
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 26 June 2020
Sec. Neuroepidemiology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00501
This article is part of the Research TopicEpidemiology of Atypical Demyelinating DiseasesView all 15 articles
 Jyh Yung Hor1*
Jyh Yung Hor1* Nasrin Asgari2
Nasrin Asgari2 Ichiro Nakashima3
Ichiro Nakashima3 Simon A. Broadley4,5
Simon A. Broadley4,5 M. Isabel Leite6
M. Isabel Leite6 Najib Kissani7
Najib Kissani7 Anu Jacob8,9
Anu Jacob8,9 Romain Marignier10
Romain Marignier10 Brian G. Weinshenker11
Brian G. Weinshenker11 Friedemann Paul12
Friedemann Paul12 Sean J. Pittock11
Sean J. Pittock11 Jacqueline Palace6
Jacqueline Palace6 Dean M. Wingerchuk13
Dean M. Wingerchuk13 Jacinta M. Behne14
Jacinta M. Behne14 Michael R. Yeaman15
Michael R. Yeaman15 Kazuo Fujihara16* on behalf of the Guthy-Jackson Charitable Foundation International Clinical Consortium for NMOSD†
Kazuo Fujihara16* on behalf of the Guthy-Jackson Charitable Foundation International Clinical Consortium for NMOSD†Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon inflammatory disease of the central nervous system, manifesting clinically as optic neuritis, myelitis, and certain brain and brainstem syndromes. Cases clinically diagnosed as NMOSD may include aquaporin 4 (AQP4)-antibody-seropositive autoimmune astrocytopathic disease, myelin oligodendrocyte glycoprotein (MOG)-antibody-seropositive inflammatory demyelinating disease, and double-seronegative disease. AQP4-antibody disease has a high female-to-male ratio (up to 9:1), and its mean age at onset of ~40 years is later than that seen in multiple sclerosis. For MOG-antibody disease, its gender ratio is closer to 1:1, and it is more common in children than in adults. Its clinical phenotypes differ but overlap with those of AQP4-antibody disease and include acute disseminated encephalomyelitis, brainstem and cerebral cortical encephalitis, as well as optic neuritis and myelitis. Double-seronegative disease requires further research and clarification. Population-based studies over the past two decades report the prevalence and incidence of NMOSD in different populations worldwide. One relevant finding is the varying prevalence observed in different racial groups. Consistently, the prevalence of NMOSD among Whites is ~1/100,000 population, with an annual incidence of <1/million population. Among East Asians, the prevalence is higher, at ~3.5/100,000 population, while the prevalence in Blacks may be up to 10/100,000 population. For MOG-antibody disease, hospital-based studies largely do not observe any significant racial preponderance so far. This disorder comprises a significant proportion of NMOSD cases that are AQP4-antibody-seronegative. A recent Dutch nationwide study reported the annual incidence of MOG-antibody disease as 1.6/million population (adult: 1.3/million, children: 3.1/million). Clinical and radiological differences between AQP4-antibody and MOG-antibody associated diseases have led to interest in the revisions of NMOSD definition and expanded stratification based on detection of a specific autoantibody biomarker. More population-based studies in different geographical regions and racial groups will be useful to further inform the prevalence and incidence of NMOSD and their antibody-specific subgroups. Accessibility to AQP4-antibody and MOG-antibody testing, which is limited in many centers, is a challenge to overcome. Environmental and genetic studies will be useful accompaniments to identify other potential pathogenetic factors and specific biomarkers in NMOSD.
Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon inflammatory disease of the central nervous system, with clinical features of optic neuritis, myelitis, and certain brain, and brainstem syndromes. Although it had long been debated whether NMOSD is a severe variant of multiple sclerosis (MS), the discovery of NMOSD-specific aquaporin 4 (AQP4) antibody, and the subsequent clinical, immunological, and pathological data have established that NMOSD is indeed a distinct entity (1–3). Currently, cases clinically diagnosed as NMOSD may include AQP4-antibody-seropositive autoimmune astrocytopathic disease, myelin oligodendrocyte glycoprotein (MOG)-antibody-seropositive inflammatory demyelinating disease, and double-seronegative disease (4).
AQP4-antibody-seropositive NMOSD has a high female-to-male ratio (up to 9:1) (5), and its mean age at onset is around 40 years (6, 7), older than in MS. Pathologically, it is primarily an astrocytopathic disease rather than a demyelinating disease (3, 8). For MOG-antibody disease, the sex ratio is close to 1:1, and it is more common in children than in adults (9, 10). Its clinical manifestations overlap with those of AQP4-antibody disease but there are differences about which there is emerging consensus. Besides optic neuritis and myelitis, its clinical phenotypes also include acute or multiphasic disseminated encephalomyelitis (ADEM/MDEM), brainstem and cerebral cortical encephalitis, and cranial nerve involvement (11–14). Double (AQP4- and MOG-antibodies)-seronegative disease is enigmatic at present and requires further clinical and laboratory research for specific classification.
There have been several editions of the diagnostic criteria for NMOSD since 1999 (15, 16), with the latest being the 2015 International Panel on NMO Diagnosis (IPND) criteria (17). In the meantime, laboratory assays for AQP4 antibody and MOG antibody have also improved over time, with increased sensitivity and specificity (18, 19). These factors have contributed to the improvement in the accuracy of the diagnosis of NMOSD cases.
In this article, we review current data on the worldwide epidemiology of NMOSD, specifically on the population-based studies of NMOSD to determine its prevalence and incidence among different populations and racial groups. We emphasize that the field of NMOSD is undergoing a rapid evolution, making epidemiological estimates tentative. Additionally, different levels of diagnostic rigor to exclude NMOSD mimics and access to medical care in study populations can bias the epidemiological survey results in the disease, which makes the interpretation and comparison of the findings in and across the studies difficult. Nonetheless, the best known of current knowledge is being presented.
The PubMed database was searched for population-based studies on NMOSD with prevalence data, from 1st January 2000 till 11th March 2020. A combination of the following search terms was used: “neuromyelitis optica,” “NMO,” “NMOSD,” “aquaporin 4,” “AQP4,” “myelin oligodendrocyte glycoprotein,” “MOG,” “optico-spinal multiple sclerosis,” “OSMS,” “idiopathic inflammatory demyelinating disease,” “IIDD,” “epidemiology,” “prevalence,” “population,” and “demographic.” The reference lists in published articles on NMOSD were also queried to identify further studies. Additionally, recent conference proceedings of major neurology and MS congresses, including the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the Pan-Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS), were searched for relevant abstracts where the full studies are not yet published. Population-based studies with information on the prevalence of NMOSD in English language were reviewed. The final list of publications was selected on the basis of relevance to the topic.
The prevalence range of NMOSD is ~0.5–4/100,000, and may be up to 10/100,000 in certain racial groups. Nevertheless, this prevalence range is rather small relative to that of MS, which ranges from 1–2/100,000 in the equatorial region, to 150–200/100,000 in Canada and northern part of Europe (20, 21).
Over the past two decades, population-based studies of NMOSD have provided important insights into its prevalence. The earliest population-based studies were conducted in French West Indies (Martinique) (22, 23), Cuba (24), Denmark (25), and Tokachi Province on Hokkaido Island in Japan (26). Interestingly, the majority of these early studies were conducted on island populations, which facilitate population-based studies by providing well-delimited boundaries of the study area. Two of the studies (Martinique and Hokkaido) (22, 23, 26) have since been updated by the original groups of researchers.
Since 2017, several new population-based studies were published, expanding knowledge of NMOSD in diverse populations around the world. Inter-racial variation in prevalence, as summarized in Table 1, is notable and consistent across geographical regions. More recently, the Australia/New Zealand group has also re-analyzed the data from their 2017 study (47) to provide further information with regards to the prevalence among different racial groups in their large continent (46). Figure 1 is a map showing population-based prevalence studies of NMOSD around the world.
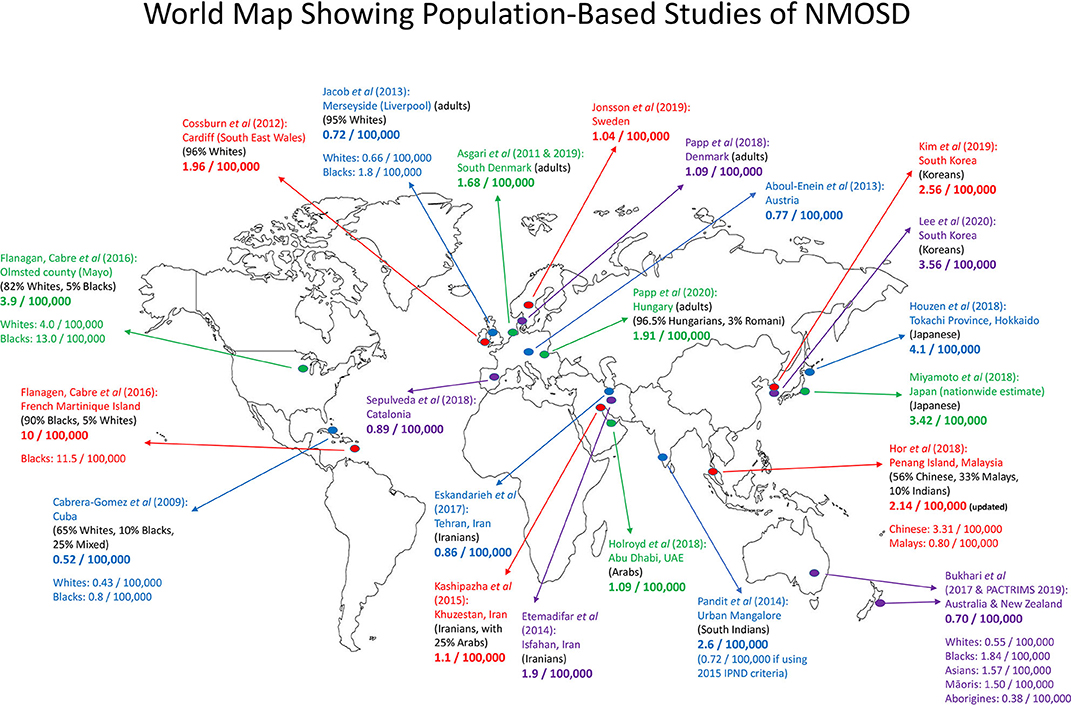
Figure 1. Map showing population-based prevalence studies of NMOSD around the world. There were eight studies in Europe, 10 in Asia, one in Oceania, and two in the Americas (one in Cuba and one joint study in the USA and Martinique Island). Numbers given were prevalence per 100,000 population. In certain studies, the prevalence according to racial groups was given. Adults, only adult population was studied.
East Asians (Japanese, Chinese, and Koreans) appear to have a higher prevalence of NMOSD (around 3.5/100,000) as compared to Whites and other Asian racial groups. The study in Hokkaido, Japan recorded a prevalence of 4.1/100,000 (36), while the Japanese nationwide survey estimated a prevalence of 3.42/100,000 (40). Meanwhile, a study conducted in the multi-racial population in Penang Island, Malaysia showed that the prevalence among Chinese was 3.31/100,000 (39). These results were in line with the genetic studies that showed that Japanese and Chinese share the same HLA risk genes for NMOSD, namely, HLA-DPB1*05:01 and HLA-DRB1*16:02 (49–51). In a very recent study from South Korea, by using a nationwide health insurance research dataset, it was calculated that the prevalence among Koreans was 3.56/100,000 in 2017 (48). More studies, especially from China, Taiwan, and Hong Kong will be useful to further inform the prevalence of NMOSD among the East Asians.
In 1971, a study conducted in a single hospital in the sub-Saharan African city of Ibadan (Nigeria) reported 95 cases of NMO, 22 cases of acute transverse myelitis, 11 cases of bilateral retrobulbar neuritis, and only two cases of MS over 12 years of hospital admissions (1957–1969) (52). During the same period, there were nine cases of non-Nigerians with MS (in eight Europeans and one Indian). It estimated that NMO cases made up 0.43/1000 (or 430/100,000) of the hospital population.
Population-based studies over the past two decades showed that Blacks also have a higher NMOSD prevalence than Whites. A study conducted in Liverpool, UK reported a prevalence rate of 1.8/100,000 among Blacks (29). The Australia/New Zealand study estimated a prevalence rate of 1.84/100,000 in those with African ancestry (46). The study conducted in the French Martinique Island in the Caribbean reported a very high prevalence of 11.5/100,000 among its Black population (34), and this was the highest prevalence reported so far. In population-based studies, within the same localities, prevalence among Blacks is always higher than in Whites, as seen in Cuba (24), Liverpool (UK) (29), Olmsted county (USA) (34), Martinique Island (34), and Australia/New Zealand (46).
As Blacks are genetically diverse, more data from different geographical regions are needed, and especially those from the African continent. Although no population-based studies of NMOSD have been published from Africa, recently there have been reports of NMOSD cases from various African countries that are to be compiled and reported elsewhere.
In recent nationwide and region-wide studies, the prevalence of NMOSD among Whites has consistently been ~1/100,000. The prevalence was 0.55/100,000 in Australia and New Zealand (46, 47), 0.89/100,000 in Catalonia (38), 1.09/100,000 in Denmark (42), and 1.04/100,000 in Sweden (43). Also recently, a re-analysis of the data of an earlier study from South Denmark has reported the prevalence of AQP4-antibody-positive NMOSD as 1.68/100,000, and the prevalence of the total clinical phenotype including AQP4-antibody-negative and MOG-antibody-positive subsets was 4.4/100,000 (25, 27).
Interestingly, the prevalence among Hungarians was slightly higher, at 1.91/100,000 (45). This has brought up the notion of whether there are some admixtures of Asian genes (from North East Asia) among the Hungarians (53). Furthermore, there is scarcity of prevalence data from Central Asia, and such data from this region will be informative.
If the 2015 IPND criteria were applied, the prevalence among South Indians in Mangalore was 0.72/100,000 (31). No cases were found among the 10% South Indian population in Penang Island, Malaysia (39), suggesting a low prevalence.
The Austronesian peoples reside in the Philippines, Malaysia, Indonesia, the Pacific Islands (Polynesia, Micronesia, and Hawaii), down to New Zealand, and also to the west in Madagascar. The study conducted in the multi-racial Penang Island, Malaysia (39) found that the prevalence of an Austronesian group, the Malays, was ~0.80/100,000 (this was revised from 0.43/100,000 as reported earlier, after a new case was diagnosed). The prevalence data from another Austronesian group was available recently, namely, the Māoris in New Zealand, with an estimated prevalence of 1.50/100,000 (46). Nevertheless, in the same study, no cases of NMOSD were found among the ~295,000 Pacific Islanders (Pasifika) (46). More data from other Austronesian groups in other localities will be useful to clarify this.
A study from Abu Dhabi, United Arab Emirates reported six cases of NMOSD among its citizens, consistent with a prevalence of 1.09/100,000 (AQP4-antibody seropositivity: 83%, all six cases were females) (41). If only adult citizens aged ≥20 years were considered (a total of five cases), the prevalence is higher at 1.76/100,000. Data on Arabs in other regions of Middle East and North Africa will be very informative.
The Australian Aborigines are one of the oldest populations in the world, with their ancestors having migrated to Australia around 50,000 years ago. There is evidence of some admixture of Denisovan genes in the Aborigines (Denisovans are an extinct species or subspecies of humans of the genus Homo). It is interesting to note that MS rarely exists in the Aborigines (54, 55). Recent data showed that NMOSD is also rare among the Aborigines, with a prevalence of 0.38/100,000 (46). However, the paper cautioned whether inequality in health care access may lead to this low figure.
MS is less common among Native Americans than in Whites in North America. Prior to AQP4-antibody discovery, a study conducted among the Native Canadians in Manitoba (56) found seven cases of “MS,” of which five cases were of NMO phenotypes, while the other two had brainstem involvement. Autopsy of one patient showed eosinophil infiltration in the cervical cord lesion, and retrospectively, this pathological finding suggests that this case was likely to be NMOSD. Genetically, Native Americans may be more closely related to early East Asians, and thus they may also have a higher prevalence than Whites. A re-look at these native populations will be helpful to confirm the results, though may be practically difficult.
After the arrival of Europeans in the 1500's, the indigenous populations of Latin American had dwindled rapidly. Today, along with the indigenous peoples, there is a large proportion of Whites, Blacks, and mixed races in Latin America.
In an earlier study from a tertiary hospital in Mexico City (57), using 1999 Wingerchuk criteria, a total of 34 cases of NMO were identified, with all patients being Mestizos (mixed race). By calculating the ratio of MS and NMO in the hospital, and by using the estimated MS prevalence in the country at that time, it was extrapolated that the prevalence of NMO among Mexican Mestizos was around 1.3/100,000. With the availability of AQP4-antibody assays, and the newer diagnostic criteria that include NMOSD cases, this prevalence rate is likely to be higher.
From the preliminary findings of a recent study involving seven general hospitals in Venezuela presented at a conference (58), it was estimated that the prevalence of NMOSD in Venezuela was 2.2/100,000, with a female-to-male ratio of 4:1, and again Mestizos formed the majority of those patients.
Studies from other representative populations will be useful to further inform the prevalence of NMOSD in Latin America.
The populations of North Africa consist mainly of Amazighs (Berbers) and Arabs. As in Whites/Caucasian populations, there appear to be much higher number of MS than NMOSD cases in North Africa (59, 60). There have been no population-based studies on NMOSD in North Africa so far. There is only one population-based study on Arabs in the Middle East (Abu Dhabi) (41), and the prevalence data among Amazighs are awaited.
Table 1 summarizes the incidence reported in the available population-based studies. Among Whites, the annual incidence of NMOSD is generally reported to be around 0.5–0.8/million (30, 38, 42, 43). In populations with a higher prevalence, the incidence is also higher. For instance, Blacks in Martinique have a high prevalence of 11.5/100,000, and its incidence was also reported to be high, at 7.3/million (34). Recently, the data from South Korea also showed a high incidence, ranging from 4.1 to 7.3/million for the period 2013–2017 (44, 48). Other populations with a prevalence higher than 1/100,000 also reported an incidence higher than 1/million [for example, 1.16/million in Arabs (41), and 1.32/million in Hungarians (45)].
A limitation regarding incidence calculation is that, if a new antibody test becomes available in the study region, or when there is increased awareness among clinicians, then the number of newly diagnosed cases in that particular year will be higher, leading to a higher incidence rate, even though the disease could have started many years earlier in some cases. Nevertheless, if researchers are able to calculate the incidence rates over the past few years (e.g., past 5 years) and average them, it is likely to be more accurate.
For pediatric NMOSD, there were two recent nationwide/region-wide studies that reported on its incidence. In the Danish study, the incidence of pediatric NMOSD was calculated as 0.31/million (61). In the Taiwanese study using the national health insurance research database, over the period from 2011 to 2015, the average annual incidence was reported as 1.1/million (62). Again, this higher incidence in Taiwan as compared to Denmark is not surprising as NMOSD is more prevalent among East Asians than Whites.
Some studies have analyzed how the clinical features and disability are affected by onset age and racial differences. Patients with young-onset NMOSD were more likely to have optic neuritis as onset attack, while older-onset patients often developed myelitis as the initial presentation (63). Furthermore, young-onset patients with optic neuritis were more likely to develop not only recurrent optic neuritis but also higher likelihood of developing blindness, as compared to older-onset patients with optic neuritis (63, 64). Conversely, older-onset patients with myelitis often had poor recovery, while most young-onset patients with myelitis recovered well without permanent motor disability (63, 64).
There also appears to be some differences in the clinical features of NMOSD among different races. Blacks and Asians tended to have lower mean ages at onset than Whites (Blacks: around 28–33 years, Asians: 35–40 years, vs. Whites: 44 years) (63, 65). Black and Asian patients were more likely to have brain and brainstem attacks and abnormalities on brain MRI as compared to Whites (64, 65). Overall, the risk of relapse was lowest in Japanese than in Whites and Blacks (63, 64).
Blacks were found to have a greater likelihood of developing visual disability with time than Whites and Japanese (63, 64). On the other hand, Whites had a higher probability of developing severe motor disability or wheelchair dependence as compared to Japanese (63). Severe attacks were more frequent in Blacks than in Asians and Whites, and therefore Blacks were at a higher risk of severe disability in the early course of the disease (65). In a study from the USA, patients with African ancestry were also found to have a higher mortality rate (15.4%) as compared to the overall mortality rate (7.0%) (66). Nonetheless, while race affected the clinical phenotype, age at onset, and severity of attacks, the overall outcomes were mostly dependent on early and effective immunosuppressive treatment (65).
After the discovery of the AQP4 antibody, a majority of NMO cases have been found positive for this antibody. Nevertheless, there is still a proportion of cases with an NMO phenotype that are persistently tested negative for AQP4 antibody, despite using the most sensitive cell-based assays available. It was later realized that some of these AQP4-antibody-negative NMOSD cases were in fact seropositive for MOG antibody. This so-called MOG-antibody-associated disease consists of a significant proportion of NMOSD cases that are AQP4-antibody seronegative, ranging from 7 to 42% (7, 67–70).
Interestingly, for MOG-antibody-associated disease, besides NMO phenotype, optic neuritis, and myelitis, some of these MOG-antibody-positive cases also have clinical phenotypes beyond the current NMOSD spectrum, such as ADEM/MDEM-like presentation (71), cerebral cortical encephalitis (12), and cranial nerve involvement (14). Pathologically, MOG-antibody-associated disease is a type of demyelinating disease, as opposed to astrocytopathic disease seen in AQP4-antibody-positive NMOSD (72, 73).
A recent Dutch nationwide study reported the incidence of MOG-antibody-associated disease as 1.6/million, with 1.3/million in adults, and a higher incidence of 3.1/million in children (74). It should be noted that this incidence rate of 1.6/million is higher than the incidence rate of 0.5–0.8/million in NMOSD (mostly AQP4-antibody-positive) among Whites.
So far, hospital-based studies largely did not observe any significant racial preponderance for MOG-antibody-associated disease. For instance, in the UK cohort, the racial breakdown was as expected in the general population (9). Nevertheless, from the annual report of the Oxford NMO Service, there were 145 patients with AQP4-antibody-positive NMOSD, 111 patients with MOG-antibody disease, and 28 patients who were double-seronegative. The proportion of MOG-antibody disease within the NMOSD spectrum was rather significant (75). Additionally, a study from Mayo Clinic on AQP4- and MOG-antibody testing for 15,598 patients showed higher positivity rate for MOG antibody (1291 patients, 8.3%) than for AQP4 antibody (387 patients, 2.3%). Of the adults, 6.5% were MOG-antibody positive vs. 2.6% for AQP4 antibody, while in children, 21.1% were positive for MOG antibody as compared to 1.9% for AQP4 antibody (76). Similarly, one study in Sri Lanka, in collaboration with the Mayo Clinic, also reported more MOG-antibody-positive cases (126 patients) than AQP4-antibody-positive cases (36 patients) (77). On the other hand, MOG-antibody-associated disease was relatively uncommon in the non-Caucasian population in Rio de Janeiro (Brazil) (70).
The preliminary findings of a population-based prevalence study of MOG-antibody-associated disease, jointly conducted at Olmsted county (USA) and Martinique Island, were recently presented at a conference (ECTRIMS 2019) (78). In Olmsted county, the prevalence was calculated to be 3.42/100,000, with an incidence of 2.39/million, while at Martinique, the prevalence was 1.6/100,000, with an incidence of 1.12/million.
In the Catalonia NMOSD prevalence study, 12% of cases were MOG-antibody-positive (38). However, the cases in this study were required to strictly fulfill the 2015 IPND criteria, and thus only those with an NMO phenotype were analyzed (The prevalence of MOG-antibody-positive NMO was calculated to be 0.11/100,000.). Needless to say, if MOG-antibody-positive cases with optic neuritis alone or myelitis alone and those with ADEM-like presentation are included, the prevalence of MOG-antibody disease is likely to be higher.
More data from different geographical areas are clearly in need to further inform about the prevalence and incidence of MOG-antibody-associated disease.
Some demographic and epidemiological data and clinical features of AQP4-antibody-positive NMOSD and MOG-antibody-associated disease in comparison with MS are shown in Table 2.
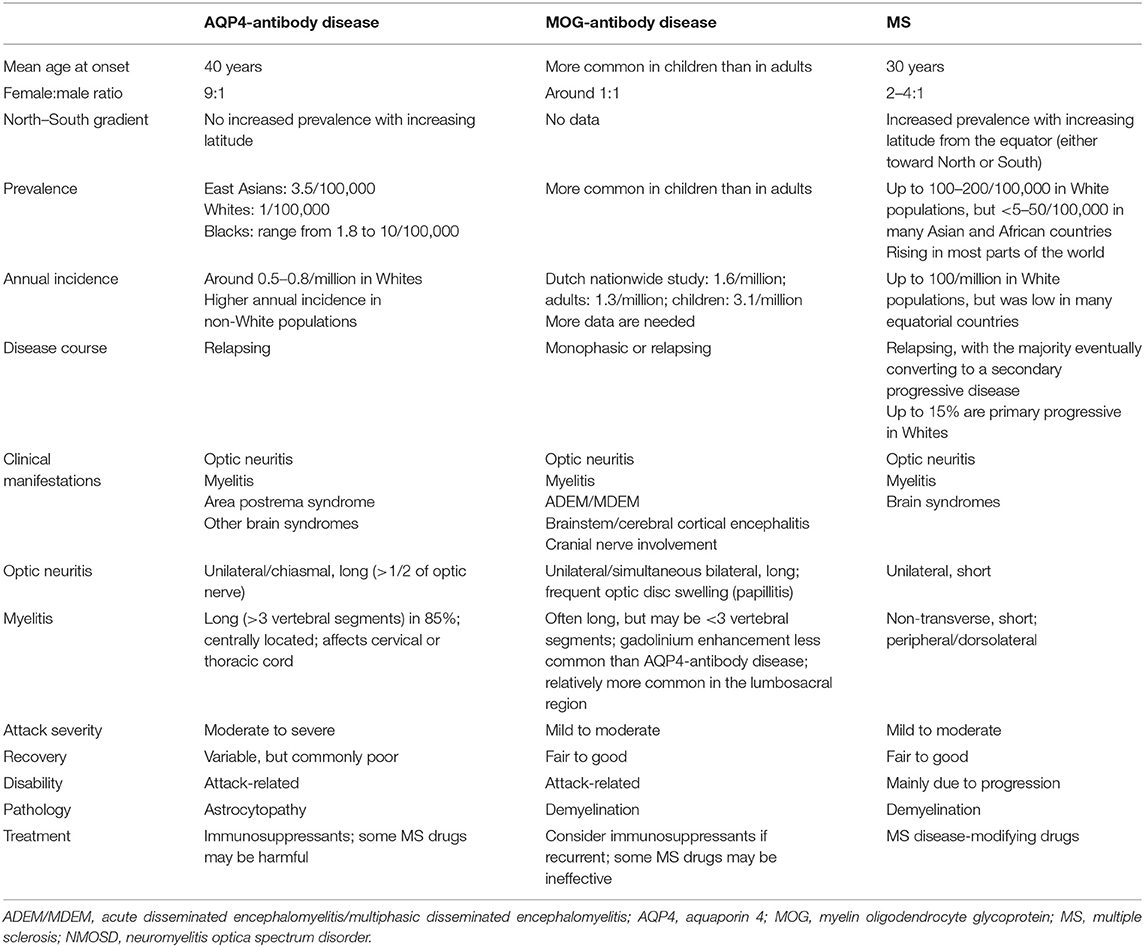
Table 2. Epidemiological and clinical comparison between AQP4-antibody-seropositive NMOSD, MOG-antibody disease, and MS.
There appears to be varying prevalence rates of NMOSD, most cases of which are AQP4-antibody-positive cases, among the different racial groups worldwide, with East Asians and Blacks having a higher prevalence than Whites. In most regions, these prevalence rates are lower than that of MS. In AQP4-antibody-positive NMOSD, female preponderance is definite (up to 90%) and the majority of the cases are adults. Moreover, the clinical features of NMOSD and disability accrual may be influenced by onset age and race. The data suggest that certain genetic and environmental factors associated with race may be involved in the pathogenesis of NMOSD. More well-designed population-based and longitudinal studies in different geographical areas and racial groups will be useful to clarify the issue, and to shed new lights onto this unique neuroinflammatory disease. Among AQP4-antibody-negative NMOSD, some patients are MOG-antibody-positive, and unlike AQP4-antibody-positive NMOSD, males, and females are equally affected by MOG-antibody-associated disease and the prevalence may be higher in children than in adults. However, the prevalence data of MOG-antibody-associated disease including the ones with an NMOSD phenotype are still insufficient and being accumulated. Accessibility to AQP4-antibody and MOG-antibody testing, which is currently limited in many regions, is a challenge to overcome.
JH conceived and designed the study, drafted the manuscript, contributed to data acquisition, and critically revised the manuscript for intellectual content. NA, IN, SB, ML, NK, AJ, RM, BW, FP, SP, JP, DW, JB, and MY made substantial contribution to the intellectual content, contributed to data acquisition, and critically revised the manuscript for intellectual content. KF supervised the study, conceived and designed the study, drafted the manuscript, contributed to data acquisition, and critically revised the manuscript for intellectual content. All authors approved the final manuscript.
JH, NA, NK, AJ, RM, and JB report no disclosures related to this work. IN has received speaker honoraria and travel funding from Mitsubishi Tanabe Pharma, Biogen Japan, and Novartis Pharmaceuticals, and received research support from LSI Medience Corporation, and has been funded by JSPS KAKENHI Grant Number 17K09772. SB has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Scherring, Biogen-Idec, Merck-Serono, Novartis, and Sanofi-Genzyme; has received speakers honoraria from Biogen-Idec and Genzyme; is an investigator in clinical trials sponsored by Biogen Idec, Novartis, and Genzyme; and was the recipient of an unencumbered research grant from Biogen-Idec. ML was partly supported by an NHS England highly specialized commissioning group for a neuromyelitis service. She has received support for scientific meetings and honoraria for presentations from Biogen Idec and Novartis and for advisory work from Viela Bio. BW has received royalties from RSR Ltd, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen GbR for a patent of NMO-IgG as a diagnostic test for NMO and related disorders, served on adjudication committee for clinical trials in NMO being conducted by MedImmune and Alexion, and consulted for Chugai, Mitsubishi-Tanabe regarding a clinical trial for NMO. FP has received research support from DFG, BMBF, KKNMS, and the Guthy-Jackson Charitable Foundation. He serves on steering committees of the OCTIMS study (Novartis) and the N-Momentum study (Viela Bio) and has received personal compensation and research support from Alexion, Bayer, Biogen, Roche, Merck, Teva, Shire, Celgene, Novartis, and Sanofi Genzyme. He is an associate editor of Neurology: Neuroimmunology and Neuroinflammation. SP has received grants, personal fees, non-financial support, honorarium, and travel expenses for speaking from Alexion Pharmaceuticals and the Guthy-Jackson Charitable Foundation paid to his institution; grants from Grifols, the National Institutes of Health, and Autoimmune Encephalitis Alliance paid to his institution; consulting fees from Euroimmun paid to his institution; and grants, personal fees, non-financial support, and honorarium from MedImmune; served on the advisory board of Alexion Pharmaceuticals; holds patent 8,889,102 and 9,891,219B2; and has patents pending for GFAP-IgG, Septin-5-IgG, MAP1B-IgG, Kelch-like protein 11, and PDE10A. JP was partly funded by highly specialized services to run a national congenital myasthenia service and a neuromyelitis service. She has received support for scientific meetings and honoraria for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai, Bayer Schering, Alexion, Roche, Genzyme, MedImmune, EuroImmun, MedDay, Abide ARGENX, UCB, and Viela Bio, and grants from Merck Serono, Novartis, Biogen Idec, Teva, Abide, MedImmune, Bayer Schering, Genzyme, Chugai, and Alexion. She has received grants from the MS society, Guthy-Jackson Charitable Foundation, NIHR, Oxford Health Services Research Committee, EDEN, MRC, GMSI, John Fell, and Myaware for research studies. DW has received research support from Alexion and TerumoBCT and honoraria from MedImmune, Novartis, Biogen, Celgene, Genentech, TG Therapeutics, Arcus Medica, Third Rock Ventures, and Reistone. MY is founder of NovaDigm Therapeutics, Inc, and Metacin, Inc. He is a member of the Genentech Scientific Advisory Committee, and has received travel expenses or honoraria from Genentech and Alexion. KF has received grants from Ministry of Education, Science and Technology of Japan and Ministry of Health, Welfare, and Labor of Japan, and received honoraria, and/or travel expenses for speaking, and/or advisory boards from Mitsubishi Tanabe, Biogen, Bayer, Takeda, Novartis, Alexion, VielaBio, Asahi Kasei, Dainihon Sumitomo, Eisai, Teijin, Ono, Roche, and Chugai.
Active members of the Guthy-Jackson Charitable Foundation (GJCF) International Clinical Consortium for NMOSD (in alphabetical order as of March 2020): Hesham Abboud (Case Western Reserve University, Cleveland, OH, USA); Orhan Aktas (Institute of Neuroimmunology, Düsseldorf, Germany); Raed Alroughani (Amiri Hospital, Kuwait City, Kuwait); Ayse Altintas (Koc University School of Medicine, Istanbul, Turkey); Lilyana Amezcua (University of Southern California, Los Angeles, CA, USA); Metha Apiwattanakul (Prasat Neurological Institute, Bangkok, Thailand); Nasrin Asgari (University of Southern Denmark, Odense, Denmark); Brenda Banwell (The Children's Hospital of Philadelphia, University of Pennsylvania, PA, USA); Terrence M. Blaschke [Stanford University (Emeritus), Palo Alto, CA, USA]; Jeffrey Bennett (University of Colorado, Denver, CO, USA); Denis Bichuetti (Universidade Federal de São Paulo, São Paulo, Brazil); James Bowen (Swedish Neuroscience Institute, Seattle, WA, USA); Alexey Boyko (Prigov's Russian Scientific Research Medical University, Moscow, Russia); Alexander Brandt (University of California, Irvine, CA, USA); Simon A. Broadley (Griffith University, Southport, QLD, Australia); Wolfgang Brück (University Medical Center Göttingen, Göttingen, Germany); Edgar Carnero Contentti (Hospital Alemán, Buenos Aires, Argentina); Robert Carruthers (University of British Columbia, Vancouver, Canada); Tanuja Chitnis (Brigham and Women's Hospital and Massachusetts General Hospital, Boston, MA, USA); Jeffrey Cohen (Cleveland Clinic, Cleveland, OH, USA); Guillermo Delgado-García (National Autonomous University of Mexico, Mexico City, Mexico); Irena Dujmovic Basuroski (UNC School of Medicine, Chapel Hill, NC, USA); Nikos Evangelou (University of Nottingham, Nottingham, UK); Kazuo Fujihara (Fukushima Medical University, Fukushima, Japan); Andrew Goodman (University of Rochester, Rochester, NY, USA); Benjamin Greenberg (University of Texas Southwestern Medical Center at Dallas, Dallas, TX, USA); Yael Hacohen (Great Ormond Street Hospital/UCL, London, UK); May Han (Stanford University School of Medicine, Stanford, CA, USA); Joachim Havla (Ludwig-Maximilians University, Munich, Germany); Kerstin Hellwig (St. Josef Hospital Bochum, Bochum, Germany); Jyh Yung Hor (Penang General Hospital, Penang, Malaysia); Raffaele Iorio (Institute of Neurology, Catholic University of Sacred Heart, Rome, Italy); Anu Jacob (Walton Centre, Liverpool, UK); Sven Jarius (University of Heidelberg, Heidelberg, Germany); Jorge Andres Jimenez Arango (Universidad de Antioquia, Medellin, Colombia); Ilana Katz-Sand (Icahn School of Medicine at Mount Sinai, New York, NY, USA); Ho Jin Kim (National Cancer Center, Goyang, Republic of Korea); Sung Min Kim (Seoul National University Hospital, Seoul, Republic of Korea); Dorlan Kimbrough (Brigham and Women's Hospital and Massachusetts General Hospital, Boston, MA, USA); Najib Kissani (Neurology Department, University Hospital, Marrakech, Morocco); Ilya Kister (NYU, Langone Medical Center, NY, USA); Eric Klawiter (Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA); Ingo Kleiter (Marianne-Strauß-Klinik, Berg, Germany); Marco Lana-Peixoto (Universidade Federal de Minas Gerais, Belo Horizonte, Brazil); M. Isabel Leite (University of Oxford, Oxford, UK); Michael Levy (Massachusetts General Hospital, Boston, MA, USA); Yaou Liu (Xuanwu Hospital, Capital Medical University, Beijing, China); Fred Lublin (Icahn School of Medicine at Mount Sinai, New York, NY, USA); Youssoufa Maiga (Teaching Hospital Gabriel Touré, Bamako, Mali); Yang Mao-Draayer (University of Michigan, Ann Arbor, MI, USA); Romain Marignier (CHU Lyon, Lyon, France); Sara Mariotto (University of Verona, Verona, Italy); Marcelo Matiello (Brigham and Women's Hospital and Massachusetts General, Boston, MA, USA); Maureen Mealy (Viela Bio, Baltimore, MD, USA); Esther Melamed (UT Austin, Austin, TX, USA); Callene Momtazee (University of California Los Angeles, CA, USA); Ichiro Nakashima (Tohoku Medical and Pharmaceutical University, Sendai, Japan); Jayne Ness (Children's of Alabama—Neurology, AL, USA); Kevin O'Connor (Yale University School of Medicine, New Haven, CT, USA); Celia Oreja-Guevara (Hospital Clínico San Carlos, Madrid, Spain); Jacqueline Palace (University of Oxford, Oxford, UK); Lekha Pandit (K S Hegde Medical Academy, Mangalore, India); Friedemann Paul (Charité University Medicine Berlin, Berlin, Germany); Sarah Planchon Pope (Cleveland Clinic, Cleveland, OH, USA); Anne-Katrin Pröbstel (University Hospital Basel, Basel, Switzerland); Peiqing Qian (Swedish Neuroscience Institute, Seattle, WA, USA); Chao Quan (Huashan Hospital, Shanghai, China); Pavle Repovic (Swedish Neuroscience Institute, Seattle, WA, USA); Claire Riley (Columbia University, New York, NY, USA); Marius Ringelstein (Heinrich-Heine-University, Düsselforf, Germany); Victor Rivera (Houston Methodist Hospital, Houston, TX, USA); Dalia Rotstein (St. Michael's Hospital, Toronto, Canada); Klemens Ruprecht (Charité University Medicine Berlin, Berlin, Germany); Maria José Sá (Centro Hospitalar São João, Porto, Portugal); Albert Saiz (IDIBAPS, Hospital Clinic of Barcelona, Barcelona, Spain); Douglas Sato (Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil); Ché Serguera (MIRCen INSERM/CEA, Paris, France); Eslam Shosha (Prince Sultan Military Medical City, Riyadh, Saudi Arabia); Nancy Sicotte (Cedars Sinai, Los Angeles, CA, USA); Sasitorn Siritho (Mahidol University, Bangkok, Thailand); Aksel Siva (Istanbul University, Cerrahpaşa School of Medicine, Istanbul, Turkey); Terry J. Smith (University of Michigan, Ann Arbor, MI, USA); Ibis Soto de Castillo (Hospital Universitario de Maracaibo, Maracaibo, Venezuela); Silvia Tenembaum (Hospital de Pediatria, Buenos-Aires, Argentina); Leticia Tornes (University of Miami, FL, USA); Pablo Villoslada (Stanford University School of Medicine, Stanford, CA, USA); Brian G. Weinshenker (Mayo Clinic, Rochester, MN, USA); Dean M. Wingerchuk (Mayo Clinic, Scottsdale, AZ, USA); Jens Würfel (MIAC AG, Basel, Switzerland); Bassem Yamout (American University of Beirut Medical Center, Beirut, Lebanon); Michael R. Yeaman (University of California, Los Angeles, Los Angeles, CA, USA); Ann Yeh (Sick Kids Hospital, Toronto, Canada); and Scott Zamvil (University of California San Francisco, CA, USA).
1. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
2. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. (2005) 202:473–7. doi: 10.1084/jem.20050304
3. Fujihara K, Misu T, Nakashima I, Takahashi T, Bradl M, Lassmann H, et al. Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol. (2012) 3:58–73. doi: 10.1111/j.1759-1961.2012.00030.x
4. Fujihara K. Neuromyelitis optica spectrum disorders: still evolving and broadening. Curr Opin Neurol. (2019) 32:385–94. doi: 10.1097/WCO.0000000000000694
5. Gold SM, Willing A, Leypoldt F, Paul F, Friese MA. Sex differences in autoimmune disorders of the central nervous system. Semin Immunopathol. (2019) 41:177–88. doi: 10.1007/s00281-018-0723-8
6. Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. (2014) 71:276–83. doi: 10.1001/jamaneurol.2013.5857
7. Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haider Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. (2014) 82:474–81. doi: 10.1212/WNL.0000000000000101
8. Kawachi I, Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry. (2017) 88:137–45. doi: 10.1136/jnnp-2016-313300
9. Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. (2017) 140:3128–38. doi: 10.1093/brain/awx276
10. Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. (2018)90:e1858–69. doi: 10.1212/WNL.0000000000005560
11. Nakashima I. Anti-myelin oligodendrocyte glycoprotein antibody in demyelinating diseases. Clin Exp Neuroimmunol. (2015) 6(Suppl.1):59–63. doi: 10.1111/cen3.12262
12. Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e322. doi: 10.1212/NXI.0000000000000322
13. Fujihara K, Sato DK, Nakashima I, Takahashi T, Kaneko K, Ogawa R, et al. Myelin oligodendrocyte glycoprotein immunoglobulin G-associated disease: an overview. Clin Exp Neuroimmunol. (2018) 9(Suppl.1):48–55. doi: 10.1111/cen3.12434
14. Cobo-Calvo A, Ayrignac X, Kerschen P, Horellou P, Cotton F, Labauge P, et al. Cranial nerve involvement in patients with MOG antibody-associated disease. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e543. doi: 10.1212/NXI.0000000000000543
15. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. (1999) 53:1107–14.
16. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. (2006) 66:1485–9.
17. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
18. Waters P, Reindl M, Saiz A, Schanda K, Tuller F, Kral V, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. (2016) 87:1005–15. doi: 10.1136/jnnp-2015-312601
19. Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e674. doi: 10.1212/NXI.0000000000000674
20. Evans C, Beland SG, Kulaga S, Wolfson C, Kingwell E, Marriott J, et al. Incidence and prevalence of multiple sclerosis in the Americas: a systematic review. Neuroepidemiology. (2013) 40:195–210. doi: 10.1159/000342779
21. Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, et al. Incidence and prevalence of multiple sclerosis in europe: a systematic review. BMC Neurol. (2013) 13:128. doi: 10.1186/1471-2377-13-128
22. Cabre P, Heinzlef O, Merle H, Buisson GG, Bera O, Bellance R, et al. MS and neuromyelitis optica in Martinique (French West Indies). Neurology. (2001) 56:507–14. doi: 10.1212/wnl.56.4.507
23. Cabre P, Gonzalez-Quevedo A, Lannuzel A, Bonnan M, Merle H, Olindo S, et al. Descriptive epidemiology of neuromyelitis optica in the caribbean basin [article in french, with english abstract]. Rev Neurol. (2009) 165:676–83. doi: 10.1016/j.neurol.2009.02.012
24. Cabrera-Gómez JA, Kurtzke JF, Gonzalez-Quevedo A, Lara-Rodríguez R. An epidemiological study on neuromyelitis optica in Cuba. J Neurol. (2009) 256:35–44. doi: 10.1007/s00415-009-0009-0
25. Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO. A population-based study of neuromyelitis optica in Caucasians. Neurology. (2011)76:1589–95. doi: 10.1212/WNL.0b013e3182190f74
26. Houzen H, Niino M, Hirotani M, Fukazawa T, Kikuchi S, Tanaka K, et al. Increased prevalence, incidence, and female predominance of multiple sclerosis in northern Japan. J Neurol Sci. (2012) 323:117–22. doi: 10.1016/j.jns.2012.08.032
27. Asgari N, Lillevang ST, Skejoe HP, Kyvik KO. Epidemiology of neuromyelitis optica spectrum disorder in Denmark (1998–2008, 2007–2014). Brain Behav. (2019) 9:e01338. doi: 10.1002/brb3.1338
28. Cossburn M, Tackley G, Baker K, Ingram G, Burtonwood M, Malik G, et al. The prevalence of neuromyelitis optica in south east wales. Eur J Neurol. (2012) 19:655–9. doi: 10.1111/j.1468-1331.2011.03529.x
29. Jacob A, Panicker J, Lythgoe D, Elsone L, Mutch K, Wilson M, et al. The epidemiology of neuromyelitis optica amongst adults in the merseyside county of United Kingdom. J Neurol. (2013) 260:2134–7. doi: 10.1007/s00415-013-6926-y
30. Aboul-Enein F, Seifert-Held T, Mader S, Kuenz B, Lutterotti A, Rauschka H, et al. Neuromyelitis optica in Austria in 2011: to bridge the gap between neuroepidemiological research and practice in a study population of 8.4 million people. PLoS ONE. (2013) 8:e79649. doi: 10.1371/journal.pone.0079649
31. Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. (2014) 20:1651–3. doi: 10.1177/1352458514521503
32. Etemadifar M, Dashti M, Vosoughi R, Abtahi SH, Ramagopalan SV, Nasr Z. An epidemiological study of neuromyelitis optica in Isfahan. Mult Scler. (2014) 20:1920–2. doi: 10.1177/1352458514537699
33. Kashipazha D, Mohammadianinejad SE, Majdinasab N, Azizi M, Jafari M. A descriptive study of prevalence, clinical features and other findings of neuromyelitis optica and neuromyelitis optica spectrum disorder in khuzestan province, Iran. Iran J Neurol. (2015) 14:204–10.
34. Flanagan EP, Cabre P, Weinshenker BG, St Sauver J, Jacobson DJ, Majed M, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol. (2016) 79:775–83. doi: 10.1002/ana.24617
35. van Pelt ED, Wong YY, Ketelslegers IA, Siepman DA, Hamann D, Hintzen RQ. Incidence of AQP4-IgG seropositive neuromyelitis optica spectrum disorders in the Netherlands: about one in a million. Mult Scler Exp Transl Clin. (2016) 2:2055217315625652. doi: 10.1177/2055217315625652
36. Houzen H, Kondo K, Niino M, Horiuchi K, Takahashi T, Nakashima I, et al. Prevalence and clinical features of neuromyelitis optica spectrum disorders in northern Japan. Neurology. (2017) 89:1995–2001. doi: 10.1212/WNL.0000000000004611
37. Eskandarieh S, Nedjat S, Azimi AR, Moghadasi AN, Sahraian MA. Neuromyelitis optica spectrum disorders in Iran. Mult Scler Relat Disord. (2017) 18:209–12. doi: 10.1016/j.msard.2017.10.007
38. Sepúlveda M, Aldea M, Escudero D, Llufriu S, Arrambide G, Otero-Romero S, et al. Epidemiology of NMOSD in catalonia: influence of the new 2015 criteria in incidence and prevalence estimates. Mult Scler. (2018) 24:1843–51. doi: 10.1177/1352458517735191
39. Hor JY, Lim TT, Chia YK, Ching YM, Cheah CF, Tan K, et al. Prevalence of neuromyelitis optica spectrum disorder in the multi-ethnic penang island, malaysia, and a review of worldwide prevalence. Mult Scler Relat Disord. (2018) 19:20–4. doi: 10.1016/j.msard.2017.10.015
40. Miyamoto K, Fujihara K, Kira JI, Kuriyama N, Matsui M, Tamakoshi A, et al. Nationwide epidemiological study of neuromyelitis optica in Japan. J Neurol Neurosurg Psychiatry. (2018) 89:667–8. doi: 10.1136/jnnp-2017-317321
41. Holroyd KB, Aziz F, Szolics M, Alsaadi T, Levy M, Schiess N. Prevalence and characteristics of transverse myelitis and neuromyelitis optica spectrum disorders in the United Arab Emirates: a multicenter, retrospective study. Clin Exp Neuroimmunol. (2018) 9:155–61. doi: 10.1111/cen3.12458
42. Papp V, Illes Z, Magyari M, Koch-Henriksen N, Kant M, Pfleger CC, et al. Nationwide prevalence and incidence study of neuromyelitis optica spectrum disorder in Denmark. Neurology. (2018) 91:e2265–75. doi: 10.1212/WNL.0000000000006645
43. Jonsson DI, Sveinsson O, Hakim R, Brundin L. Epidemiology of NMOSD in Sweden from 1987 to 2013: a nationwide population-based study. Neurology. (2019) 93:e181–9. doi: 10.1212/WNL.0000000000007746
44. Kim JE, Park SH, Han K, Kim HJ, Shin DW, Kim SM. Prevalence and incidence of neuromyelitis optica spectrum disorder and multiple sclerosis in Korea. Mult Scler. (2019) 352458519888609. doi: 10.1177/1352458519888609. [Epub ahead of print].
45. Papp V, Iljicsov A, Rajda C, Magyari M, Koch-Henriksen N, Petersen T, et al. A population-based epidemiological study of neuromyelitis optica spectrum disorder in hungary. Eur J Neurol. (2020) 27:308–17. doi: 10.1111/ene.14079
46. Bukhari W, Khalilidehkordi E, Mason D, Barnett M, Taylor B, Fabis-Pedrini M, et al. NMOSD and MS prevalence in the indigenous populations of Australia and New Zealand. Presented at the 12th Congress of the Pan-Asian Committee for Treatment and Research in Multiple Sclerosis (PACTRIMS), Singapore (2019).
47. Bukhari W, Prain KM, Waters P, Woodhall M, O'Gorman CM, Clarke L, et al. Incidence and prevalence of NMOSD in Australia and New Zealand. J Neurol Neurosurg Psychiatry. (2017)88:632–8. doi: 10.1136/jnnp-2016-314839
48. Lee HL, Kim JY, Seok JM, Hong YH, Lim NG, Shin HY, et at. Prevalence and incidence of neuromyelitis optica spectrum disorder in Korea: population based study. J Korean Med Sci. (2020) 35:e115. doi: 10.3346/jkms.2020.35.e115
49. Matsushita T, Matsuoka T, Isobe N, Kawano Y, Minohara M, Shi N, et al. Association of the HLA-DPB1*0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders. Tissue Antigens. (2009) 73:171–6. doi: 10.1111/j.1399-0039.2008.01172.x
50. Wang H, Dai Y, Qiu W, Zhong X, Wu A, Wang Y, et al. HLA-DPB1*0501 is associated with susceptibility to anti-aquaporin-4 antibodies positive neuromyelitis optica in Southern Han Chinese. J Neuroimmunol. (2011) 233:181–4. doi: 10.1016/j.jneuroim.2010.11.004
51. Yoshimura S, Isobe N, Matsushita T, Yonekawa T, Masaki K, Sato S, et al. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin-4 antibody status. J Neurol Neurosurg Psychiatry. (2013) 84:29–34. doi: 10.1136/jnnp-2012-302925
52. Osuntokun BO. The pattern of neurological illness in tropical Africa: experience at Ibadan, Nigeria. J Neurol Sci. (1971) 12:417–42.
53. Hellenthal G, Busby GB, Band G, Wilson JF, Capelli C, Falush D, et al. A genetic atlas of human admixture history. Science. (2014) 343:747–51. doi: 10.1126/science.1243518
54. Hammond SR, de Wytt C, Maxwell IC, Landy PJ, English D, McLeod JG, et al. The epidemiology of multiple sclerosis in Queensland, Australia. J Neurol Sci. (1987) 80:185–204.
55. McLeod JG, Hammond SR, Hallpike JF. Epidemiology of multiple sclerosis in Australia: with NSW and SA survey results. Med J Aust. (1994) 160:117–22.
56. Mirsattari SM, Johnston JB, McKenna R, Del Bigio MR, Orr P, Ross RT, et al. Aboriginals with multiple sclerosis: HLA types and predominance of neuromyelitis optica. Neurology. (2001) 56:317–23.
57. Rivera JF, Kurtzke JF, Booth VJ, Corona T. Characteristics of devic's disease (neuromyelitis optica) in Mexico. J Neurol. (2008) 255:710–5. doi: 10.1007/s00415-008-0781-2
58. Soto de Castillo I, Molina O, Soto A, Armas E, Mendoza S, Castillo MC, et al. Neuromyelitis optica spectrum disorders: epidemiologic study in Venezuela. Presented at the 11th International NMO Roundtable Conference, Los Angeles (2019).
59. Daoudi S, Bouzar M. Neuromyelitis optica spectrum disorders in algeria: a preliminary study in the region of tizi ouzou. Mult Scler Relat Disord. (2016) 6:37–40. doi: 10.1016/j.msard.2015.12.005
60. Salama S, Marouf H, Reda MI, Mansour AR, El Khory O, Levy M. Clinical and radiological characteristics of neuromyelitis optica spectrum disorder in the North Egyptian Nile Delta. J Neuroimmunol. (2018) 324:22–5. doi: 10.1016/j.jneuroim.2018.08.014
61. Boesen MS, Jensen PE, Born AP, Magyari M, Nilsson AC, Hoei-Hansen C, et al. Incidence of pediatric neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody-associated disease in Denmark 2008 - 2018: a nationwide, population-based cohort study. Mult Scler Relat Disord. (2019) 33:162–7. doi: 10.1016/j.msard.2019.06.002
62. Lin WS, Wang HP, Chen HM, Lin JW, Lee WT. Epidemiology of pediatric multiple sclerosis, neuromyelitis optica, and optic neuritis in Taiwan. J Neurol. (2019). 267:925–32. doi: 10.1007/s00415-019-09647-9
63. Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. (2012) 135:1834–49. doi: 10.1093/brain/aws109
64. Palace J, Lin DY, Zeng D, Majed M, Elsone L, Hamid S, et al. Outcome prediction models in AQP4-IgG positive neuromyelitis optica spectrums disorders. Brain. (2019) 142:1310–23. doi: 10.1093/brain/awz054
65. Kim SH, Mealy MA, Levy M, Schmidt F, Ruprecht K, Paul F, et al. Racial differences in neuromyelitis optica spectrum disorder. Neurology. (2018) 91:e2089–99. doi: 10.1212/WNL.0000000000006574
66. Mealy MA, Kessler RA, Rimler Z, Reid A, Totonis L, Cutter G, et al. Mortality in neuromyelitis optica is strongly associated with African ancestry. Neurol Neuroimmunol Neuroinflamm. (2018) 5:e468. doi: 10.1212/NXI.0000000000000468
67. Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. (2012) 79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e
68. Siritho S, Sato DK, Kaneko K, Fujihara K, Prayoonwiwat N. The clinical spectrum associated with myelin oligodendrocyte glycoprotein antibodies (anti-MOG-Ab) in Thai patients. Mult Scler. (2016) 22:964–8. doi: 10.1177/1352458515614093
69. Hamid SH, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. (2017) 264:2088–94. doi: 10.1007/s00415-017-8596-7
70. Papais-Alvarenga RM, Neri VC, de Araújo e Araújo AC, da Silva EB, Alvarenga MP, Pereira AB, et al. Lower frequency of antibodies to MOG in Brazilian patients with demyelinating diseases: an ethnicity influence? Mult Scler Relat Disord. (2018) 25:87–94. doi: 10.1016/j.msard.2018.07.026
71. Misu T, Fujihara K. Neuromyelitis optica spectrum and myelin oligodendrocyte glycoprotein antibody-related disseminated encephalomyelitis. Clin Exp Neuroimmunol. (2019) 10:9–17. doi: 10.1111/cen3.12491
72. Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. (2015) 2:e62. doi: 10.1212/NXI.0000000000000062
73. Narayan R, Simpson A, Fritsche K, Salama S, Pardo S, Mealy M, et al. MOG antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. (2018) 25:66–72. doi: 10.1016/j.msard.2018.07.025
74. de Mol CL, Wong YY, van Pelt ED, Wokke BH, Siepman TA, Neuteboom RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. (2019). 1352458519845112. doi: 10.1177/1352458519845112. [Epub ahead of print].
75. Oxford University Hospitals NHS Foundation Trust. Diagnostic and Advisory Service for Neuromyelitis Optica (NMO) Annual Report 2018. Oxford University Hospitals NHS Foundation Trust (2018).
76. Kunchok A, Chen JJ, McKeon A, Mills JR, Flanagan EP, Pittock SJ. Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurol. (2020) 77:257–9. doi: 10.1001/jamaneurol.2019.3656
77. Senanayake B, Jitprapaikulsan J, Aravinthan M, Wijesekera JC, Ranawaka UK, Riffsy MT, et al. Seroprevalence and clinical phenotype of MOG-IgG-associated disorders in Sri Lanka. J Neurol Neurosurg Psychiatry. (2019) 90:1381–3. doi: 10.1136/jnnp-2018-320243
Keywords: neuromyelitis optica spectrum disorder, NMOSD, AQP4, MOG, prevalence, incidence, population study, epidemiology
Citation: Hor JY, Asgari N, Nakashima I, Broadley SA, Leite MI, Kissani N, Jacob A, Marignier R, Weinshenker BG, Paul F, Pittock SJ, Palace J, Wingerchuk DM, Behne JM, Yeaman MR and Fujihara K (2020) Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front. Neurol. 11:501. doi: 10.3389/fneur.2020.00501
Received: 15 March 2020; Accepted: 07 May 2020;
Published: 26 June 2020.
Edited by:
Su-Hyun Kim, National Cancer Center, South KoreaReviewed by:
Kok Pin Yong, National Neuroscience Institute (NNI), SingaporeCopyright © 2020 Hor, Asgari, Nakashima, Broadley, Leite, Kissani, Jacob, Marignier, Weinshenker, Paul, Pittock, Palace, Wingerchuk, Behne, Yeaman and Fujihara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jyh Yung Hor, aG9yanlAeWFob28uY29t; Kazuo Fujihara, ZnVqaWthenVAbWVkLnRvaG9rdS5hYy5qcA==
†Active members of the Guthy-Jackson Charitable Foundation (GJCF) International Clinical Consortium for NMOSD (in alphabetical order as of March 2020) are listed in the Acknowledgments
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.