- 1Neuroscience Research Australia, Sydney, NSW, Australia
- 2School of Psychology, Macquarie University, Sydney, NSW, Australia
- 3School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
- 4Graduate School of Health, University of Technology Sydney, Sydney, NSW, Australia
- 5School of Psychology, University of New South Wales, Sydney, NSW, Australia
A decrease in glutamate in the medial prefrontal cortex (mPFC) has been extensively found in animal models of chronic pain. Given that the mPFC is implicated in emotional appraisal, cognition and extinction of fear, could a potential decrease in glutamate be associated with increased pessimistic thinking, fear and worry symptoms commonly found in people with chronic pain? To clarify this question, 19 chronic pain subjects and 19 age- and gender-matched control subjects without pain underwent magnetic resonance spectroscopy. Both groups also completed the Temperament and Character, the Beck Depression and the State Anxiety Inventories to measure levels of harm avoidance, depression, and anxiety, respectively. People with chronic pain had significantly higher scores in harm avoidance, depression and anxiety compared to control subjects without pain. High levels of harm avoidance are characterized by excessive worry, pessimism, fear, doubt and fatigue. Individuals with chronic pain showed a significant decrease in mPFC glutamate levels compared to control subjects without pain. In people with chronic pain mPFC glutamate levels were significantly negatively correlated with harm avoidance scores. This means that the lower the concentration of glutamate in the mPFC, the greater the total scores of harm avoidance. High scores are associated with fearfulness, pessimism, and fatigue-proneness. We suggest that chronic pain, particularly the stress-induced release of glucocorticoids, induces changes in glutamate transmission in the mPFC, thereby influencing cognitive, and emotional processing. Thus, in people with chronic pain, regulation of fear, worry, negative thinking and fatigue is impaired.
Introduction
Brain morphological changes are known to occur in chronic pain (1, 2). Although different types of chronic pain, e.g., nociceptive and neuropathic pain, differ in their pattern of gray matter changes (3–5), they show a substantial overlap in the medial prefrontal cortex (mPFC), where a decrease in gray matter volume has been widely demonstrated (2, 6–9). While a logical explanation for a decrease in mPFC gray matter volume is neuronal loss, there is no evidence to confirm this (10). Instead, we recently proposed that a decrease in mPFC gray matter volume reflects many changes, including vascular alterations caused by a change in metabolic activity, e.g., glutamate (10). In detail, a change in glutamate concentration effects microvasculature through neurovascular signaling and activation of pericytes which are contractile cells that line capillaries, controlling their diameter size through contraction or dilation (11).
An alteration in mPFC glutamate levels has been shown in animal models of chronic pain but has never been reported in humans with chronic pain (12–18). In animal models of chronic pain, it has been suggested that during the acute stage of pain there is an initial increase in glutamate, which is followed by a decline during the progression from acute to chronic pain (13). Thus, we would expect a decrease in mPFC glutamate in people with chronic pain.
Another important question refers to the clinical impact of such metabolic changes. Given that the mPFC is implicated in emotional appraisal (19–21), cognition (19–22) and extinction of fear (19–21), could a decrease in glutamate be associated with the increased pessimistic thinking, fear and worry symptoms commonly found in people with chronic pain? Indeed spectroscopy studies have revealed that glutamate mediates the behavioral sequelae associated with anxiety and stress (23) as well as the sequelae of pain perception itself (24, 25).
While as many as 50% of people with chronic pain suffer from anxiety and depression (26, 27), it is not known if these state factors relate to a potential decrease in mPFC glutamate. Unfortunately, no published studies have examined mPFC glutamate levels in individuals with chronic pain. As alterations in mPFC gray matter volume represent changes in mPFC glutamate levels in people with chronic pain (10), studies investigating the relationship between mPFC gray matter volume and state anxiety and depression may provide evidence about whether these state factors are related to decreased mPFC glutamate in chronic pain sufferers.
Decreased mPFC gray matter volume has been reported in anxiety disorders (28–30) and depression (31–34). To our knowledge, there are two published studies which directly link the decrease in mPFC gray matter volume to state anxiety and depression symptoms in people with chronic pain (35, 36). However, five other studies found no such relationship between mPFC gray matter decrease and state anxiety and depression in chronic pain sufferers (37–41). This suggests that there may be other emotional, cognitive and behavioral factors that are linked to a decrease in mPFC gray matter volume and hence, to a potential decline in mPFC glutamate.
Evidence is accumulating that a high level of the temperament “harm avoidance” is the most distinguishing multidimensional trait of chronic pain sufferers (42–45). Elevated harm avoidance, as per Cloninger's Temperament and Character Inventory (46), comprises cognitive, emotional, and behavioral factors characterized by excessive worry, pessimism, fear, doubt, apprehension and fatigue. In the chronic pain context, high harm avoidance usually manifests as persistent, excessive fear and worry about pain (47). Harm avoidance has previously been linked directly to mPFC metabolic and neuronal activity (48–52). Hence, we suggest that a potential decrease in mPFC glutamate may be associated with high levels of harm avoidance amongst chronic pain sufferers.
We used magnetic resonance spectroscopy to determine: (1) if individuals with chronic pain show a decrease in mPFC glutamate and (2) if this decline in mPFC glutamate is associated with negative affective state factors such as depression and anxiety or multidimensional trait factors such as harm avoidance. Metabolites including glutamate, N-acetylaspartate, creatine, and myo-inositol were compared between chronic pain subjects and age- and gender- matched healthy controls. Both groups also completed the Beck Depression Inventory, the State-Trait Anxiety Inventory and the Temperament and Character Inventory which measure depression, anxiety and harm avoidance, respectively. We hypothesized that individuals with chronic pain would demonstrate a decrease in glutamate within the mPFC compared to age and gender matched individuals without pain. Further, we hypothesized that a decline in glutamate levels would be linked to higher levels of harm avoidance in individuals with chronic pain. That is, the higher the reports of multidimensional trait of harm avoidance, e.g., the higher the ongoing fear and worry about pain, the lower the levels of mPFC glutamate.
Methods
Subjects
Nineteen subjects with chronic pain (9 males; mean [± standard deviation (SD)] age 51 ± 13 years) and 19 age- and gender- matched control subjects without pain (9 males; mean [±SD] age 49 ± 14 years) were recruited for the study. Five out of 19 chronic pain sufferers were diagnosed with painful temporomandibular disorder (TMD) using the Research Diagnostic Criteria for TMD (53). Two people with chronic pain suffered from trigeminal neuropathy (TNP), one person suffered from trigeminal post-herpetic neuralgia, and one individual had atypical trigeminal neuralgia assessed by the Liverpool Criteria (54). Ten people with chronic pain suffered from neuropathic pain after spinal cord injury (SCI) as defined by the International Association for the Study of Pain SCI pain taxonomy (55) (Table 1).
TMD is mainly a nociceptive pain condition (56) affecting the temporomandibular joint and mastication muscles. TMD is primarily related to the trigeminal nerve; however, symptoms can also occur around the neck, head and ears. TMD is characterized by ongoing aching pain and tenderness (53). TNP is a neuropathic pain condition occurring in one or more branches of the trigeminal nerve. It features continuous or long periods of background aching and burning pain with episodic sharp stabbing pain (54). Trigeminal post-herpetic neuralgia is a unilateral neuropathic pain syndrome characterized by ongoing deep aching or burning pain occurring in one or more branches of the trigeminal nerve, caused by herpes zoster (57, 58). Atypical trigeminal neuralgia is a unilateral neuropathic pain condition of a branch or branches of the trigeminal nerve, featuring constant, or long periods of mild, background burning pain with abrupt onset sharp, stabbing pain (54). All SCI subjects had complete thoracic injury with continuous shooting or burning pain in the area of sensory loss which was minimum three segments below the neurological level of injury. The International standards for neurological classification of spinal cord injury (ISNCSCI) examination (59) was used to assess the extent of spinal cord damage. Specifically, we determined the most caudal level of the spinal cord with normal sensory and motor function on both sides of the body (neurological level of injury). When there was no sensory or motor function in the sacral segments, we specified the injury as complete. The Human Research Ethics Committees of the University of New South Wales and University of Sydney approved the research and all subjects gave their informed written consent in accordance with the Declaration of Helsinki.
Psychometric Measures
During the MRI scanning session, subjects rated their present pain intensity (Table 1). A pain diary was also completed to assess participant pain intensity during the week before the scanning session. Subjects rated their pain three times daily by making a vertical pen stroke on a 10 cm horizontal line (0 cm reflecting no pain to 10 cm reflecting maximum pain imaginable). The values were averaged to reflect the subject's chronic pain intensity. Each participant also completed the revised Temperament and Character Inventory (TCI-R) (46) to assess their level of harm avoidance. The TCI-R comprises 240 items and measures four temperament traits (Novelty Seeking, Harm Avoidance, Reward Dependence, and Persistence) and three character traits (Self-Directeness, Cooperativeness, and Self-Transcendence). Percentile scores were determined from the raw scores for harm avoidance using the following ranges: 84–100%, very high; 67–83.3%, high; 34–66.7%, average; 17–33%, low; 0–16.7%, very low. The State Anxiety Inventory (32, 60) was also completed by each participant. This measure has 20 items with scores ranging from 20 to 80. A cut-off score of 39–40 has been suggested to detect clinically significant symptoms for state anxiety (61). State anxiety reflects a temporary condition characterized by autonomic nervous system activation and feelings of fear, nervousness and tension in response to a perceived threat. Moreover, to assess depressive symptoms, the Beck Depression Inventory (62) was completed by each participant. This is a valid and reliable measure of depression in chronic pain patients (63), with scores ranging from 0–63. The standard cut-off scores are as follows, 0–9 indicates minimal depression, 10–18 indicates mild depression, 19–29 indicates moderate depression, and 30–63 indicates severe depression (64). All psychometric variables were continuous in nature.
Proton Magnetic Resonance Spectroscopy (1H-MRS) Acquisition
Subjects lay supine head first on the bed of a 3T MRI scanner (Achieva TX Philips Healthcare, Best, Netherlands) with their head immobilized in a tight-fitting 32 channel head coil. One high resolution T1-weighted volumetric image covering the whole brain was acquired for each participant (turbo field echo; echo time = 2.5 ms, repetition time = 5,600 ms, flip angle = 8°, voxel size = 0.8 mm3). For voxel positioning, we used multi-planar (axial, sagittal, coronal) reconstructions. In the right mPFC (65), a voxel (20 × 30 × 30 mm3) was placed to collect proton magnetic spectra (Figure 2A) using the PRESS sequence (TR: 2,000 ms, TE: 32 ms, 1,024 acquisition points, bandwidth of 2 kHz, 64 averages, water suppression technique “excitation”). From the same voxel position, we also collected an unsuppressed water spectrum with 8 averages. All voxel placements were positioned based on anatomical and histological features. This was done by reference to BioImage Suite by Yale University (https://bioimagesuiteweb.github.io/webapp/) in the MNI2TAL application, against a 1.5T high resolution (1 mm) brain. Further to this, the atlas of the Human Brain (65) was used to verify final placement of the voxel to ensure that the target voxel was placed within the mPFC and not within the anterior cingulate, orbitofrontal, or dorsolateral prefrontal cortex. The voxel was targeted to land on the Superior Frontal Gyrus, Medial Part or the Superior Frontopolar Gyrus, both well within the mPFC.
1H-MRS Analysis
Java-based magnetic resonance user's interface (jMRUI 4.1, European Union project) was used for the analysis of the MRS data in the time domain. First, the Hankel Lanczos Singular Value Decomposition algorithm was employed to remove the dominant water resonance. QUEST was then used to quantify N-acetylaspartate (NAA), creatine (Cr), glutamate (Glu), and myo-inositol (MI) resonances using a 32 ms TE metabolite basis set including NAA, Cr, Glu, MI, glutamine, and glycerophosphorylcholine generated using the NMR-SCOPE tool in jMRUI using coupling constant and chemical shift information from Gasparovic et al. (66). Ratios were calculated for NAA, Cr, Glu, and MI relative to H2O which was measured from the unsuppressed water reference spectrum. H2O ratios have been used because H2O provides a more robust reference compared to Cr which can vary across the brain even in non-disease states and particularly in healthy aging (67–70).
Spectral Quality Assessment and Voxel Tissue Content Analysis
Variances were calculated from the peak areas and the standard deviations of the fit for each metabolite in each subject to assess the goodness of fit. Average linewidths and signal to noise ratios (SNR) were also examined. Signal-to-noise ratios were measured using the peak amplitudes of NAA in the water suppressed spectrum compared to the peak amplitude of the noise from a signal free section of the spectrum around 10 ppm in each subject. Spectroscopy region of interests (ROIs) were assessed for relative fraction of cerebrospinal fluid, gray and white matter using a tool for partial volume estimation of Philips data (66).
Statistical Methods
All data was analyzed using SPSS statistical software (version 25). The Shapiro-Wilk test was used to assess the normal distribution of all data. Data identified as parametric was analyzed with two-tailed independent t-tests to assess for between group comparisons. Two-tailed Pearson correlations were also used to determine any significant correlations between parametric data variables. A stepwise sequential regression model [including Akaike's Information Criterion with small-sample correction (AICc) fit and forward selection with harm avoidance as the outcome and NAA and glutamate as predictors] was used to assess the influence of NAA and glutamate on harm avoidance. During the study, a significance level of p < 0.05 was employed, with the Bonferroni-Holm correction used for multiple comparisons and respective cumulative α error.
Results
On average (mean ± SD), chronic pain subjects had on-going pain intensity of 3.9 ± 2.3 (diary pain), pain intensity during scanning of 3.1 ± 1.9 (scan pain), and an average pain duration of 16.5 ± 13 years. Table 1 shows the individual and mean chronic pain participant characteristics. Seventeen out of 19 chronic pain subjects had bilateral pain and two had left-sided pain. Fourteen out of 19 chronic pain subjects had neuropathic pain and five had non-neuropathic pain (myofascial pain). Ten out of 19 chronic pain sufferers had neuropathic pain after SCI and nine had orofacial pain (Table 1). There was no significant difference in age for chronic pain subjects compared to controls without pain (mean [±SD] age: chronic pain subjects: 51 ± 13; control subjects without pain: 49 ± 14; p = 0.68, computed test statistic (t) = 0.43, degrees of freedom (df) = 36).
Psychometric Measures
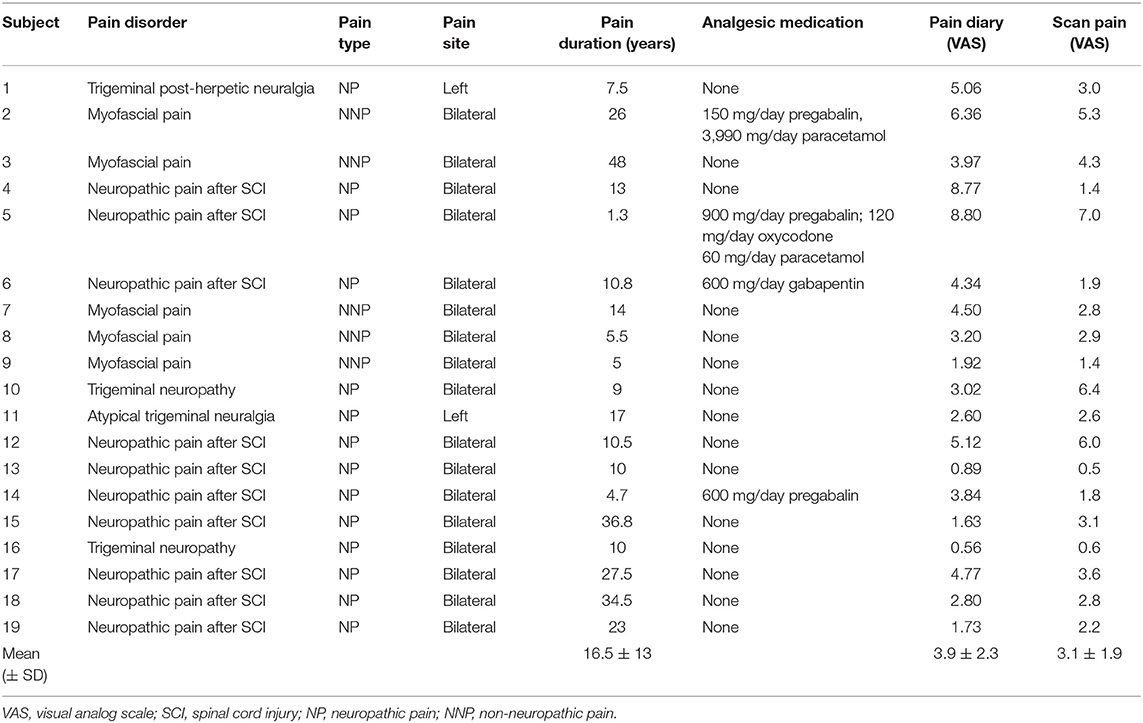
Chronic pain subjects had significantly higher scores in harm avoidance compared to the age and gender matched control group without pain (mean [±SD] harm avoidance: chronic pain subjects: 74 ± 22; control subjects without pain: 34 ± 23; p < 0.001, t = 5.32, df = 36; Figure 1A). In addition, chronic pain subjects had high values in harm avoidance when compared to a standard community sample of 300 normal adult individuals (71). In contrast, the control group had average levels of harm avoidance compared to the standard community sample (71). There was no significant difference in harm avoidance levels between subjects with neuropathic pain (n = 14) and subjects with non-neuropathic pain (myofascial pain, n = 5) (mean [±SD] harm avoidance: neuropathic pain subjects: 69 ± 23; non-neuropathic pain subjects: 86 ± 17; p = 0.14, t = −1.77, df = 9).
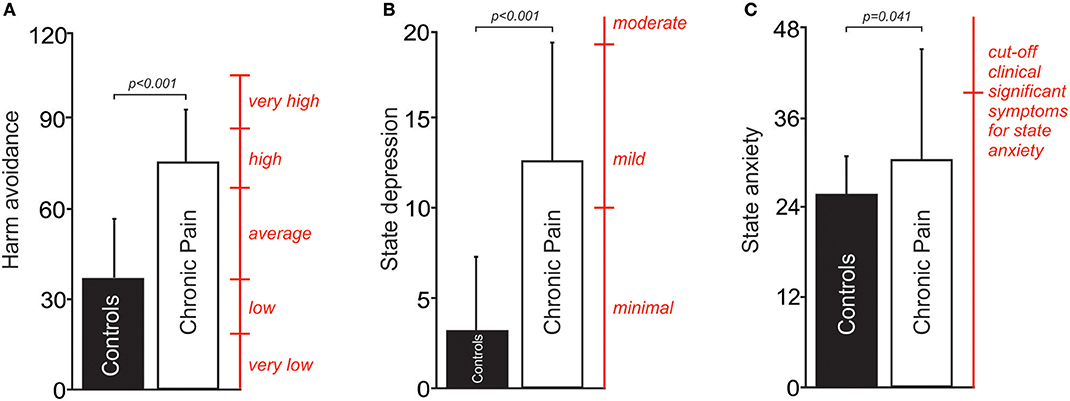
Figure 1. (A) A plot of mean (±SD) levels of harm avoidance in people with chronic pain and pain-free controls. Percentile scores (red) derived from a standard community sample of 300 normal adult individuals; 84–100%, very high; 67–83.3%, high; 34–66.7%, average; 17–33%, low; 0–16.7%, very low (71). (B) A plot of mean (±SD) depression scores in people with chronic pain and pain-free controls. Cut-off scores (red) of 0–9 indicate minimal depression, 10–18 indicate mild depression, and 19–29 indicate moderate depression (64). (C) A plot of mean (±SD) anxiety scores in people with chronic pain and pain-free controls. A cut-off score (red) of 39–40 has been suggested to detect clinically significant symptoms for state anxiety (61).
Chronic pain subjects had significantly higher depression scores when compared to the age and gender matched control group without pain (mean [±SD] depression scores: chronic pain subjects: 12 ± 7; control subjects without pain: 3 ± 4; p < 0.001, t = 4.50, df = 26; Figure 1B). There was no significant difference in depression scores between subjects with neuropathic pain (n = 14) and subjects with non-neuropathic pain (myofascial pain, n = 5) (mean [±SD] depression scores: neuropathic pain subjects: 11 ± 7; non-neuropathic pain subjects: 14 ± 8; p = 0.33, t = −1.01, df = 17).
Finally, chronic pain subjects had significantly higher scores in state anxiety when compared to the age and gender matched control group without pain (mean [±SD] state anxiety score: chronic pain subjects: 31 ± 12; control subjects without pain: 25 ± 5; p = 0.041, t = 2.20, df = 36; Figure 1C). There was no significant difference in anxiety scores between subjects with neuropathic pain (n = 14) and subjects with non-neuropathic pain (myofascial pain, n = 5) (mean [±SD] anxiety scores: neuropathic pain subjects: 28 ± 8; non-neuropathic pain subjects: 39 ± 16; p = 0.17, t = −1.60, df = 5).
Differences in mPFC Resonance Levels Between Chronic Pain and Control Subjects
The mPFC voxel from which 1H-MRS spectra was acquired is shown in Figure 2A. Chronic pain subjects had significantly lower mPFC glutamate levels compared with age and gender matched healthy control subjects (mean [±SD] Glu/H2O ratio: chronic pain subjects: 0.18 ± 0.04; control subjects without pain: 0.22 ± 0.05; p = 0.013, t = −2.65, df = 35; Figure 2B). There was no significant difference in mPFC glutamate levels between subjects with neuropathic pain (n = 14) and subjects with non-neuropathic pain (myofascial pain, n = 5) (mean [±SD] Glu/H2O ratio: neuropathic pain subjects: 0.18 ± 0.04; non-neuropathic pain subjects: 0.19 ± 0.03; p = 0.46, t = −0.75, df = 17).
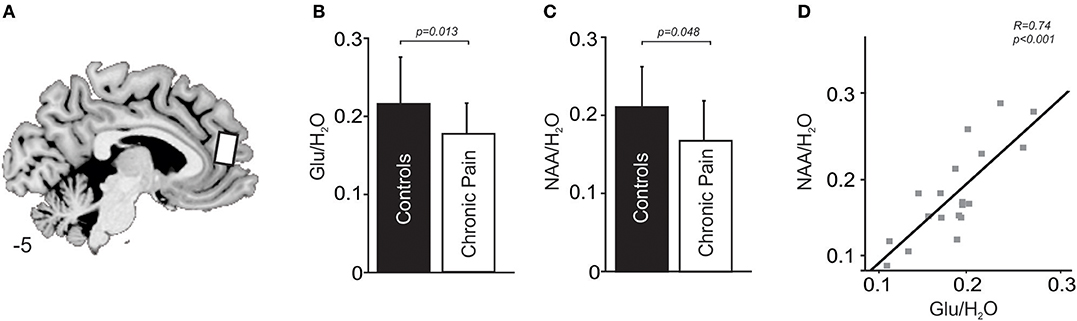
Figure 2. (A) Sagittal slice showing location from which proton spectroscopy was performed in the right medial prefrontal cortex in people with chronic pain and pain-free controls. Slice location in Montreal Neurological Institute space is indicated at the lower left of the image. (B) A plot of mean (±SD) Glu/ H2O ratios in the medial prefrontal cortex in people with chronic pain and pain-free controls. (C) A plot of mean (±SD) NAA/H2O ratios in the medial prefrontal cortex in people with chronic pain and pain-free controls. (D) A plot of Glu/ H2O ratios in people with chronic pain against NAA/ H2O ratios in the medial prefrontal cortex.
Chronic pain subjects had significantly lower mPFC NAA levels compared with control subjects without pain (mean [±SD] NAA/H2O ratio: chronic pain subjects: 0.17 ± 0.05; control subjects without pain: 0.21 ± 0.05; p = 0.048, t = −2.05, df = 36; Figure 2C). There was no significant difference in mPFC NAA levels between subjects with neuropathic pain (n = 14) and subjects with non-neuropathic pain (myofascial pain, n = 5) (mean [±SD] NAA/H2O ratio: neuropathic pain subjects: 0.17 ± 0.06; non-neuropathic pain subjects: 0.17 ± 0.04; p = 0.82, t = 0.24, df = 17).
Finally, chronic pain subjects had similar mPFC MI and Cr levels compared with control subjects (mean [±SD] MI/H2O ratio: chronic pain subjects: 0.20 ± 0.04; control subjects without pain: 0.20 ± 0.08; p = 0.61, t = −0.52, df = 36; mean [±SD] Cr/H2O ratio: chronic pain subjects: 0.22 ± 0.04; control subjects without pain: 0.24 ± 0.06; p = 0.36, t = −0.92, df = 36).
Relationship Between mPFC Resonances and Psychometric Measures
In the chronic pain group, Glu/H2O ratios were negatively correlated to levels of harm avoidance (R = −0.5, p = 0.03), that is the greater the reduction in medial prefrontal cortex glutamate levels, the higher the levels in harm avoidance (Figure 3A). In contrast, no significant correlation between Glu/H2O ratios and levels of harm avoidance was found in controls without pain (R = −0.188, p = 0.46). Furthermore, in chronic pain subjects Glu/H2O ratios were not correlated to either diary pain (R = 0.03, p = 0.90), scan pain (R = 0.4, p = 0.87), pain duration (R = 0.34, p = 0.16), state depression (R = −0.20, p = 0.42), or state anxiety (R = −0.27, p = 0.27). In control subjects without pain, Glu/H2O ratios were also not correlated to either state anxiety (R = −0.13, p = 0.62) or state depression (R = −0.40, p = 0.10).
Figure 3. (A) A plot of Glu/ H2O ratios in the medial prefrontal cortex in people with chronic pain against levels of harm avoidance. (B) A plot of NAA/H2O ratios in the medial prefrontal cortex in people with chronic pain against levels of harm avoidance.
In chronic pain subjects, NAA/H2O ratios were negatively correlated to levels of harm avoidance (R = −0.7, p = 0.001); that is, the greater the reduction in medial prefrontal cortex NAA levels, the higher the levels of harm avoidance (Figure 3B). In contrast, no significant correlation between NAA/H2O ratios and levels of harm avoidance was found in controls without pain (R = −0.01, p = 0.96). Furthermore, in chronic pain subjects NAA/H2O ratios were not correlated to either diary pain (R = 0.09, p = 0.18), scan pain (R = −1.25, p = 0.61), pain duration (R = 0.44, p = 0.06), state depression (R = 0.09, p = 0.72), or state anxiety (R = −0.04, p = 0.88). In control subjects without pain, NAA/H2O ratios were also not correlated to either state anxiety (R = 0.42, p = 0.07) or state depression (R = 0.22, p = 0.36).
In chronic pain subjects, MI/H2O ratios were not correlated to levels of harm avoidance (R = 0.48, p = 0.85), diary pain (R = −0.07, p = 0.77), scan pain (R = −0.10, p = 0.65), pain duration (R = 0.10, p = 0.97), state depression (R = −0.13, p = 0.60), or state anxiety (R = −0.19, p = 0.44). In controls without pain MI/H2O ratios were also not correlated to either state anxiety (R = −0.39, p = 0.10), state depression (R = −0.02, p = 0.94), or harm avoidance (R = 0.015, p = 0.952).
In chronic pain subjects, Cr/H2O ratios were not correlated to levels of harm avoidance (R = −0.35, p = 0.14), diary pain (R = −0.07, p = 0.77), scan pain (R = −0.10, p = 0.65), pain duration (R = 0.35, p = 0.14), state depression (R = 0.02, p = 0.94), or state anxiety (R = −0.20, p = 0.41). In control subjects without pain, MI/H2O ratios were also not correlated to levels of harm avoidance (R = −0.25, p = 0.30), state anxiety (R = −0.23, p = 0.36), or state depression (R = −0.12, p = 0.66).
In both chronic pain subjects and control subjects, Glu/H2O ratios were positively correlated to NAA/H2O ratios (chronic pain subjects: R = 0.74, p < 0.001, Figure 2D; control subjects: R = 0.61, p = 0.008). Furthermore, in chronic pain subjects, the relationship of NAA to harm avoidance remained significant when we accounted for glutamate in a sequential regression model [p = 0.009, R2 = 0.49 (AICc = 165.6)]. In contrast, the relationship of glutamate to harm avoidance was no longer significant when we accounted for NAA in a sequential regression model [p = 0.88, R2 = 0.049 (AICc = 169.7)].
1H-MRS Partial Volume Makeup
There was no significant difference in the fraction of cerebrospinal fluid, gray, and white matter within the mPFC voxel between chronic pain and control subjects (gray matter percentage within the mPFC voxel: chronic pain subjects: 0.23 ± 0.16 mean [±SD]; control subjects: 0.24 ± 0.19 mean [±SD]; t = −0.07, df = 35, p = 0.94; white matter fraction within the mPFC voxel: chronic pain subjects: 0.06 ± 0.05 mean [±SD]; control subjects: 0.18 ± 0.26, t = −1.92 mean [±SD], df = 19.41, p = 0.07; mean [±SD]; cerebrospinal fluid fraction within the mPFC voxel: chronic pain subjects: 0.70 ± 0.15 mean [±SD]; control subjects: 0.58 ± 0.34 mean [±SD]; t = 1.42, df = 25.17, p = 0.17).
Spectral Quality Assessment
According to the consensus on clinical proton MRS of the brain (72) the linewidths, SNR and variances of the metabolites were all well within acceptable limits for data quality. Line widths for all spectra were <10 Hz after automatic shimming (pencil beam auto second order option). Furthermore, there was no significant difference in each metabolite's mean variance (%) between chronic pain and control subjects [NAA variance mean ± SD: chronic pain subjects: 8.2 ± 4.8% (minimum 3.5; maximum 19.0); control subjects: 7.0 ± 2.0% (minimum 4.2; maximum 11.0); t = 0.97, df = 25, p = 0.34; Glu variance mean ± SD: chronic pain subjects: 15.0 ± 5.8% (minimum 3.8; maximum 19.8); control subjects: 15.4 ± 5.2% (minimum 3.4; maximum 19.9); t = −0.24, df = 35, p = 0.81; MI variance mean ± SD: chronic pain subjects: 7.6 ± 2.6% (minimum 3.4; maximum 12.0); control subjects: 8.1 ± 4.6% (minimum 4.2; maximum 19.4); t = −39, df = 35, p = 0.70; Cr variance mean ± SD: chronic pain subjects: 5.0 ± 2.0% (minimum 2.4; maximum 9.1); control subjects: 5.2 ± 2.1% (minimum 2.6; maximum 11.0); t = −0.37, df = 35, p = 0.72]. There was also no significant difference in mean SNR ratios between chronic pain and control subjects [SNR ratios mean ± SD: chronic pain subjects: 30 ± 14.7 (minimum 7.7; maximum 54); control subjects: 24 ± 12.1 (minimum 3.5; maximum 54); t = 1.39, df = 35, p = 0.17].
Discussion
This study demonstrates that chronic pain is associated with a significant reduction in glutamate in the mPFC. Glutamate is known as the major excitatory neurotransmitter in the brain (73). Furthermore, the study revealed that people with chronic pain show a decrease in NAA, a marker of neuronal integrity (74), in the mPFC, compared to age- and gender- matched individuals without pain. Additionally, in chronic pain subjects, both metabolites glutamate and NAA were significantly negatively correlated to harm avoidance. That is, the higher the multidimensional trait of harm avoidance, e.g., the higher the ongoing fear and worry about pain, the lower the levels of mPFC glutamate and NAA. In contrast, no significant relationship between either metabolite and harm avoidance was found in healthy subjects. Moreover, no significant relationship was found between either metabolite and state anxiety and depression in healthy or chronic pain subjects. Finally, we found a significant positive relationship between glutamate and NAA.
NAA is well-known to be a marker of both neuronal loss and mitochondrial activity (69). In our study, we argue that the decline in mPFC NAA represents a decrease in mitochondrial activity rather than neuronal loss because (1) we also found a reduction in mPFC glutamate which declines with decreased mitochondrial activity in a linear manner (69, 75, 76) and (2) we found a significant positive correlation between NAA and glutamate. In line with this argument, evidence is arising that a reduction in NAA is related to glutamate dysfunction (77, 78). The positive association between NAA and glutamate may reflect NAA's involvement in facilitating energy metabolism in neuronal mitochondria from glutamate (78). Furthermore, multimodal brain imaging studies point away from neuronal loss as the likely explanation for mPFC gray matter volume decline in people with chronic pain (79). Indeed, we recently suggested that in individuals with chronic pain, a decrease in mPFC gray matter volume does not represent neuronal loss but rather a dysregulation in glutamate metabolism (10).
Our study revealed no significant difference between the fraction of gray matter within the mPFC voxel between control and chronic pain subjects. This could be explained by the variance (>30%) in the amount of gray matter across the age range from 23 to 68 studied here, being greater than the reported changes in mPFC gray matter [12%, (80)] and whole brain gray matter volume [5.4%, (81)] in chronic pain subjects compared to healthy control subjects. Hence, the chances of finding a difference in gray matter within the mPFC box between both groups studied here is minimal.
The mPFC is rich in glutamatergic cells and innervation (82, 83). Both increased (12, 14, 16, 18) and reduced mPFC glutamate (13, 15, 17) have been shown in the animal model of chronic pain. Furthermore, reduced glutamate has been found in the anterior cingulate cortex in individuals with both acute and chronic pain (25, 84–86). Guida et al. suggested that an initial glutamate increase during the acute phase of pain is followed by a decline during its progression to chronicity (13). This suggestion aligns with findings, as we presented here, of a decrease in mPFC glutamate in people with long-term, chronic pain. The interesting question to ask is which cellular mechanisms underlie this decrease in mPFC glutamate in people with chronic pain. It is well-known that the mPFC is specifically vulnerable to the effects of stress (87–89). It is also well-established that acute pain can be perceived as an acute stressor which can evoke a physiological stress response, e.g., release of glucocorticoids such as cortisol (73, 90). In acute pain, the stress-induced release of glucocorticoids rapidly increases glutamate release in the mPFC (91, 92). In particular, acute stress induces a rise of readily releasable glutamate vesicles in the mPFC (73). In contrast, the effects of chronic stress, and hence chronic pain, on glutamate release are mostly unknown (73). It is thought that chronic pain results in sustained mPFC glial cell changes (93–95), which alter glutamate neurotransmission in the mPFC (73, 96, 97). In particular, high levels of glucocorticoids result in glia activation (97) leading to the production of cytokines, which downregulate glutamate function (98). The downregulation of mPFC glutamate function may reflect a decrease in mPFC glutamate, as measured by magnetic resonance spectroscopy in our study.
The sequential regression model used in our study (including harm avoidance as outcome and NAA and glutamate as predictors) revealed that NAA may be key in the relationship between glutamate and harm avoidance. This is not surprising as it has been proposed that NAA may act as a reservoir of glutamate (77). Particularly, Clark et al. suggested that NAA is converted to aspartate in oligodendrocytes, which can then be converted to glutamate through the TCA cycle with an energetically favorable set of reactions (77). Thus, NAA in neuronal tissue may serve as a large reservoir for refilling glutamate (77), and hence may be key in shaping the relationship between glutamate and harm avoidance in times of stress.
Another important question refers to the clinical impact of a decrease in mPFC metabolic activity. In individuals with chronic pain, both glutamate and NAA were significantly negatively correlated with harm avoidance, but not with state depression and anxiety scores. As anxiety and depression scores were not correlated to glutamate and NAA levels, it seems that the decrease in glutamate and NAA is associated with different constructs than state anxiety and depression in individuals with chronic pain. The mPFC is implicated in complex cognitive functions such as learning and memory (21, 99), decision making (22), executive control (100), and emotional processing (19) such as extinction of fear (20). Given this, it is conceivable that changes in concentration of these metabolites may alter more complex multidimensional trait factors.
Harm avoidance is a multidimensional trait comprising cognitive, emotional, and behavioral characteristics and aligns with the psychological complexity of chronic pain presentations (47, 101). Harm avoidance refers to the psychological correlates of fear and worry, but it also refers to other symptom characteristics which compound adjustment to chronic pain such as pessimistic thinking, chronic tiredness, fatigue-proneness, and sensitivity to criticism and punishment (46).
Both rodent and human studies have revealed that the mPFC is critical in fear conditioning (20, 102–105). Chronic pain sufferers habitually experience pain as a threat from which they need to escape and at any time possibly avoid (106). This experience results in conditioned fear where fear constitutes an adaptive response to immediate threat (107). Glutamate mediates conditioned fear responses which can lead to maladaptive behavior (23), which in turn can manifest in fatigue (108), mood disorders (109, 110), and anxiety (20). This aligns with the current study, which reveals that chronic pain is associated with reduced mPFC glutamate content, which in turn is significantly correlated to fearfulness, worry, pessimism, fatigue-proneness and sensitivity to criticism and punishment. That is, the more mPFC glutamate decreases the more people with chronic pain tend to anticipate pain with fearful and pessimistic thoughts, resulting in maladaptive behavior such as fatigue-proneness (111) and avoidance of feared activities (47, 101). We suggest that the mPFC has lost its ability to extinguish fears and worries due to the decrease in mPFC glutamate concentration, resulting in an ongoing tendency toward fearfulness, pessimism, and fatigue-proneness. Indeed, alterations in mPFC glutamate concentrations directly affect mPFC glutamateric projections to the periaqueductal gray (PAG) (112). These projections are known to be critical in the cortical modulation of pain and fear responses mediated by the PAG (113, 114). Thus, changes in mPFC glutamateric projections to the PAG result in persistent fear and worry.
Furthermore, in individuals with chronic pain, mPFC NAA concentration was significantly negatively correlated with levels of harm avoidance. As described above, we argue that NAA can be seen as a marker of metabolic activity in our study. Therefore, this negative association supports our result that the lower mPFC metabolic activity, e.g., glutamate and NAA concentration, the higher the multidimensional trait of harm avoidance, e.g., more fear, worry, and pessimistic thinking.
In healthy controls, harm avoidance scores were not correlated with mPFC glutamate and NAA concentration. The lack of relationships may be because of an insufficient range in harm avoidance scores in the healthy control group. In contrast, it may suggest important causal effects. If harm avoidance was itself associated with levels of mPFC glutamate and NAA, then a similar relationship between these metabolites and harm avoidance would have occurred in both control and chronic pain subjects. The unique relationship between mPFC glutamate, NAA and harm avoidance in only chronic pain subjects suggests that any association between mPFC glutamate, NAA and harm avoidance likely appears after the development of chronic pain. Notably, we found the same pattern in a previous study—trait depression scores were only correlated to changes in gray matter volume in the thalamus, the cingulate, the dorsolateral prefrontal and hippocampal cortices in chronic pain subjects, but not in age and gender matched healthy controls (115).
Thus, with the onset of chronic pain, changes in mPFC metabolic activity may be induced. In particular, chronic pain may result in mPFC glia activation (93–95). This may lead to the production of cytokines, which downregulate glutamate function in the mPFC (73, 96, 97) that in turn may negatively affect chronic pain suffers' behavioral and emotional traits. For example, pain sufferers show the following negative behavioral and emotional characteristics: fearfulness, pessimism and fatigue-proneness. Indeed, we recently revealed that subtle alterations in prefrontal brain structure and metabolism can change an individual's personality trait in chronic pain (116).
Limitations
Our sample size was small and therefore our results should be validated in a larger sample. We are confident that our results accurately reflect the nature of mPFC metabolic changes associated with chronic pain as, although our subject numbers were limited, both groups of chronic pain and healthy subjects were comparable on age and gender demographics. This is particularly important as we know that the mPFC changes its structure and function across the lifespan (117). It is possible that subtle differences between dissimilar types of chronic pain may emerge in a larger sample. In our sample there was no significant difference between people with neuropathic and non-neuropathic (nociceptive) pain in mPFC glutamate and NAA concentration, as well as harm avoidance, anxiety and depression scores. Indeed, we recently demonstrated that high levels of harm avoidance (113), state anxiety and depression (118) are independent of chronic pain type, e.g., neuropathic and non-neuropathic (nociceptive). A decrease in mPFC gray matter volume which may reflect a decline in mPFC glutamate (10) has been consistently shown in various pain disorders (6). Therefore, alterations in mPFC glutamate may also be independent of chronic pain type. Notably, in our study both neuropathic and non-neuropathic groups, were not significantly different in mean age, pain duration, scan pain and diary pain. Further, it is possible that some analgesics may have an effect on mPFC metabolic activity as well as on harm avoidance, depression and anxiety levels. For example, monoaminergic-based antidepressants have been shown to affect glutamate system function (119). Further, in healthy subjects, painful stimulation during treatment with morphine has resulted in decreased Glu/Cr, MI/Cr, and NAA/Cr ratios in the anterior cingulate cortex (74). Another study in healthy subjects revealed that Glu/Cr ratio in the anterior cingulate cortex/mPFC, insula and prefrontal cortex was reduced after 5 days of taking an opioid or a serotonin and norepinephrine reuptake inhibitor (120). Acute and chronic effects of medications in the mPFC needs to be tested in a larger sample size. A larger sample size may also identify differences relating to pain phenotype. In our study, the limited sample size may also explain the lack of association between mPFC NAA concentration and pain intensity and mPFC NAA concentration and pain duration. Previous studies have revealed that NAA concentration within the anterior cingulate cortex is associated with both pain intensity and duration in individuals with chronic pain (25, 86).
Finally, it is important to acknowledge that at 3T, glutamate and glutamine overlap (121). The fitting algorithm used here gives an estimation of glutamate concentration that is within generally acceptable error but estimation of glutamine by this method at 3T using short-echo PRESS is problematic. Given that glutamate concentrations are far greater (up to 5x higher) than glutamine (69), the uncertainty in the glutamine estimation is of limited concern.
Conclusion
This study reveals for the first time a significant decrease in mPFC glutamate in individuals with chronic pain. Furthermore, the decrease in glutamate is significantly negatively correlated with harm avoidance. This means that the greater the mPFC glutamate decrease, the more chronic pain sufferers show the following characteristics: fearfulness, pessimism, fatigue-proneness, and sensitivity to criticism and punishment.
We suggest that chronic pain, particularly the stress-induced release of glucocorticoids, induces changes in glutamate transmission in the mPFC, thereby influencing cognitive, and emotional processing. Thus, regulation of fear, worry, negative thinking, and fatigue is impaired.
Data Availability Statement
The datasets for this manuscript are not publicly available because the study participants did not give consent to make the data publicly available. Requests to access the datasets should be directed to SG,cy5ndXN0aW5AdW5zdy5lZHUuYXU=.
Ethics Statement
The Human Research Ethics Committees of the University of New South Wales and University of Sydney approved the research and all participants gave their informed written consent in accordance with the Declaration of Helsinki.
Author Contributions
SG designed the study. SG and BN recruited subjects, collected, analyzed data, and wrote the manuscript. NH-S, TN-J, JM, SB, and CR provided substantial contributions to the interpretation of the findings and critically revised the manuscript. All authors provided approval for publication of the content and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Health and Medical Research Council of Australia (GNT1084240, GNT1087045) and the Rebecca L. Cooper Medical Research Foundation. The MRUI software package was kindly provided by the participants of the EU Network programmes: Human Capital and Mobility [CHRX-CT94-0432] and Training and Mobility of Researchers [ERB-FMRX-CT970160].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank A/Prof. Paul Wrigley, University of Sydney, Australia, for his kind support in the recruitment and diagnosis of people with neuropathic pain following spinal cord injury. The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at Neuroscience Research Australia. The Partial Volume code for Philips MRS data was kindly provided by Dr. Nia Goulden and Dr. Paul Mullins from Bangor University.
References
1. May A. Structural brain imaging: a window into chronic pain. Neuroscientist. (2011) 17:209–20. doi: 10.1177/1073858410396220
2. Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. (2013) 14:663–75. doi: 10.1016/j.jpain.2013.03.001
3. Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS ONE. (2011) 6:e26010. doi: 10.1371/journal.pone.0026010
4. Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. (2012) 32:14874–84. doi: 10.1523/JNEUROSCI.1733-12.2012
5. Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. (2011) 31:5956–64. doi: 10.1523/JNEUROSCI.5980-10.2011
6. Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, et al. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuro Image Clin. (2014) 4:676–86. doi: 10.1016/j.nicl.2014.04.007
7. Lin C, Lee S-H, Weng H-H. Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. Bio Med Res Int. (2016) 2016:7296125. doi: 10.1155/2016/7296125
8. Rauschecker JP, May ES, Maudoux A, Ploner M. Frontostriatal gating of tinnitus and chronic pain. Trends Cogn Sci. (2015) 19:567–78. doi: 10.1016/j.tics.2015.08.002
9. Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum. (2016) 46:330–7. doi: 10.1016/j.semarthrit.2016.06.002
10. Kang D, McAuley JH, Kassem MS, Gatt JM, Gustin SM. What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain? Eur J Pain. (2018) 23:203–19. doi: 10.1002/ejp.1304
11. Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. (2010) 468:232–43. doi: 10.1038/nature09613
12. Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immunity. (2016) 56:397–411. doi: 10.1016/j.bbi.2016.04.012
13. Guida F, Luongo L, Marmo F, Romano R, Iannotta M, Napolitano F, et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol Brain. (2015) 8:47. doi: 10.1186/s13041-015-0139-5
14. Hung KL, Wang SJ, Wang YC, Chiang TR, Wang CC. Upregulation of presynaptic proteins and protein kinases associated with enhanced glutamate release from axonal terminals (synaptosomes) of the medial prefrontal cortex in rats with neuropathic pain. Pain. (2014) 155:377–87. doi: 10.1016/j.pain.2013.10.026
15. Kelly CJ, Huang M, Meltzer H, Martina M. Reduced glutamatergic currents and dendritic branching of layer 5 pyramidal cells contribute to medial prefrontal cortex deactivation in a rat model of neuropathic pain. Front Cell Neurosci. (2016) 10:133. doi: 10.3389/fncel.2016.00133
16. Metz AE, Yau H-J, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA. (2009) 106:2423–8. doi: 10.1073/pnas.0809897106
17. Palazzo E, Luongo L, Guida F, Marabese I, Romano R, Iannotta M, et al. D-Aspartate drinking solution alleviates pain and cognitive impairment in neuropathic mice. Amino Acids. (2016) 48:1553–67. doi: 10.1007/s00726-016-2205-4
18. Xu H, Wu L-J, Wang H, Zhang X, Vadakkan KI, Kim SS, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. (2008) 28:7445–53. doi: 10.1523/JNEUROSCI.1812-08.2008
19. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. (2011) 15:85–93. doi: 10.1016/j.tics.2010.11.004
20. Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. (2009) 16:279–88. doi: 10.1101/lm.1041309
21. Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. (2011) 70:1054–69. doi: 10.1016/j.neuron.2011.05.014
22. Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ Behav. (2005) 52:336–72. doi: 10.1016/j.geb.2004.06.010
23. Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. (2005) 10:820–30. doi: 10.1017/S1092852900010427
24. Auvichayapat P, Keeratitanont K, Janyachareon T, Auvichayapat N. The effects of transcranial direct current stimulation on metabolite changes at the anterior cingulate cortex in neuropathic pain: a pilot study. J Pain Res. (2018) 11:2301–9. doi: 10.2147/JPR.S172920
25. Mullins PG, Rowland LM, Jung RE, Sibbitt WL Jr. A novel technique to study the brain's response to pain: proton magnetic resonance spectroscopy. Neuroimage. (2005) 26:642–6. doi: 10.1016/j.neuroimage.2005.02.001
26. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. PAIN. (2003) 106:127–33. doi: 10.1016/S0304-3959(03)00301-4
27. Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. (2008) 9:883–91. doi: 10.1016/j.jpain.2008.05.005
28. Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. (2012) 72:57–64. doi: 10.1016/j.biopsych.2011.11.022
29. Gorka AX, Hanson JL, Radtke SR, Hariri AR. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol Mood Anxiety Dis. (2014) 4:12. doi: 10.1186/2045-5380-4-12
30. Huey ED, Lee S, Brickman AM, Manoochehri M, Griffith E, Devanand DP, et al. Neuropsychiatric effects of neurodegeneration of the medial versus lateral ventral prefrontal cortex in humans. Cortex. (2015) 73:1–9. doi: 10.1016/j.cortex.2015.08.002
31. Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Dis. (2012) 138:9–18. doi: 10.1016/j.jad.2011.03.049
32. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. (2002) 51:273–9. doi: 10.1016/S0006-3223(01)01336-1
33. Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immunity. (2016) 53:39–48. doi: 10.1016/j.bbi.2015.11.003
34. Zhao YJ, Du MY, Huang XQ, Lui S, Chen ZQ, Liu J, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. (2014) 44:2927–37. doi: 10.1017/S0033291714000518
35. Diaz-Piedra C, Guzman MA, Buela-Casal G, Catena A. The impact of fibromyalgia symptoms on brain morphometry. Brain Imaging Behav. (2016) 10:1184–97. doi: 10.1007/s11682-015-9485-2
36. Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. (2010) 139:48–57.e2. doi: 10.1053/j.gastro.2010.03.049
37. Ivo R, Nicklas A, Dargel J, Sobottke R, Delank K-S, Eysel P, et al. Brain structural and psychometric alterations in chronic low back pain. Eur Spine J. (2013) 22:1958–64. doi: 10.1007/s00586-013-2692-x
38. Mansour ZM, Lepping RJ, Honea RA, Brooks WM, Yeh H-W, Burns JM, et al. Structural brain imaging in people with low back pain. Spine. (2017) 42:726–32. doi: 10.1097/BRS.0000000000001915
39. Niddam DM, Lee SH, Su YT, Chan RC. Brain structural changes in patients with chronic myofascial pain. Eur J Pain. (2017) 21:148–58. doi: 10.1002/ejp.911
40. Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. (2011) 12:436–43. doi: 10.1016/j.jpain.2010.10.003
41. Valet M, Gündel H, Sprenger T, Sorg C, Mühlau M, Zimmer C, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosomat Med. (2009) 71:49–56. doi: 10.1097/PSY.0b013e31818d1e02
42. Abbate-Daga G, Fassino S, Rossana Lo G, Rainero I, Gramaglia C, Marech L, et al. Anger, depression and personality dimensions in patients with migraine without aura. Psychother Psychosomat. (2007) 76:122–8. doi: 10.1159/000097971
43. Conrad R, Schilling G, Bausch C, Nadstawek J, Wartenberg HC, Wegener I, et al. Temperament and character personality profiles and personality disorders in chronic pain patients. PAIN. (2007) 133:197–209. doi: 10.1016/j.pain.2007.07.024
44. Conrad R, Wegener I, Geiser F, Kleiman A. Temperament, character, and personality disorders in chronic pain. Curr Pain Headache Rep. (2013) 17:1–9. doi: 10.1007/s11916-012-0318-3
45. Gustin SM, Burke LA, Peck CC, Murray GM, Henderson LA. Pain and personality: do individuals with different forms of chronic pain exhibit a mutual personality? Pain Pract. (2016) 16:486–94. doi: 10.1111/papr.12297
46. Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A Guide to Its Development and Use. Vol. 1. St Louis, MO: Washington University; Center for Psychobiology of Personality (1994). doi: 10.1037/t03902-000
47. Naylor B, Boag S, Gustin SM. New evidence for a pain personality? A critical review of the last 120 years of pain and personality. Scandinav J Pain. (2017) 17:58–67. doi: 10.1016/j.sjpain.2017.07.011
48. Hakamata Y, Iwase M, Iwata H, Kobayashi T, Tamaki T, Nishio M, et al. Regional brain cerebral glucose metabolism and temperament: a positron emission tomography study. Neurosci Lett. (2006) 396:33–7. doi: 10.1016/j.neulet.2005.11.017
49. Nakao T, Matsumoto T, Shimizu D, Morita M, Yoshimura S, Northoff G, et al. Resting state low-frequency fluctuations in prefrontal cortex reflect degrees of harm avoidance and novelty seeking: an exploratory NIRS study. Front Sys Neurosci. (2013) 7:115. doi: 10.3389/fnsys.2013.00115
50. O'Gorman RL, Kumari V, Williams SCR, Zelaya FO, Connor SEJ, Alsop DC, et al. Personality factors correlate with regional cerebral perfusion. Neuro Image. (2006) 31:489–95. doi: 10.1016/j.neuroimage.2005.12.048
51. Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, et al. Correlation between human personality and neural activity in cerebral cortex. NeuroImage. (2000) 11:541–6. doi: 10.1006/nimg.2000.0564
52. Youn T, Lyoo IK, Kim JJ, Park HJ, Ha KS, Lee DS, et al. Relationship between personality trait and regional cerebral glucose metabolism assessed with positron emission tomography. Biol Psychol. (2002) 60:109–20. doi: 10.1016/S0301-0511(02)00047-9
53. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. (1992) 6:301–55.
54. Nurmikko TJ, Eldridge PR. Trigeminal neuralgia–pathophysiology, diagnosis and current treatment. Br J Anaesth. (2001) 87:117–32. doi: 10.1093/bja/87.1.117
55. Bryce T, Biering-Sørensen F, Finnerup NB, Cardenas D, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. (2012) 50:413–7. doi: 10.1038/sc.2011.156
56. Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs. (2005) 180:69–75. doi: 10.1159/000086200
57. Kost RG, Straus SE. Postherpetic neuralgia — pathogenesis, treatment, and prevention. N w Engl J Med. (1996) 335:32–42. doi: 10.1056/NEJM199607043350107
58. Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthcare. (2016) 9:447–54. doi: 10.2147/JMDH.S106340
59. Kirshblum S, Biering-Sorensen F, Betz R, Burns S, Donovan W, Graves D, et al. International standards for neurological classification of spinal cord injury: cases with classification challenges. J Spinal Cord Med. (2014) 37:120–7. doi: 10.1179/2045772314Y.0000000196
60. Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists' Press (1970).
61. Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br J Clin Psychol. (1983) 22:245–9. doi: 10.1111/j.2044-8260.1983.tb00610.x
62. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
63. Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. (1997) 13:163–170. doi: 10.1097/00002508-199706000-00011
64. Beck AT, Steer RA, Carbin MG. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. (1988) 8:77–100. doi: 10.1016/0272-7358(88)90050-5
66. Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnet Reson Med. (2006) 55:1219–26. doi: 10.1002/mrm.20901
67. Gruber S, Pinker K, Riederer F, Chmelík M, Stadlbauer A, Bittšanský M, et al. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur J Radiol. (2008) 68:320–7. doi: 10.1016/j.ejrad.2007.08.038
68. Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. (2009) 30:353–63. doi: 10.1016/j.neurobiolaging.2007.07.005
69. Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. (2014) 39:1–36. doi: 10.1007/s11064-013-1199-5
70. Rae CD, Bröer S. Creatine as a booster for human brain function. How might it work? Neurochem Int. (2015) 89:249–59. doi: 10.1016/j.neuint.2015.08.010
71. Cloninger C, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. (1993) 50:975–90. doi: 10.1001/archpsyc.1993.01820240059008
72. Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magnet Reson Med. (2019) 82:527–50. doi: 10.1002/mrm.27742
73. Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. (2012) 13:22–37. doi: 10.1038/nrn3138
74. Hansen TM, Olesen AE, Simonsen CW, Drewes AM, Frøkjær JB. Cingulate metabolites during pain and morphine treatment as assessed by magnetic resonance spectroscopy. J Pain Res. (2014) 7:269–76. doi: 10.2147/JPR.S61193
75. Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. (1998) 95:316–21. doi: 10.1073/pnas.95.1.316
76. Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. (2011) 589:5845–55. doi: 10.1113/jphysiol.2011.216978
77. Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. (2006) 67:506–12. doi: 10.1016/j.mehy.2006.02.047
78. Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progr Neurobiol. (2007) 81:89–131. doi: 10.1016/j.pneurobio.2006.12.003
79. Pomares F, Funck T, Feier N, Roy S, Daigle-Martel A, Ceko M, et al. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J Neurosci. (2016) 37:1090–1101. doi: 10.1523/JNEUROSCI.2619-16.2016
80. Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. (2008) 60:570–81. doi: 10.1016/j.neuron.2008.08.022
81. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. (2004) 24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004
82. Bozkurt A, Zilles K, Schleicher A, Kamper L, Arigita ES, Uylings HB, et al. Distributions of transmitter receptors in the macaque cingulate cortex. Neuroimage. (2005) 25:219–29. doi: 10.1016/j.neuroimage.2004.10.040
83. Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapping. (2009) 30:2336–55. doi: 10.1002/hbm.20667
84. Becerra L, Veggeberg R, Prescot A, Jensen J, Renshaw P, Scrivani S, et al. A ‘complex'of brain metabolites distinguish altered chemistry in the cingulate cortex of episodic migraine patients. Neuro Image Clin. (2016) 11:588–94. doi: 10.1016/j.nicl.2016.03.020
85. Gussew A, Rzanny R, Güllmar D, Scholle H-C, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage. (2011) 54:1315–23. doi: 10.1016/j.neuroimage.2010.09.039
86. Kameda T, Fukui S, Tominaga R, Sekiguchi M, Iwashita N, Ito K, et al. Brain metabolite changes in the anterior cingulate cortex of chronic low back pain patients and correlations between metabolites and psychological state. Clin J Pain. (2018) 34:657–63. doi: 10.1097/AJP.0000000000000583
87. Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. (2009) 164:798–808. doi: 10.1016/j.neuroscience.2009.08.053
88. Holmes A, Wellman CLJN, Reviews B. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. (2009) 33:773–83. doi: 10.1016/j.neubiorev.2008.11.005
89. Perez-Cruz C, Müller-Keuker JI, Heilbronner U, Fuchs E, Flügge G. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plast. (2007) 2007:46276. doi: 10.1155/2007/46276
90. Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. (2014) 94:1816–25. doi: 10.2522/ptj.20130597
91. Bagley J, Moghaddam BJN. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. (1997) 77:65–73. doi: 10.1016/S0306-4522(96)00435-6
92. Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. (1993) 60:1650–7. doi: 10.1111/j.1471-4159.1993.tb13387.x
93. Chen FL, Dong YL, Zhang ZJ, Cao DL, Xu J, Hui J, et al. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res Bull. (2012) 87:60–6. doi: 10.1016/j.brainresbull.2011.09.022
94. Ikeda H, Mochizuki K, Murase KJP. Astrocytes are involved in long-term facilitation of neuronal excitation in the anterior cingulate cortex of mice with inflammatory pain. Pain. (2013) 154:2836–43. doi: 10.1016/j.pain.2013.08.023
95. Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. (2015) 138:604–15. doi: 10.1093/brain/awu377
96. Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. (2010) 16:519–31. doi: 10.1177/1073858409360822
97. Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Perez O, Gonzalez-Castañeda R, Garcia-Estrada J, Luquin SJ. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. (2010) 6:195–204. doi: 10.2174/157339510791823790
98. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. (2009) 10:23–36. doi: 10.1038/nrn2533
99. Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. (2004) 304:881–3. doi: 10.1126/science.1094804
100. Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. (2007) 7:391–5. doi: 10.3758/CABN.7.4.391
101. Boersma K Is the search for a “pain personality” of added value to the Fear-Avoidance-Model (FAM) of chronic pain? Scand J Pain. (2017) 17:226–7. doi: 10.1016/j.sjpain.2017.08.019
102. Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosc. (2015) 9:298. doi: 10.3389/fnbeh.2015.00298
103. Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. (2010) 67:199–207. doi: 10.1016/j.biopsych.2009.08.026
104. Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog Brain Res. (2007) 161:195–203. doi: 10.1016/S0079-6123(06)61013-3
105. Moghaddam BJN. Bringing order to the glutamate chaos in schizophrenia. Neuron. (2003) 40:881–4. doi: 10.1016/S0896-6273(03)00757-8
106. De Peuterl S, Van Diestl I, Vansteenwegenl D, Van den Berghl O, Vlaeyenl JW. Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. Eur J Pain. (2011) 15:889–94. doi: 10.1016/j.ejpain.2011.03.002
107. Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. (2007) 11:307–16. doi: 10.1016/j.tics.2007.05.008
108. Rönnbäck L, Hansson E. On the potential role of glutamate transport in mental fatigue. J Neuroinflammation. (2004) 1:22. doi: 10.1186/1742-2094-1-22
109. Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Archiv Gen Psychiatry. (2007) 64:193–200. doi: 10.1001/archpsyc.64.2.193
110. Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. (2012) 62:63–77. doi: 10.1016/j.neuropharm.2011.07.036
111. Clauw DJ, Chrousos GP. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. (1997) 4:134–53. doi: 10.1159/000097332
112. Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, et al. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS ONE. (2013) 8:e60312. doi: 10.1371/annotation/8a1feb12-d70a-4fb2-8dcb-a9cad56c3afd
113. Coutinho MR, Menescal-de-Oliveira L. Role of homocysteic acid in the guinea pig (Cavia porcellus) anterior cingulate cortex in tonic immobility and the influence of NMDA receptors on the dorsal PAG. Behav Brain Res. (2010) 208:237–42. doi: 10.1016/j.bbr.2009.11.047
114. Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. (2011) 70:838–45. doi: 10.1002/ana.22537
115. Gustin SM, Peck CC, Macey PM, Murray GM, Henderson LA. Unraveling the effects of plasticity and pain on personality. J Pain. (2013) 14:1642–52. doi: 10.1016/j.jpain.2013.08.005
116. Gustin SM, McKay JG, Petersen ET, Peck CC, Murray GM, Henderson LA. Subtle alterations in brain anatomy may change an Individual's personality in chronic pain. PLoS ONE. (2014) 9:e109664. doi: 10.1371/journal.pone.0109664
117. Fjell AM, Sneve MH, Grydeland H, Storsve AB, Amlien IK, Yendiki A, et al. Relationship between structural and functional connectivity change across the adult lifespan: a longitudinal investigation. Hum Brain Mapping. (2017) 38:561–73. doi: 10.1002/hbm.23403
118. Gustin SM, Wilcox SL, Peck CC, Murray GM, Henderson LA. Similarity of suffering: equivalence of psychological and psychosocial factors in neuropathic and non-neuropathic orofacial pain patients. PAIN. (2011) 152:825–32. doi: 10.1016/j.pain.2010.12.033
119. Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry. (2013) 73:1180–8. doi: 10.1016/j.biopsych.2012.11.009
120. Hansen TM, Olesen AE, Simonsen CW, Fischer IW, Lelic D, Drewes AM, et al. Acute metabolic changes associated with analgesic drugs: an MR spectroscopy study. J Neuroimaging. (2016) 26:545–51. doi: 10.1111/jon.12345
121. Lally N, An L, Banerjee D, Niciu MJ, Luckenbaugh DA, Richards EM, et al. Reliability of 7T 1H-MRS measured human prefrontal cortex glutamate, glutamine, and glutathione signals using an adapted echo time optimized PRESS sequence: A between-and within-sessions investigation. J Magnet Reson Imaging. (2016) 43:88–98. doi: 10.1002/jmri.24970
Keywords: medial prefrontal cortex, chronic pain, spectroscopy, glutamate, N-acetylaspartate, harm avoidance, emotional dysregulation
Citation: Naylor B, Hesam-Shariati N, McAuley JH, Boag S, Newton-John T, Rae CD and Gustin SM (2019) Reduced Glutamate in the Medial Prefrontal Cortex Is Associated With Emotional and Cognitive Dysregulation in People With Chronic Pain. Front. Neurol. 10:1110. doi: 10.3389/fneur.2019.01110
Received: 14 March 2019; Accepted: 03 October 2019;
Published: 03 December 2019.
Edited by:
Freimut Dankwart Juengling, Universität Bern, SwitzerlandReviewed by:
Timothy Joseph Meeker, Johns Hopkins Medicine, United StatesMatt Howard, King's College London, United Kingdom
Copyright © 2019 Naylor, Hesam-Shariati, McAuley, Boag, Newton-John, Rae and Gustin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvia M. Gustin, cy5ndXN0aW5AdW5zdy5lZHUuYXU=
 Brooke Naylor1,2
Brooke Naylor1,2 Negin Hesam-Shariati
Negin Hesam-Shariati Simon Boag
Simon Boag Toby Newton-John
Toby Newton-John Caroline D. Rae
Caroline D. Rae Sylvia M. Gustin
Sylvia M. Gustin