
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 19 December 2019
Sec. Multiple Sclerosis and Neuroimmunology
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.01339
 Shunichiro Takano1
Shunichiro Takano1 Aya Hanabusa1
Aya Hanabusa1 Yuji Yoshikawa1
Yuji Yoshikawa1 Kaori Sassa2
Kaori Sassa2 Airi Shimura1
Airi Shimura1 Takuhei Shoji1
Takuhei Shoji1 Hisao Ohde3
Hisao Ohde3 Kei Shinoda1*
Kei Shinoda1* Hideo Yamanouchi2
Hideo Yamanouchi2Purpose: To describe a Japanese girl with unilateral optic neuritis who was seropositive for the anti-myelin-oligodendrocyte glycoprotein (MOG). Serial recordings of the pattern visual evoked potentials (pVEPs) were made to follow the dynamic changes of the disease activity.
Observations: A 5-year-old girl developed a sudden reduction of vision and deep ocular pain in her right eye. On examination at our university hospital, the best-corrected visual acuity (BCVA) was light perception, and a swelling of the optic disc and tortuous vessels at the posterior pole of the right eye were observed. MRI demonstrated that her right optic nerve was hyperintense on short TI inversion recovery (STIR) sequence. A diagnosis of right papillitis was made, and she was treated with steroid pulse therapy followed by a gradual tapering of oral prednisolone. The visual acuity decreased to no light perception and plasmapheresis combined with high-dose intravenous immunoglobulin therapy was performed. The decimal visual acuity rapidly improved and recovered to 1.2, and no recurrence was observed for at least 1 year. On day 19, she was found to be anti-MOG antibody positive and anti-Aquaporin 4 antibody negative. pVEPs were recorded during the course of the disease process which showed the dynamic changes of the physiology of the visual pathways. The implicit times of the N75 and P100 components were prolonged in the right eye in the acute phase. The right visual acuity remained at 1.2 for at least 1 year, but the implicit times of the N75 and P100 components of the pVEPs of the right eye were still prolonged compared to left eye.
Conclusion: Our findings indicate a positive relationship between the anti-MOG antibodies-positivity and the prolonged pVEPs. Further analyses of the pVEPs and other clinical findings of the optic neuritis are needed to establish the clinical significance of the anti-MOG antibodies positivity and optic neuritis for the diagnosis, treatment, and prognosis for this disease.
Myelin-oligodendrocyte glycoprotein (MOG) is a surface protein located on the oligodendrocytes of the central nervous system (CNS) and the optic nerves (1, 2). Autoantibodies against MOG are associated with acute disseminated encephalomyelitis (ADEM) in children and the opticospinal type of multiple sclerosis (MS) in adults (3–5). In addition, anti-MOG antibodies are frequently detected in patients with recurrent optic neuritis at ages ≤ 18-years (6). The optic neuritis can be a part of ADEM, MS, and the neuromyelitis optica spectrum disorders (NMOSDs). Although anti-aquaporin 4 (AQP4) antibody-positivity is a key in the diagnosis of NMOSD, anti-MOG antibodies have played an important role in NMO because a proportion of anti-AQP4 antibody-negative cases have anti-MOG antibody positivity (7, 8). Kim et al. reported that the predominant demyelinating disease found in anti-MOG antibody-positive patients was optic neuritis (83%) (3). Because patients with anti-MOG antibody may show a relapsing-remitting disease course, and MRI evidence shows a dissemination in space and time, anti-MOG antibody positive optic neuritis might have been diagnosed as optic neuritis associated with multiple sclerosis (9–11).
Although the characteristics of the visual evoked potentials (VEPs) are not used to diagnose MS, they have been helpful for the diagnosis and monitoring of the optic neuritis in patients with MS. The prolongation of the P100 implicit times is accepted as a pathognomonic sign of optic nerve demyelination in MS (12–15).
We present our findings in a young Japanese girl who developed acute optic neuritis and was anti-MOG antibody-positive. Pattern visually evoked potentials were recorded during the recovery course.
A 5-year-old girl developed a sudden reduction in her vision and had deep ocular pain in her right eye. She visited a private eye clinic on the following day. She had no noteworthy medical and family histories. Her decimal visual acuity (VA) was 0.7 OD and 1.2 OS with a relative afferent pupillary defect in the right eye on day 2. She was referred to the Saitama Medical University Hospital, and ophthalmoscopy and optical coherence tomography showed a swelling of the optic disc and tortuous vessels at posterior pole of the right eye on day 3 (Figure 1). Neurological and general examinations were within normal limits. MRI demonstrated hyperintensity of her right optic nerve on short TI inversion recovery (STIR) sequence and no cerebral lesions (Figure 2). On the initial visit to Saitama Medical University, patient's blood was drawn for laboratory examinations and for serum antibodies against AQP4 and MOG. Laboratory examinations showed that the blood and cerebrospinal fluid tests were within the normal limits except a few items (Supplemental Table 1). Spinal MRI showed no abnormalities in the cervical, thoracic, and lumbar spinal cord.
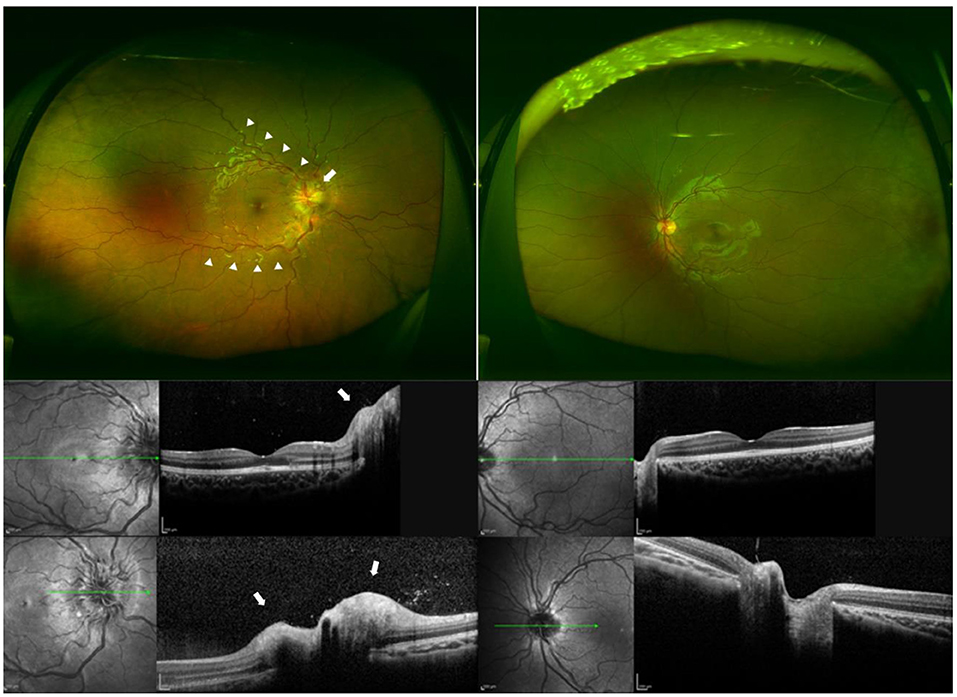
Figure 1. Fundus photographs and optical coherence tomographic (OCT) images of a patient with unilateral optic neuritis and seropositivity to anti-myelin oligodendrocyte glycoprotein (MOG) antibody. (Top) Ultra-widefield fundus photographs of each eye at the initial visit showing tortuous arcade vessels (arrowheads) and optic disc swelling (arrow) in the right eye. The decimal best-corrected visual acuity (BCVA) was light perception in the right eye and 1.2 in the left eye. (Middle) Optical coherence tomographic (OCT) images of the posterior pole of each eye at the initial visit showing optic disc swelling in the right eye (arrow). Left, right eye; right, left eye. (Bottom) OCT images of the optic disc of each eye at the initial visit showing optic disc swelling in the right eye (arrow). Left, right eye; right, left eye.
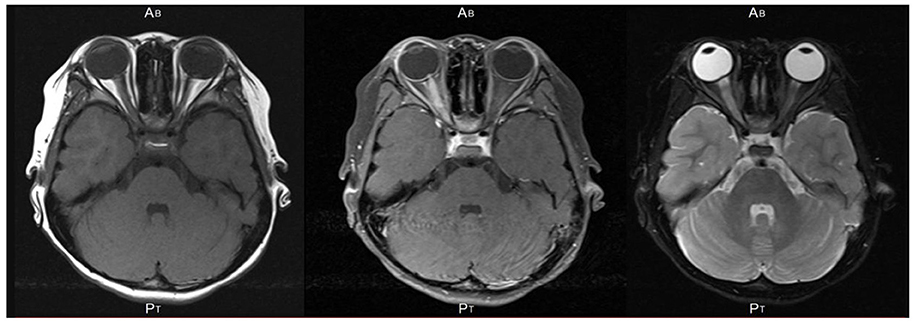
Figure 2. An axial section of magnetic resonance images of the brain and orbits in the patient showing hyperintensity of the right optic nerve on short TI inversion recovery (STIR) sequence (Left), T1-weighted image with gadolinium enhancement (Middle), and T2-weighted image (Right). No cerebral lesion was detected. An axial section of MRI of the brain and orbits in the patient showing hyperintensity of the right optic nerve on short TI inversion recovery (STIR) sequence and no cerebral lesion.
Ischemic optic neuropathy, traumatic neuropathy, toxic-nutritional optic neuropathy, rhinogenic optic neuritis, and hereditary optic neuropathy were considered as a differential diagnosis and were denied from various clinical findings. She was diagnosed with right papillitis and treated with 450 mg of intravenous methylprednisolone pulses for 3 days (days 3–5) followed by a gradual tapering of oral prednisolone (Table 1). On day 5, the best-corrected visual acuity (BCVA) decreased to no light perception, and plasmapheresis was performed for 3 days (days 6, 9, and 12) combined with high dose intravenous immunoglobulin therapy (160 mg/kg, total 2.5 g) for 1 day (day 10) (Table 1). Because the patient's vision loss was so severe, we started systemic treatment to prevent a relapse after discussion with patient's mother. The decimal visual acuity rapidly improved and reached 1.0 on day 13 [Table 1; (16)]. The swelling of the optic disc and tortuosity of the retinal vessel of the right eye disappeared on days 35 and 63, respectively. From the examination of the blood drawn on the initial visit, it turned out on day 9 that she was anti-AQP4 antibody-negative and on day 19 that anti-MOG antibody-positive.
Pattern VEPs (pVEPs) were recorded several times during the course of the disease with the recording parameters conforming to the International Society of Clinical Electrophysiology of Vision (ISCEV) standards except that the checkerboard size was ~2° (17). The findings represented the dynamic changes in the physiology of the visual pathways objectively (Figure 3; Table 2). The implicit times of the N75 and P100 components of the pVEPs of the right eye were prolonged compared to that of the normal fellow eye when the VEPs were elicited by stimulating the right eye throughout the follow-up period. The implicit times of the right eye became shorter on day 109 and remained prolonged on day 316.
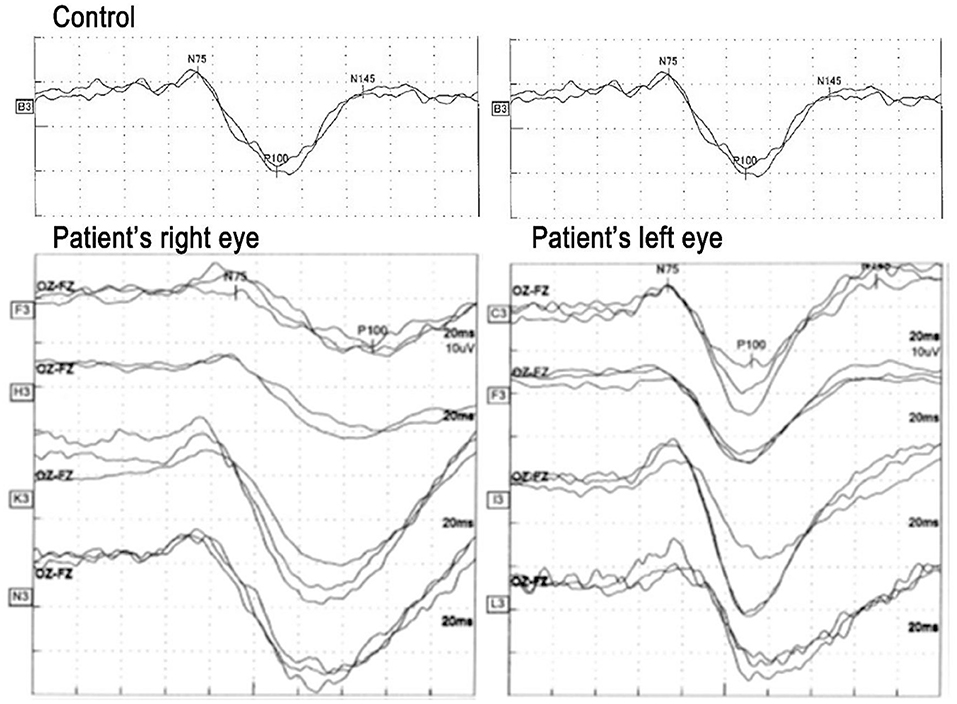
Figure 3. Pattern visual evoked potentials (pVEPs) recorded several times during the experimental period. Left pVEPs elicited by stimulating the right eye and that elicited by stimulating the left eye. Top pVEPs recorded on day 14 when visual acuity was 1.0, second row: on day 21 when visual acuity was 1.2, third row: on day 109 when visual acuity was 1.2, and bottom: on day 305 when visual acuity was 1.2. The implicit time of N75 was prolonged until day 21 in the right eye compared to the left eye, and thereafter it seems to normalize and stable for almost 1 year. On the other hand, the implicit time of the P100 component was prolonged in the right eye compared to the left eye throughout of the following period. The implicit time in the right eye became shorter with time and stabilized between the day 109 and 305 while the implicit time in the left eye was stable during entire period. An example from normal control is shown in the top.
Although the patient reported that she felt that the image of the right eye was relative dark compared to the left eye, the decimal BCVA of the right eye has remained at 1.2 for at least 1 year. The implicit time of N75 was prolonged in the right eye compared to the left eye until day 21, and thereafter it seemed to normalize and become stable for almost 1 year. On the other hand, the implicit time of the P100 component was prolonged in the right eye compared to the left eye throughout the follow-up period.
Five milligram of oral prednisolone has been used every other day, and no recurrences have been observed for at least 1 year.
Anti-MOG antibodies have been recognized to be specific biomarkers for different disease entities, such as anti-AQP4 antibodies for NMO, but their pathogenic role has not been fully determined (2–4, 11). The diseases associated with MOG antibody are now recognized as a distinct nosological entity with specific management and therapeutic requirements (18). Because patients with optic neuritis often have a severe reduction of visual acuity that develops in a short time, a rapid decision on the therapeutic strategy is required before seropositivity or negativity for anti-MOG and anti-AQP4 antibodies becomes clear. Several clinical features that are useful for the diagnosis have been reported, and the pVEP findings are very important especially in children (15, 18–20).
The prolongation of the implicit times of the pVEPs represents the neurotransmission rate, and its delay is a good biomarker to evaluate functional damage of the demyelinated optic neuritis. In our case, the prolongation was present in spite of the recovery of the fundus appearance and the BCVA. The prolongation stabilized in the chronic phase and did not return to that of the healthy fellow eye. Our findings are from single case and cannot be expanded for other optic neuritis. It has been reported that there were no significant differences in the incidence of eyes with prolonged P100 implicit time between the anti-MOG antibody-positive patients and anti-AQP4 antibody-positive patients; i.e., 57% of anti-MOG antibody-positive patients and 50% of anti-AQP4 antibody-positive patients had prolonged implicit times (15). A more accurate comparison such as in the degree of the implicit time delay between the two groups might differentiate patients with optic neuritis and anti-MOG antibody-positivity from other optic neuritis. Further investigations on the pVEPs for the possibility of differentiating anti-MOG antibody-positive optic neuritis from anti-AQP4 antibody-positive optic neuritis will be important. In addition, the use of the implicit times as an indicator of the effectiveness of a new therapeutic regimen should be examined.
We began our treatment with steroid pulse therapy using the same regimen used to treat anti-AQP4 antibody-positive optic neuritis followed by post-therapy administration of oral steroids plus immunotherapy. Because systematic treatment to prevent a relapse of anti-MOG positive pathologies is not well-accepted, we, the pediatrician and ophthalmologist, discussed this treatment with the patient's mother. Because this was prior to the detection of the sero-positivity or negativity of anti-MOG antibody and anti-AQP4 antibody and the patient's vision loss was so severe, we started systemic treatment to try to prevent a relapse. Anti-MOG antibody-positive optic neuritis and anti-AQP4 antibody-positive optic neuritis have different clinical features and different optic nerve damage. Thus, different therapeutic strategies needed to be determined (15, 18–20). The determinations of the clinical characteristics and analyses of the pVEPs of these different clinical entities should be helpful in the understanding of the pathology and establishing the best therapeutic regimen (11, 21). Our case had persistent VEP abnormalities despite clinical recovery in the right eye. Jarius et al. reported that some anti-MOG antibody-positive patients with only transverse myelitis and no optic neuritis had delays in the implicit times of P100 of the pVEP This suggested a subclinical optic nerve dysfunction (11). Further studies are necessary to determine the implications of the prolonged P100 implicit time as to whether it is only a sign of old optic neuritis, a sign of relapse, or an indicative marker for the anti-MOG antibody activity. In conclusion, we present our findings in a 5-year-old Japanese patient with unilateral optic neuritis and seropositive to anti-MOG antibodies. Steroid pulse therapy followed by post-therapy administration of oral steroids plus immunotherapy led to a recovery of the appearance of the fundus. However, the implicit times of the pVEPs remained longer than that of the normal fellow eye. Additional case reports and further analyses of the pVEPs should provide new insights into anti-MOG antibodies-related diseases and whether the implicit time of the pVEPs can be a marker for eyes with subclinical unilateral optic neuritis and seropositive to anti-MOG antibodies.
All datasets generated for this study are included in the article/Supplementary Material.
Written informed consent to publish this case report and any accommodating data and images was obtained from the patient's next of kin.
AH, KSa, HY, and KSh cared for the patient, including during assessment and treatment throughout and in the follow up period. ST, YY, AS, TS, and KSh collected data, analyzed the ophthalmological findings, and gave critical suggestions. ST, AH, and KSh prepared figures. KSh prepared a draft. ST, YY, TS, HO, and HY revised and finalized it. All authors agree to be accountable for all aspects of work. All authors attest that they meet the current ICMJE criteria for authorship.
This study was supported in part by the Japan Society for the Promotion of Science (JSPS; KAKENHI Grant No. 17K11430).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Prof. Duco Hamasaki for editing the manuscript and insightful comments. We thank Dr. Keiko Tanaka in Department of Animal Model Development, Brain Research Institute, Niigata University for the measurement of the anti-MOG and anti-AQP4 antibodies.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.01339/full#supplementary-material
1. Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 30-phosphodiesterase in the CNS of adult rats. J Neurochem. (1989) 52:296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x
2. Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. (2013) 9:455–61. doi: 10.1038/nrneurol.2013.118
3. Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e163.10. doi: 10.1212/NXI.0000000000000163
4. Weber M, Derfuss T, Metz I, Brück W. Defining distinct features of anti-MOG associated central nervous system demyelination. Ther Adv Neurol Disord. (2018) 11:1–15. doi: 10.1177/1756286418762083
5. Ramanthan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmune Rev. (2016) 15:307–24. doi: 10.1016/j.autrev.2015.12.004
6. Rostasy K, Mader S, Schanda K, Huppke P, Gartner J, Kraus V, et al. Anti–myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. (2012) 69:752–6. doi: 10.1001/archneurol.2011.2956
7. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 14:177–89. doi: 10.1212/WNL.0000000000001729
8. Probstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflamm. (2015) 12:46–53. doi: 10.1186/s12974-015-0256-1
9. Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. (2013) 19:1261–7. doi: 10.1177/1352458513484547
10. Tsuburaya RS, Miki N, Tanaka K, Kageyama T, Irahara K, Mukaida S, et al. Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in a Japanese boy with recurrent optic neuritis. Brain Dev. (2015) 37:145–8. doi: 10.1016/j.braindev.2014.02.002
11. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm. (2016) 13:280. doi: 10.1186/s12974-016-0718-0
12. Halliday AM, McDonald WI, Mushin J. Visual evoked response in diagnosis of multiple sclerosis. Br Med J. (1973) 4:661–4. doi: 10.1136/bmj.4.5893.661
13. Kjaer M. Evoked potentials. With special reference to the diagnostic value in multiple sclerosis. Acta Neurol Scand. (1983) 67:67–89. doi: 10.1111/j.1600-0404.1983.tb04547.x
14. Matthews WB, Small DG, Small M, Pountney E. Pattern reversal evoked visual potential in the diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry. (1977) 40:1009–14. doi: 10.1136/jnnp.40.10.1009
15. Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflamm. (2016) 13:282. doi: 10.1186/s12974-016-0720-6
16. Grover S, Fishman GA, Anderson RJ, Tozatti MS, Heckenlively JR, Weleber RG, et al. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. (1999) 106:1780–5. doi: 10.1016/S0161-6420(99)90342-1
17. Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A, et al. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. (2016) 133:1–9. doi: 10.1007/s10633-016-9553-y
18. Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. (2019) 266:1280–6. doi: 10.1007/s00415-018-9122-2
19. Kezuka T, Tanaka K, Matsunaga Y, Goto H. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. (2014) 83:475. doi: 10.1212/WNL.0000000000000636
20. Kezuka T, Ishikawa H. Diagnosis and treatment of anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis. Jpn J Ophthalmol. (2018) 62:101–8. doi: 10.1007/s10384-018-0561-1
Keywords: optic neuritis, myelin-oligodendrocyte glycoprotein (MOG), anti-MOG antibody, aquaporin 4 (AQP4), anti-AQP4 antibody, pattern visual-evoked potentials
Citation: Takano S, Hanabusa A, Yoshikawa Y, Sassa K, Shimura A, Shoji T, Ohde H, Shinoda K and Yamanouchi H (2019) Pattern Visually Evoked Potentials in Japanese Girl With Optic Neuritis and Seropositive to Anti-myelin Oligodendrocyte Glycoprotein (MOG) Antibody. Front. Neurol. 10:1339. doi: 10.3389/fneur.2019.01339
Received: 24 July 2019; Accepted: 04 December 2019;
Published: 19 December 2019.
Edited by:
Maria K. Houtchens, Brigham and Women's Hospital, Harvard Medical School, United StatesReviewed by:
Michael Levy, Massachusetts General Hospital, Harvard Medical School, United StatesCopyright © 2019 Takano, Hanabusa, Yoshikawa, Sassa, Shimura, Shoji, Ohde, Shinoda and Yamanouchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kei Shinoda, c2hpbm9rQHNhaXRhbWEtbWVkLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.