. Notably, patients with AIS exhibited a significantly higher prevalence of APS panel positivity than those without AIS (83.3 vs. 26.9%, p < 0.05). Moreover, patients with AIS were more likely to have a higher myoglobulin level, and a lower hemoglobin level (Table 2). The cTnI and NT-proBNP levels seemed to be higher in patients with AIS, although there was no statistically significant difference between the two groups. All patients with AIS received anticoagulant therapy. Five of six patients with AIS were alive until the end of the follow-up period, and the median survival duration was 66.5 days for these patients.
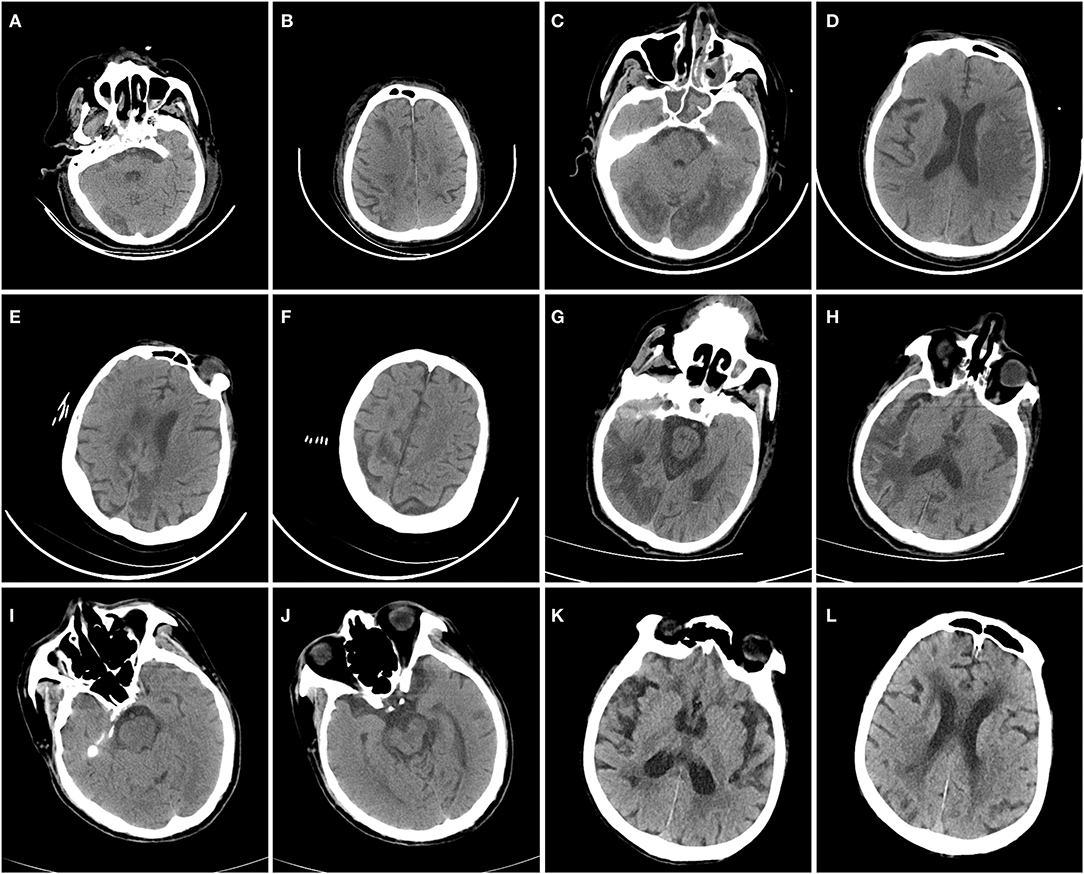
Figure 1. Head CT scans of coronavirus disease 2019 patients with acute ischemic stroke. In Case 1 (A,B), head CT revealed low-density lesions in the right occipital lobe and bilateral frontal and parietal lobes. In Case 2 (C,D), head CT revealed low-density lesions in the bilateral occipital and temporal lobes and the left hemisphere. In Case 3 (E,F), head CT revealed low-density lesions in the bilateral frontal and parietal lobes. In Case 4 (G,H), head CT revealed low-density lesions in the right hemisphere. In Case 5 (I,J), head CT revealed low-density lesions in the left midbrain. In Case 6 (K,L), head CT revealed low-density lesions on the right side of the periventricular area.
Neurological complications of the peripheral nervous system and musculature were also observed in critically ill patients with COVID-19. Persistent flaccid paralysis was observed in four patients after withdrawal of sedation. Two patients had rhabdomyolysis.
Other Notable Neurological Evaluation
Lumbar puncture was performed in two critically ill patients with COVID-19. Protein levels were slightly elevated in one patient with persistent flaccid paralysis, while the opening pressure, white blood cell count and glucose levels were normal. SARS-CoV-2 was not detected in these two patients, either on RT-PCR or on metagenomic next-generation sequencing (Supplementary Table 1).
Discussion
This retrospective study yields new insight into neurological manifestations in the critically ill patients with COVID-19. Of the 86 critically ill patients with COVID-19 included in this study, 65% presented with at least one neurological symptom. The clinical spectrum of neurological complications in critically ill patients with COVID-19 was broad, including delirium, acute ischemic stroke, intracerebral hemorrhage, hypoxic-ischemic brain injury, flaccid paralysis and rhabdomyolysis. Notably, that cerebrovascular disease was a common comorbidity, and the prevalence of previous stroke in our study was 14%. Moreover, 8% of patients exhibited new stroke during the course of disease, and most strokes were ischemic. Positivity of antiphospholipid antibodies was highly prevalent in patients with ischemic stroke.
CNS symptoms were the main neurological complications in critically ill patients with COVID-19. Only two types of human coronaviruses, namely HCoV-OC43 and E299 were found to be neuroinvasive and can spread from the respiratory tract to the CNS (30). Invasion of CNS by SARS-CoV-2 has been suggested by researchers from the University of Yamanashi, and SARS-CoV-2 RNA can be detected in the CSF of patients with COVID-19 (18). Furthermore, autopsy reports have revealed the presence of virus in neural and capillary endothelial cells in frontal lobe tissue (31), as well as secondary brain damage and neuronal degeneration without evidence of viral encephalitis (32, 33). Recent studies illustrated that COVID-19 has the potential to cause nervous system damage. We performed lumbar puncture in two patients with COVID-19 and neurological manifestations in our ICU; however, neither patient showed signs of significant inflammation in the CSF. Furthermore, RT-PCR assays of the virus and metagenomic next-generation sequencing in the CSF samples were negative. Our findings were consistent with the previous observational report on severe COVID-19 patients, which indicated that RT-PCR assays of the CSF samples were negative for SARS-CoV-2 in all 7 tested patients (23). Whether and how CNS involvement is related to the direct invasion of the virus remains to be addressed in future studies.
Accumulating evidence suggested that neuroimaging features of hospitalized COVID-19 patients were variable, dominated by acute ischemic infarction and intracranial hemorrhages (19–24). Besides, leptomeningeal enhancement, hypoxic-ischemic brain injury, cortical signal abnormalities that may be caused by systemic toxemia were also reported (23, 34, 35). Hypodensities localized in multiple brain areas on CT scans, which were in line with vascular origin were observed in case 1 to case 6 in our series. The lesions of five patients (case 1–5) indicated large artery involvement, while four of them had multiterritory infarcts. Case 6 presented with sudden onset of focal neurological deficit (slurred speech) after admission with a moderate background of cerebral small vessel disease on head CT scan, ischemic stroke of small vessel disease subtype was diagnosed.
Stroke is not uncommon in patients with coronavirus infection. AIS has been reported in patients with SARS and MERS (36–39). To date, 2.3–13.5% of patients with severe COVID-19 have been reported to have comorbid cerebrovascular disease (3, 9). Although stroke has been recognized as a complication of COVID-19 (usually in the severe cases), the exact incidence is not fully investigated (2, 3, 9, 10, 12, 40, 41). Data from Wuhan, China, reported that acute cerebrovascular disease (mainly ischemic stroke) was more common among 88 patients with severe COVID-19 than those with non-severe disease (5.7 vs. 0.8%) (13). In recent case series, ischemic stroke of both large artery- and small vessel- etiology have been reported (19–24). In the present study, stroke was diagnosed in 7 of 86 critically ill patients with COVID-19 and 6 cases were classified as ischemic stroke. This incidence might be higher because neuroimaging examinations were not performed for all patients with acute focal neurologic deficits because of a rapid deterioration of the conditions the result in death. The exact mechanism of ischemic stroke in COVID-19 remains under investigation. Possible explanations include the following.
First, abnormal coagulation results, especially markedly elevated D-dimer and fibrin degradation product, are quite prevalent in critically ill patients with COVID-19, which indicates a common coagulation activation and secondary hyperfibrinolysis condition (42). We also found coagulopathy and antiphospholipid antibodies in critically ill patients with COVID-19 in our cohort (29). Our results indicated that five of the six cases of ischemic stroke had large artery or embolic origin. Similarly, in a previous report of COVID-19 patients with ischemic stroke, all six stroke patients had large-vessel occlusion and three of them had multiterritory infarcts (21). The high incidence of thrombotic complications and the principal subtypes of ischemic strokes verified the existence of a pro-coagulant state in critically ill patients with COVID-19. D-Dimer levels were repeatedly measured in some patients in our study and showed a trend of decreasing, which might be related to anticoagulant therapy. Furthermore, compared with patients without a cerebrovascular event, a significantly higher prevalence of antiphospholipid antibodies was observed in stroke patients. Previous studies have shown an increased risk of developing antiphospholipid antibodies in various viral infections (43). Our results indicate that clinicians should be aware of the increased risk and consider testing for antiphospholipid antibodies in patients with COVID-19 infection and clinical manifestations suggestive of APS. All of the six patients complicated with ischemic stroke received anticoagulant therapy, and five improved or stabilized, which may indicate that critically ill patients with COVID-19 with ischemic stroke may benefit from anticoagulant therapy. Previous studies have also suggested that anticoagulant treatment was necessary and beneficial for severe COVID-19 patients with coagulopathy (44–46).
Second, virus-induced vascular inflammation might be responsible for stroke. In patients with COVID-19, the imbalanced response among T helper cell subtypes could precipitate a cytokine storm syndrome (36). Our results indicated that inflammatory markers were markedly elevated in most critically ill patients with COVID-19. Viral infection and the subsequent immune responses could cause lymphocytic infiltration, necrosis of smooth muscle, endothelial dysfunction and occlusion of large vessel walls. Furthermore, angiotensin-converting enzyme 2 (ACE2), which is a cardio-cerebral vascular protection factor, has been identified as the functional target for SARS-CoV-2 (47). The virus could interact with ACE2 expressed in the endothelium and further attack the vascular system. For patients with underlying cardiovascular disease, SARS-CoV-2 infection can further damage vessel walls through reduction of cerebral blood flow, decreases in oxygen supply and destabilization of arterial plaque. However, we have not been able to demonstrate an association of COVID-19 with vessel wall damage.
Third, there is evidence suggesting that patients with myocardial injury have an increased risk of occurrence of future cerebrovascular events compared with those without myocardial injury (48). Myocardial injury, evidenced by elevated cardiac biomarkers or new electrocardiogram or echocardiographic abnormalities, was recognized among early COVID-19 cases in China (8). In our cohort, more than 50% of critically ill patients had elevated high-sensitivity troponin I and NT-proBNP levels. Myoglobulin level was significantly higher in patients with AIS. Although no statistically significant difference was found because of the small number of stroke patients, higher levels of both cTnI and NT-proBNP levels were observed in patients with COVID-19 with incident ischemic stroke than in those without this event. Furthermore, 18% of the patients were complicated with atrial fibrillation. We speculated that myocardial injury and the concomitant atrial fibrillation may further contribute to the occurrence of ischemic stroke.
Finally, a higher prevalence of anemia was also observed in patients with ischemic stroke in our cohort. Anemia is associated with an increased risk of cerebrovascular events because of decreased tissue oxygen delivery as well as a hyperkinetic state, which disturbs endothelial function and may lead to thrombus formation. Our results indicated that correcting anemia in critically ill patients with COVID-19 might have positive effect on stroke prevention.
In addition to CNS involvement, neuromuscular manifestations, including persistent flaccid paralysis and rhabdomyolysis, were also observed in patients with severe coronavirus infection, which have previously been reported for SARS and MERS (37–39, 49). In a case series study consisting of four patients with SARS who had concomitant neuromuscular problems, the neuromuscular involvement was considered to be critical-illness polyneuropathy or myopathy (50). Significantly elevated inflammatory cytokine levels and immune activation may play a role in neuromuscular injury. We noticed that the prevalence of flaccid paralysis was higher in patient with AIS (66.7%), compared with those without AIS (1.3%). Possible explanations included the longer time at ICU and the more serious clinical conditions for stroke patients. On the other hand, the mortality rate of non-AIS group was high, which limited our ability to withdraw sedatives and determine whether flaccid paralysis exists in these patients. Further electrophysiological and pathological studies are necessary to determine the relationship between COVID-19 and neuromuscular involvement.
Our study has several limitations. First, only 86 patients with confirmed COVID-19 were included in the present analysis, and a large, multi-center study is warranted to verify the neurological manifestations of COVID-19. Second, most of the critically ill patients in our ICU were receiving intensive sedation because of invasive MV, which may have resulted in underestimation of the incidence of neurological complications. Third, some specific information regarding neurological complications, such as brain MRI, imaging evaluations of large intracranial arteries, electrophysiological examinations and CSF profiles were not available. The data were incomplete because of the highly infectious nature of COVID-19, the serious clinical conditions of critically ill patients and the limited conditions for examination in the isolation ward. Thus, we restricted examinations to only those that could have a direct effect on patient management. Fourth, the relatively small number of stroke patients limited the accurate comparisons between patients with AIS and those without. Finally, some patients with neurological complications were still hospitalized at the time of analysis, which may limit the assessment of the ultimate clinical outcome and natural course of the disease, and further long-term observation is needed.
Conclusions
Stroke, delirium and neuromuscular diseases are major neurological complications of COVID-19. Neurological manifestations might be underestimated in critically ill patients with COVID-19, and physicians should pay close attention to neurological complications. Patients with COVID-19 complicated with ischemic stroke might benefit from anticoagulant therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Peking Union Medical College Hospital (No. S-K1151). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HG and SZ supervised, coordinated and designed the study. SF and FH drafted the manuscript. SF, MX, PX, XB, HC, HZhang, XD, HZhao, JZ, XS, WJ, CW, WC, FG, RT, PG, WW, JM, DZ, and YC collected the clinical data. SF, MX, FH, and JX participated in the interpretation of the data. SF and TG participated in statistical analysis. DW, ZL, XZ, JW, YQ, TL, YX, YL, XY, YZ, BP, and LC supervised and coordinated the study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China, Grant No. 2016YFC0901500.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patients and people in Wuhan.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00806/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
4. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. (2020) 141:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
5. World Health Organization. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19. (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (accessed April 2, 2020).
6. World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_6 (accessed April 2, 2020).
7. Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. (2020) 69:1002–9. doi: 10.1136/gutjnl-2020-320926
8. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular Implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 27:e201017. doi: 10.1001/jamacardio.2020.1017
9. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
10. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. (2020) 368:m606. doi: 10.1136/bmj.m606
11. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
12. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
13. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:1–9. doi: 10.1001/jamaneurol.2020.1127
14. Eliezer M, Hautefort C, Hamel A L, Verillaud B, Herman P, Houdart E, et al. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. (2020). doi: 10.1001/jamaoto.2020.0832. [Epub ahead of print].
15. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. (2020). doi: 10.1148/radiol.2020201187. [Epub ahead of print].
16. Sun T, Guan J. Novel coronavirus and central nervous system. Eur J Neurol. (2020). doi: 10.1111/ene.14227. [Epub ahead of print].
17. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
18. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
19. Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. (2020) 51:e124–7. doi: 10.1161/STROKEAHA.120.030153
20. Morassi M, Bagatto D, Cobelli M, D'Agostini S, Gigli GL, Bnà C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. (2020) 20:1–8. doi: 10.1007/s00415-020-09885-2
21. Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiaty. (2020). doi: 10.1136/jnnp-2020-323586. [Epub ahead of print].
22. Tunç A, Ünlübaş Y, Alemdar M, Akyüz E. Coexistence of Covid-19 and acute ischemic stroke report of four cases. J Clin Neurosci. (2020) 77:227–9. doi: 10.1016/j.jocn.2020.05.018
23. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
24. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. (2020) 382:e60. doi: 10.1056/NEJMc2009787
25. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. (2020). doi: 10.1001/jamaneurol.2020.2065. [Epub ahead of print].
26. National Health Commission of the People's Republic of China. Chinese Management Guideline for COVID-19 (version 7.0). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed April 2, 2020).
27. Fan S, Ren H, Wei Y, Mao C, Ma Z, Zhang L, et al. Next-generation sequencing of the cerebrospinal fluid in the diagnosis of neurobrucellosis. Int J Infect Dis. (2018) 67:20–4. doi: 10.1016/j.ijid.2017.11.028
28. Guan H, Shen A, Lv X, Yang X, Ren H, Zhao Y, et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol. (2016) 22:240–5. doi: 10.1007/s13365-015-0390-7
29. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. (2020) 382:e38. doi: 10.1056/NEJMc2007575
30. Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. (2016) 375:497–8. doi: 10.1056/NEJMc1509458
31. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus−2 (SARS-CoV-2). J Med Virol. (2020) 92:699–702. doi: 10.1002/jmv.25915
32. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
33. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological Features of Covid-19. N Engl J Med. (2020). doi: 10.1056/NEJMc2019373. [Epub ahead of print].
34. Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. (2020). doi: 10.1148/radiol.2020201697. [Epub ahead of print].
35. Mahammedi A, Saba L, Vagal A, Leali M, Rossi A, Gaskill M, et al. Imaging in neurological disease of hospitalized COVID-19 Patients: an italian multicenter retrospective observational study. Radiology. (2020). doi: 10.1148/radiol.2020201933. [Epub ahead of print].
36. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. (2020) 87:18–22. doi: 10.1016/j.bbi.2020.03.031
37. Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. (2017) 13:227–33. doi: 10.3988/jcn.2017.13.3.227
38. Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. (2016) 2016:3502683. doi: 10.1155/2016/3502683
39. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. (2005) 14:113–9.
40. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
41. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am J Respir Crit Care Med. (2020) 201:1372–9. doi: 10.1164/rccm.202003-0543OC
42. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:844–7. doi: 10.1111/jth.14768
43. Abdel-Wahab N, Talathi S, Lopez-Olivo MA, Suarez-Almazor ME. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. (2018) 27:572–83. doi: 10.1177/0961203317731532
44. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. (2020) 18:1094–9. doi: 10.1111/jth.14817
45. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
46. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. (2020). doi: 10.1097/CCM.0000000000004458. [Epub ahead of print].
47. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
48. Rydén L, Roos A, Holzmann MJ. chronic myocardial injury and risk for stroke. Am J Med. (2019) 132:833–9. doi: 10.1016/j.amjmed.2019.01.027
49. Stainsby B, Howitt S, Porr J. Neuromusculoskeletal disorders following SARS: a case series. J Can Chiropr Assoc. (2011) 55:32–9.
Keywords: COVID-19, neurological manifestations, critically ill, stroke, neuromuscular diseases
Citation: Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, Zhang H, Ding X, Zhao H, Zhao J, Sun X, Jiang W, Wang C, Cao W, Guo F, Tian R, Gao P, Wu W, Ma J, Wu D, Liu Z, Zhou X, Wang J, Guan T, Qin Y, Li T, Xu Y, Zhang D, Chen Y, Xie J, Li Y, Yan X, Zhu Y, Peng B, Cui L, Zhang S and Guan H (2020) Neurological Manifestations in Critically Ill Patients With COVID-19: A Retrospective Study. Front. Neurol. 11:806. doi: 10.3389/fneur.2020.00806
Received: 05 May 2020; Accepted: 29 June 2020;
Published: 10 July 2020.
Edited by:
Jesús Porta-Etessam, Hospital Clínico San Carlos, SpainReviewed by:
Rodrigo Guerrero, University of the Andes, ChileJulián Benito León, University Hospital October 12, Spain
Copyright © 2020 Fan, Xiao, Han, Xia, Bai, Chen, Zhang, Ding, Zhao, Zhao, Sun, Jiang, Wang, Cao, Guo, Tian, Gao, Wu, Ma, Wu, Liu, Zhou, Wang, Guan, Qin, Li, Xu, Zhang, Chen, Xie, Li, Yan, Zhu, Peng, Cui, Zhang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyang Zhang, c2h1eWFuZ3poYW5nMTAzQDE2My5jb20=; Hongzhi Guan, cHVtY2hnaHpAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Siyuan Fan
Siyuan Fan Meng Xiao
Meng Xiao Fei Han
Fei Han Peng Xia3
Peng Xia3 Chunyao Wang
Chunyao Wang Wei Cao
Wei Cao Wei Wu
Wei Wu Yan Qin
Yan Qin Yingchun Xu
Yingchun Xu Yicheng Zhu
Yicheng Zhu Bin Peng
Bin Peng Liying Cui
Liying Cui