- Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
Muscle-specific tyrosine kinase (MuSK) myasthenia gravis (MG) is a rare, frequently more severe, subtype of MG with different pathogenesis, and peculiar clinical features. The prevalence varies among countries and ethnic groups, affecting 5–8% of all MG patients. MuSK-MG usually has an acute onset affecting mainly the facial-bulbar muscles. The symptoms usually progress rapidly, within a few weeks. Early respiratory crises are frequent. The disease may lead to generalized muscle weakness up to muscle atrophy. The main bulbar involvement, the absence of significant thymus alterations, and the association with HLA class II DR14, DR16, and DQ5 alleles have been confirmed. Atypical onset, such as ocular involvement, lack of symptom fluctuations, acetylcholinesterase inhibitors failure, and negative results of electrophysiologic testing, if not specifically performed in the mainly involved muscle groups, makes MuSK-MG diagnosis challenging. In most cases, steroids are effective. Conventional immunosuppressants are not commonly able to replace steroids in maintaining a satisfactory long-term control of symptoms. However, the majority of MuSK-MG patients are refractory to treatment. In these cases, the use of rituximab showed promising results, resulting in sustained symptom control.
Introduction
In 2001, serum antibodies against muscle-specific tyrosine kinase (MuSK-Abs) were identified for the first time as cause of myasthenia gravis (MG) (1), opening the way to the description of a distinct peculiar subtype of MG disease (2–5). A reliable neuromuscular junction (NMJ) transmission is guaranteed by both morphological NMJ appropriate structure and NMJ transmission efficacy. NMJ transmission efficacy is strictly related to the “safety factor,” which refers to the ability of the NMJ to remain effective under several conditions. This is possible mainly because each nerve impulse releases more transmitter than is required to excite the muscle fiber, ensuring that the transmission does not fail (6). The role of muscle-specific tyrosine kinase (MuSK) in determining NMJ efficacy has been recently clarified (7). A tetrameric complex on the postsynaptic membrane results from the association between MuSK and the low-density lipoprotein receptor-related protein 4 (LRP4). The MuSK–LRP4 tetramer is phosphorylated by agrin and recruits downstream of kinases 7, which further enhances MuSK activation for postsynaptic differentiation and acetylcholine receptor (AChR) clustering. Furthermore, an interaction between MuSK and matrix proteins, such as collagen Q (ColQ), which contributes to synapsis stabilization, has been demonstrated in vitro (8, 9). Recently, Huijbers et al. confirmed MuSK-Abs as pathogenetic (10). MuSK-Abs belongs mostly to the IgG4 class of immunoglobulins, which acts by the direct inhibition of protein function. In particular, MuSK-Abs interfere with MuSK–LRP4 complex and, consequently, AChR clustering is inhibited (11). The aim of this mini-review is to report on the epidemiological and major clinical features, diagnostic approach, and treatment of MuSK-MG subtype.
Epidemiology
MuSK-MG is reported in about 5–8% of MG patients. Its prevalence varies among countries and ethnic groups, with a higher percentage in Southern Europe, and it is clearly predominant in females, actually constituting more than 70% of patients in all studies reviewed (9, 12).
The disease has an early age of onset, with a peak of incidence in the late 3rd decade, and it rarely occurs after 70 years of age. Cohorts from different countries confirm the association with HLA class II DR14, DR16, and DQ5 (9). No significant thymus alterations have been reported in MuSK-MG patients as related to the disease (9, 12, 13).
Clinical Features
A peculiar clinical onset picture has been described from several groups for MuSK-MG. The disease typically has an acute onset, with rapid progression within a few weeks. In the majority of cases, bulbar involvement appears in the first stage and the presenting symptoms are ptosis and diplopia.
However, some peculiarities have been demonstrated about ocular manifestations which are observed in the early stages of the disease, consisting in symmetrical ophtalmoparesis of horizontal gaze and, more rarely, of vertical gaze with rapid remittance of diplopia. Furthermore, the typical fluctuation of myasthenic symptoms may not be evident in MuSK-MG patients. Commonly, a purely ocular onset generalizes in 2–3 weeks (14–17).
Bulbar impairment has been demonstrated in up to 80% of MuSK-MG patients, consisting of dysarthria, dysphonia with nasal voice, dysphagia, and masticatory difficulty. Bulbar onset is usually related to rapid deterioration, frequently leading to respiratory crisis. Generalized weakness and fatigue have also been described as onset syndrome, resembling anti-AChR-associated MG (AChR-MG). Furthermore, MuSK-MG patients have a higher risk of myasthenic crisis (3). Usually, axial muscle weakness involves neck extensor, which may present as head drop, and it can be the only presenting sign, without bulbar involvement. Neck extensor weakness is more frequent in MuSK- MG, whereas neck flexors could be only mildly involved (18).
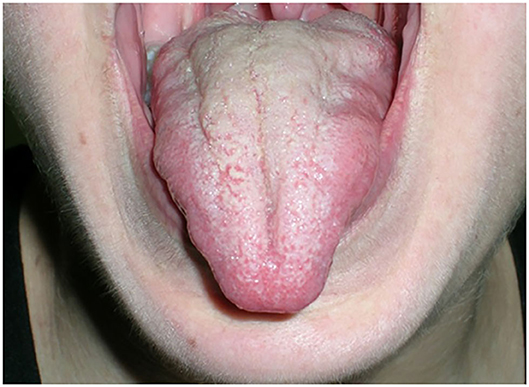
An unusual but distinct feature of MuSK-MG is muscle atrophy. In particular, the mainly involved muscle groups are facial muscles and the tongue (Figure 1). Muscular atrophy can also be observed at shoulder girdle muscles, limb, and paraspinal muscles, resulting in severe scoliosis, as reported in a few cases in literature (19).
Electromyography (EMG) on atrophic muscles reveals a myopathic pattern and magnetic resonance imaging confirms muscle thinning and documents fatty replacement. There are evidences that corticosteroid treatment can improve muscle wasting; however, in some cases, atrophy becomes chronic and a significant cause of severe disability (20). The majority of MuSK-MG patients do not present relevant thymus alterations (21, 22). Hyperplasia is rarely described. Case reports incidentally documented thymoma treated with thymectomy (23). There are few data and no consensus on the role of thymectomy in MuSK-MG. In AChR-MG, a randomized, controlled trial of thymectomy in non-thymomatous acetylcholine receptor patients demonstrated a significant improvement in clinical outcomes after thymectomy, as well as a decreased requirement for immunosuppression (24). Conversely, available studies on thymectomy in MuSK-MG outline a limited improvement in clinical outcomes or immunosuppression management after thymectomy (21–24). Moreover, it has been reported that the outcome in MuSK-MG after thymectomy may not be beneficial (25). Therefore, thymectomy in MuSK-MG should not be considered as a therapeutic option.
Diagnostic Approach
MuSK-MG diagnosis might be challenging. In fact, muscle atrophy, dysphagia, dysarthria, and neck extensor weakness as onset clinical picture may be easily misdiagnosed, for example, with bulbar onset of amyotrophic lateral sclerosis, oculopharyngeal muscular dystrophy, and mitochondrial myopathy. The diagnostic procedure includes MuSK-Ab testing, edrophonium/neostigmine test, and electroneurophysiological studies such as repetitive nerve stimulation (RNS), single-fiber electromyography (SFEMG), and needle EMG.
A positive result for MuSK-Ab, sustained by clinical evidences, supports the diagnosis of MuSK-MG. Detection of MuSK-Ab is usually a second step for AChR-Abs-negative patients or individuals positive for AChR-Ab who do not respond to treatment. It has been recently proposed that radio immunological assay-negative MG sera should be tested for IgG-specific antibodies by MuSK-cell-based assay to increase the detection of antibodies (26). Edrophonium or neostigmine tests, although non-routine, resulted positive in 40–75% of MuSK-MG patients; however, these tests demonstrated a higher sensitivity (97–100%) for AChR-MG diagnosis (27).
RNS sensitivity appears to be lower in MuSK-MG compared with AChR-MG, especially when performed on distal limb muscles. However, it has been reported that it is possible to increase the diagnostic sensitivity of RNS in MuSK-MG by testing proximal muscles, in particular the facial muscles, reaching a diagnostic sensitivity of 75–85% (28, 29). In AChR-MG, RNS usually show a partial recovery of the compound muscle action potential amplitude after a transient decrement during the first responses to low-frequency RNS (U-shaped pattern), not reported in MuSK-MG. On the contrary, a progressive decremental pattern after the fourth or fifth stimulation is typically revealed in Lambert–Eaton myasthenic syndrome (LEMS). It has been demonstrated that a similar pattern is usually found also in MuSK-MG, probably due to an underlying presynaptic dysfunction in MuSK-MG patients, as in the LEMS ones (28).
Needle EMG in patients with MuSK-MG may show myopathic features, rarely observed in AChR-MG, in particular in the facial muscles. These non-specific findings only partially contribute to define diagnosis (26). In cases with evocative clinical manifestations of MuSK-MG, associated with borderline antibody values, SFEMG is mandatory to diagnose MuSK-MG. Furthermore, it is worth to underline the importance to focus SFEMG on the mainly affected muscles to precociously detect alterations. In fact, in MuSK-MG, SFEMG of cervical paraspinals, deltoid, frontalis, and orbicularis oculi, which are usually the first and more frequently involved muscles, may be noticeably abnormal since the beginning of the disease. These patients may conversely have normal jitter in clinically uncompromised muscles (30). Stickler et al. reported cases of normal jitter in the extensor digitorum muscle and frontalis but markedly increased jitter with blocking in neck extensors (29). Cases with normal SFEMG at orbicularis oculi but with abnormal jitter in paraspinal muscles have been described by Padua et al. (31).
Treatment
Long-term pharmacological treatment is usually required to achieve an effective control of symptoms in MuSK-MG; however, it could be at least challenging.
The symptomatic treatment with acetylcholinesterase inhibitors is generally unsatisfactory and may be deleterious in MuSK-MG. Moreover, the response to pyridostigmine standard doses, used for AChR-MG, lacks efficacy and has poor tolerance because of side effects (26). Among symptomatic drugs for MuSK-MG, recently 3,4-diaminopyridine (3,4-DAP), ephedrine, and albuterol have been considered. The use of 3,4-DAP in MuSK- MG patients has been described as mildly to moderately effective, with no remarkable side effects (32). There is only a report demonstrating a clinical improvement in MuSK-MG due to the administration of both ephedrine and albuterol, two sympathomimetics agents commonly used to treat some phenotypes of congenital myasthenic syndromes (33).
Immunosuppression
Immunosuppression still represents the mainstay of therapy for MuSK-MG. It is well-known that steroids have a prompt and effective response, but they are burdened by long-term side effects.
A high dose of prednisone, in combination with plasma exchange, is generally recommended for patients experiencing life-threatening weakness or suffering from severe disease deterioration. In these patients, intravenous immunoglobulin should also be considered (27).
Traditional immunosuppressants (azathioprine, mycophenolate, tacrolimus, methotrexate, and cyclosporine), in common clinical practice, have been administered with success in MuSK-MG patients as steroid-sparing agents, but it is usually more difficult to achieve and to ensure long-term and complete control of symptoms (34). It is important to consider that 10–15% of MuSK- MG patients have a refractory disease or suffer from relapses on tapering immunosuppressive medication.
The management of this percentage of patients who do not respond to steroids or traditional immunosuppressants is often difficult. In the previous years, clinical trials and evidences from observational prospective studies encourage the use of monoclonal Ab such as rituximab (RTX), a chimeric anti-CD20 monoclonal Ab (35–37). A significant number of MuSK-MG patients showed a greater and sustained improvement of symptoms after RTX administration, compared to those patients who do not receive RTX administration (37). Immunosuppressants can be reduced or even stopped (37). Topakian et al. confirmed the safety and efficacy of RTX in a large cohort of both AChR-MG and MuSK-MG patients; furthermore, these authors demonstrated a significantly higher rate of remission in patients with MuSK-MG compared to AChR-MG ones (38).
In light of common clinical practice and of the above-mentioned results, a recent consensus recommends RTX as an early therapeutic option in MuSK-MG, suggesting its possible role as a steroid-sparing agent since the beginning of the disease (39). RTX has a good safety profile; however, side effects such as myocardial infarction, spondylodiscitis, agranulocytosis, and two cases of progressive multifocal leukoencephalopathy in MG patients have been reported (40, 41).
Summary
MuSK-MG is a distinctive, frequently more severe, subtype of MG. Onset is usually acute and typically bulbar, with rapid progression of symptoms within a few weeks. Clinical presentation can be atypical: neck weakness, for example, as onset symptom could be misleading, causing a delay in diagnosis. MuSK-Ab testing confirms the diagnosis when the clinical picture is highly suggestive. SFEMG plays an important role in diagnosing MuSK-MG, and we underline the importance to focus it on the mainly affected muscles to precociously detect alterations.
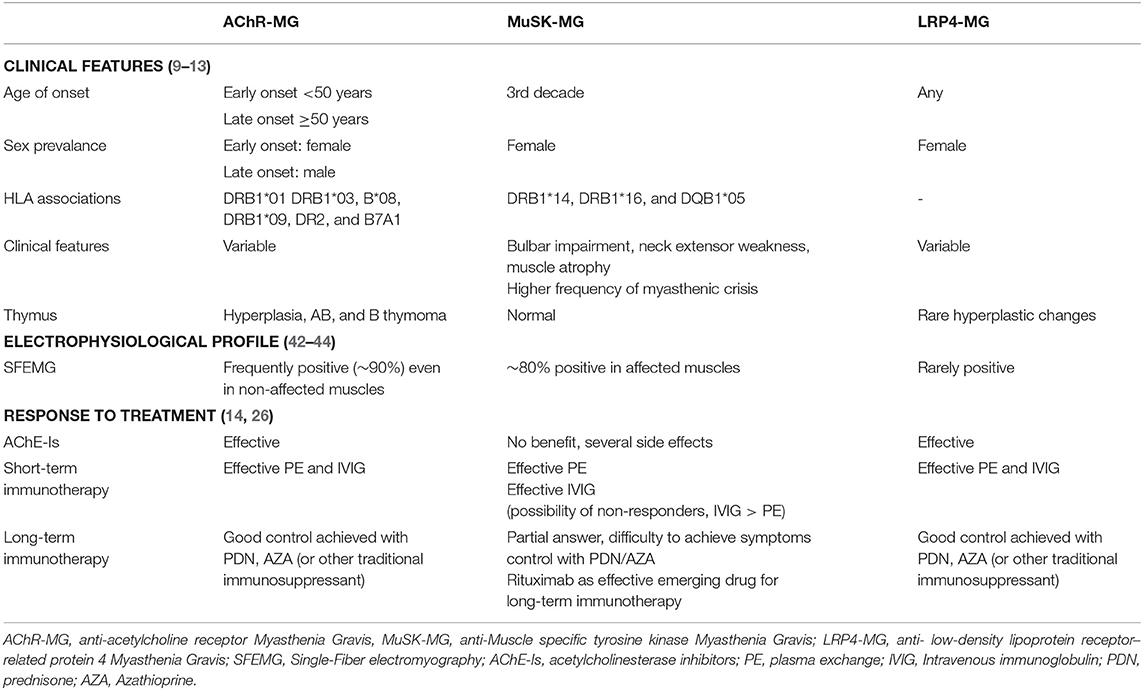
Response to treatment is often different from that expected in MG patients and achieving a regression of symptoms could be quite challenging. Among immunotherapies, prednisone, plasmapheresis, and RTX are the cornerstones of treatment for MuSK-MG. The main features of MuSK-MG are summarized and compared to the main other subtypes of MG (AChR-MG and LRP4-MG) in Table 1.
Consent for Publication
Written informed consent was obtained from the patient to acquire and publish photos.
Author Contributions
CR conceived the review. CR and CB were equally involved in literature search, figure and table preparation, and drafted and wrote the manuscript. AT and GV reviewed and revised the final draft of the manuscript. All authors have both approved the submitted version of the manuscript and agreed to be personally accountable for their own contributions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. (2001) 7:365–8. doi: 10.1038/85520
2. Evoli A, Tonali PA, Padua L, Lo Monaco M, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. (2003) 126:2304–11. doi: 10.1093/brain/awg223
3. Sanders DB, el-Salem K, Massey JM, Vincent A. Muscle specific tyrosine kinase (MuSK) positive, seronegative myasthenia gravis. clinical characteristics and response to therapy. Neurology. (2003) 60(supp l1):A418. doi: 10.1212/01.WNL.0000065882.63904.53
4. Lavrnic D, Losen M, de Baets M, Hajdukovic LJ, Stojanovic V, Trikic R, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. (2005) 76:1099–102. doi: 10.1136/jnnp.2004.052415
5. Deymeer F, Gungor-Tuncer O, Yilmaz V, Parman Y, Serdaroglu P, Ozdemir C, et al. Clinical comparison of anti-MuSK- vs anti-AChRpositive and seronegative myasthenia gravis. Neurology. (2007) 68:609–11. doi: 10.1212/01.wnl.0000254620.45529.97
6. Slater CR. The structure of human neuromuscular junctions: some unanswered molecular questions. Int J Mol Sci. (2017) 18:2183. doi: 10.3390/ijms18102183
7. Burden SJ, Yumoto N, Zhang W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb Perspect Biol. (2013) 5:a009167. doi: 10.1101/cshperspect.a009167
8. Ohno K, Otsuka K, Ito M. Roles of collagen Q in MuSK antibody-positive myasthenia gravis. Chem Biol Interact. (2016) 259:266–70. doi: 10.1016/j.cbi.2016.04.019
9. Evoli A, Alboini PE, Damato V, Iorio R, Provenzano C, Bartoccioni E, et al. Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci. (2018) 1412:82–9. doi: 10.1111/nyas.13518
10. Huijbers MG, Vergoossen DL, Fillié-Grijpma YE, van Es IE, Koning MT, Slot LM, et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e547. doi: 10.1212/NXI.0000000000000547
11. Koneczny I, Stevens JA, De Rosa A, Huda S, Huijbers MG, Saxena A, et al. IgG4 autoantibodies against muscle-specific kinase undergo fabarm exchange in myasthenia gravis patients. J. Autoimmun. (2017) 77:104–15. doi: 10.1016/j.jaut.2016.11.005
12. Evoli A, Lindstrom J. Myasthenia gravis with antibodies to MuSK: another step toward solving mystery? Neurology. (2011) 77:1783–4. doi: 10.1212/WNL.0b013e3182377fa6
13. Pasnoor M, Wolfe GI, Nations S, Trivedi J, Barohn RJ, Herbelin L, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a U.S. experience. Muscle Nerve. (2010) 41:370–4. doi: 10.1002/mus.21533
14. Guptill J, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. (2011) 44:36–40. doi: 10.1002/mus.22006
15. Skjei KL, Lennon VA, Kuntz NL. Muscle specific kinase autoimmune myasthenia gravis in children: a case series. Neuromuscul Disord. (2013) 23:874–82. doi: 10.1016/j.nmd.2013.07.010
16. Bennett DL, Mills KR, Riordan-Eva P, Barnes PRJ, Rose MR. Anti- MuSK antibodies in a case of ocular myasthenia gravis. J Neurol Neurosurg Psychiatry. (2006) 77:564–5. doi: 10.1136/jnnp.2005.075812
17. Evoli A, Alboini PE, Iorio R, Damato V, Bartoccioni E. Pattern of ocular involvement in myasthenia gravis with MuSK antibodies. J Neurol Neurosurg Psychiatry. (2017) 88:761–3. doi: 10.1136/jnnp-2017-315782
18. Rodolico C, Parisi D, Portaro S, Biasini F, Sinicropi S, Ciranni A, et al. Myasthenia gravis: unusual presentations and diagnostic pitfalls. J Neuromuscul Dis. (2016) 3:413–8. doi: 10.3233/JND-160148
19. Zouvelou V, Rentzos M, Toulas P, Evdokimidis I. MRI evidence of early muscle atrophy in MuSK positive myasthenia gravis. J Neuroimaging. (2011) 21:303–5. doi: 10.1111/j.1552-6569.2009.00456.x
20. Sanders DB, Juel VC. MuSK-antibody positive myasthenia gravis: questions from the clinic. J Neuroimmunol. (2008) 201–202:85–9. doi: 10.1016/j.jneuroim.2008.05.032
21. Lauriola L, Ranelletti F, Maggiano N, Guerriero M, Punzi C, Marsili F, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. (2005) 64:536–8. doi: 10.1212/01.WNL.0000150587.71497.B6
22. Leite MI, Strobel P, Jones M, Micklem K, Moritz R, Gold R, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. (2005) 57:444–8. doi: 10.1002/ana.20386
23. Ito A, Sasaki R, Ii Y, Nakayama S, Motomura M, Tomimoto H. A case of thymoma associated myasthenia gravis with anti-MuSK antibodies [in Japanese]. Rinsho Shinkeigaku. (2013) 53:372–5. doi: 10.5692/clinicalneurol.53.372
24. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized trial of thymectomy in myasthenia gravis. New Engl J Med. (2016) 375:511–22. doi: 10.1056/NEJMc1611704
25. Clifford KM, Hobson-Webb LD, Benatar M, Burns TM, Barnett C, Silvestri NJ, et al. Thymectomy may not be associated with clinical improvement in MuSK myasthenia gravis. Muscle Nerve. (2019) 59:404–10. doi: 10.1002/mus.26404
26. Huda S, Waters P, Woodhall M, Leite MI, Jacobson L, De Rosa A, et al. IgG-specific cell-based assay detects potentially pathogenic MuSK-Abs in seronegative MG. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e357. doi: 10.1212/NXI.0000000000000357
27. Morren J, Li Y. Myasthenia gravis with muscle-specific tyrosine kinase antibodies: a narrative review. Muscle Nerve. (2018) 58:344–58. doi: 10.1002/mus.26107
28. Sanders DB, Cao L, Massey JM, Juel VC, Hobson-Webb L, Guptill JT. Is the decremental pattern in lambert-eaton syndrome different from that in myasthenia gravis? Clin Neurophysiol. (2014) 125:1274–7. doi: 10.1016/j.clinph.2013.11.007
29. Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. (2005) 116:2065–8. doi: 10.1016/j.clinph.2005.06.003
30. Kuwabara S, Nemoto Y, Misawa S, Takahashi H, Kawaguchi N, Hattori T. Anti-MuSK-positive myasthenia gravis: neuromuscular transmission failure in facial and limb muscles. Acta Neurol Scand. (2007) 115:126–8. doi: 10.1111/j.1600-0404.2006.00721.x
31. Padua L, Tonali P, Aprile I, Caliandro P, Bartoccioni E, Evoli A. Seronegative myasthenia gravis: comparison of neurophysiological picture in MuSK1 and MuSK2 patients. Eur J Neurol. (2006) 13:273–76. doi: 10.1111/j.1468-1331.2006.01214.x
32. Evoli A, Alboini PE, Damato V, Iorio R. 3,4- Diaminopyridine may improve myasthenia gravis with MuSK antibodies. Neurology. (2016) 86:1070–1. doi: 10.1212/WNL.0000000000002466
33. Haran M, Schattner A, Mate A, Starobin D, Haran G, Shtalrid M. Can a rare form of myasthenia gravis shed additional light on disease mechanisms? Clin Neurol Neurosurg. (2013) 115:562–6. doi: 10.1016/j.clineuro.2012.06.038
34. Reddel SW, Morsch M, Phillips WD. Clinical and scientific aspects of muscle-specific tyrosine kinase-related myasthenia gravis. Curr Opin Neurol. (2014) 27:558–65. doi: 10.1097/WCO.0000000000000136
35. Díaz-Manera J, Martínez-Hernández E, Querol L, Klooster R, Rojas-García R, Suárez-Calvet X, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. (2012) 78:189–93. doi: 10.1212/WNL.0b013e3182407982
36. Anderson D, Phan C, Johnston WS, Siddiqi ZA. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol. (2016) 3:552–5. doi: 10.1002/acn3.314
37. Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF Jr, et al. Rituximab as treatment for anti-MuSK myasthenia gravis: multicenter blinded prospective review. Neurology. (2017) 89:1069–77. doi: 10.1212/WNL.0000000000004341
38. Topakian R, Zimprich F, Iglseder S, Embacher N, Guger M, Stieglbauer K, et al. High efficacy of rituximab for myasthenia gravis: a comprehensive nationwide study in Austria. J Neurol. (2019) 266:699–706. doi: 10.1007/s00415-019-09191-6
39. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87:4419–25. doi: 10.1212/WNL.0000000000002790
40. Kanth KM, Solorzano GE, Goldman MD. PML in a patient with myasthenia gravis treated with multiple immunosuppressing agents. Neurol Clin Pract. (2016) 6:e17–9. doi: 10.1212/CPJ.0000000000000202
41. Afanasiev V, Demeret S, Bolgert F, Eymard B, Laforêt P, Benveniste O. Resistant myasthenia gravis and rituximab: a monocentric retrospective study of 28 patients. Neuromuscul Disord. (2017) 27:251–8. doi: 10.1016/j.nmd.2016.12.004
42. Padua L, Caliandro P, Di Iasi G, Pazzaglia C, Ciaraffa F, Evoli A. Reliability of SFEMG in diagnosing myasthenia gravis: sensitivity and specificity calculated on 100 prospective cases. Clin Neurophysiol. (2014) 125:1270–3. doi: 10.1016/j.clinph.2013.11.005
43. Nikolic A, Basta I, Stojanovic VR, Stevic Z, Lavrnic D. Electrophysiological profile of the patients with MuSK positive myasthenia gravis. Neurol Res. (2014) 36:945–9. doi: 10.1179/1743132814Y.0000000387
Keywords: muscle-specific tyrosine kinase, atypical onset, tongue atrophy, MuSK-MG therapy, rituximab
Citation: Rodolico C, Bonanno C, Toscano A and Vita G (2020) MuSK-Associated Myasthenia Gravis: Clinical Features and Management. Front. Neurol. 11:660. doi: 10.3389/fneur.2020.00660
Received: 09 April 2020; Accepted: 02 June 2020;
Published: 23 July 2020.
Edited by:
Amelia Evoli, Catholic University of the Sacred Heart, ItalyReviewed by:
Marcus Vinicius Pinto, Mayo Clinic, United StatesPaola Sandroni, Mayo Clinic, United States
Copyright © 2020 Rodolico, Bonanno, Toscano and Vita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmelo Rodolico, Y3JvZG9saWNvQHVuaW1lLml0
†These authors have contributed equally to this work
 Carmelo Rodolico
Carmelo Rodolico Carmen Bonanno
Carmen Bonanno Antonio Toscano
Antonio Toscano Giuseppe Vita
Giuseppe Vita