
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 February 2020
Sec. Epilepsy
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00068
 Chaorong Liu1
Chaorong Liu1 Yanmin Song2
Yanmin Song2 Ying Yuan3
Ying Yuan3 Ying Peng4
Ying Peng4 Nan Pang5
Nan Pang5 Ranhui Duan6
Ranhui Duan6 Wen Huang6
Wen Huang6 Xuehui Qin7
Xuehui Qin7 Wenbiao Xiao1
Wenbiao Xiao1 Hongyu Long1
Hongyu Long1 Sha Huang1
Sha Huang1 Pinting Zhou1
Pinting Zhou1 Lili Long1*
Lili Long1* Bo Xiao1*
Bo Xiao1*Benign adult familial myoclonic epilepsy (BAFME) is an autosomal dominant disorder characterized by adult-onset cortical myoclonus with or without seizures. Recently, it was reported to be associated with intronic TTTTA/TTTCA expansions. To investigate whether these abnormal expansions are involved in our new pedigree from China, whole exome sequencing (WES) and repeat-primed polymerase chain reaction (RP-PCR) analysis were performed to detect potential mutation in pedigree members. Neither causal mutations cosegregated with the disease in the family nor any novel mutation was identified through WES, while an abnormal TTTCA expansion in SAMD12 was identified by RP-PCR and then proved to be cosegregated in the pedigree. All the 12 alive affected individuals (M/F = 4/8; average age = 46.7 years old, range from 27 to 66) showed typical characteristics of BAFME. In addition, maternal clinical anticipation was observed in six mother/child pairs. In conclusion, our study offered the evidence of intronic pentanucleotide expansions in SAMD12 from a new Chinese BAFME pedigree, which further confirmed the association between this expansion and the pathogenesis of BAFME.
Benign adult familial myoclonic epilepsy (BAFME), also known as familial cortical myoclonic tremor with epilepsy (FCMTE) and autosomal dominant cortical myoclonus and epilepsy (ADCME), was first reported in the 1990's in Japan (1, 2). It is an autosomal dominant disease featured by adult-onset cortical myoclonus with or without seizures and developing with a benign course. Giant somatosensory-evoked potential and long-latency cortical reflex can be detected by electromyography in patients with BAFME, which collectively support the cortical origin of the cortical tremor (3). Seven subtypes have been reported so far, including BAFME1 (8q24, OMIM 601068) (4), BAFME 2 (2p11.1–q12.2, OMIM 607876) (5), BAFME 3 (5p15.31–p15.1, OMIM 613608) (6), BAFME 4 (3q26.32–3q28, OMIM 615127) (7), BAFME 5 (1q32.1, OMIM 615400) (8), BAFME 6 (16p12.1, OMOM 618074) (4), and BAFME 7 (4q32.1, OMIM 618075) (4). Thus, BAFME showed genetic heterogeneity among different families. Recently, pentanucleotide expansions of intronic TTTCA and TTTTA were found to be involved in the pathogenesis of BAFME 1 in Japanese pedigrees regardless of the gene location (4). Three more recent reports also confirmed TTTCA pentanucleotide expansions in BAFME 1 families of another ethic background (9–11). Subsequently, TTTCA repeat insertions in an intron of YEATS2 in BAFME 4 was confirmed in a Thailand pedigree (7). ATTTC repeat expansions in STARD7 and unstable TTTTA/TTTCA expansions in MARCH6 were also found in European families (5, 6). Here, we performed mutation analysis in a new BAFME family from central China and detected whether abnormal insertion of TTTTA and TTTCA expansions were associated with this BAFME pedigree.
The family was a four-generation Chinese pedigree with 80 members, which presents as autosomal dominant inheritance (Figure 1). All available individuals underwent detailed medical history collection and physical examination by two experienced neurologists. Brain MRI, electroencephalogram (EEG), and electromyogram (EMG) examinations were collected at the same time; Unified Myoclonus Rating Scale (UMRS) and Fahn Tremor Rating Scale (FTRS) were assessed to evaluate the severity of myoclonus and tremor, respectively. Mini-Mental State Examination (MMSE) and Digit Symbol Test (DST) were used to evaluate the cognitive function. Ten gender- and age-matched unrelated healthy controls were recruited to detect whether there were cognitive dysfunction in BAFME patients. Genomic DNA was extracted from peripheral venous blood using the Genomic DNA kit from Aidlab. This study was approved by the Ethics Committee of Xiangya Hospital, Central South University. Written informed consent was obtained from all enrolled participants.
Five members (IV19, III13, III14, III10, and IV6) from the pedigree were performed whole-exome sequence (WES) analysis using HiSeq2000 (Illumina, PE150) following the manufacturer's protocol. Sequenced reads were aligned to GRCh37/hg19 using Burrows–Wheeler Aligner (BWA) (v.0.7.12) with default parameters. SAMtools (v0.1.12) was used to call the Variants and RefSeq Genomes database; 1000 Genomes database (http://www.1000genomes.org/), and ExAC database (http://exac.broadinstitute.org/) were used to annotate the Variants.
Repeat-primed polymerase chain reaction (RP-PCR) analysis targeting TTTTA and TTTCA expansions in SAMD12 was performed using the primers (listed in Supplementary Table 1) with ABI3730xl DNA Analyzer. The reaction mixture (10 μl) included 1 × GC buffer I (Takara), 0.3 mM deoxynucleoside triphosphate (dNTP), 0.2 μM of each primer, 0.3 U HotStarTaq polymerase (Takara), and 1 μl template DNA. The cycling program was 95°C, 2 min; 11 cycles × (94°C, 20 s, 56–0.5°C/cycle 40 s, 72°C, 3 min); 25 cycles × (94°C, 20 s; 57°C, 30 s; 72°C, 3 min); 72°C, 10 min; 4°C forever. PCR product was analyzed on the ABI3730XL sequencer. The results were analyzed with Genemapper (v5.0) software and combined with manual proofreading. Every experiment was performed three times (primers used for RP-PCR are listed in Supplementary Table 1).
Continuous variables are presented as means (± standard deviation), and categorical variables are shown as numbers (%). SPSS version 23.0 for Windows (SPSS Inc., Chicago, IL, United States) was used for calculation. Pearson correlation analysis was conducted to evaluate the relationship between the severity (FTRS, UMRS) and the duration of the disease. Two-sample t-test was used to assess the cognitive difference between BAFME patients and unrelated healthy controls. Significance was set at p < 0.05.
All the 12 alive affected individuals (M/F = 4/8; average age = 46.7 years old, range from 27 to 66) showed typical characteristics of BAFME. Two affected individuals presented only tremor symptoms (IV-23 and IV-24), and one with both tremor and myoclonus (IV-6), while another two members showed seizures only (III-8 and IV-18); the remaining seven patients manifested both tremor and infrequent epilepsy, among which four presented myoclonus together. Among the seven individuals, tremor appeared earlier than epilepsy in four individuals by an average of 4 years (50%), two appeared in the same year (25%), while for the remaining two patients, seizure appeared in advance with 2.5 years (25%). Tremor was present in adults, with an average age of onset of 35.4 ± 8.68 years in all affected members. Myoclonus appeared in the same year with tremor (3/4) or 2 years after it (1/4). Neither the severity of tremor nor myoclonus is correlated to its duration (for FTRS, r = 0.42, p = 0.196; for UMRS, r = −0.93, p = 0.071). Seizures were also present in adults, with an average age of onset of 35 ± 5.52 years, and all manifested as generalized tonic–clonic seizure, with a frequency of once to three times a year. Six patients took antiepilepsy drugs phenobarbital, of which all became seizure free.
No significant difference was found in the age between patients and unrelated healthy controls (p = 0.079, two-sample t-test). There was significant difference between the two groups in MMSE score (28.2 ± 8.03 vs. 29.7 ± 0.46, p = 0.005) and Verbal Fluency Test (VFT) (14.3 ± 3.71 vs. 20.1 ± 4.67, p = 0.018), whereas no significant difference was found in DST (41.3 ± 24.10 vs. 52.5 ± 24.94, p = 0.093). No significant correlation was found between minimum repeat size estimated by RP-PCR and the onset age of BAFME (r = −0.477, p = 0.163) (Supplementary Table 3).
Clinical anticipation was observed in both symptoms of tremor and seizure, with an average anticipation of 14 and 5 years, respectively (Table 1).
There were no other neurological or psychiatric diseases presented in all affected individuals. Drawing test of Archimedes' spiral showed mild to severe irregularity. Brain MRI showed no obvious abnormality. EMG represented a giant somatosensory-evoked potential (SEP) and C-reflex by stimulating the median nerve in bilateral wrist. EEG showed bilateral bursts with high-amplitude θ-wave; spikes and incompletely sharp complex waves were recorded mainly in the right frontal region and left posterior temporal region. These results indicated the cortical origin of the tremors or myoclonus. Detailed clinical information is displayed in Table 2 and Figure 2.
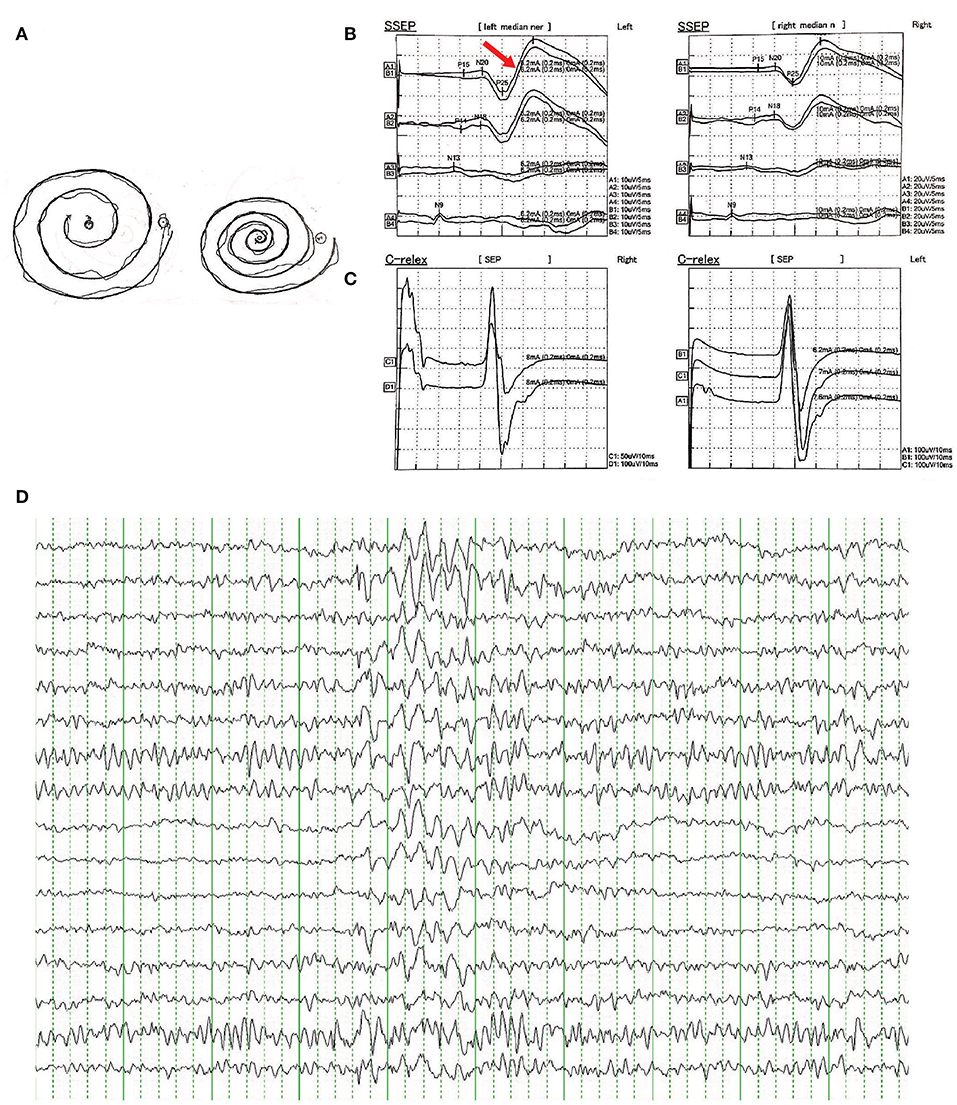
Figure 2. (A) Free-hand drawing test of Archimedes' spiral showed mild irregularity in patients of the family. (B) Representative result of somatosensory-evoked potential (SEPs) in bilateral median nerve of wrist showed enhanced cortical components (giant potentials and increase in N20-P25 indicated by red arrow). (C) Representative result of C-reflex. (D) Representative result of epileptiform discharges recorded in EEG showed bilateral bursts with high-amplitude θ-wave, spikes, and incompletely sharp complex waves were recorded mainly in the right frontal region and left posterior temporal region.
Neither causal mutations cosegregated with the disease in the family nor any novel mutation was identified through WES (sequence depth of WES for individuals in the pedigree listed in Supplementary Figure 1), while an abnormal TTTCA expansion in SAMD12 was identified by RP-PCR and then proved to be cosegregated in the pedigree. Minimum repeat size of TTTCA and TTTTA expansion for each patient estimated by RP-PCR are listed in Supplementary Table 2. Although TTTTA repeats were detected in all TTTCA-positive patients, there were still two unaffected individuals (III-21, IV-25) showing abnormal TTTTA expansions (Figure 3).
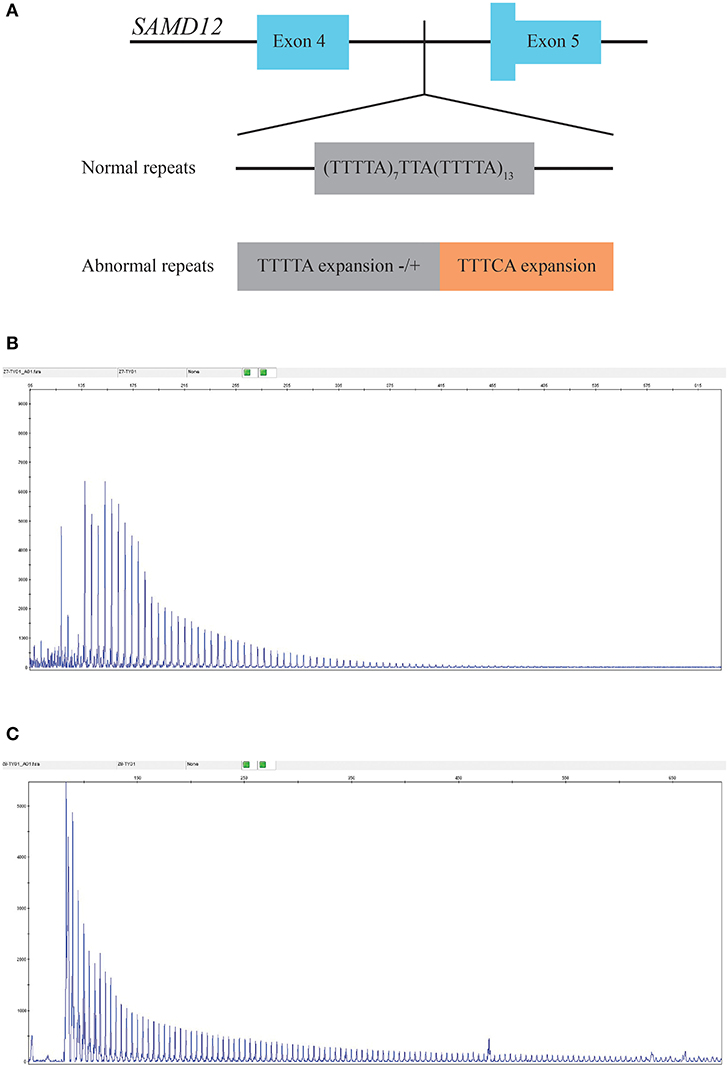
Figure 3. (A) Structure of the mutation in SAMD12. (B) RP-PCR indicated the existence of abnormal TTTTA expansion of III-7. (C) Repeat-primed PCR (RP-PCR) indicated the existence of abnormal TTTCA expansion of III-7.
Since the first report from Japan in the 1990's, several pedigrees are reported worldwide and has been given various names including benign adult familial myoclonic epilepsy (BAFME), familial cortical myoclonic tremor with epilepsy (FCMTE), familial adult myoclonus epilepsy (FAME), and autosomal dominant cortical myoclonus and epilepsy (ADCME). BAFME is now known as a benign epilepsy syndrome with considerable clinical and genetic heterogeneity. van Rootselaar et al. summarized the characteristics and put forward diagnostic criteria of this disease (3). Subsequent researches further improved our understanding of this disorder. Our study offered the evidence of intronic pentanucleotide expansions in SAMD12 from a new Chinese BAFME pedigree, which further confirmed the association between this expansion and the pathogenesis of BAFME.
In our pedigree, patients all met the diagnostic criteria proposed in 2005. Still, there were something special with their phenotype. First, there were only five (5/12) individuals presenting cortical tremor earlier than seizure, which was different from the conclusion that tremor was usually the first symptom (12). Besides, cognitive impairment was found by MMSE and VFT, which was basically consistent with our previous research (13). In addition, maternal clinical anticipation was observed in six mother/child pair. Since there was no alive father–child patient pair in this family, all were maternal, which varies from reports from another Chinese pedigree but consistent with that of Japanese (12, 14). No psychomotor development disorder or other disease such as night blindness reported in other BAFME pedigrees was witnessed. Our report further confirmed that BAFME was a non-progressing neurological disorder, but accompanied with cognitive impairment especially in language usage. Clinical anticipation did not occur in the severity of the disease but only in the age of onset. Larger samples are needed to confirm the cognitive impairment in the future.
Intronic pentanucleotide TTTCA and TTTTA repeat insertion of SAMD12 gene were reported to be associated with BAFME recently (4). Our research in a new Chinese family confirmed again that the TTTCA abnormal expansion of SAMD12 was the cause of BAFME, and the number of TTTCA expansion tended to differ from one to another, which showed instability of the expansions.
A recent report of BAFME pedigree identified interindividual instability of the pentanucleotide repeats and inversely correlated with age at onset of myoclonic tremor and seizure (11). Our study confirmed the interindividual instability, but minimum repeat size estimated by RP-PCR shows no relation to the age of onset in this BAFME family. Further studies such as Southern blot are needed to confirm the definite number of repeats. In addition, we also found that TTTCA insertions were accompanied by the abnormal TTTTA expansions in this Chinese pedigree, yet there were two unaffected individuals who showed abnormal TTTTA expansion, which indicated that TTTTA expansion may not be the cause of BAFME. Cen et al. found that some affected members had only 25–44 TTTTA repeats on the allele containing (TTTCA)n insertion, while in contrast, some unaffected individuals had alleles with 800 repeats in long-range PCR. They also observed an TTTTA pentanucleotide with more than 80 repeats in the healthy control, which was consistent with the report from Japan (4, 9). Our results further confirmed that TTTTA expansion might not be the cause of BAFME.
The mechanism how the expanded TTTCA and TTTTA repeats cause BAFME remains unclear up till now. However, similar repeat expansion has been proven to be the cause of many other neurodegenerative diseases such as fragile X syndrome (FXS) (15), several types of spinocerebellar ataxia (SCA31 and SCA37) (16, 17), C9orf72-related disorder (18), and, most recently, neuronal intranuclear inclusion disease (NIID) (19). These expansions are thought to cause disease through RNA-mediated toxicity. Repeated insertions expressed in brain and RNA lesions in the nucleus of the Purkinje cells were observed in SCA31 patient (20). While in FXS and C9orf72-related diseases, a loss of RNA and protein expression was observed and thought to be a result of hypermethylation and silencing of the promoter or nuclear RNA foci formation and haploid deficiency (21). According to previous studies, BAFME patients showed elevated choline/creatine ratio in the cerebellar cortex (22). The existence of cerebellar dysfunction was then proved in a Chinese BAFME pedigree along with altered cerebellar-cerebral functional connectivity (13, 23). In the report of the Japanese BAFME pedigrees, there were no obvious alterations in SAMD12 transcript 1 levels observed in patient's brain, but RNA lesions were observed in cortical neurons and Purkinje cells, which was consistent with MRI findings, suggesting that RNA-mediated toxicity might be the basis of the pathogenesis in BAFME.
In conclusion, BAFME is a non-progressing neurological disorder accompanied with cognitive impairment especially in language usage. Our study offered the evidence of intronic pentanucleotide expansions in SAMD12 from a new Chinese BAFME pedigree, which further confirmed the association between this expansion and the pathogenesis of BAFME. The underlying mechanism of this disorder remains to be elucidated.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Ethics Committee of Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL, LL, and BX: conception and design. CL, YS, XQ, WX, HL, SH, PZ, LL, and BX: collection and assembly of data. CL, YP, NP, RD, and WH: data analysis and interpretation. CL, YY, and LL: manuscript writing. All authors have approved the final manuscript.
This study was supported financially by National Natural Science Foundation of China (Nos. 81201001 and 81671300 to LL, No. 81671299 to BX) and Clinical Research Fund Project of Xiangya Hospital to LL (No. 2016L08), Xiangya Hospital–Peking University Weiming Clinical and Rehabilitation Research Fund to BX.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Foremost, we thank the patients, their families, and healthy controls for their cooperation and participation in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00068/full#supplementary-material
1. Striano P, Zara F, Striano S. Autosomal dominant cortical tremor, myoclonus and epilepsy: many syndromes, one phenotype. Acta Neurol Scand. (2005) 111:211–7. doi: 10.1111/j.1600-0404.2005.00385.x
2. Ikeda A, Kakigi R, Funai N, Neshige R, Kuroda Y, Shibasaki H. Cortical tremor: a variant of cortical reflex myoclonus. Neurology. (1990) 40:1561–5. doi: 10.1212/WNL.40.10.1561
3. van Rootselaar AF, van Schaik IN, van den Maagdenberg AM, Koelman JH, Callenbach PM, Tijssen MA. Familial cortical myoclonic tremor with epilepsy: a single syndromic classification for a group of pedigrees bearing common features. Mov Disord. (2005) 20:665–73. doi: 10.1002/mds.20413
4. Ishiura H, Doi K, Mitsui J, Yoshimura J, Matsukawa MK, Fujiyama A, et al. Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat Genet. (2018) 50:581–90. doi: 10.1038/s41588-018-0067-2
5. Corbett MA, Kroes T, Veneziano L, Bennett MF, Florian R, Schneider AL, et al. Intronic ATTTC repeat expansions in STARD7 in familial adult myoclonic epilepsy linked to chromosome 2. Nat Commun. (2019) 10:4920. doi: 10.1038/s41467-019-12671-y
6. Florian RT, Kraft F, Leitão E, Kaya S, Klebe S, Magnin E, et al. Unstable TTTTA/TTTCA expansions in MARCH6 are associated with familial adult myoclonic epilepsy type 3. Nat Commun. (2019) 10:4919. doi: 10.1038/s41467-019-12763-9
7. Yeetong P, Pongpanich M, Srichomthong C, Assawapitaksakul A, Shotelersuk V, Tantirukdham N, et al. TTTCA repeat insertions in an intron of YEATS2 in benign adult familial myoclonic epilepsy type 4. Brain. (2019) 142:3360–6. doi: 10.1093/brain/awz267
8. Stogmann E, Reinthaler E, Eltawil S, El Etribi MA, Hemeda M, El Nahhas N, et al. Autosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2. Brain. (2013) 136:1155–60. doi: 10.1093/brain/awt068
9. Cen Z, Jiang Z, Chen Y, Zheng X, Xie F, Yang X, et al. Intronic pentanucleotide TTTCA repeat insertion in the SAMD12 gene causes familial cortical myoclonic tremor with epilepsy type 1. Brain. (2018) 141:2280–8. doi: 10.1093/brain/awy160
10. Zeng S, Zhang MY, Wang XJ, Hu ZM, Li JC, Li N, et al. Long-read sequencing identified intronic repeat expansions in SAMD12 from Chinese pedigrees affected with familial cortical myoclonic tremor with epilepsy. J Med Genet. (2019) 56:265–70. doi: 10.1136/jmedgenet-2018-105484
11. Lei XX, Liu Q, Lu Q, Huang Y, Zhou XQ, Sun HY, et al. TTTCA repeat expansion causes familial cortical myoclonic tremor with epilepsy. Eur J Neurol. (2019) 26:513–8. doi: 10.1111/ene.13848
12. Cen Z, Huang C, Yin H, Ding X, Xie F, Lu X, et al. Clinical and neurophysiological features of familial cortical myoclonic tremor with epilepsy. Mov Disord. (2016) 31:1704–10. doi: 10.1002/mds.26756
13. Long L, Zeng LL, Song Y, Shen H, Fang P, Zhang L, et al. Altered cerebellar-cerebral functional connectivity in benign adult familial myoclonic epilepsy. Epilepsia. (2016) 57:941–8. doi: 10.1111/epi.13372
14. Hitomi T, Kondo T, Kobayashi K, Matsumoto R, Takahashi R, Ikeda A. Clinical anticipation in Japanese families of benign adult familial myoclonus epilepsy. Epilepsia. (2012) 53:e33–6. doi: 10.1111/j.1528-1167.2011.03349.x
15. Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. (1991) 65:905–14. doi: 10.1016/0092-8674(91)90397-H
16. Sato N, Amino T, Kobayashi K, Asakawa S, Ishiguro T, Tsunemi T, et al. Spinocerebellar ataxia type 31 is associated with inserted penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet. (2009) 85:544–57. doi: 10.1016/j.ajhg.2009.09.019
17. Corral-Juan M, Serrano-Munuera C, Rabano A, Cota-Gonzalez D, Segarra-Roca A, Ispierto L, et al. Clinical, genetic and neuropathological characterization of spinocerebellar ataxia type 37. Brain. (2018) 141:1981–97. doi: 10.1093/brain/awy137
18. Stepto A, Gallo JM, Shaw CE, Hirth F. Modelling C9ORF72 hexanucleotide repeat expansion in amyotrophic lateral sclerosis and frontotemporal dementia. Acta Neuropathol. (2014) 127:377–89. doi: 10.1007/s00401-013-1235-1
19. Tian Y, Wang JL, Huang W, Zeng S, Jiao B, Liu Z, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. (2019) 105:166–76. doi: 10.1016/j.ajhg.2019.05.013
20. Niimi Y, Takahashi M, Sugawara E, Umeda S, Obayashi M, Sato N, et al. Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)n in the purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis. Neuropathology. (2013) 33:600–11. doi: 10.1111/neup.12032
21. Rohilla KJ, Gagnon KT. RNA biology of disease-associated microsatellite repeat expansions. Acta Neuropathol Comm. (2017) 5:63. doi: 10.1186/s40478-017-0468-y
22. Striano P, Caranci F, Di Benedetto R, Tortora F, Zara F, Striano S. (1)H-MR spectroscopy indicates prominent cerebellar dysfunction in benign adult familial myoclonic epilepsy. Epilepsia. (2009) 50:1491–7. doi: 10.1111/j.1528-1167.2008.01900.x
Keywords: benign adult familial myoclonic epilepsy, SAMD12, pentanucleotide, cognitive impairment, TTTCA expansion
Citation: Liu C, Song Y, Yuan Y, Peng Y, Pang N, Duan R, Huang W, Qin X, Xiao W, Long H, Huang S, Zhou P, Long L and Xiao B (2020) TTTCA Repeat Expansion of SAMD12 in a New Benign Adult Familial Myoclonic Epilepsy Pedigree. Front. Neurol. 11:68. doi: 10.3389/fneur.2020.00068
Received: 11 October 2019; Accepted: 20 January 2020;
Published: 26 February 2020.
Edited by:
Jose F. Tellez-Zenteno, University of Saskatchewan, CanadaReviewed by:
Maheedhar Kodali, Texas A&M Health Science Center, United StatesCopyright © 2020 Liu, Song, Yuan, Peng, Pang, Duan, Huang, Qin, Xiao, Long, Huang, Zhou, Long and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Long, MTAzNTM2NTRAcXEuY29t; Bo Xiao, bWRib3hpYW9AMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.