
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 10 October 2019
Sec. Neurotrauma
Volume 10 - 2019 | https://doi.org/10.3389/fneur.2019.01063
This article is part of the Research Topic Decompressive Craniectomy in the Management of Neurological Emergencies View all 11 articles
Introduction: Traumatic brain injury (TBI) is a global epidemic. The incidence of TBI in low and middle-income countries (LMICs) is three times greater than in high-income countries (HICs). Decompressive craniectomy (DC) is a surgical procedure to reduce intracranial pressure (ICP) and prevent secondary injury. Multiple comparative studies, and several randomized controlled trials (RCTs) have been conducted to investigate the influence of DC for patients with severe TBI on outcomes such as mortality, ICP, neurological outcomes, and intensive care unit (ICU) and hospital length of stay. The results of these studies are inconsistent. Systematic reviews and meta-analyses have been conducted in an effort to aggregate the data from the individual studies, and perhaps derive reliable conclusions. The purpose of this project was to conduct a review of the reviews about the effectiveness of DC to improve outcomes.
Methods: We conducted a systematic search of the literature to identify reviews and meta-analyses that met our pre-determined criteria. We used the AMSTAR 2 instrument to assess the quality of each of the included reviews, and determine the level of confidence.
Results: Of 973 citations from the original search, five publications were included in our review. Four of them included meta-analyses. For mortality, three reviews found a positive effect of DC compared to medical management and two found no significant difference between groups. The four reviews that measured neurological outcome found no benefit of DC. The two reviews that assessed ICP both found DC to be beneficial in reducing ICP. DC demonstrated a significant reduction in ICU length of stay in the one study that measured it, and a significant reduction in hospital length of stay in the two studies that measured it. According to the AMSTAR 2 criteria, the five reviews ranged in levels of confidence from low to critically low.
Conclusion: Systematic reviews and meta-analyses are important approaches for aggregating information from multiple studies. Clinicians rely of these methods for concise interpretation of scientific literature. Standards for quality of systematic reviews and meta-analyses have been established to support the quality of the reviews being produced. In the case of DC, more attention must be paid to quality standards, in the generation of both individual studies and reviews.
Traumatic brain injury (TBI) remains one of the most serious public health problems worldwide, and in particular in low- and middle-income countries (LMICs) (1). Decompressive craniectomy (DC) has been used for the management of intracranial pressure (ICP) with severe TBI patients as a primary or prophylactic intervention, or as a secondary intervention when first-line therapies fail (2–4). Some studies in TBI populations have shown that DC improves ICP and cerebral perfusion pressure (CPP), contributing to improved long-term functional outcomes and reduction in costs (5–12). However, other studies show opposite results (13–15). Given the variation in results, leading to uncertainty about the actual benefit or not of the procedure, multiple systematic reviews and meta-analyses have been conducted to synthesize the results of the individual studies. However, in order to use the information from these reviews to make treatment and policy decisions, the findings must be critically considered within the context of the quality of the reviews.
Standards have been established for the assessment of the quality of systematic reviews and meta-analyses. One instrument that is widely used is AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews) (16). The instrument contains 16 individual domains, with 7 of them being “critical domains.” It was developed to provide health professionals and policy makers with a practical critical appraisal instrument to assess systematic reviews and meta-analyses that include randomized controlled trials (RCTs) as well as non-randomized studies (NRSs).
We conducted a literature search to identify systematic reviews and meta-analyses that compare the outcome for patients with severe TBI who receive DC with patients who receive standard medical management. We used the AMSTAR 2 instrument to assess the included publications. The purpose of this project was to summarize the findings of the publications in light of their AMSTAR 2 scores, and to identify potential improvements in the conduct of systematic reviews about DC that could contribute to the confidence in the findings. Thus, the emphasis in this paper is to critically assess the included systematic reviews/meta-analyses.
The search included systematic reviews (SRs) and meta-analyses (MAs) published on the topic of DC in the treatment of severe TBI patients. A search strategy was developed including mesh terms and all field terms but also free text searches in search engines. The main strategy included: “brain injuries, traumatic”[MeSH Terms] OR “craniocerebral trauma”[MeSH Terms] AND (“decompressive craniectomy”[MeSH Terms] OR “decompressive craniectomy”[All Fields]) OR “decompressive craniotomy”[All Fields], filtering by study types of meta-analysis and systematic review (excluding all other types of studies). Systematic reviews/meta-analyses that included pathologies other than TBI, and those that focused on interventions other than DC specifically, were excluded.
Two investigators independently reviewed abstracts and full text articles. Discrepancies were resolved through consensus of three investigators.
Nine hundred seventy three citations were obtained, most of which were not specific to the topic or did not meet the inclusion criteria. Six publications were retrieved that met the pre-determined inclusion criteria (17–22). We eliminated Sahuquillo (17) because it included only one study (23), which was included also in four of the other included reviews (18, 20–22). Thus, five SRs/MAs were included in this review.
The five reviews included 9 RCTs and 16 NRSs (see Table 1). Four of the five studies included both RCTs and NRSs, and one (21) included only RCTs. Three of the five studies used only RCTs in their MAs (18, 20, 21), one included both RCTs and NRSs in the MA (22), and one did not conduct a MA (19).
The following summarizes each review with an emphasis on findings from the MA (when utilized) and RCTs, and presents the results of the AMSTAR 2 assessments.
Wang et al. (18) conducted a SR and MA to investigate the effect of early DC on mortality, ICP reduction, and hospital stay. They included three RCTs and five NRSs in their review, and used only the RCTs for the MA (see Table 1). For mortality, the pooled odds ratio (OR) was 0.531 [95% confidence interval (CI) 0.209–1.350, Z = 1.95, p = 0.183]. There was a significant reduction in ICP for the DC group compared to the non-DC group (pooled difference in means −2.081, 95% CI −2.796 to −1.366, p < 0.001). Also, the DC group had significantly fewer days in hospital than the non-DC group (pooled difference in means −9.907, 95% CI −16.250 to −3.565, p = 0.002). Thus, the findings from the pooled analysis indicate no significant effect of DC on mortality, and significantly reduced ICP and days in hospital.
Applying the AMSTAR 2 assessment criteria, the confidence in the findings from this review is critically low. They sustained violations in 5 of the 7 critical domains, and a partial violation for one additional critical domain (see Table 2).
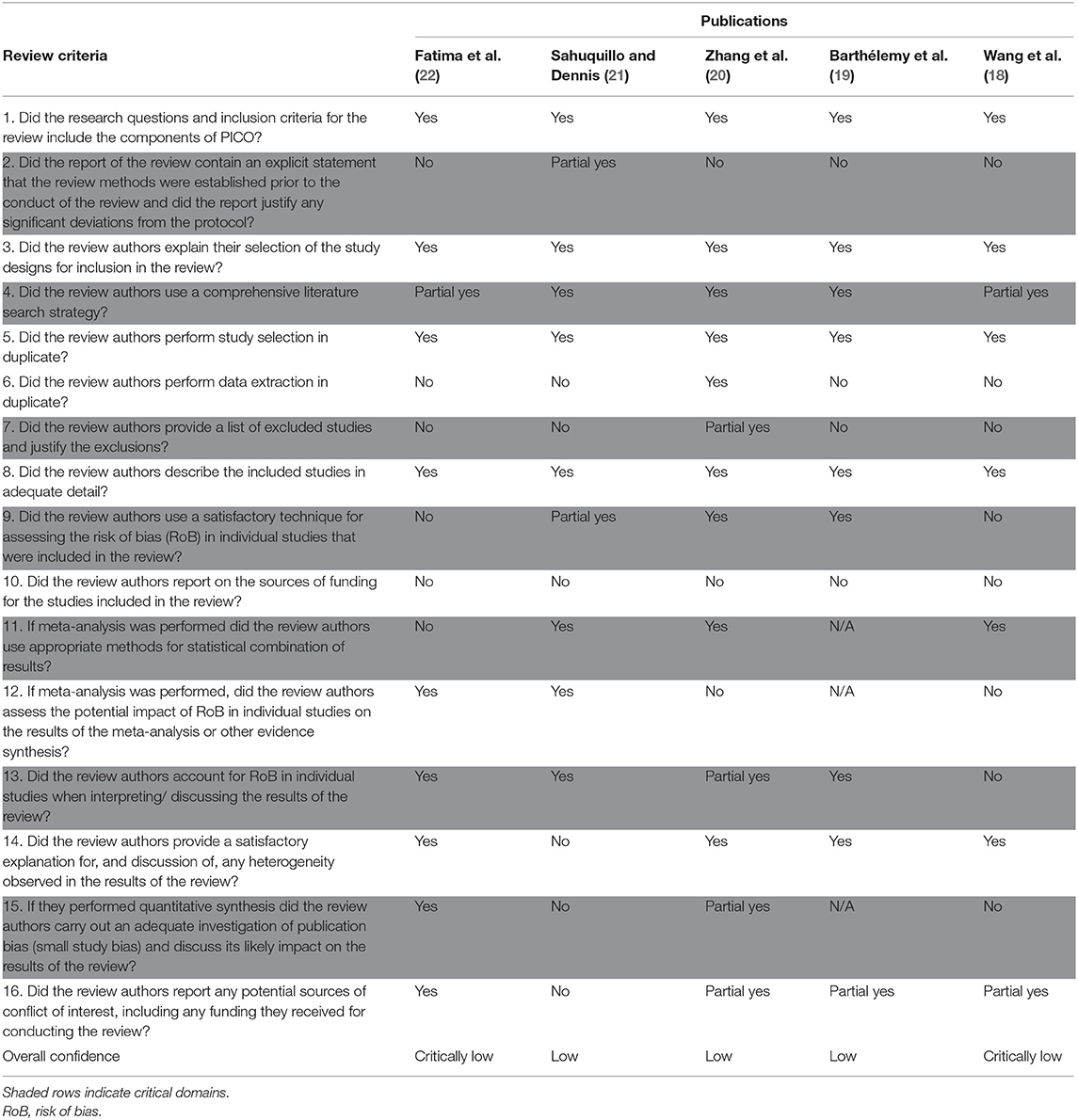
Table 2. AMSTAR 2 individual domains and overall confidence scores for systematic reviews/meta analyses about decpompressive craniectomy.
Barthelemy et al. (19) conducted a SR of studies that compared DC to medical management or to alternative means of surgical decompression, and reported on mortality, neurological outcomes measured by the Glasgow Outcome Scale (GOS), and ICP. The alternative means of decompression included craniotomy with controlled decompression and DC with multiple dural stabs (MDS). Four RCTs and eight NRSs were included in the review (see Table 1), which did not utilize a MA to combine data. Thus, the reported results and conclusions were derived from findings from the individual studies, rather than from pooled quantitative data. Among the RCTs, no significant benefits were found in mortality or neurological outcomes between the DC group and the medical management group, or between the DC group and the controlled decompressive craniectomy group. One study (28) found significantly lower mortality and higher function at discharge for patients who received MDS compared to DC. Of the two trials that reported on ICP, one (29) showed no benefit of DC and one (14) showed significant reduction in ICP with DC.
The AMSTAR 2 rating for this review is low confidence. There were violations in 2 of the 7 critical domains (see Table 2).
Zhang et al. (20) conducted a SR and MA to compare DC to medical management, and reported on mortality, neurological outcomes measured by the GOS, length of stay in the intensive care unit (ICU), length of stay in hospital, and complications. Of the ten included studies, four were RCTs and six were NRSs; the RCTs were included in the MA (see Table 1). For mortality, patients in the DC group had significantly lower risk of death compared to patients who received only medical management [Risk Ratio (RR) 0.59, 95% CI 0.47–0.74, Z = 4.60, p < 0.001]. Subgroup analysis showed a significant benefit for mortality with the early DC group (p < 0.001) but no difference for late DC (p = 0.89). For neurological outcomes, no significant difference was found between groups on the GOS or GOS-E (Extended GOS) at 6 months follow-up (RR 0.85, 95% CI 0.61–1.18, Z = 0.97, p = 0.33). However, the subgroup analysis of early DC showed a significant benefit in neurological outcome compared to late DC (RR 0.74, 95% CI 0.56–0.99, Z = 2.02, p = 0.04). Compared to medical management, DC significantly reduced ICP [mean difference (MD) −2.12 mmHg, 95% CI −2.81 to −1.43, Z = 6.03, p < 0.001]; significantly reduced length of ICU stay (MD −4.63 days, 95% CI −6.62 to −2.65, Z = 4.57, p < 0.001); and significantly reduced length of stay in hospital (MD −14.39 days, 95% CI −26.00 to −2.78, Z = 2.43, p = 0.02). The DC group sustained significantly more complications than the medical management group (RR 1.94, 95% CI 1.31–2.87, Z = 3.33, p = 0.0009). In sum, the DC group had significantly lower mortality, ICP, and length of ICU and hospital stay than the medical management group, and had significantly more complications. There was no difference between groups in neurological outcomes.
The AMSTAR 2 rating for this review is low confidence. There was a violation of 1 of the 7 critical domains, and partial violations of 3 critical domains.
Sahuquillo and Dennis (21) limited their SR and MA to only RCTs comparing DC to medical management. They reported on mortality and neurological outcomes measured by the GOS-E. Three trials were included in the review. Pooled results indicated significantly lower mortality for the DC group compared to the medical management group (RR 0.61, 95% CI 0.48–0.78, I2 = 38%). There was no significant difference between groups in neurological outcome measured at 6 months follow-up (RR 1.08, 95% CI 0.93–1.20, I2 = 78%). Authors reported DC was superior to medical management in reducing ICP, but did not provide quantitative data. To summarize, this review found that DC reduces the risk of mortality compared to medical management, reduces ICP, and does not reduce the risk of unfavorable neurological outcomes.
The AMSTAR 2 rating for this review is low confidence. There was violation of 2 of the 7 critical domains, and partial violation of 2 of the critical domains.
Fatima et al. (22) conducted a SR and MA to compare outcomes from early DC with those from medical management with or without (±) late DC. They reported on mortality and neurological outcomes measured by the GOS. Of seven included reviews, five were RCTs and two were NRSs (see Table 1). All studies were included in the MA. There was significantly lower mortality for the early DC group compared to the medical management ± late DC group (RR 0.62, 95% CI 0.40–0.94, p = 0.03). There was no difference between groups for neurological outcomes (OR 1.00, 95% CI 0.75–1.34, p = 0.99). A subgroup analysis indicated a significant reduction in mortality for the early DC group compared to the late DC group (RR 0.43, 95% CI 0.26–0.71, p = 0.0009), but no difference in neurological outcomes (OR 1.30, 95% CI 0.75–2.27, p = 0.35). In sum, when early DC is compared to medical management ± late DC, there is a significantly lower risk of mortality with early DC but no difference in neurological outcomes; the findings are the same in subgroup analysis that compares early DC to late DC.
The AMSTAR 2 rating for this review is critically low. They sustained violations in 4 of the 7 critical domains and a partial violation in 1 of the critical domains.
For mortality, three reviews found a positive effect of DC compared to medical management and two found no significant difference between groups. The four reviews that measured neurological outcome found no benefit of DC. The two reviews that assessed ICP both found DC to be beneficial in reducing ICP. DC demonstrated a significant reduction in ICU length of stay in the one study that measured it, and a significant reduction in hospital length of stay in the two studies that measured it.
Subgroup analyses showed the following: early DC reduced mortality compared to late DC, but did not improve neurological outcomes in one study; in another study, DC was associated with significantly more complications; in a third study that assessed alternative means of decompression, dural stabs improved mortality and neurological function compared to open dural flap.
The scoring system for the AMSTAR 2 instrument is in Table 3. As stated earlier, there are 16 domains that constitute the instrument, with 7 designated as “critical domains.” The shaded columns in Table 2 are the critical domains for the instrument.
According to the AMSTAR 2 criteria, the five reviews ranged in levels of confidence from low to critically low. The most common violations were in critical domain #2, “Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol?” and in critical domain #7, “Did the review authors provide a list of excluded studies and justify the exclusions?” None of the reviews adhered completely to these criteria. Other violations include inadequate investigation of publication bias (domain 15) and insufficient technique for assessing risk of bias (domain 9). In light of the AMSTAR 2 scores for these reviews, confidence in the reported findings is low.
As stated in the Introduction, the purpose of this project was to summarize the findings from SRs and MAs about the effectiveness of DC to improve outcomes for patients with severe TBI, and to consider those findings in the context of their AMSTAR 2 scores. In general, the reviews report that DC can decrease mortality, reduce ICP, and minimize days in the ICU and hospital, but does not serve to improve neurological function. However, based solely on the AMSTAR 2 criteria, we report a low level of confidence in these findings. They are in part, however, consistent with findings from Class 1 RCTs (14, 41). These RCTs, as well as other literature about DC, have been the focus of intense and ongoing critical conversation (39, 45, 46), and have inspired the gathering of a formidable group of clinical experts who generated a consensus statement about DC (39).
DC is a complex and multi-faceted intervention. A key flaw in DC studies and reviews has been a lack of sufficient attention to this complexity in the conduct the studies and the analyses. Cranial decompression is a procedure with several technical variations (primary vs. secondary, early vs. delayed, bifrontal vs. unilateral). Furthermore, timing of the DC is a source of heterogeneity within and across studies. The SRs and MAs mixed these variations in the DC intervention in pooled analyses.
The findings for the effect of DC on mortality from the five SRs/MAs included in this review were mixed; three found a positive effect and two found no difference between groups. However, all four SRs/MAs that measured neurological outcomes concluded no benefit from DC. To consider this finding, we focus on the factor of the timing of the DC procedure from the two Class 1 trials included in this review—DECRA (14) and RESCUEicp (41). Both trials aimed to treat patients with refractory elevated intracranial pressure. The median time from injury to surgery in the DECRA trial was 38.1 h [interquartile range (IQR) 27.1–55.0]. Timing for the RESCUEicp trial was reported as follows: time from injury to initial treatment: <12 h. N = 120, >12 h. N = 76; median time from initial treatment to randomization 44.3 h (IQR 16.8–80.9); median time from randomization to surgery 2.2 h IQR 1.3–5.1, mean 7.5 h (95% CI 5–9.9). Thus, the timing of the DC procedure in these trials ranged from hours to days, being technically studies of secondary DC.
Some neurosurgeons believe that DC is best performed as a last ditch procedure, as it is drastic and it has a high complication rate. However, in the setting of potentially intractable ICP, perhaps the delay in timing—meant to be a conservative approach—is at least in part a source of the observed poor outcomes. Are poor outcomes an inevitable result of delayed surgery, and overly conservative surgical approaches? To date, a trial of early DC with a pre-specified, controlled surgical approach has not been conducted. Such a trial could run the risk of over-aggressive use of DC. The next step might be a systematic review and report of the evidence for patient and injury characteristics that are indicators of the need for immediate surgery; then a trial randomizing this subset of patients to DC or medical management.
Timing is only one factor that varies across studies, and is used here as an example of the possible sources of study and SR/MA heterogeneity.
Systematic reviews and meta-analyses are important approaches for aggregating information from multiple studies but are susceptible to misinterpretation of the results due to methodological flaws. Clinicians rely on these methods for concise interpretation of scientific literature. Standards for assessing SRs and MAs have been established to support the quality of the reviews being produced. In the case of DC, more attention must be paid to quality standards, in the analysis of both individual studies and reviews. In the included reviews, the procedure was found to decrease mortality, reduce ICP, and minimize days in the ICU and hospital, but was not found to improve neurological function. However, according to the assessment of the reviews utilizing a validated instrument, these conclusions have a low level of confidence.
AR, NC, AK, and MA contributed equally to the conception, writing, and preparation of the manuscript.
This work was supported by the NIHR Global Health Research Group on Neurotrauma, which was commissioned by the National Institute for Health Research (NIHR) using UK aid from the UK Government Grant: RG89187 from NIHR and University of Cambridge (to AR and AK).
This research was commissioned by the National Institute of Health Research using Official Development Assistance (ODA) funding. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, National Institute for Health Research or the Department of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors acknowledge the support of the NIHR Global Health Research Group on Neurotrauma and the University of Cambridge in the preparation and publication of the article.
1. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2018) 130:1039–408. doi: 10.3171/2017.10.JNS17352
2. Al-Jishi A, Saluja RS, Al-Jehani H, Lamoureux J, Maleki M, Marcoux J. Primary or secondary decompressive craniectomy: different indication and outcome. Can J Neurol Sci. (2011) 38:612–20. doi: 10.1017/S0317167100012154
3. Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nat Rev Neurol. (2013) 9:405–15. doi: 10.1038/nrneurol.2013.106
4. Kolias AG, Adams H, Timofeev I, Czosnyka M, Corteen EA, Pickard JD, et al. Decompressive craniectomy following traumatic brain injury: developing the evidence base. Br J Neurosurg. (2016) 30:246–50. doi: 10.3109/02688697.2016.1159655
5. Qiu W, Guo C, Shen H, Chen K, Wen L, Huang H, et al. Effects of unilateral decompressive craniectomy on patients with unilateral acute post-traumatic brain swelling after severe traumatic brain injury. Crit Care. (2009) 13:R185. doi: 10.1186/cc8178
6. Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, De Oliveira MM, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. (2012) 117:589–96. doi: 10.3171/2012.6.JNS101400
7. García Vicente E, Garnelo Rey V, Manikon M, Ashworth S, Wilson MH. Does early decompressive craniectomy improve outcome? Experience from an Active UK Recruiter Centre. Case Rep Crit Care. (2013) 2013:714945. doi: 10.1155/2013/714945
8. Honeybul S, Ho KM, Lind CR. What can be learned from the DECRA study. World Neurosurg. (2013) 79:159–61. doi: 10.1016/j.wneu.2012.08.012
9. Alali AS, Burton K, Fowler RA, Naimark DM, Scales DC, Mainprize TG, et al. Economic evaluations in the diagnosis and management of traumatic brain injury: a systematic review and analysis of quality. Value Health. (2015) 18:721–34. doi: 10.1016/j.jval.2015.04.012
10. Nambiar M, Macisaac C, Grabinski R, Liew D, Kavar B. Outcomes of decompressive craniectomy in patients after traumatic brain injury. Crit Care Resusc. (2015) 17:67–72. Available online at: https://pdfs.semanticscholar.org/fca3/3c411e738da3b5192d04249cd32638db6dc1.pdf
11. Sinha S, Raheja A, Garg M, Moorthy S, Agrawal D, Gupta DK, et al. Decompressive craniectomy in traumatic brain injury: a single-center, multivariate analysis of 1,236 patients at a tertiary care hospital in India. Neurol India. (2015) 63:175–83. doi: 10.4103/0028-3886.156277
12. Gouello G, Hamel O, Asehnoune K, Bord E, Robert R, Buffenoir K. Study of the long-term results of decompressive craniectomy after severe traumatic brain injury based on a series of 60 consecutive cases. Sci World J. (2014) 2014:207585. doi: 10.1155/2014/207585
13. Nirula R, Millar D, Greene T, Mcfadden M, Shah L, Scalea TM, et al. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. J Trauma Acute Care Surg. (2014) 76:944–52; discussion 952–5. doi: 10.1097/TA.0000000000000194
14. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. (2011) 364:1493–502. doi: 10.1056/NEJMoa1102077
15. Ho KM, Honeybul S, Litton E. Delayed neurological recovery after decompressive craniectomy for severe nonpenetrating traumatic brain injury. Crit Care Med. (2011) 39:2495–500. doi: 10.1097/CCM.0b013e318225764e
16. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
17. Sahuquillo J. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database of Syst Rev. (2008). CD003983. doi: 10.1002/14651858.CD003983
18. Wang R, Li M, Gao WW, Guo Y, Chen J, Tian HL. Outcomes of early decompressive craniectomy versus conventional medical management after severe traumatic brain injury: a systematic review and meta-analysis. Medicine (Baltimore). (2015) 94:e1733. doi: 10.1097/MD.0000000000001733
19. Barthélemy EJ, Melis M, Gordon E, Ullman JS, Germano IM. Decompressive craniectomy for severe traumatic brain injury: a systematic review. World Neurosurg. (2016) 88:411–20. doi: 10.1016/j.wneu.2015.12.044
20. Zhang D, Xue Q, Chen J, Dong Y, Hou L, Jiang Y, Wang J. Decompressive craniectomy in the management of intracranial hypertension after trumatic brain injury: a systematic review and meta-analysis. Nature. (2017) 7:8800. doi: 10.1038/s41598-017-08959-y
21. Sahuquillo J, Dennis J. Outcome following decompressive craniectomy for managing high intracranial pressure in traumatic brain injury patients: a systematic review. In: Aarabi B, Simard JM, editors. Decompressive Craniectomy. Hauppauge, NY: Nova Science Publishers, Inc. (2018). p. 237–64.
22. Fatima N, Al Rumaihi G, Shuaib A, Saqqur M. The role of decompressive craniectomy in traumatic brain injury: A systematic review and meta-analysis. Asian J Neurosurg. (2019) 14:371–81. doi: 10.4103/ajns.AJNS_289_18
23. Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. (2001) 17:154–62. doi: 10.1007/s003810000410
24. Josan VA, Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv Syst. (2006) 22:1268–74. doi: 10.1007/s00381-006-0064-0
25. Soustiel JF, Sviri GE, Mahamid E, Shik V, Abeshaus S, Zaaroor M. Cerebral blood flow and metabolism following decompressive craniectomy for control of increased intracranial pressure. Neurosurgery. (2010) 67:65–72; discussion 72. doi: 10.1227/01.NEU.0000370604.30037.F5
26. Rubiano AM, Villarreal W, Hakim EJ, Aristizabal J, Hakim F, Dìez JC, et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulus Travma Acil Cerrahi Derg. (2009) 15:28–38. Available online at: http://tjtes.org/eng/jvi.aspx?un=UTD-33154
27. Olivecrona M, Rodling-Wahlstrom M, Naredi S, et al. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. (2007) 24:927–35. doi: 10.1089/neu.2005.356E
28. Bhat AR, Kirmani AR, Wani MA. Decompressive craniectomy with multi-dural stabs - A combined (SKIMS) technique to evacuate acute subdural hematoma with underlying severe traumatic brain edema. Asian J Neurosurg. (2013) 8:15–20. doi: 10.4103/1793-5482.110275
29. Wang Y, Wang C, Yang L, Cai S, Cai X, Dong J, et al. Controlled decompression for the treatment of severe head injury: a preliminary study. Turk Neurosurg. (2014) 24:214–20. doi: 10.5137/1019-5149.JTN.8135-13.1
30. Moein H, Sanati MA, Abbasi FS, Moein P, Hasheminasab SM. Outcome of decompressive craniectomy in patients with severe head injury: a pilot randomized clinical trial. Neurosurg Q. (2012) 22:149–52. doi: 10.1097/WNQ.0b013e318240f1e0
31. Galal A. Outcome after decompressive craniectomy in severe head injured patients with acute subdural hematoma. Egypt J Neurol Psychiatry Neurosurg. (2013) 50:293–9. Available online at: https://www.researchgate.net/publication/282916752_Outcome_after_decompressive_craniectomy_in_severe_head_injured_patients_with_acute_subdural_hematoma
32. Schulz CL, Mauer U. [Postoperative course after acute traumatic subdural hematoma in the elderly. Does the extent of craniotomy influence outcome,? Z Gerontol Geriatr. (2011) 44:177–80. doi: 10.1007/s00391-011-0168-3
33. Agrawal D, Joshua SP, Gupta D, Sinha S, Satyarthee GD. Can Glasgow score at discharge represent final outcome in severe head injury,? J Emerg Trauma Shock. (2012) 5:217–9. doi: 10.4103/0974-2700.99685
34. Gong JB, Wen L, Zhan RY, Zhou HJ, Wang F, Li G, et al. Early decompressing craniectomy in patients with traumatic brain injury and cerebral edema. Asian Biomed. (2014) 8:53–9. doi: 10.5372/1905-7415.0801.261
35. Chen S-H, Chen Y, Fang W-K, Huang D-W, Huang K-C, Tseng S-H. Comparison of craniotomy and decompressive craniectomy in severely head-injured patients with acute subdural hematoma. J Trauma. (2011) 71:1632–6. doi: 10.1097/TA.0b013e3182367b3c
36. Girotto D, Ledi CD, Bajek G, Jerkovi CR, Dragicevi CS. Efficancy of decompressive craniectomy in treatment of severe brain injury at the Rijeka University Hospital Centre. Coll Antropol. (2011) 35:255–8.
37. Goksu E, Ucar T, Akyuz M, Yilmaz M, Kazan S. Effects of decompressive surgery in patients with severe traumatic brain injury and bilateral nonreactive dilated pupils. Ulus Travma ve Acil Cerrahi Derg. (2012) 18:231–8. doi: 10.5505/tjtes.2012.79059
38. Limpastan K, Norasetthada T, Watcharasaksilp W, Vaniyapong T. Factors influencing the outcome of decompressive craniectomy used in the treatment of severe traumatic brain injury. J Med Assoc Thai. (2013) 96:678–82.
39. Hutchinson PJ, Kolias AG, Tajsic T, Adeleye A, Aklilu AT, Apriawan T, et al. Consensus statement from the International Consensus Meeting on the role of decompressive craniectomy in the management of traumatic brain injury. Acta Neurochir. (2019) 161:1261–74. doi: 10.1007/s00701-019-03936-y
40. Thomale UW, Graetz D, Vajkoczy P, Sarrafzadeh AS. Severe traumatic brain injury in children–a single center experience regarding therapy and long-term outcome. Childs Nerv Syst. (2010) 26:1563–73. doi: 10.1007/s00381-010-1103-4
41. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. (2016) 375:1119–30. doi: 10.1056/NEJMoa1605215
42. Wettervik TS, Lenell S, Nyholm L, Howells T, Lewén A, Enblad P, et al. Decompressive craniectomy in traumatic brain injury: usage and clinical outcome in a single centre. Acta Neurochir (Wien). (2018) 160:229–37. doi: 10.1007/s00701-017-3418-3
43. Mendelow AD, Gregson BA, Rowan EN, Francis R, Mccoll E, Mcnamee P, et al. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH[Trauma]): The first randomized trial. J Neurotrauma. (2015) 32:1312–23. doi: 10.1089/neu.2014.3644
44. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. (1999) 90:187–96. doi: 10.3171/jns.1999.90.2.0187
45. Shutter L, Timmons S. Intracranial pressure rescued by decompressive surgery after traumatic brain injury. N Engl J Med. (2016) 375:1183–84. doi: 10.1056/NEJMe1609722
Keywords: brain injury, head trauma, decompressive craniectomy, ICP, TBI
Citation: Rubiano AM, Carney N, Khan AA and Ammirati M (2019) The Role of Decompressive Craniectomy in the Context of Severe Traumatic Brain Injury: Summary of Results and Analysis of the Confidence Level of Conclusions From Systematic Reviews and Meta-Analyses. Front. Neurol. 10:1063. doi: 10.3389/fneur.2019.01063
Received: 07 September 2018; Accepted: 20 September 2019;
Published: 10 October 2019.
Edited by:
Kwok Ming Ho, Royal Perth Hospital, AustraliaReviewed by:
Rita Formisano, Santa Lucia Foundation (IRCCS), ItalyCopyright © 2019 Rubiano, Carney, Khan and Ammirati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrés M. Rubiano, YW5kcmVzcnViaWFub0Bhb2wuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.