
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 July 2020
Sec. Stroke
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00636
Background: After a first-ever-in-a-lifetime stroke (FELS), hospital readmissions are common and associated with increased mortality and morbidity of stroke survivors, thus, raising the overall health burden of stroke. Population-based stroke studies on hospital readmissions are scarce despite it being an important healthcare service quality indicator. We evaluated unplanned readmissions or death during the first year after a FELS and their potential factors, based on a Portuguese community register.
Methods: Data were retrieved from a population-based prospective register undertaken in Northern Portugal (ACIN2) in 2009–2011. Retrospective information about unplanned hospital readmissions and case fatality within 1 year after FELS index hospitalization (FELS-IH) was evaluated. Readmission/death-free survival 1 year after discharge was estimated using the Kaplan–Meyer method. Independent risk factors for readmission/death were identified using Cox proportional hazard models.
Results: Unplanned readmission/death within 1 year occurred in 120 (31.6%) of the 389 hospitalized FELS survivors. In 31.2% and 33.5% of the cases, it occurred after ischemic stroke or intracerebral hemorrhage, respectively. Infections and cerebrovascular and cardiovascular diseases were the main causes of readmission. Of the readmissions, 65.3% and 52.5% were potentially avoidable or stroke related, respectively. The main cause of potentially avoidable readmissions was the continuation/recurrence of the event responsible for the initial admission or a closely related condition (71.2%). Male sex, age, previous and post-stroke functional status, and FELS-IH length of stay were independent factors of readmission/death within 1 year.
Conclusions: Almost one-third of FELS survivors were readmitted/dead 1 year after their FELS-IH. This outcome persisted after the first months after stroke hospitalization in all stroke subtypes. More than half of readmissions were considered potentially avoidable or stroke related.
After a first-ever-in-a-lifetime stroke (FELS) or transient ischemic attack (TIA), the use of hospital emergency services or hospital readmissions is common and associated with increased stroke mortality and morbidity, thus, raising the overall health burden of stroke (1). Also, despite some well-characterized limitations (2), readmissions are currently a measure of the hospital's performance and quality of care (3).
Several risk factors for stroke readmissions have been described. However, many meaningful clinical associations may have been ignored since most studies only rely on large administrative or single-hospital databases, particular subtypes of stroke, or readmissions in the first 3 months after stroke (1, 4, 5). This assertion is especially true in Portugal, where, to our knowledge, there are no population-based stroke readmission studies, and therefore, the corresponding information is scarce.
We aimed to study unplanned readmissions or death during the first year after a FELS and to identify their potential factors, based on a Portuguese community register.
The sample was obtained from the second population-based register undertaken in Northern Portugal (ACIN2), comprising all FELS recorded between October 2009 and September 2011 in the population registered in the Health Centers Group of Western Porto main city (190,000 persons) and two health centers in rural regions in Northern Portugal (Mirandela and Vila Pouca de Aguiar, involving about 46,000 persons) (6). Multiple sources of information were used to identify all patients with a FELS using a record-linkage methodology based on the National Health Number, a unique identifier for residents in Portugal to contact the National Health Service (NHS). Hot-pursuit and cold-pursuit ascertainment involving community-based and hospital-based information sources were used (6). Hot-pursuit encompassed a daily review of emergency admissions and referrals to the project out-patient clinic at Hospital de Santo António. Cold pursuit was used to check for completeness of hot-pursuit identification (7). Patients were examined as soon as possible after symptoms' onset at the emergency room, during their hospital stay or at the project out-patient clinic, within 1 month and then were followed-up until 3 months after a stroke. More detailed information is described elsewhere (6). This study includes all patients from Porto admitted to the hospital after a FELS. The information about readmissions after the 3-months follow-up period was collected retrospectively. The ethics committee of the Centro Hospitalar Universitário de São João and the Centro Hospitalar Universitário do Porto approved this study.
The World Health Organization's “stroke” definition and Sudlow's and Warlow's stroke pathological types—ischemic stroke (IS), intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH)—were considered for the corresponding concepts (8, 9). Brain image (computerized tomography scan/magnetic resonance imaging) was used to confirm stroke types. The TOAST criteria were used to define IS etiology and the Bamford Oxfordshire classification to define clinical IS syndromes (10, 11).
Stroke severity at the first medical evaluation was characterized as mild, moderate, or severe based on the National Institutes of Health Stroke Scale (NIHSS) (12) (NIHSS ≤7, 8–16, or ≥17, respectively), except for SAH. Whenever the NIHSS was unavailable, the score was estimated retrospectively from the patients' clinical records, if valid for that purpose (13). The pre and post-stroke (~28 days after stroke) functional outcome was assessed with the modified Rankin Scale (mRS) (14).
The following criteria were considered as pre-stroke risk factors: (a) history of hypertension or antihypertensive treatment; (b) previous diagnosis/treatment of diabetes mellitus with oral antidiabetic agent/insulin or fasting glycemia >126 mg/dl, postprandial glycemia ≥200 mg/dl, and/or ≥200 mg/dl in the 2-h glucose tolerance test; (c) evidence of atrial fibrillation in electrocardiogram or documented in the patient's records; (d) previous diagnosis/treatment of hypercholesterolemia; (e) history of myocardial infarction; (f) current smoking habits (if patients had smoked at all in the preceding 12 months) (6). Other pre-stroke comorbidities such as congestive heart failure, dementia, HIV infection, and malignant neoplasm were included after reviewing patients' medical records using the International Classification of Diseases 9th Revision (ICD-9) diagnosis code.
Planned readmissions were defined as readmissions to perform a scheduled procedure (e.g., carotid endarterectomy or stenting, patent foramen ovale closure, cardiac planned procedures, and cranioplasty) and planned hospitalizations (rehabilitation, chemo- or radiotherapy treatment, major organ transplant, or obstetrical delivery) (15).
Unplanned readmissions were defined as >24-h hospitalizations due to unexpected causes and emergency episodes leading to death that did not fulfill any planned readmission criterion that had occurred within 1 year of the FELS index hospitalization (FELS-IH) (15). Unplanned readmissions after a planned admission were also acknowledged.
Patients who died during their FELS-IH were excluded. Two neurology study investigators (including a stroke neurologist) reviewed the patients' medical records using the ICD-9 diagnosis code to obtain and validate the unplanned readmission causes. The main unplanned readmission diagnosis-related group code was identified for statistical data and subgroup analyses. A composite outcome event of unplanned first-ever readmission or death without readmission within 1 year after FELS was considered to capture all negative health outcomes (3).
Potentially avoidable readmissions (PAR) were defined as causes that could have been prevented or modified during the FELS-IH, and their clinical plausibility was defined using Goldfield et al.'s criteria (16): (a) medical readmission for a continuation or recurrence of the reason for the initial admission, or for a closely related condition; (b) medical readmission for an acute decompensation of a chronic problem that was not the reason for the initial admission, but was plausibly related to care either during or immediately after the initial admission; (c) medical readmission for an acute medical complication plausibly related to care during the initial admission; (d) readmission for a surgical procedure to address a continuation or a recurrence of the problem causing the initial admission; (e) readmission for a surgical procedure to address a complication resulting from care during the initial admission. In case of disagreement, the study investigators reached a consensus for the readmission classification.
Stroke-related readmissions were defined as recurrent vascular events and complications that warranted readmission, including stroke, pneumonia, urinary tract infection, peripheral and coronary artery disease, hip fracture, and pulmonary embolism (17, 18).
Sociodemographic characteristics were summarized using descriptive statistics. The baseline and clinical characteristics of readmitted vs. non-readmitted patients were compared using the Chi-square or the Fisher exact test when adequate for categorical variables and the t-test or the Mann–Whitney U test for continuous variables (normality of distributions was assessed using the Shapiro–Wilk test). The overall cumulative readmission/death-free survival and PAR-free survival over 12 months was estimated using the Kaplan–Meyer method. Independent risk factors for readmission were evaluated using Cox proportional hazard models. ICH and SAH were combined as hemorrhagic stroke (HS) for the Kaplan–Meyer survival estimation (Figure 2) and the description of characteristics and reasons for all-cause readmissions by sub-groups (Table 3). A value of p = 0.05 was considered as the limit to wrongly reject the null hypothesis. Data analysis was performed using SPSS Statistics v24.
Figure 1 shows the study design for the cohort follow-up. From the initial cohort of 720 FELS patients in the ACIN2 database, we excluded 258 not hospitalized in the index event and 73 that died during the FELS-IH; 389 FELS patients at risk of an unplanned readmission/death were included. This cohort had a mean age of 70 years and 208 (53.5%) women; 317 (81.5%) had an IS in the index event, 58 (14.9%) an ICH, and 14 (3.6%) an SAH. All patients had a brain image performed.
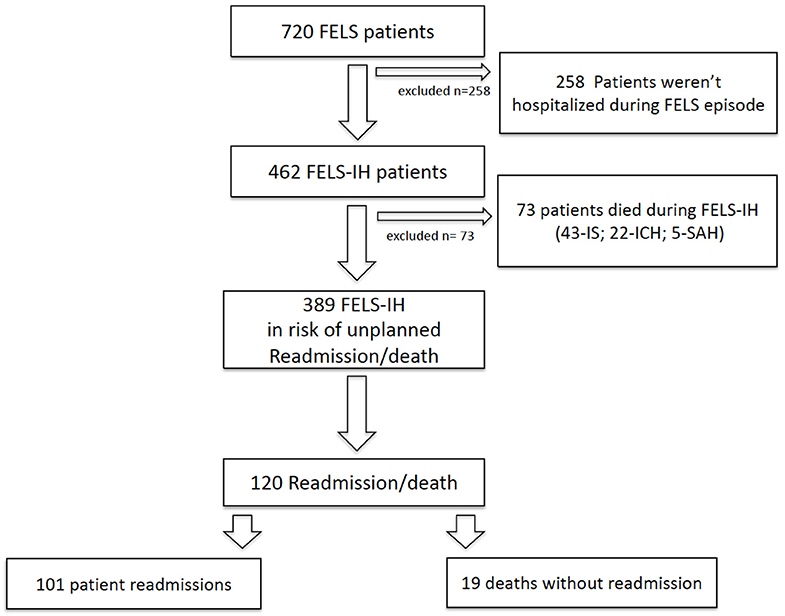
Figure 1. Study design for cohort follow-up. FELS, first-ever-in-a-lifetime stroke; FELS-IH, first-ever-in-a-lifetime-stroke index hospitalization; IS, ischemic stroke, ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Table 1 shows the baseline characteristics of non-readmitted and readmitted/deceased patients. Readmitted/deceased patients were older (77.1 vs. 67.1, p < 0.001), had higher pre-stroke dependency (mRS ≥ 2, 50.8% vs. 25.7%, p < 0.001), and had more often hypertension (83.3% vs. 73.2%, p = 0.03), atrial fibrillation (AF; 32.5% vs. 20.1%, p = 0.008), and congestive heart failure (25.0% vs. 16.4%, p = 0.045). They also had had more total anterior circulation infarcts and less lacunar and posterior circulation infarcts (p = 0.008), more cardioembolic strokes and less small vessels or other determined infarcts (p = 0.019). Moreover, readmitted/deceased patients had a higher NIHSS score (9 vs. 4, p < 0.001), more moderate or severe strokes (p < 0.001), higher post-stroke dependency levels (mRS ≥ 2, 95.0% vs. 71.4%, p < 0.001), higher likelihood of having had a previous hospital admission (18.3% vs. 8.9%, p = 0.008), and a higher median in-hospital length of stay (LoS) (11 vs. 8 days, p = 0.008). We found no differences between non-readmitted and readmitted/deceased patients regarding other baseline characteristics.
Figure 2 shows the cumulative readmission or death rates. The all-cause readmission/death rate was 9.8, 23.2, and 31.6% at 30 days, 180 days, and 1 year, respectively. In cases of IS and HS, the rate was, respectively, 9.5 and 11.1% at 30 days, 22.2% and 27.8% at 180 days, and 31.2% and 33.5% at 1 year.
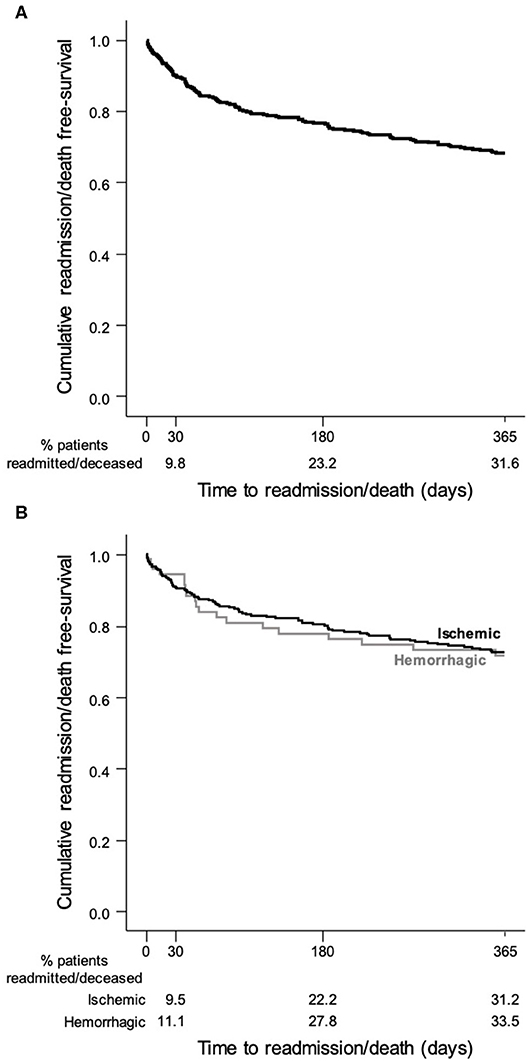
Figure 2. Kaplan–Meier survival curve showing the probability of a stroke patient will remain free of readmission/death after discharge: (A) all stroke patients; (B) stroke patients stratified by pathological type.
Overall, 120 patients were readmitted/deceased within 1 year: 101 (84.2%) unplanned readmissions and 19 (15.8%) deaths without readmission (Figure 1). Cumulative rates of readmission were similar to the readmission/death rates (data not shown). Of the 19 patients who had deceased without readmission at 1 year, 1/19 died within 30 days, 12/19 within 30–180 days, and 6/19 within 180–365 days.
Tables 2–4 summarize the patients' characteristics and the reasons for the hospital readmissions within the first year. The three most common reasons for readmission were infectious diseases (39.6%), cerebrovascular diseases (15.8%)—particularly IS (9.9%), and cardiovascular diseases (8.9%). The median time for readmission after FELS-IH was 59 days (interquartile range (IQR): 23–183 days). Of the unplanned readmissions, 32.7% occurred within 30 days, 41.6% within 30–180 days, and 25.7% within 180–365 days. The median in-hospital LoS of readmissions was 7 days (IQR: 3–17 days). Nineteen patients (18.8%) died during readmission: 7/19 within 30 days, 9/19 within 30–180 days, and 3/19 within 180–365 days.
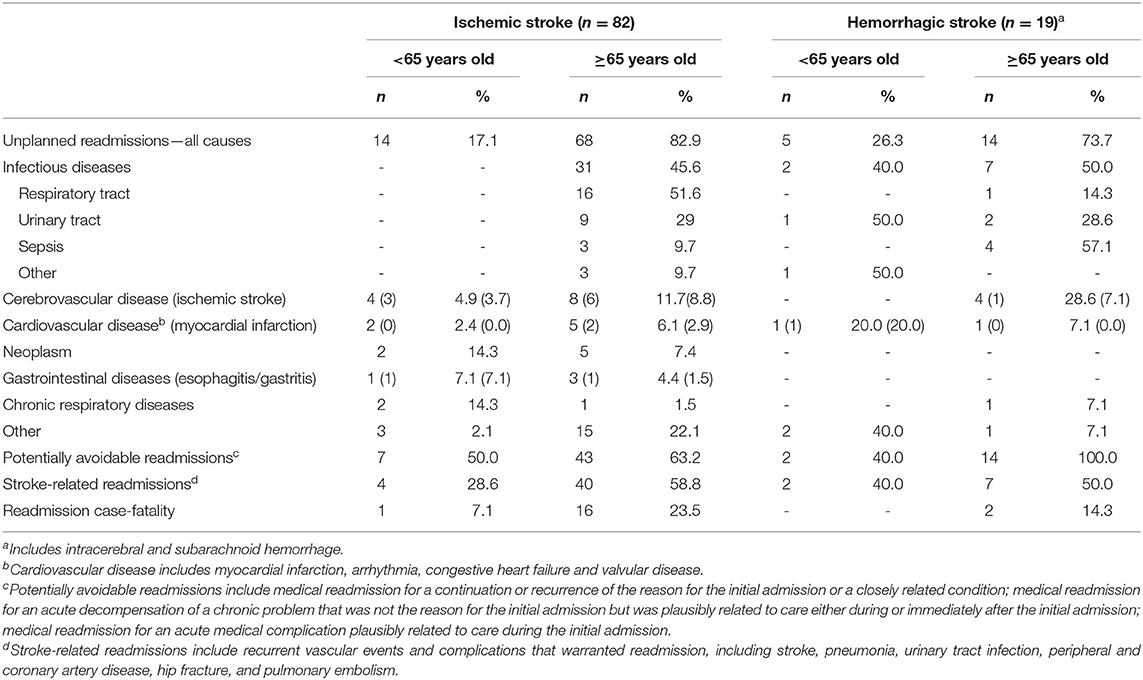
Table 3. Characteristics and causes of patients' all-cause readmissions within 1 year, by sub-groups: stroke type and age.
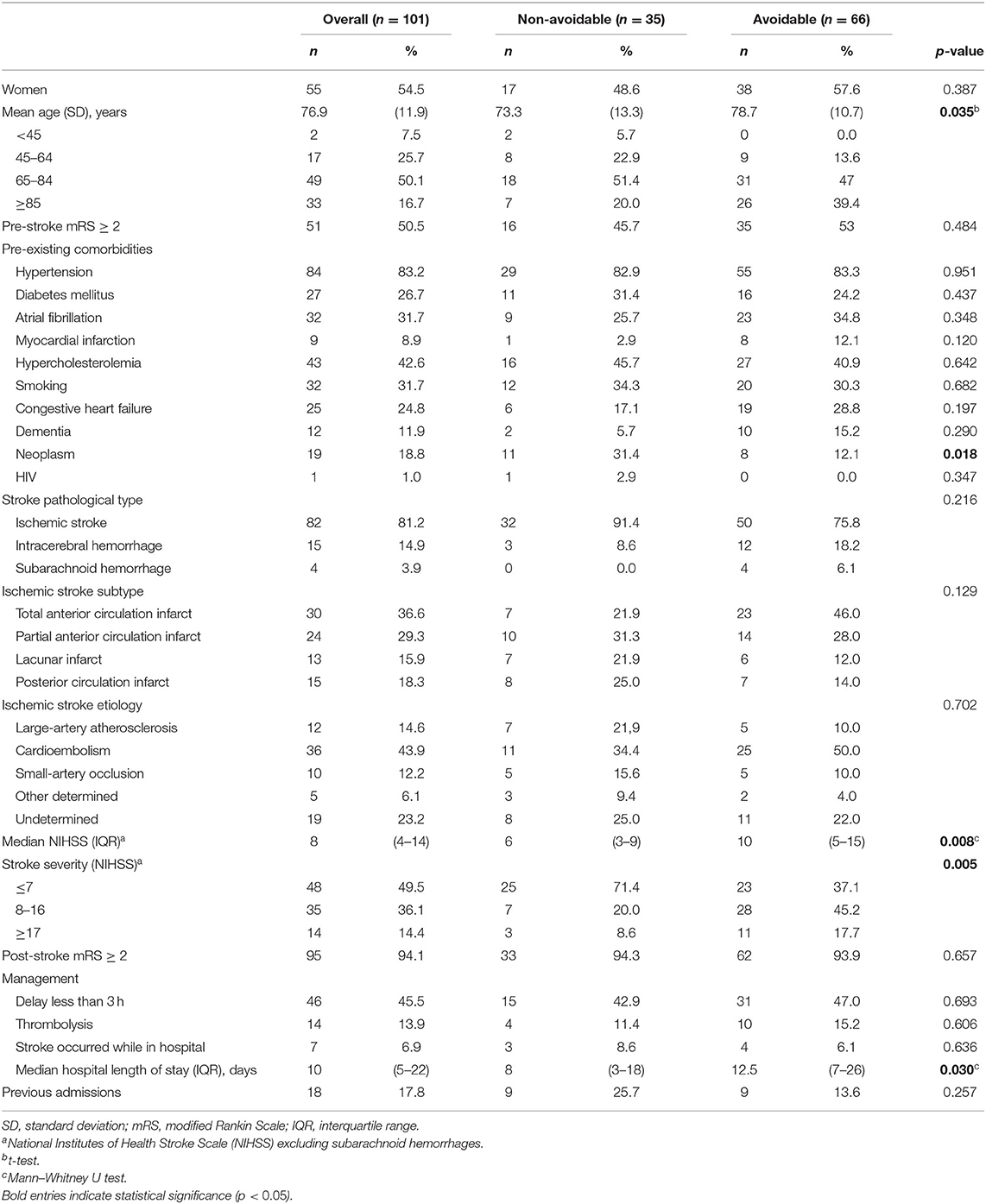
Table 4. Baseline characteristics of patients' readmitted within 1 year, by sub-groups: avoidable and non-avoidable.
PAR occurred in 66 (65.3%) of the readmitted patients: 47 (71.2%) due to medical readmission for a continuation or recurrence of the reason for the initial admission, or for a closely related condition; 6 (9.1%) due to medical readmission for an acute decompensation of a chronic problem that was not the reason for the initial admission but was plausibly related to care either during or immediately after the initial admission; and 13 (19.7%) due to a medical readmission for an acute medical complication plausibly related to care during the initial admission; and no readmissions for a surgical procedure were observed. The PAR rate was 6.0, 15.1, and 18.5% at 30 days, 180 days, and 1 year, respectively (Figure 3). Compared to non-avoidable readmissions (Table 4), PAR patients were older (78.7 vs. 73.3 years old, p = 0.035), had less neoplasms (31.4% vs. 12.1%, p = 0.018), a higher NIHSS score (10 vs. 6, p = 0.008), more moderate or severe strokes (p = 0.008), and a higher median in-hospital LoS (13 vs. 8 days, p = 0.030).
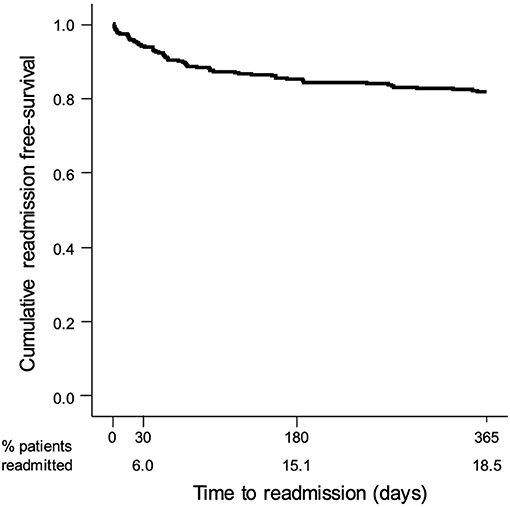
Figure 3. Kaplan–Meier survival curve showing the probability of a stroke patient remaining free of non-avoidable readmissions after discharge.
Stroke-related readmissions represented 52.5% of readmissions and occurred in 48.5, 61.9, and 42.3% of the patients readmitted within 30 days, 30–180 days, and 180–365 days, respectively (Table 2).
Table 5 shows the univariate and multivariate analyses for all-cause readmission or death within 1 year. Age, previous functional status, hypertension, AF, congestive heart failure, initial NIHSS score, post-stroke functional status, disabling stroke, FELS-IH LoS, and previous admissions were significantly associated with readmission or death within 1 year, in the univariate model analysis. Conversely, being a current smoker was negatively associated with this outcome. In the multivariate regression analysis, only the male gender, age, previous and post-stroke functional status, and FELS-IH LoS remained independent factors of readmission/death within 1 year.
Our study showed that 31.6% of FELS-IH survivors were readmitted/dead within 1 year. Infections, stroke recurrence, and cardiovascular diseases were the most common causes. More than half of the readmissions were PAR or stroke-related. Only the male gender, age, previous and post-stroke functional status, and FELS-IH LoS were independent factors of readmission/death.
In literature, the 1-year stroke readmission/death rate varies between 13 and 62% (4, 5, 19). Such a wide variation reflects different study methodologies and national health realities. Our results are within this range. Likewise, our readmission/death rate by stroke type confirms the lower-end range of previously reported readmission/death rates regarding IS (31 to 49%) (20, 21) and ICH (33 to 44.7%) (22–24), particularly in two Portuguese single-data center studies (IS, 34%; ICH, 33%) (22, 25).
The main differences between non-readmitted and readmitted/deceased patients (e.g., age, stroke comorbidities, stroke type/etiology, pre- and post-stroke disability, LoS, and previous admissions) fit the pattern of other readmission studies' cohorts, such as those identified by Koennecke et al., of in-hospital worse stroke outcome/morbidity (26). Hence, these factors may help recognize individuals who are more vulnerable to readmission and must be considered in the clinical setting (1, 4, 26, 27). One-third of readmissions/deaths in our cohort occurred within 30 days after discharge. Nevertheless, the readmission/death risk endured after this period, as reported in other studies (18). Also, readmission case fatality at 30 days was higher than that reported for the general all-hospital in a Portuguese readmission administrative database study (21 vs. 9.5%) (28). As in the French Dijon Stroke registry cohort, this finding shows that readmission negatively affected survival (29).
In our study, the main causes of readmission were infections, stroke recurrence, and cardiovascular diseases, as in other cohorts (1). While infectious diseases dominated the 1-year readmission causes, over the year, readmissions due to cerebrovascular diseases decreased, and those due to cardiovascular diseases increased; this suggests an increasing importance of cardiac disease overtime after stroke (21, 30).
More than half of our readmissions were PAR, and these were highest within 180 days after FELS-IH. The few reports addressing this issue in stroke cohorts study PAR only within a 30-days period, and comparatively, PAR at 30 days were higher in our study (31–34). Nonetheless, this difference may reflect a different methodology for PAR report more than a contrasting stroke treatment reality (34). Moreover, we identified differences in the characteristics of PAR patients compared to non-avoidable readmission patients that may explain their proneness to this type of readmission; e.g., they were older, had higher NIHSS scores, more severe strokes, and higher median in-hospital LoS.
Theoretically, most of our readmissions were preventable since their causes rely on the transitional and outpatient quality of care after the initial hospitalization, including secondary prevention measures (24). On the other hand, as explained by Bjerkreim et al. (20), severe stroke patients, even when appropriately treated, are prone to infections due to repeated pulmonary aspiration and urinary catheterizations, and because strokes may affect the immunological status (20, 35). Furthermore, the natural disease history and the stroke early phase prothrombotic state may explain some readmissions due to vascular events, despite proper secondary prevention measures (20, 27).
Most stroke-related readmissions also occurred shortly after FELS-IH and persisted over the year. This finding is coherent with the aforementioned temporal pattern of readmission causes and highlights the need for more targeted specific interventions (34).
Hypertension, AF, and congestive heart failure are well-recognized stroke risk factors (27, 29) and were identified in our cohort univariate analysis as independent readmission/death risk factors. Thus, in order to prevent subsequent readmissions/deaths, besides the constant monitoring of stroke secondary prevention treatments, patients must also be aware of stroke warning signs and educated about the importance of controlling stroke risk factors and adhering to the recommended medication and behavioral changes in the long term (29, 36–39). This last goal may be achieved in the aftercare of stroke with well-designed and targeted multifactorial intervention programs of support, as described in the INSPiRE-TMS study (38).
Although smoking habits are considered a deleterious stroke risk factor (40), the univariate analysis showed that being a current smoker may be a protective factor of readmission/death. As explained elsewhere (41), this may be due to potential misreporting of patients' smoking status, or a bias since the sickest individuals (most prone to readmission/death) might had already stopped smoking because of their morbidity status.
Our study's multivariate analysis reinforced the evidence that age, previous and post-stroke functional status, and FELS-IH LoS are important independent factors of readmission/death (1, 5).
Although seldom referred in the literature (1, 4), the male sex was also an independent predictor of readmission/death in our study; this might be due to their proneness to recurrent IS (42); nevertheless, this hypothesis is not consensual in the literature (43).
Our study has some limitations. Since information about readmissions/death was collected retrospectively 3 months after FELS, there is an inherent data collection bias, which we mitigated using information from the medical records instead of only from administrative data. Also, our definitions for the vascular risk factors may have led to a decreased report rate, e.g., patients with no information regarding their smoking habits were considered non-smokers. We might have underestimated the true proportion of readmissions by not collecting data from private hospitals, but this information was probably registered posteriorly in the patients' NHS medical records, which we analyzed. Last, we did not include some complications in the FELS-IH or the type of discharge destination, which in other studies were linked to the readmission risk (1).
Almost one-third of FELS survivors were readmitted/dead 1 year after their FELS-IH. This outcome persisted after the first months after stroke hospitalization in all stroke subtypes. More than half of readmissions were considered potentially avoidable or stroke related, and the main cause of potentially avoidable readmissions was continuation/recurrence of the event responsible for the initial admission or a closely related condition. Identifying potentially modifiable causes of readmissions and stroke survivors more prone to readmissions, as we have done in this study, may help organizations allocate resources and implement targeted readmission reduction policies.
The data that support the findings of this study is available from the population-based register (ACIN2) which is managed by its main investigators. The dataset used and analyzed in this study are available from the corresponding author on reasonable request and with permission of the ACIN2 investigators.
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and with the approval of the Ethics Committee of the Centro Hospitalar Universitário de São João and the Centro Hospitalar Universitário do Porto where it was performed.
PA was responsible for the study conceptualization, data acquisition, part of the statistical analysis and table elaboration, and drafting of the main manuscript. RM was the main responsible for the statistical analyses and table and figures elaboration, contributed to the study conceptualization/methodology, data acquisition, and article draft/review. DB contributed to the data acquisition and study conceptualization. EA, MS, and MC critically reviewed this article and contributed to the study conceptualization/methodology and article draft.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FELS, first-ever-in-a-lifetime stroke; FELS-IH, first-ever-in-a-lifetime stroke index hospitalization; NHS, National Health Service; TIA, transient ischemic attack; IS, ischemic stroke; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; TOAST, Trial of Org 10172 in Acute Stroke Treatment; NIHSS, National Institutes of Health Stroke Scale; mRS, Modified Rankin Scale; ICD-9, International Classification of Diseases 9th Revision; PAR, potentially avoidable readmissions; HS, hemorrhagic stroke; LoS, length of stay; IQR, interquartile range; MI, myocardial infarction; SD, standard deviation.
1. Lichtman JH, Leifheit-Limson EC, Jones SB, Watanabe E, Bernheim SM, Phipps MS, et al. Predictors of hospital readmission after stroke: a systematic review. Stroke. (2010) 41:2525–33. doi: 10.1161/STROKEAHA.110.599159
2. Fischer C, Lingsma HF, Marang-van de Mheen PJ, Kringos DS, Klazinga NS, Steyerberg EW. Is the readmission rate a valid quality indicator? A review of the evidence. PLoS ONE. (2014) 9:e112282. doi: 10.1371/journal.pone.0112282
3. Rumball-Smith J, Hider P. The validity of readmission rate as a marker of the quality of hospital care, and a recommendation for its definition. N Z Med J. (2009) 122 (1289):63–70.
4. Zhong W, Geng N, Wang P, Li Z, Cao L. Prevalence, causes and risk factors of hospital readmissions after acute stroke and transient ischemic attack: a systematic review and meta-analysis. Neurol Sci. (2016) 37:1195–202. doi: 10.1007/s10072-016-2570-5
5. Rao A, Barrow E, Vuik S, Darzi A, Aylin P. Systematic review of hospital readmissions in stroke patients. Stroke Res Treat. (2016) 2016:9325368. doi: 10.1155/2016/9325368
6. Correia M, Magalhaes R, Felgueiras R, Quintas C, Guimaraes L, Silva MC. Changes in stroke incidence, outcome, and associated factors in porto between 1998 and 2011. Int J Stroke. (2017) 12:169–79. doi: 10.1177/1747493016669846
7. Correia M, Silva MR, Matos I, Magalhaes R, Lopes JC, Ferro JM, et al. Prospective community-based study of stroke in Northern Portugal: incidence and case fatality in rural and urban populations. Stroke. (2004) 35:2048–53. doi: 10.1161/01.STR.0000137606.34301.13
8. Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. (1997) 28:491–9. doi: 10.1161/01.STR.28.3.491
9. Sudlow CL, Warlow CP. Comparing stroke incidence worldwide: what makes studies comparable? Stroke. (1996) 27:550–8. doi: 10.1161/01.STR.27.3.550
10. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
11. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
12. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
13. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH stroke scale. Stroke. (2000) 31:858–62. doi: 10.1161/01.STR.31.4.858
14. Uk-Tia Study Group. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. Br Med J. (1988). 296:316–20. doi: 10.1136/bmj.296.6618.316
15. Horwitz LI, Grady JN, Cohen DB, Lin Z, Volpe M, Ngo CK, et al. Development and validation of an algorithm to identify planned readmissions from claims data. J Hosp Med. (2015) 10:670–7. doi: 10.1002/jhm.2416
16. Goldfield NI, McCullough EC, Hughes JS, Tang AM, Eastman B, Rawlins LK, et al. Identifying potentially preventable readmissions. Health Care Financ Rev. (2008) 30:75–91.
17. Lichtman JH, Allen NB, Wang Y, Watanabe E, Jones SB, Goldstein LB. Stroke patient outcomes in US hospitals before the start of the joint commission primary stroke center certification program. Stroke. (2009) 40:3574–9. doi: 10.1161/STROKEAHA.109.561472
18. Lakshminarayan K, Schissel C, Anderson DC, Vazquez G, Jacobs DR Jr, Ezzeddine M, et al. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke: medicare linkage study. Stroke. (2011) 42:1556–62. doi: 10.1161/STROKEAHA.110.605600
19. Kilkenny MF, Dewey HM, Sundararajan V, Andrew NE, Lannin N, Anderson CS, et al. Readmissions after stroke: linked data from the Australian stroke clinical registry and hospital databases. Med J Aust. (2015) 203:102–6. doi: 10.5694/mja15.00021
20. Bjerkreim AT, Thomassen L, Brogger J, Waje-Andreassen U, Naess H. Causes and predictors for hospital readmission after ischemic stroke. Cerebrovasc Dis. (2015) 24:2095–101. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.019
21. Bravata DM, Ho SY, Meehan TP, Brass LM, Concato J. Readmission and death after hospitalization for acute ischemic stroke: 5-year follow-up in the medicare population. Stroke. (2007) 38:1899–904. doi: 10.1161/STROKEAHA.106.481465
22. Nzwalo H, Nogueira J, Guilherme P, Abreu P, Felix C, Ferreira F, et al. Hospital readmissions after spontaneous intracerebral hemorrhage in Southern Portugal. Clin Neurol Neurosurg. (2018) 169:144–8. doi: 10.1016/j.clineuro.2018.04.015
23. Bjerkreim AT, Thomassen L, Waje-Andreassen U, Selvik HA, Naess H. Hospital readmission after Intracerebral Hemorrhage. Cerebrovasc Dis. (2016) 25:157–62. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.009
24. Lee HC, Chang KC, Huang YC, Hung JW, Chiu HH, Chen JJ, et al. Readmission, mortality, and first-year medical costs after stroke. J Chin Med Assoc. (2013) 76:703–14. doi: 10.1016/j.jcma.2013.08.003
25. Leitao A, Brito A, Pinho J, Alves JN, Costa R, Amorim JM, et al. Predictors of hospital readmission 1 year after ischemic stroke. Intern Emerg Med. (2017) 12:63–8. doi: 10.1007/s11739-016-1519-2
26. Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. (2011) 77:965–72. doi: 10.1212/WNL.0b013e31822dc795
27. Lin HJ, Chang WL, Tseng MC. Readmission after stroke in a hospital-based registry: risk, etiologies, and risk factors. Neurology. (2011) 76:438–43. doi: 10.1212/WNL.0b013e31820a0cd8
28. Sousa-Pinto B, Gomes AR, Oliveira A, Ivo C, Costa G, Ramos J, et al. Hospital readmissions in Portugal over the last decade. Acta Med Port. (2013) 26:711–20.
29. Lainay C, Benzenine E, Durier J, Daubail B, Giroud M, Quantin C, et al. Hospitalization within the first year after stroke: the dijon stroke registry. Stroke. (2015) 46:190–6. doi: 10.1161/STROKEAHA.114.007429
30. Christensen MC, Munro V. Ischemic stroke and intracerebral hemorrhage: the latest evidence on mortality, readmissions and hospital costs from Scotland. Neuroepidemiology. (2008) 30:239–46. doi: 10.1159/000128323
31. Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. Preventable readmissions within 30 days of ischemic stroke among medicare beneficiaries. Stroke. (2013) 44:3429–35. doi: 10.1161/STROKEAHA.113.003165
32. Liang JW, Cifrese L, Ostojic LV, Shah SO, Dhamoon MS. Preventable readmissions and predictors of readmission after subarachnoid hemorrhage. Neurocrit Care. (2018) 29:336–43. doi: 10.1007/s12028-018-0557-1
33. Vahidy FS, Donnelly JP, McCullough LD, Tyson JE, Miller CC, Boehme AK, et al. Nationwide estimates of 30-day readmission in patients with ischemic stroke. Stroke. (2017) 48:1386–8. doi: 10.1161/STROKEAHA.116.016085
34. Bambhroliya AB, Donnelly JP, Thomas EJ, Tyson JE, Miller CC, McCullough LD, et al. Estimates and temporal trend for us nationwide 30-day hospital readmission among patients with ischemic and hemorrhagic stroke. JAMA Network Open. (2018) 1:e181190. doi: 10.1001/jamanetworkopen.2018.1190
35. Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, et al. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. (2009) 158:1184–93. doi: 10.1016/j.neuroscience.2008.07.044
36. Stroebele N, Muller-Riemenschneider F, Nolte CH, Muller-Nordhorn J, Bockelbrink A, Willich SN. Knowledge of risk factors, and warning signs of stroke: a systematic review from a gender perspective. Int J Stroke. (2011) 6:60–6. doi: 10.1111/j.1747-4949.2010.00540.x
37. Nolte CH, Jungehulsing GJ, Rossnagel K, Roll S, Haeusler KG, Reich A, et al. Vascular risk factor awareness before and pharmacological treatment before and after stroke and TIA. Eur J Neurol. (2009) 16:678–83. doi: 10.1111/j.1468-1331.2009.02562.x
38. Ahmadi M, Laumeier I, Ihl T, Steinicke M, Ferse C, Endres M, et al. A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:49–60. doi: 10.1016/S1474-4422(19)30369-2
39. Moreira E, Correia M, Magalhaes R, Silva MC. Stroke awareness in urban and rural populations from northern Portugal: knowledge and action are independent. Neuroepidemiology. (2011) 36:265–73. doi: 10.1159/000328867
40. Levine DA, Walter JM, Karve SJ, Skolarus LE, Levine SR, Mulhorn KA. Smoking and mortality in stroke survivors: can we eliminate the paradox? Cerebrovasc Dis. (2014) 23:1282–90. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.026
41. Baptista D, Abreu P, Azevedo E, Magalhaes R, Correia M. Sex differences in stroke incidence in a portuguese community-based study. Cerebrovasc Dis. (2018) 27:3115–23. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.005
42. Hsieh CY, Lin HJ, Hu YH, Sung SF. Stroke severity may predict causes of readmission within one year in patients with first ischemic stroke event. J Neurol Sci. (2017) 372:21–7. doi: 10.1016/j.jns.2016.11.026
Keywords: stroke readmissions, epidemiology, outcome, mortality, community-based study
Citation: Abreu P, Magalhães R, Baptista D, Azevedo E, Silva MC and Correia M (2020) Readmissions and Mortality During the First Year After Stroke—Data From a Population-Based Incidence Study. Front. Neurol. 11:636. doi: 10.3389/fneur.2020.00636
Received: 10 April 2020; Accepted: 28 May 2020;
Published: 24 July 2020.
Edited by:
Antonio Arauz, Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez, MexicoReviewed by:
Maurice Giroud, Centre Hospitalier Regional Universitaire De Dijon, FranceCopyright © 2020 Abreu, Magalhães, Baptista, Azevedo, Silva and Correia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Abreu, cG1hYnJldUBuZXRjYWJvLnB0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.